Abstract
Mossy fiber synapses act as the critical mediators of highly dynamic communication between hippocampal granule cells in the dentate gyrus and CA3 pyramidal neurons. Excitatory synaptic strength at mossy fiber to CA3 pyramidal cell synapses is potentiated rapidly and reversibly by brief trains of low-frequency stimulation of mossy fiber axons. We show that slight modifications to the pattern of stimulation convert this short-term potentiation into prolonged synaptic strengthening lasting tens of minutes in rodent hippocampal slices. This low-frequency potentiation of mossy fiber EPSCs requires postsynaptic mGlu1 receptors for induction but is expressed presynaptically as an increased release probability and therefore impacts both AMPA and NMDA components of the mossy fiber EPSC. A nonconventional signaling pathway initiated by mGlu1 receptors contributes to induction of plasticity, because EPSC potentiation was prevented by a tyrosine kinase inhibitor and only partially reduced by guanosine 5′-O-(2-thiodiphosphate). A slowly reversible state of enhanced synaptic efficacy could serve as a mechanism for altering the integrative properties of this synapse within a relatively broad temporal window.
Introduction
Efficacy of excitatory transmission at the hippocampal mossy fiber–CA3 (mf-CA3) pyramidal neuron synapse is acutely sensitive to dynamic changes in the frequency of granule cell firing. Robust forms of short- and long-term synaptic plasticity are thought to be integral components of the “conditional detonator” function of mossy fibers in triggering pyramidal neuron excitation (Henze et al., 2002; Nicoll and Schmitz, 2005; Bischofberger et al., 2006). Short-term potentiation of excitatory transmission by as much as 1 order of magnitude results from increases in presynaptic release probability after modest elevations of action potential firing frequency (Salin et al., 1996). This form of plasticity, referred to as frequency-dependent facilitation, rapidly reverses after resumption of basal stimulation frequencies. Conventional long-term potentiation (LTP) of mf-CA3 pyramidal neurons also has a presynaptic locus of expression (Xiang et al., 1994; Weisskopf and Nicoll, 1995).
Classically defined LTP of mossy fiber EPSCs (mf-EPSCs) is independent of NMDA receptor activation (Harris and Cotman, 1986; Zalutsky and Nicoll, 1990) in contrast to the prototypical form of NMDA receptor-dependent LTP found at the Schaffer collateral–CA1 synapse (Kerchner and Nicoll, 2008). The signaling pathways that underlie induction are still a matter of debate, with evidence both for and against postsynaptic mechanisms such as mobilization of calcium from internal stores or via entry through voltage-gated channels (Williams and Johnston, 1989; Zalutsky and Nicoll, 1990; Yeckel et al., 1999; Mellor and Nicoll, 2001). Because expression of conventional mf-LTP occurs presynaptically, any postsynaptic contributions to induction must be transduced through a retrograde messenger system; the bidirectional Eph receptor–ephrin signaling system was proposed to serve this role (Contractor et al., 2002; Armstrong et al., 2006). Presynaptic expression of mf-LTP is known to engage cAMP and its downstream effectors (Weisskopf et al., 1994), which increase release probability through poorly defined actions in mossy fiber boutons (Nicoll and Schmitz, 2005). More recently, additional forms of LTP were described in which NMDA EPSCs could be potentiated by activation of postsynaptic group I metabotropic glutamate (mGlu) receptors or adenosine A2A receptors (Kwon and Castillo, 2008b; Rebola et al., 2008), which then serve as a metaplastic switch for induction of a postsynaptic form of plasticity of AMPA EPSCs previously not observed at mossy fiber synapses (Rebola et al., 2011).
