Abstract
The objective of this study was to identify racial differences in willingness to participate in a population with previous exposure to clinical research. A survey instrument was administered to community-dwelling whites and African Americans who were voluntarily receiving a lay research and health education newsletter from a local Boston geriatric clinical research institution. The survey instrument assessed willingness to participate in 3 hypothetical clinical trials (diet trial for obesity, medication trial for hypertension [HTN], chemotherapy trial for cancer). Surveys were received from 473 whites and 279 African Americans (53% response rate) with mean age 74 (SD ± 9). In multivariate models, race was not significantly related to willingness to participate in the multivariate models for any of the 3 trials. Previous trial participation was related to a higher odds of willingness to participate in the diet trial only (OR 1.8, 95% CI 1.2,2.6). Lower levels of trust in one’s primary care physician were associated with a lower odds of willingness to participate in clinical trials for the diet and HTN trials (OR 0.5, 95% CI 0.3,0.8 and OR 0.6, 95% CI 0.3,0.9, respectively). These findings suggest that, within populations previously exposed to clinical research, African Americans are no less willing to participate in clinical trials compared to whites.
Keywords: Minorities, Clinical Trials, Patient Selection
Despite National Institutes of Health (NIH) mandates in 1993 for the inclusion of racial and ethnic minorities in clinical trials, African Americans and other minority groups continue to be underrepresented in clinical trials. Minority under-representation raises concerns that trial results will lack generalizability to these populations (Freedman et al., 1995; NIH, 2001). Previous studies have suggested that lower enrollment in trials among African Americans may be due to a lower willingness to participate (Baquet, Commiskey, Daniel Mullins, & Mishra, 2006; Braunstein, Sherber, Schulman, Ding, & Powe, 2008; Brown & Topcu, 2003; Buchbinder et al., 2004; V. L. Shavers, Lynch, & Burmeister, 2002; Stone, Mauch, Steger, Janas, & Craven, 1997; Unson et al., 2004).
Racial differences in personal preferences for participation may be attributable to multiple factors including socioeconomic status, logistical barriers and concerns about a lack of personal benefit (Comis, Miller, Aldige, Krebs, & Stoval, 2003; Roberson, 1994; Ross et al., 1999; Swanson & Ward, 1995). However, distrust in clinical research is the most commonly cited contributor to a lower willingness to participate among African Americans (Braunstein et al., 2008; Buchbinder et al., 2004; el-Sadr & Capps, 1992; Yancey, Ortega, & Kumanyika, 2006). In a study of African Americans and whites receiving care at 13 different primary care and cardiology clinics, investigators found that racial differences in willingness to participate were most attributable to higher levels of distrust in clinical research among African Americans, even when taking into account other factors such as socioeconomic status and access to care (Braunstein et al., 2008). This distrust in clinical research among African Americans is thought to stem from the legacy of the U.S. Public Health Service Tuskegee Syphilis Study and other past research abuses (Corbie-Smith, Thomas, Williams, & Moody-Ayers, 1999; Smith et al., 2007). Potential study participants are often referred through the health care system because of easy access and a presumption of some familiarity with the health care enterprise (UyBico, Pavel, & Gross, 2007). Yet, racial differences in distrust and willingness to participate can be observed even in those populations that are actively engaged in the health care system (Braunstein et al., 2008; Creel et al., 2005; Stone et al., 1997).
Less is known about racial differences in willingness to participate among persons who have been exposed to clinical research in some manner. Some studies have shown that those persons knowledgeable about research may be more likely to participate in clinical trials (Baquet et al., 2006; el-Sadr & Capps, 1992). To take advantage of previous exposure to clinical research among African Americans’, some observers have advocated using registries of active or potential trial participants to enhance minority recruitment (Easterbrook, 1992; Rogers, 2002; Royal et al., 2000; Wilson et al., 2006). It is unclear, however, whether racial differences in willingness to participate in clinical research trials persist in those populations that are already engaged with the clinical research enterprise.
To address this question, the primary objective of this study was to examine racial differences in willingness to participate among community-dwelling seniors who have been recruited to be on the mailing list for a research newsletter published by a local clinical research institution. Another objective was to identify the relative impact of other factors, including health status, attitudes toward research participation, and trust in clinical research, on preferences for trial participation in this population.
METHODS
Survey Sample and Procedures
Study participants were recruited from a group of community-dwelling adults aged 50 and over in the Boston metropolitan area who were participating in the Harvard Cooperative Program on Aging (HCPOA). The program is jointly sponsored by the Harvard Older Americans Independence Center and the Massachusetts Alzheimer’s Disease Research Center. The HPCOA provides education to the lay community on health and aging issues and each program participant receives a research newsletter published by a local geriatric clinical and research institution. The quarterly newsletter contains information on healthy aging and lay reports on research pertinent to the elderly. Participants were recruited to be on the list through community outreach efforts including community presentations, fliers, and word-of-mouth referrals. A subset of this population also agreed to be on a registry for consideration for future clinical trials.
From 3,314 participants in the mailing list, all the African Americans (n= 798) and an equal random sample of whites (n=793) were recruited for participation in the survey. The survey instrument was first tested using cognitive interviews with 5 white and 5 African American adults over the age of 50, and the instrument was modified based on feedback from these interviews. Each potential respondent was mailed a cover letter explaining the study and the survey instrument. A reminder postcard offering the option to complete the survey by telephone was sent to all non-respondents after 3 weeks. A final mailing, including another cover letter and a survey, was sent to all the remaining non-responders after another 3 weeks. Completed surveys were returned in a stamped, pre-addressed envelope provided with the survey.
Survey instrument
A mail survey instrument was used assessing respondents’ willingness to participate in clinical trials as well as respondents’ characteristics in the following domains: 1) sociodemographic characteristics; 2) trust; 3) access to care; 4) health / functional status; 5) previous exposure to clinical research; 6) incentives and beliefs related to research participation; and 7) history of perceived discrimination and / or awareness of the Tuskegee Syphilis Study. The survey instrument assessed willingness to participate in three hypothetical clinical trials (diet for weight loss, treatment of hypertension, and treatment of cancer) on a scale from 1 (“Not willing”) to 5 (“Very willing”). The three vignettes are presented in Table 1.
Table 1.