The frequency of mossy fiber stimulation is a key parameter in induction of plasticity. mf-LTP requires short or sustained bursts of high-frequency stimulation (HFS), at 25–50 Hz, whereas low-frequency stimulation (LFS) is associated with long-term depression (LTD) at mossy fiber synapses (Kobayashi et al., 1996). Here we discovered that paired stimulation patterns with LFS elicited a slowly deprecating potentiation of mf-EPSCs, which we refer to as mossy fiber low-frequency potentiation (mf-LFP) because it is prolonged but ultimately fades after approximately 1 h. This form of potentiation occurred downstream of bimodal mGlu1-mediated activation of both G-protein-dependent pathways and noncanonical signaling through tyrosine kinase cascades. Potentiation of mossy fiber synaptic efficacy for tens of minutes could provide a broad window for temporal integration underlying pattern completion in the CA3 network.
Materials and Methods
Hippocampal slice preparation.
Horizontal hippocampal slices (350 μm) were made from postnatal day 15–21 wild-type 129SvEv, GluK2−/−, or mGlu1−/− gene-targeted mice of either sex using a Vibratome 3000 Plus (Vibratome). mGlu1−/− mice were a generous gift from Dr. K. Huber (University of Texas Southwestern Medical Center, Dallas, TX). Mice were rapidly decapitated after isoflurane anesthesia, and the brain was removed under ice-cold sucrose-rich slicing solution (SRSS) equilibrated with 95% O2/5% CO2 containing 85 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 25 mm glucose, 75 mm sucrose, 25 mm NaHCO3, 10 μm dl-APV, 100 μm kynurenate, 0.5 mm CaCl2, and 4 mm MgCl2. Slices were slowly warmed to 30°C and allowed to return to room temperature while exchanging the SRSS for oxygenated artificial CSF (ACSF) solution containing 125 mm NaCl, 2.4 mm KCl, 1.2 mm NaH2PO4, 25 mm NaHCO3, 25 mm glucose, 1 mm CaCl2, 2 mm MgCl2, 10 μm dl-APV, and 100 μm kynurenate.
Electrophysiological recordings.
After a 1 h incubation period, slices were transferred to a recording chamber and continuously perfused with oxygenated ACSF solution containing 2 mm CaCl2 and 1 mm MgCl2 at 25°C. Glass electrodes were pulled from borosilicate glass to resistances of 3–6 ΜΩ and filled with an internal solution containing 95 mm CsF, 25 mm CsCl, 10 mm Cs-HEPES, 10 mm Cs-EGTA, 2 mm NaCl, 2 mm Mg-ATP, 10 mm QX-314, 5 mm TEA-Cl, and 5 mm 4-AP. The pH was adjusted to 7.3 with CsOH, and osmolarity was maintained at 290 mOsm. Whole-cell patch-clamp recordings were made from visually identified CA3b or CA3c pyramidal cells in the hippocampus. All cells were held at a potential of −70 mV, and the series resistance was monitored continuously and compensated to 50–70%. Mossy fiber EPSCs, which are predominantly composed of AMPA receptor currents, were evoked at a basal stimulation frequency of 0.05 Hz in the presence of the GABAA antagonists, bicuculline (10 μm) and picrotoxin (50 μm), and the NMDA receptor antagonist, d-AP5 (50 μm). Low-frequency stimuli with varying parameters (i.e., 1 Hz paired pulse, 40 ms intervals) were delivered by a monopolar glass electrode filled with ACSF positioned in the inner 50 μm of the stratum lucidum. The amplitude of the evoked mf-EPSCs and paired-pulse ratio (PPR) was measured during basal activity and after induction of each LFS mf-LFP protocol. Mossy fiber responses were verified based on their robust short-term facilitation, large paired-pulse facilitation (>2.0 at 40 ms intervals), short and stable latency, and inhibition (>70%) by the group II metabotropic glutamate receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG-IV) (1 μm).
Our standard paired-pulse low-frequency stimulation (PP-LFS) protocol consisted of first recording mf-EPSCs under basal conditions at a frequency of 0.05 Hz with paired stimuli (separated by an interstimulus interval of 40 ms) for 10 min, followed by a 1 Hz train of paired stimuli over 2 min. mf-EPSCs were then further recorded for 30 min at the 0.05 Hz basal rate. Experimental modifications to this standard PP-LFS induction protocol included varying the paired-pulse interval to 200 or 500 ms, the frequency of the train between 0.2 and 2 Hz, the duration of the train between 30 s and 2 min, and the number of stimuli during each burst in the LFS train (one, two, or three). For the experiments where the frequency was changed, the total number of stimuli delivered during the train remained at 240 resulting in longer train durations (e.g., 240 stimuli at 0.2 Hz for 10 min). During all pharmacology experiments, drugs were applied for 10 min after the basal control stimulation and continued during the induction protocol. The magnitude of mf-LFP was determined by measuring the mf-EPSC amplitude over the last 5 min of each recording relative to the 10 min average of control EPSC amplitudes before the LFS.
Data acquisition and analysis.
Data were acquired with pClamp 10.2 software (Molecular Devices) and analyzed with Origin 7.0 (OriginLab). Values were represented as mean ± SEM, and n values represent the number of recordings from individual slice preparations. Only a single recording was taken from each slice preparation. Statistical significance was tested on raw data using a student's paired t test to compare the control amplitudes before LFS stimulation with the last 5 min of each recording. Cumulative probability histograms were tested with a Kolmogorov–Smirnov nonparametric test. Coefficients of variation (CV) of mf-EPSCs were compared for 5 min of recording preceding and 30 min after the mf-LFP induction protocol. The CV was corrected for the background noise. For clarity and illustrative purposes, every third data point was plotted in each graph.
Compounds.
The following compounds were bath applied with external ACSF during the pharmacological experiments: SNX-482 (500 nm), isradipine (5 μm), JNJ 16259685 (500 nm), LY 367385 (100 μm), MPEP (10 μm), genistein (30 μm), genistin (30 μm), and EphB2 Fc chimeric protein (5 μg/ml). BAPTA (20 mm) and guanosine 5′-O-(2-thiodiphosphate) (GDPβS) (2 mm) were included in the recording pipette. All chemicals, including inorganic salts, were purchased from Sigma-Aldrich (St. Louis, MO), except JNJ 16259685, LY 367385, MPEP (Tocris Cookson, Bristol, UK), and SNX-482 (Peptides International). GDPβS was purchased from BIOMOL Research Laboratories, and the recombinant mouse EphB2 fusion protein was purchased from R&D Systems.
Results
Patterned low-frequency stimulation elicits a quasi-stable potentiation of mossy fiber EPSCs
PP-LFS produces duration-dependent plasticity of Schaffer collateral–CA1 pyramidal cell ESPCs (Huang and Kandel, 2006), but the effect of similar stimulation paradigms on mf-EPSCs is unknown. We explored how alterations in the pattern of mossy fiber stimulation within low-frequency ranges (primarily 1 Hz) impacted synaptic efficacy of mf-EPSCs arising from AMPA and kainate receptor activation. In initial experiments, a long-lasting potentiation of mf-EPSCs was observed if paired stimuli were delivered during the 1 Hz train rather than single events used in the classical frequency facilitation paradigm; the increased mf-EPSCs was accompanied by an apparent reduction in the paired-pulse ratio (a representative example is shown in Fig. 1A). In the PP-LFS train protocol used in these experiments, we evoked pairs of mf-EPSC stimuli with an interstimulus interval (ISI) of 40 ms both at the basal frequency of 0.05 Hz and during the 1 Hz train of 2 min duration (i.e., 240 stimuli in the train). Each experiment was concluded with the application of the group II mGlu agonist DCG-IV (1 μm), which was one of our standard criteria for validating that the EPSCs arose from mossy fiber inputs.
Figure 1.