Clinical trial vignettes describing 3 hypothetical trials and response scale for assessment of primary outcome.
| Diet Vignette | ||||
| Suppose you are overweight. You are asked to volunteer for a study looking at how well a new diet may help people lose weight. Half of the people who join the study will be placed on the new diet. The other half will be placed on a more standard diet for weight loss (for example Weight Watchers). The two groups will then be compared to one another. Your participation should not inconvenience you or increase the costs of your meals at home. | ||||
| Hypertension Vignette | ||||
| Suppose that you are newly diagnosed with high blood pressure. You are asked to volunteer for a study looking at how well a new pill treats high blood pressure. Half of the people who join the study will take the new pill. The other half will take a commonly used pill. The two groups will then be compared to one another. You won’t know which type of pill you received until the trial is over. The new pill is not expected to cause side effects. | ||||
| Cancer Vignette | ||||
| Suppose you have been diagnosed with a life-threatening cancer. You are asked to volunteer for a study looking at a new treatment for cancer. Half of the people who join the study will take the new treatment while the other half will receive usual care. The two groups will then be compared to one another. You won’t know which treatment you received until the trial is over. Both types of treatment might cause side effects such as nausea. | ||||
| How Willing would you be to participate in this clinical research trial? | ||||
| Response Scale a | ||||
| Not willing | Somewhat willing | Very willing | ||
| 1 | 2 | 3 | 4 | 5 |
Responses dichotomized into categories of “willing to participate” (responses 4 or 5) and “unwilling to participate” (responses 1, 2, or 3)
Trust was assessed using two validated scales. The first, the 7-item Corbie-Smith Distrust in Clinical Research Index, examined respondents’ perceptions of potential abuses in clinical research (Corbie-Smith, Thomas, & St. George, 2002). The second, an 8-item trust subscale taken from Primary Care Assessment Survey (PCAS), measured trust in one’s primary care physician (PCP) (Safran et al., 1998). This survey administration yielded comparable Cronbach’s alphas of 0.6 and 0.8 for the Corbie-Smith Distrust in Clinical Research Index and PCAS trust subscale, respectively, relative to previously observed values of 0.7 and 0.9, respectively [G. Corbie-Smith, Personal communication, 8 / 31 / 09] (Safran et al., 1998). To characterize each respondent’s access to care, the survey inquired about insurance status and whether the respondent had a single physician or nurse primarily responsible for his / her care. Respondents were also asked how many outpatient visits they had with that same provider in the previous 12 months. Respondents’ health and functional status was assessed by asking about chronic medical conditions, the number of hospitalizations in the previous 12 months, the number of prescription medications taken daily, and the ability to complete, independently, basic activities of daily living (ADLs).
Exposure to clinical research was assessed by asking respondents whether they, a loved one, or a friend had previously participated in a clinical trial. Respondents were also asked about their awareness of the Tuskegee Syphilis Study as well as their own personal history of general perceived discrimination or perceived discrimination in health care. Respondents rated the potential importance, on a scale from 1 (not important) to 5 (extremely important), of incentives (e.g. monetary compensation or free transportation to trial sites) and beliefs about clinical research in the decision to participate in a clinical trial. The incentives and beliefs related to research participation were selected based on a review of previous literature (Corbie-Smith, Moody-Ayers, & Thrasher, 2004; Corbie-Smith et al., 2003; Fouad et al, 2000; V. L. Shavers et al., 2002). Responses were collapsed into two categories of “more important” (responses of 4 or 5) and “less important” (responses of 1, 2, or 3). Finally, sociodemographic information including age, gender, race / ethnicity, and education level was also collected.
Analyses
Each vignette was analyzed independently. Responses on the 5-point scale were dichotomized into categories of “willing to participate” (response of 4 or 5) and “not willing to participate” (responses of 1, 2, or 3). Based on their responses to the distrust in clinical research index, participants were categorized as either “distrustful” (≥ 5 of 7 responses endorsing a negative perception of clinical research) or “not distrustful” (< 5 of 7 negative responses) (Corbie-Smith et al., 2002). The PCAS trust subscale was scored on a continuous scale from 0–100 (0=lowest trust, 100=highest trust) (Dana Gelb Safran, 1998). Scored responses were categorized into quartiles. To test the sensitivity of our results to these categorizations, the analyses were repeated with an alternate definition of willingness to participate (e.g. responses of 3 or higher on willingness scale) and distrust in clinical research (≥ 4 of 7 negative responses) with no substantive differences in our findings.
Bivariable analyses were performed using chi-square or t-tests as appropriate to identify relationships between willingness to participate in each of the three trials and the independent variables. There were few differences in the bivariable analyses by race, so the results are presented for the entire study population. Using those covariates associated (p≤ 0.05) with willingness to participate in the bivariable analyses, separate multivariate logistic regression models for each vignette were created to determine the independent relationship between race and the outcome for each vignette while controlling for potential confounders.
For each vignette, a base model was created that included race alone. The candidate variables from each domain of the survey were then added sequentially to explore for mediating effects. The final model included race as well as all of the candidate variables from each domain. There were no significant differences observed for any of the coefficients across these models so only data from the final full models for each vignette are presented. There was little evidence for race as an effect modifier in the relationships between other covariates (e.g. education level, age, awareness of the Tuskegee Syphilis Study, and history of target disease) and willingness to participate, so the results for multivariable analyses are presented for the entire study population without interaction terms included. Data were analyzed using SAS version 9.1 (SAS, 2004).
RESULTS
Respondents’ Characteristics and Beliefs
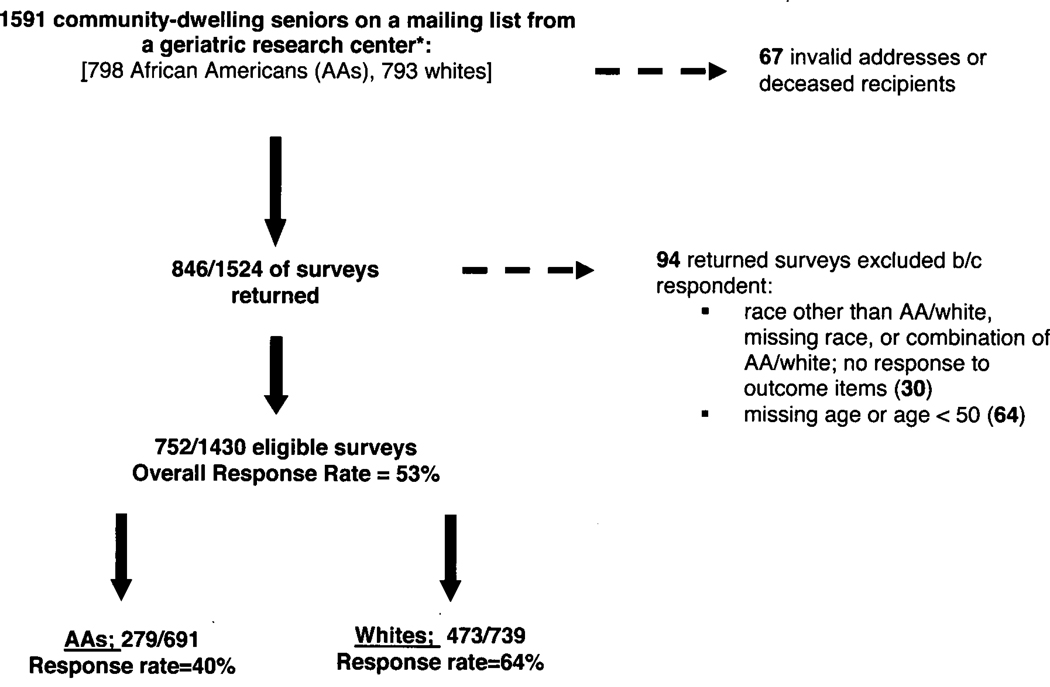
Of the 1591 surveys mailed, 846 completed surveys were received. Sixty-seven were returned either because of an invalid address or because the intended recipient was deceased (Figure). Thirty additional surveys were excluded because the respondent either reported a race other than black or white, reported a combination of black and white race, or did not respond to any of the items assessing willingness to participate. Finally, 64 surveys were excluded because the respondent reported an age < 50 or no age at all. Following these exclusions, our final response rate was 53% (752 / 1430). The response rate differed by race with 64% (473 / 739) of whites responding compared to 40% (279 / 691) of African Americans.
Figure 1.
Sampling Scheme and Response Rates
* All African Americans sampled and partial random sample of whites from original group (N=1591)
The population was elderly (mean age 73.8, S.D. 9.3 years) and well-educated (48% [359 / 752] with college degree or better) and had good access to health care (2% [15 / 752] without health insurance or public assistance). Compared to whites, African American respondents were younger (mean age 70 vs. 76, p<0.0001) and less well-educated (35% [95 / 279] vs. 57% [264 / 473] with college degree or better, p< 0.0001) (Table 2). Meanwhile, African Americans were almost as likely to report having previously participated in a clinical trial (50% [139 / 279] of African American respondents vs. 55% [261 / 473] of white respondents, p=0.4). There were no significant racial differences in the level of trust in one’s PCP (mean PCAS trust subscale score 78 vs. 77 among African Americans and whites, respectively, p=0.3) or differences in the proportions of African Americans and whites expressing distrust in clinical research (3% among both African Americans [9 / 279] and whites [14 / 473], p=0.8). African Americans were more likely than whites to believe that monetary incentives (74% [193 / 260] vs. 63% [291 / 460], p=0.003), free transportation to trial sites (71% [188 / 263] vs. 64% [291 / 454], p=0.04), and racial concordance with members of the research team (36% [94 / 260] vs. 13% [57 / 454], p<0.0001) were important when making decisions about trial participation. Conversely, African American and white respondents’ were equally likely to identify equal sharing of risks (89% [236 / 265] vs. 88% [397 / 450], p=0.7) and benefits (92% [239 / 260] vs. 93% [423 / 457], p=0.8) among different races in trial participation as important considerations when deciding whether or not to participate.
Table 2.
Description of study population
| Characteristics | Black n=279 n (%) or mean ± SD |
White n=473 n (%) or mean ± SD |
p-value |
|---|---|---|---|
| Sociodemographics | |||
| Female gender | 225 (81) | 313 (66) | <0.0001 |
| Age (years) | 70.0 ± 9.0 | 76.0 ± 8.8 | <0.0001 |
| Education - College graduate or higher | 95 (35) | 264 (57) | <0.0001 |
| Retired | 202 (76) | 374 (82) | 0.05 |
| Health/Functional status | |||
| Excellent, very good, or good health | 200 (74) | 386 (82) | 0.005 |
| Medical problems: | |||
| Overweight/obesity | 111 (40) | 87 (18) | <0.0001 |
| Hypertension | 203 (73) | 253 (53) | <0.0001 |
| Cancer | 36 (13) | 88 (19) | 0.04 |
| Number of daily medications | 4.1 ± 3.6 | 3.4 ± 2.8 | 0.0003 |
| Requires help with at least one activity of daily living | 24 (9) | 41 (9) | 0.98 |
| Access to care | |||
| Insurance type | <0.0001 | ||
| Medicare with or without other coverage | 151 (54) | 351 (74) | |
| Private insurance alone | 68 (24) | 86 (18) | |
| Medicaid/MassHealth/Free care alone | 35 (13) | 12 (3) | |
| Other | 18 (6) | 16 (3) | |
| No insurance | 7 (3) | 8 (2) | |
| Personal Doctor or Nurse | 252 (94) | 441 (95) | 0.6 |
| Number of clinic visits in the past year | <0.0001 | ||
| 0 | 32 (11) | 56 (12) | |
| 1–3 | 168 (60) | 348 (74) | |
| > 5 | 79 (28) | 69 (15) | |
| History of perceived discrimination | |||
| Discrimination in health care | 120 (43) | 163 (58) | <0.0001 |
| General discrimination | 72 (15) | 159 (34) | <0.0001 |
| Aware of Tuskegee Syphilis Study | 182 (65) | 194 (41) | <0.0001 |
| Prior participation in clinical trials | |||
| Personal participation | 139 (50) | 261 (55) | 0.4 |
| Family/friend participation, but no personal participation | 37 (13) | 55 (12) | |
| Neither personal nor family/friend prior participation | 103 (37) | 157 (33) | |
| Trust | |||
| Trust in Physicians a | 0.3 | ||
| 1st quartile (Most trust) | 60 (22) | 105 (22) | |
| 2nd quartile | 67 (24) | 107 (23) | |
| 3rd quartile | 84 (31) | 123 (26) | |
| 4th quartile (Least trust) | 63 (23) | 137 (29) | |
| Distrust in clinical researchb | 9 (3) | 14 (3) | 0.8 |
Trust in physicians measured by Primary Care Assessment Survey Trust Subscale, which is continuously scored on a scale from 0–100 with higher numbers representing higher levels of trust. We collapsed the continuous data into quartiles of “trust”.