Patterned low-frequency stimulation leads to potentiation of mossy fiber EPSCs. A, Mossy fiber EPSCs recorded from CA3 pyramidal neurons in mouse hippocampal slices were elicited at a basal frequency of 0.05 Hz and train frequency of 1 Hz (gray bar, 2 min). Paired-pulse ratios were monitored during the duration of the experiment, and mossy fiber activation was confirmed with administration of the group II mGlu receptor agonist DCG-IV. B, Stimuli were evoked at a 1 Hz frequency with single LFS (open circles) or with paired LFS with a 40 ms interstimulus interval (PP-LFS, gray squares). Mean normalized EPSC amplitudes show that mf-EPSCs returned to basal levels after single pulses were administered. In contrast, PP-LFS stimulation potentiated mf-EPSCs 30 min after train stimulation. Insets in this and other panels show representative mf-EPSCs during prestimulus control (a, black trace) and during the last 5 min of the recordings (b, gray trace). Calibration: x-axes, 10 ms; y-axes, 100 pA (single stimulation) and 150 pA (PP-LFS stimulation). C, Cumulative probability histogram for mean 1 Hz single LFS (open circles) and 1 Hz PP-LFS (gray squares). The dashed line indicates 100% control amplitude. D, Plot of the PPR during baseline and after mf-LFP induction in 1 Hz PP-LFS experiments. Gray boxes represent average PPR and SEM for pretrain control and 30 min posttrain. E, Mean normalized amplitudes of mf-EPSCs evoked singly (open circles) or in pairs (PP-LFS, gray squares) in experiments where the train frequency was 2 Hz (for 1 min). Single stimuli did not potentiate mf-EPSCs, whereas a PP-LFS paradigm effectively potentiated mean amplitudes. Calibration: x-axes, 10 ms; y-axes, 150 pA. F, Mean normalized mf-EPSC amplitudes were potentiated in 1 Hz PP-LFS experiments in which the extracellular divalent cation concentrations were raised to 4 mm Ca2+ and 4 mm Mg2+. Calibration: x-axis, 10 ms; y-axis, 250 pA. G, mf-LFP occurs at more physiological temperatures. Calibration: x-axis, 10 ms; y-axis, 250 pA. H, Recording mf-EPSCs for a longer experimental time course revealed that elevated amplitudes returned to control levels 1 h after PP-LFS induction. Inset traces in this case are taken from the baseline period (a, black) and 1 h after PP-LFS (b, gray; overlapping with the control trace). Calibration: x-axis, 10 ms; y-axis, 250 pA. I, Bar graph summarizing mf-LFP expressed as a percentage of control amplitudes within each experimental group. Asterisks represent significance of individual degrees of potentiation compared with baseline within each experimental group (*p < 0.05; **p < 0.01).
PP-LFS train stimulation caused the mf-EPSC mean amplitudes to remain potentiated by 148 ± 13% at 30 min after tetanus (Fig. 1B, gray squares; n = 10; p = 0.0056 relative to control). In contrast, mf-EPSC amplitudes rapidly returned to control levels if pairing was omitted during the 2 min train stimulation (99 ± 9% of control amplitudes 30 min after 1 Hz train; n = 6; Fig. 1B, open circles), consistent with previous reports (Salin et al., 1996). Cumulative probability histograms of normalized mf-EPSC amplitudes after conventional 1 Hz single stimulation and PP-LFS are shown in Figure 1C. A decrease in the PPR of mf-EPSCs was observed 30 min after PP-LFS when compared with initial control PPRs (3.2 ± 0.2 pretrain, 2.6 ± 0.2 posttrain) (Fig. 1D). A reduction in the mean CV of mf-EPSCs after PP-LFS also was observed (control, 0.36 ± 0.02; 30 min after PP-LFS, 0.31 ± 0.03; n = 10; p < 0.05, Student's paired t test). These data, while not conclusive, are consistent with a presynaptic locus of expression, suggesting that some mechanistic overlap could exist with LTP induced by conventional high-frequency paradigms.