Distrust in clinical research defined as ≥5 distrustful responses to 7-item Corbie-Smith Distrust Index
Race and Other Characteristics and Willingness to Participate in Clinical Trials
There were no significant differences in willingness to participate when comparing African Americans and whites for any of the three trials. Fifty-three percent (144 / 270) of African American respondents and 54% (246 / 459) of white respondents (p=0.9) were willing to participate in the diet trial whereas slightly smaller proportions of both groups were willing to participate in the hypertension and cancer trials (36% [99 / 272] vs. 37% [172 / 461], p=0.8 for HTN trial and 34% [94 / 273] vs. 41% [191 / 464], p=0.07 for cancer trial among African Americans and whites, respectively) (Table 4). These results were not sensitive to an alternate definition for willingness to participate (e.g., responses of 3 or higher on willingness scale). In addition, higher levels of trust in one’s PCP and previous personal trial participation were both associated with a higher likelihood of willingness to participate in all the three trials (Table 4).
Table 4.
Adjusted odds ratios of willingness to participate in diet, hypertension, and cancer trialsa
| Covariates | OR for willingness to participate | |||||
|---|---|---|---|---|---|---|
| Diet Trial | Hypertension Trial |
Cancer Trial |
||||
| OR | (95% Cl) | OR | (95% Cl) | OR | (95% Cl) | |
| Race | ||||||
| White (ref) | ||||||
| African American | 0.8 | 0.5,1.1 | 0.8 | 0.6,1.3 | 0.8 | 0.5,1.2 |
| Gender | ||||||
| F (ref) | ||||||
| M | 0.7 | 0.5, 1.0 | 1.8 | 1.3,2.6 | 1.9 | 1.3,2.8 |
| Age | ||||||
| < 65 (ref) | ||||||
| 65–80 | 0.8 | 0.5,1.5 | 0.7 | 0.4,1.2 | N/Ac | N/Ac |
| ≥ 80 | 0.5 | 0.3,0.98 | 0.5 | 0.2,0.9 | ||
| Self rating of health | ||||||
| Excellent/Very Good/Good (ref) | ||||||
| Fair/Poor | 0.9 | 0.6,1.4 | 0.8 | 0.5,1.2 | 0.6 | 0.4,1.0 |
| History of target diseaseb | ||||||
| N (ref) | ||||||
| Y | 1.6 | 1.03,2.3 | 1.1 | 0.8,1.5 | 1.1 | 0.7,1.8 |
| Prior participation in clinical trial | ||||||
| No prior participation (ref) | ||||||
| Personal participation | 1.8 | 1.2,2.6 | 1.4 | 1.0,2.1 | 1.3 | 0.8,2.3 |
| Family/friend participation | 0.9 | 0.5,1.6 | 0.6 | 0.3,1.1 | 1.3 | 0.7,2.4 |
| Awareness of Tuskegee Syphilis Study | ||||||
| N (ref) | ||||||
| Y | 1.6 | 1.1,2.3 | 1.1 | 0.7,1.5 | 1.6 | 1.1,2.3 |
| Trust in PCP | ||||||
| 1st quartile (More trust) (ref) | ||||||
| 2nd quartile | 0.9 | 0.5,1.4 | 1.1 | 0.7,1.8 | 0.7 | 0.4,1.2 |
| 3rd quartile | 0.6 | 0.4,1.0 | 0.9 | 0.5,1.4 | 0.7 | 0.4,1.2 |
| 4th quartile (Least trust) | 0.5 | 0.3,0.8 | 0.6 | 0.3,0.9 | 0.6 | 0.4,1.0 |
| Distrust in clinical research | ||||||
| N (ref) | ||||||
| Y | 2.1 | 0.7,6.1 | 1.0 | 0.3,2.7 | 0.6 | 0.2,1.9 |
| Receiving money as payment for your time | ||||||
| Less Important (ref) | ||||||
| More Important | 1.2 | 0.9,1.8 | 1.6 | 1.1,2.3 | 1.8 | 1.2,2.7 |
| Your belief that you will personally benefit from participating in the clinical research trial | ||||||
| Less Important (ref) | ||||||
| More Important | N/Ac | N/Ac | 1.3 | 0.8,2.1 | 1.7 | 1.04,2.8 |
| Your belief that someone else in the future will benefit from your participation in the clinical research trial | ||||||
| Less Important (ref) | ||||||
| More Important | 2.1 | 0.8,5.3 | 3.9 | 1.1,13.8 | 2.9 | 0.8,10.5 |
| Members of the research team are from the same race as you are | ||||||
| Less Important (ref) | ||||||
| More important | N/Ac | N/Ac | N/Ac | N/Ac | 0.5 | 0.3,0.8 |
Models, adjusted for sociodemographics, health/functional status, patient trust in PCP and clinical research, exposure to clinical research, attitudes about clinical research, and perceived discrimination and awareness of the Tuskegee Syphilis Study.
Target diseases are obesity, hypertension, and cancer for diet, hypertension, and cancer trials, respectively.
N/A denotes those variables that were not significantly related to the outcome (p >0.05) in bivariable analysis and thus were not included in the multivariable model.