PP-LFS with a 2 Hz train frequency also potentiated mf-EPSCs (194 ± 38%; n = 8; p = 0.041), whereas amplitudes returned to control after single train stimulation at 2 Hz (115 ± 15%) (Fig. 1E). Increasing the extracellular divalent cation concentrations to 4 mm Ca2+ and 4 mm Mg2+, an often-used experimental condition that reduces network excitability in hippocampal slice preparations, did not alter the level of potentiation observed at 30 min after induction of plasticity using the 1 Hz PP-LFS stimulation protocol (150 ± 20%; n = 10; p = 0.042 vs pretetanus amplitudes) (Fig. 1F). We also found that mf-EPSC intermediate potentiation in our standard ACSF (i.e., 2 mm Ca2+/1 mm Mg2+) was observed at a higher temperature of 30°C (140 ± 4%; n = 4; p = 0.0027) (Fig. 1G).
The potentiation of mf-EPSCs after 1 and 2 Hz pairing seemed to diminish slowly with time rather than stabilizing at a plateau level, as is evident in Figure 1, B and E. We therefore recorded mf-EPSCs for 60 min after tetanus to more fully describe the time course of low-frequency potentiation. As with earlier recordings, mf-EPSC amplitudes remained elevated 30 min after tetanus (139 ± 15%; p = 0.047 relative to control amplitudes) but returned to baseline levels within 1 h (111 ± 16%; n = 6) (Fig. 1H). The mean potentiation at 30 min for each of these experimental conditions is summarized in the bar graph in Figure 1I.
In light of suggestions that some degree of facilitation and potentiation of mossy fiber AMPA EPSCs can be attributed to enhanced postsynaptic excitability and polysynaptic activity (Kwon and Castillo, 2008b), we next tested whether EPSCs arising from synaptic NMDA receptors exhibited the same degree of mf-LFP after PP-LFS stimulation. NMDA-EPSCs were recorded in a nominally Mg2+-free external solution with glycine (10 μm) and the AMPA/kainate receptor antagonist CNQX (50 μm) at a command potential of +40 mV; however, the 1 Hz tetanic stimulation was performed at −70 mV. The mean amplitude of NMDA-EPSCs was increased to 161 ± 14% of control amplitudes by the low-frequency stimulation paradigm (n = 7; p = 0.0028; data not shown), ruling out contributions by enhanced CA3 polysynaptic activity in this form of mossy fiber plasticity.
In summary, we found that pairing stimuli during a low-frequency activation of mossy fibers transformed short-term plasticity into an elevation of synaptic efficacy that lasted for at least 30 min but no longer than 1 h. For the subsequent experiments, we used the 30 min post-1 Hz train time frame as a standard for this form of plasticity at mossy fiber synapses, referred to as mf-LFP.
Induction parameters that impact low-frequency potentiation of mf-EPSCs
Our initial studies implied that the pattern of stimulation, or interval between the paired stimuli, contained within the low-frequency burst train was a critical parameter in the induction of intermediate mf-EPSC potentiation. To test this hypothesis, we increased the ISI of pairs during the 1 Hz train to either 200 or 500 ms while maintaining the pair interval at 40 ms during the pretrain and posttrain periods. Stimulation with 200 ms intervals during the train produced a robust level of potentiation 30 min after the tetanus (179 ± 22%; n = 6; p = 0.012; Fig. 2A). In contrast, pairs of stimuli separated by 500 ms (equivalent of a train with single stimuli evoked at 2 Hz) failed to elicit potentiation (117 ± 14%; n = 7; Fig. 2A), indicating that the duration between the pairs of stimuli is a critical parameter in inducing potentiation. Mean potentiation of mf-EPSCs with different interstimulus intervals during the 1 Hz train are compared in the bar graph in Figure 2A.
Figure 2.