Adjusted Models of Willingness to Participate in Clinical Trials
In multivariate logistic regression models, race still was not significantly associated with willingness to participate in any of the three trials (Table 4). Yet, other participant characteristics were associated with willingness to participate in multivariable models. Those respondents older than 80 had a lower odds of willingness to participate in the diet (OR 0.5, 95% CI 0.3,0.98) and hypertension trials (OR 0.5, 95% CI 0.2,0.9) compared to those < 65 years old. Prior personal participation in clinical trials was also associated with a lower odds of willingness to participate in the diet clinical trial only (OR 1.8, 95% CI 1.2,2.6) (Table 4). The lowest level of trust in one’s PCP was associated with a lower odds of willingness to participate in the diet (OR 0.5, 95% CI 0.3,0.8) and hypertension trials (OR 0.6, 95% CI 0.3,0.9), respectively (Table 4). Conversely, awareness of the Tuskegee Syphilis Study was associated with a 60% higher odds of willingness to participate in the both the diet and cancer trials. Sensitivity analyses using an alternate definition of “willingness to participate” (responses of 3 or higher or on willingness scale) revealed no significant differences from the main models for each type of trial.
Those who felt that monetary compensation for one’s time was a more important factor in making a decision to participate were significantly more likely to be willing to participate in both the hypertension (OR 1.6, 95% CI 1.1,2.3) and cancer (OR 1.8, 95% CI 1.2,2.7) trials (Table 4). An endorsement of the importance of incentives such as free transportation to study sites, after-hours or weekend times for research participation, or an invitation to participate from one’s PCP was not related to willingness to participate in the multivariable models for any of the three trials.
DISCUSSION
The study results demonstrate that African American respondents, already engaged with a research institution, were not less willing than whites to participate in 3 hypothetical trials. Other factors such as trust in one’s PCP and prior trial participation were each associated with willingness to participate among whites and African Americans in at least 2 of the 3 hypothetical trials. African Americans and whites did differ somewhat in both their awareness of the Tuskegee Syphilis Study and their beliefs about clinical trial participation. Yet, these differences did not contribute to significant racial differences in willingness to participate in this population.
The findings build upon the mixed results of previous studies assessing willingness to participate in clinical trials using hypothetical scenarios. While conducting a study in a clinic-based population using the same Corbie-Smith distrust index, Braunstein et al. showed that higher distrust in clinical research, among African Americans, was related to a lower willingness to participate in a hypothetical double-blinded, placebo-controlled cardiovascular disease drug prevention trial (Braunstein et al., 2008). Like the population in the present study, the respondents in the Braunstein analysis were generally trusting in medical research with over 70% (512 / 717) providing negative responses in 2 or fewer of the 7 distrust index items. In contrast, a review of over 20 studies, with over 70,000 participants from population-, clinic-, and registry-based samples, found no racial differences in willingness to participate in clinical trials (Wendler et al., 2006). The review, however, was based on a heterogeneous group of study populations, and it is difficult to distinguish how preferences for trial participation among different racial groups may vary based on the type of sample.
The present study differs from prior studies because there were no racial differences in willingness to participate while focusing solely on a study population already engaged by the clinical research enterprise. Furthermore, this study is unique because of the added examination of the impact of other factors, such as trust in physicians, awareness of the Tuskegee Syphilis Study, and beliefs about clinical research, on preferences for trial participation. This engaged study population was part of the HCPOA, a 15-year old program designed to inform lay people in racially diverse communities about healthy aging and research studies on aging. Therefore, the absence of racial differences in willingness to participate in this study population may be due to the longitudinal efforts of the HCPOA to inform its participants in local communities about clinical research. Previous studies support this notion that efforts, such as general education about clinical trials in African American communities, involvement of community members in trial planning, and the utilization of unique channels of communication (e.g. churches and civic groups), may be helpful in engaging potential minority participants before active trial recruitment even begins (Brown, Fouad, Basen-Engquist, & Tortolero-Luna, 2000; Fitzgibbon et al., 1998; Kennedy & Burnett, 2007; Lara et al., 2001; Lewis et al., 1998; Royal et al., 2000; Saunders, Greaney, Lees, & Clark, 2003; Stallings et al., 2000; Whelton et al., 1996; Wisdom, Neighbors, Williams, Havstad, & Tilley, 2002). Attempts at community engagement, focusing solely on trial recruitment without any input or cooperation from community members, may prove less successful (Corbie-Smith et al., 2004). Though African Americans in this study were more likely to deem incentives such as monetary compensation or free transportation as important when making a decision about trial participation their endorsements of the importance of these incentives were not associated with willingness to participate in the three different trials. This finding suggests that more downstream recruitment methods and incentives may be less effective if potential participants are not engaged earlier in the recruitment pathway (Corbie-Smith et al., 2004; Corbie-Smith et al., 2003). Therefore, community engagement efforts should not be trial-specific, but instead should be aimed at creating longitudinal, mutually beneficial research partnerships.
Trust in both physicians and clinical research was generally high among our engaged respondents. Yet, our findings demonstrate that trust in physicians was still an important factor related to willingness to participate among both African American and white respondents. There was no evidence, however, that African Americans had lower trust in their physicians. The significance of individuals’ relationships with their PCPs was buttressed by the finding that African Americans and whites were also equally likely to deem a personal invitation from one’s PCP as more important when making the decision about trial participation. Yet, those who thought that it was important to receive an invitation for trial participation directly from their PCPs were no more likely to be willing to participate in any of the 3 hypothetical trials. This dichotomy suggests that among African Americans, who have been exposed to clinical research and maintain relatively high levels of trust in their PCPs, willingness to participate may not be contingent on a direct PCP referral.
Previous studies of minority recruitment have suggested that the legacy of the Tuskegee Syphilis Study and other examples of racial discrimination have led to distrust of clinical research among African Americans (Corbie-Smith et al., 1999; Freimuth et al., 2001; V. Shavers, Lynch, & Burmeister, 2001). It has been theorized that this distrust may negatively influence African Americans’ personal preferences for trial participation (Corbie-Smith et al., 1999; Freimuth et al., 2001). Contrary to this belief, awareness of Tuskegee Syphilis Study among African Americans, as well as whites, in this study was actually positively associated with willingness to participate. In addition, perceived discrimination in health care was more common among African American respondents, but perceived discrimination was not associated with willingness to participate in any of the three trials among African Americans or whites. Awareness of the Tuskegee Study may be associated with some familiarity with clinical research that favors willingness to participate among African Americans and whites who are already engaged by the clinical research enterprise (V. L. Shavers et al., 2002). We assessed only awareness of the landmark study without examining individuals’ actual knowledge of the historical facts. Previous studies have shown that while African Americans may harbor generally negative perceptions of the Tuskegee Syphilis Study, their actual knowledge of the details of the study varies widely (Corbie-Smith et al., 1999; Freimuth et al., 2001; V. L. Shavers et al., 2002). Furthermore, it is possible that African Americans with awareness of the Tuskegee Syphilis Study, regardless of their knowledge of related facts, may actually have more confidence in clinical research because they are also aware of the human subjects protections that have been implemented in the 39 years following the halt of this research abuse. Likewise, any negative influence created by perceptions of discrimination may be overcome by trust in one’s physician or prior participation in a clinical trial. Therefore, investigators should pursue potential African American participants aggressively rather than assuming that the legacy of the Tuskegee Syphilis Study or a history of perceived discrimination makes this group overwhelmingly averse to trial participation.
While the response rate to this survey was reasonably high overall, there was a differential response rate among whites and African Americans. The lower response rate among African Americans compared to whites raises concerns that those African Americans who would be less willing to participate simply did not respond to the survey, resulting in bias. In combination with the main results of our study, the differential response rate might be informative with regards to policies for enhancing minority participation in clinical trials. The results suggest that the response to the survey may represent more incremental engagement in clinical research beyond mere membership on the mailing list. Among those engaged at the level of responding to our survey, African Americans were no less willing to participate compared to whites.
This study is subject to several additional limitations. A local population of middle age and elderly respondents in Boston were surveyed, and their perspectives on trial participation may not be representative of younger individuals or persons living in other cities or regions of the country. In addition, the cohort was relatively well-educated and a substantial portion of them had previous clinical trial experiences as well as access to care. While these traits may not be as prevalent in the general population, the cohort is likely more representative of most potential participants who are screened for trials through some encounter with the health care system or a clinical research institution. Finally, the study measured hypothesized willingness to participate, and these hypothetical choices may not reflect actual decisions for trial participation. However, these approaches are widely used in survey research based on the theory of reasoned action which states that a person’s behavior is determined by his / her intent to perform the behavior (Ajen & Fishbein, 1980; Brown & Topcu, 2003).
In this large survey of community-dwelling African Americans and whites already engaged by a clinical research institution, there were no racial differences in willingness to participate in 3 hypothetical clinical trials for obesity, hypertension, and cancer. In addition, although there were associations of factors, such as physician trust and age, with willingness to participate, these did not vary by race. These results suggest that racial disparities in trial enrollment may not be due to actual differences in preferences for participation among African Americans and whites with prior exposure to clinical research. In the future, investigators should seek to engage African Americans through longitudinal community partnerships to increase their exposure to clinical research and to create more opportunities for trial participation among minorities.
Table 3.
Willingness to participate in diet, hypertension, and cancer trials according to respondent characteristics
| Participant characteristics | Willing to participatea | |||||
|---|---|---|---|---|---|---|
| Diet Trial | Hypertension Trial | Cancer Trial | ||||
| n (%) | p-value | n (%) | p-value | n (%) | p-value | |
| Race | 0.9 | 0.8 | 0.07 | |||
| White | 246 (54) | 172 (37) | 191 (41) | |||
| African American | 144 (53) | 99 (36) | 94 (34) | |||
| Gender | 0.3 | 0.0008 | <0.0001 | |||
| M | 103 (50) | 96 (47) | 105 (50) | |||
| F | 285 (55) | 175 (33) | 180 (34) | |||
| Age | <0.0001 | 0.0005 | 0.3 | |||
| < 65 | 84 (69) | 59 (50) | 53 (43) | |||
| 65–80 | 224 (55) | 154 (38) | 159 (39) | |||
| > 80 | 82 (40) | 58 (28) | 73 (35) | |||
| Education | 0.002 | 0.3 | 0.02 | |||
| Some college or less | 180 (48) | 131 (35) | 130 (35) | |||
| College graduate or more | 208 (60) | 137 (39) | 152 (43) | |||
| Retired | <0.0001 | 0.002 | 0.001 | |||
| Y | 281 (50) | 189 (34) | 200 (36) | |||
| N | 100 (69) | 69 (48) | 74 (50) | |||
| Insurance type b | 0.2 | 0.1 | 0.7 | |||
| Medicare with or without other coverage | 246 (51) | 172 (35) | 191 (39) | |||
| Private insurance alone | 93 (62) | 59 (39) | 59 (39) | |||
| Medicaid or Massachusetts “Free Care” alone | 25 (53) | 24 (53) | 18 (39) | |||
| Other | 17 (53) | 13 (39) | 14 (41) | |||
| No insurance | 9 (60) | 3 (25) | 3 (20) | |||
| Self-rating of health | 0.03 | 0.03 | 0.0001 | |||
| Excellent/Very Good/Good | 318 (56) | 224 (39) | 244 (42) | |||
| Fair/Poor | 68 (46) | 44 (30) | 38 (25) | |||
| Has target disease | 0.001 | 0.7 | 0.6 | |||
| Y | 125 (63) | 166 (37) | 50 (41) | |||
| N | 265 (50) | 105 (36) | 235 (38) | |||
| History of perceived discrimination in health care | 0.5 | 0.2 | 0.3 | |||
| Y | 105 (56) | 63 (33) | 68 (36) | |||
| N | 285 (53) | 208 (38) | 217 (40) | |||
| History of perceived general discrimination | 0.2 | 0.4 | 0.2 | |||
| Y | 176 (56) | 122 (39) | 131 (41) | |||
| N | 214 (51) | 149 (36) | 154 (37) | |||
| Prior participation in clinical trial | <0.0001 | 0.0002 | <0.0001 | |||
| Personal prior participation | 246 (63) | 171 (43) | 182 (46) | |||
| Family/friend prior participation | 41 (47) | 21 (24) | 31 (35) | |||
| No personal or family/friend prior participation | 103 (41) | 79 (31) | 72 (28) | |||
| Aware of the Tuskegee Syphilis Study | 0.0002 | 0.07 | 0.0008 | |||
| Y | 222 (60) | 149 (40) | 166 (45) | |||
| N | 168 (47) | 122 (34) | 119 (33) | |||
| Trust in PCP | 0.02 | 0.006 | 0.02 | |||
| 1st quartile (More trust) | 98 (61) | 69 (43) | 79 (48) | |||
| 2nd quartile | 98 (58) | 74 (43) | 66 (39) | |||
| 3rd quartile | 102(51) | 72 (35) | 75 (37) | |||
| 4th quartile (Least trust) | 90 (46) | 54 (28) | 63 (32) | |||
| Distrust in clinical research | 0.8 | 0.3 | 0.09 | |||
| Y | 13 (57) | 6 (26) | 5 (22) | |||
| N | 377 (53) | 265 (37) | 280 (39) | |||
| Receiving money as payment for your timec | 0.008 | 0.008 | 0.02 | |||
| More Important | 273 (58) | 197 (42) | 201 (42) | |||
| Less Important | 107 (47) | 69 (30) | 76 (33) | |||
| Your belief that you will personally benefit from participating in the clinical research trial c | 0.1 | 0.04 | 0.03 | |||
| More Important | 317 (56) | 226 (40) | 234 (41) | |||
| Less Important | 65 (49) | 42 (31) | 43 (31) | |||
| Your belief that someone else in the future will benefit from your participation in the clinical research trial c | <0.0001 | 0.0001 | 0.002 | |||
| More Important | 374 (57) | 263 (40) | 271 (41) | |||
| Less Important | 9 (21) | 4 (10) | 7 (17) | |||
| Members of the research team are from the same race as you are c | 0.5 | 0.4 | 0.004 | |||
| More Important | 76 (52) | 51 (35) | 43 (29) | |||
| Less Important | 303 (55) | 216 (39) | 232 (42) | |||
Willingness to participate defined as response of 4 or 5 on response scale from 1(“not willing”) to 5 (“very willing”)
Massachusetts Free Care is a state-run program that covers medical costs for those poor persons who can not afford care and are not covered by Medicaid. The “Other” category includes Veterans Health Administration, TRICARE
Respondents were asked to rate the importance of certain incentives or beliefs (e.g. “receiving money as payment for your time”) when making a decision about trial participation.
Acknowledgements
This research was supported by grants from HRSA (#2 T32 HP11001-15), National Institute on Aging (#5 P01 AG04390), Older Americans Independence Center (#2 P60 AG08812) and Massachusetts Alzheimer’s Disease Research Center (#2 P50 AG05134)
The authors would like to thank Patience Ortiz and Rebbecca Wilson for their efforts in the data collection for this manuscript.
Contributor Information
Raegan W. Durant, Division of Preventive Medicine, University of Alabama at Birmingham, and Birmingham Veterans Affairs Medical Center, Birmingham, AL, USA (R.W.D)..
Anna T. Legedza, Vertex Pharmaceuticals, Cambridge, MA, USA (A.T.L.)..
Edward R. Marcantonio, Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, Boston, MA, USA (E.R.M.,)..
Marcie B. Freeman, Institute for Aging Research, Hebrew Senior Life, (M.B.F.); Boston, MA, USA..
Bruce E. Landon, Department of Health Care Policy, Harvard Medical School and Beth Israel Deaconess Medical Center, (B.E.L.), Boston, MA, USA..
REFERENCES
- Ajen I, Fishbein M. Understanding attitudes and predicting social behavior. Englewood Cliffs, NJ: Prentice Hall; 1980. [Google Scholar]
- Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural / urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, Medical Researcher Distrust, Perceived Harm, and Willingness to Participate in Cardiovascular Prevention Trials. Medicine. 2008;87(1):1–9. doi: 10.1097/MD.0b013e3181625d78. [DOI] [PubMed] [Google Scholar]
- Brown DR, Fouad M, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Annals of Epidemiology. 2000;10:S13–S21. doi: 10.1016/s1047-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- Brown DR, Topcu M. Willingness to Participate in Clinical Treatment Research Among Older African Americans and Whites. The Gerontologist. 2003;43(1):62–72. doi: 10.1093/geront/43.1.62. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Metch B, Holte SE, Scheer S, Coletti A, Vittinghoff E. Determinants of enrollment in a preventive HIV vaccine trial: hypothetical versus actual willingness and barriers to participation. Acquir Immune Defic Syndr. 2004;36(1):604–612. doi: 10.1097/00126334-200405010-00009. [DOI] [PubMed] [Google Scholar]
- Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public Attitudes Toward Participation in Cancer Clinical Trials. J Clin Oncol. 2003;21(5):830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Moody-Ayers S, Thrasher AD. Closing the Circle Between Minority Inclusion in Research and Health Disparities. Archives of Internal Medicine. 2004;164(13):1362–1364. doi: 10.1001/archinte.164.13.1362. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, St. George DM. Distrust, Race and Research. Archives of Internal Medicine. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smifh G, Viscoli CM, Kernan WN, Brass LM, Sarrel P, Horwitz RI. Influence of race, clinical, and other socio-demographic features on trial participation. Journal of Clinical Epidemiology. 2003;56(4):304–309. doi: 10.1016/s0895-4356(03)00046-5. [DOI] [PubMed] [Google Scholar]
- Creel AH, Losina E, Mandl LA, Marx RJ, Mahomed NN, Martin SD, et al. An assessment of willingness to participate in a randomized trial of arthroscopic knee surgery in patients with osteoarthritis. Contemp Clin Trials. 2005;26(2):169–178. doi: 10.1016/j.cct.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Safran Dana Gelb, Kosinski Mark MA, Tarlov Alvin R, Rogers William H, Taira Deborah A., Lieberman Naomi BA, Ware John E. The Primary Care Assessment Survey: Tests of Data Quality and Measurement Performance. Medical Care. 1998;36(5):728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Easterbrook PJ. Directory of registries of clinical trials. Stat Med. 1992;11(3):345–359. [PubMed] [Google Scholar]
- el-Sadr W, Capps L. The challenge of minority recruitment in clinical trials for AIDS. JAMA. 1992;267(7):954–957. [PubMed] [Google Scholar]
- Fitzgibbon ML, Prewitt TE, Blackman LR, Simon P, Luke A, Keys LC, et al. Quantitative Assessment of Recruitment Efforts for Prevention Trials in Two Diverse Black Populations. Preventive Medicine. 1998;27(6):838–845. doi: 10.1006/pmed.1998.0367. [DOI] [PubMed] [Google Scholar]
- Fouad MN, Partridge E, Green BL, Kohler C, Wynn T, Nagy S, et al. Minority Recruitment In Clinical Trials: A Conference at Tuskegee, Researchers and the Community. Annals of Epidemiology. 2000;10(8) Supplement 1:S35–S40. doi: 10.1016/s1047-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Freedman LS, Simon R, Foulkes M, Friedman L, Geller NL, Gordon DJ, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993 - The perspective of NIH clinical trialists. Controlled Clinical Trials. 1995;16:277–285. doi: 10.1016/0197-2456(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Freimuth VS, Quinn SC, Thomas SB, Cole G, Zook E, Duncan T. African Americans’ views on research and the Tuskegee Syphilis study. Social Science & Medicine. 2001;52(5):797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Burnett M. Clinical research trials: factors that influence and hinder participation. Journal of Cultural Diversity. 2007;14(3):141–147. [PubMed] [Google Scholar]
- Lara PN, Higdon R, Lim N, Kwan K, Tanaka M, Lau DHM, et al. Prospective Evaluation of Cancer Clinical Trial Accrual Patterns: Identifying Potential Barriers to Enrollment. J Clin Oncol. 2001;19(6):1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- Lewis CE, George V, Fouad M, Porter V, Bowen D, Urban N. Recruitment strategies in the Women’s Health Trial: Feasibility study in minority populations. Controlled Clinical Trials. 1998;19:461–476. doi: 10.1016/s0197-2456(98)00031-2. [DOI] [PubMed] [Google Scholar]
- NIH. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. [Retrieved 2 / 15 / 11];2001 from http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm.
- Roberson NL. Clinical trial participation. Viewpoints from racial / ethnic groups. Cancer. 1994;74(9 Suppl):2687–2691. doi: 10.1002/1097-0142(19941101)74:9+<2687::aid-cncr2820741817>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Rogers JL. Effectiveness of Media Strategies to Increase Enrollment and Diversity in the Women’s Health Registry. Am ] Public Health. 2002;92(4):613–614. doi: 10.2105/ajph.92.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to Participation in Randomised Controlled Trials: A Systematic Review. Journal of Clinical Epidemiology. 1999;52(12):1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- Royal C, Baffoe-Bonnie A, Kittles R, Powell I, Bennett J, Hoke G, et al. Recruitment Experience in the First Phase of the African American Hereditary Prostate Cancer (AAHPC) Study. Annals of Epidemiology. 2000;10(8) Supplement 1:S68–S77. doi: 10.1016/s1047-2797(00)00194-0. [DOI] [PubMed] [Google Scholar]
- Safran DC, Kosinski M, Tarlov AR, Rogers WH, Taira DA, Lieberman N, et al. The Primary Care Assessment Survey: Tests of Data Quality and Measurement Performance. Medical Care. 1998;36(5):728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- SAS. SAS/STAT Version 9.1. Cary, NC: SAS Institute Inc.; 2004. [Google Scholar]
- Saunders SD, Greaney ML, Lees FD, Clark PG. Achieving Recruitment Goals Through Community Partnerships The SENIOR Project. Family & Community Health. 2003;26(3):194–202. doi: 10.1097/00003727-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Shavers V, Lynch C, Burmeister L. Factors that influence African-Americans’ willingness to participate in medical research studies. Cancer. 2001;91(S1):233–236. doi: 10.1002/1097-0142(20010101)91:1+<233::aid-cncr10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology. 2002;12:248–256. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Johnson A, Newman L, Greene A, Johnson T, Rogers J. Perceptions of clinical research participation among African American women. Journal of Women’s Health (15409996) 2007;16(3):423–428. doi: 10.1089/jwh.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings FL, Ford ME, Simpson NK, Fouad M, Jernigan JC, Trauth JM, et al. Black participation in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Controlled Clinical Trials. 2000;21(6) Supplement 1:379S–389S. doi: 10.1016/s0197-2456(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Stone VE, Mauch MY, Steger K, Janas SF, Craven DE. Race, Gender, Drug Use, and Participation in AIDS Clinical Trials Lessons from a Municipal Hospital Cohort. Journal of General Internal Medicine. 1997;12(3):150–157. doi: 10.1007/s11606-006-5022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson G, Ward A. Recruiting minorities into clinical trials: toward a participant- friendly system. J Natl Cancer Inst. 1995;87(23):1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- Unson CG, Ohannessian C, Kenyon L, Case A, Reisine S, Prestwood K. Barriers to Eligibility and Enrollment Among Older Women in a Clinical Trial on Osteoporosis: Effects of Ethnicity and SES. Aging Health. 2004;16(3):426–443. doi: 10.1177/0898264304264211. [DOI] [PubMed] [Google Scholar]
- UyBico S, Pavel S, Gross C. Recruiting Vulnerable Populations into Research: A Systematic Review of Recruitment Interventions. Journal of General Internal Medicine. 2007;22(6):852–863. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Wye GV, Christ-Schmidt H, Pratt LA, et al. Are Racial and Ethnic Minorities Less Willing to Participate in Health Research? PLoS Medicine. 2006;3(2) doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Lee JY, Kusek JW, Charleston J, DeBruge J, Douglas M, et al. Recruitment experience in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Control Clin Trials. 1996;17(4 Suppl):17S–33S. doi: 10.1016/s0197-2456(96)00087-6. [DOI] [PubMed] [Google Scholar]
- Wilson J, Mick R, Wei S, Rustgi A, Markowitz S, Hampshire M, et al. Clinical trial resources on the Internet must be designed to reach underrepresented minorities. Cancer Journal. 2006;12(6):475–481. doi: 10.1097/00130404-200611000-00007. [DOI] [PubMed] [Google Scholar]
- Wisdom K, Neighbors K, Williams VH, Havstad SL, Tilley BC. Recruitment of African Americans with Type 2 Diabetes to a Randomized Controlled Trial Using Three Sources. Ethnicity & Health. 2002;7(4):267–278. doi: 10.1080/1355785022000060727. [DOI] [PubMed] [Google Scholar]
- Yancey AK, Ortega AN, Kumanyika SK. Effective Recruitment and Retention of Minority Research Participants. Annual Review of Public Health. 2006;27(1):1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]