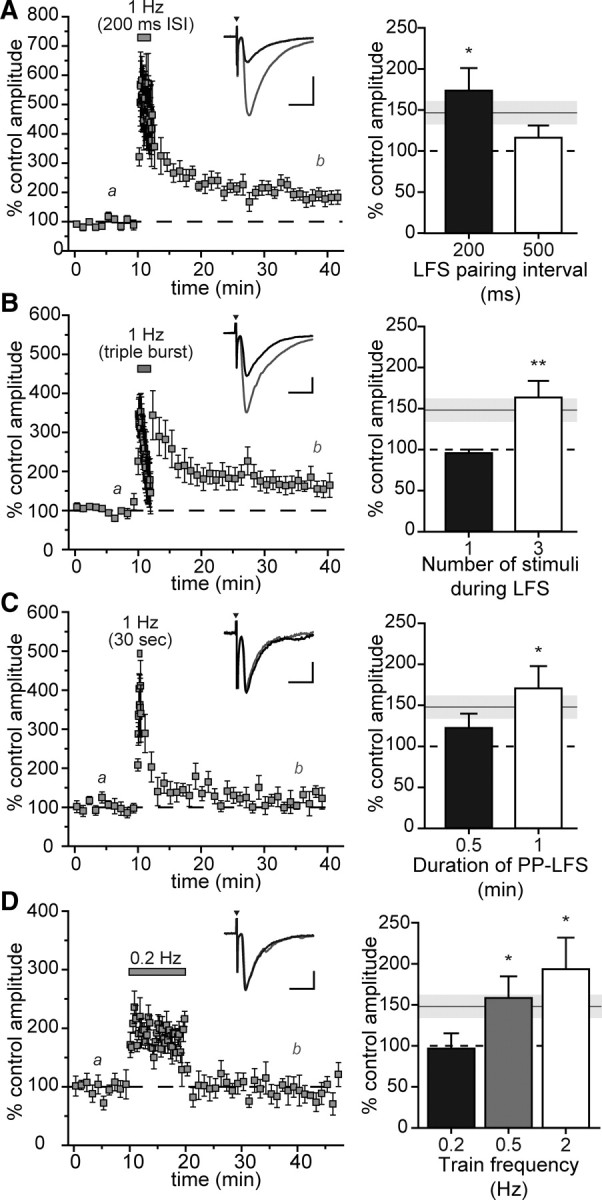
Impact of altering PP-LFS parameters on mf-LFP. A, Mean normalized mf-EPSC amplitudes were potentiated in experiments with a 200 ms interstimulus interval during the 1 Hz PP-LFS train (gray bar). Insets in this and other panels show representative mf-EPSCs during prestimulus control (black trace) and during the last 5 min of the recordings (gray trace). Calibration: x-axis, 10 ms; y-axis, 500 pA. The bar graph (right) shows mean percentage of control amplitudes after 1 Hz PP-LFS train stimulation at different interstimulus intervals. The white bar represents a standard PP-LFS induction protocol with a 500 ms interstimulus interval. The gray line and bar show mean ± SEM, respectively, of mf-LFP observed after a standard PP-LFS induction protocol. B, Addition of a third stimulus at a 40 ms interval during the 1 Hz train yields a higher degree of mf-EPSC potentiation compared with standard PP-LFS (gray bar). The bar graph summarizes the effect of varying the number of stimuli during PP-LFS induction. Calibration: x-axis, 10 ms; y-axis, 200 pA. C, The 1 Hz train duration during PP-LFS is critical for mf-LFP induction. A 30 s train failed to elicit significant potentiation when compared with the 2 min standard PP-LFS protocol. The bar graph summarizes the effect of changing the duration of the PP-LFS train from 0.5 to 1 min (white bar) and 2 min (gray bar). Calibration: x-axis, 10 ms; y-axis, 100 pA. D, The train frequency is an important determinant of mf-LFP induction. Mean normalized amplitudes of mf-EPSCs are shown from experiments in which PP-LFS induction was performed at a train frequency of 0.2 Hz (gray box), which did not induce mf-LFP. The total number of stimuli was kept constant at 240 stimuli as in the standard 1 Hz PP-LFS induction protocol. Calibration: x-axis, 10 ms; y-axis, 200 pA. The bar graph summarizes the magnitude of mf-LFP observed with different train stimulus frequencies (all delivered with pairs of stimuli). The gray line and bar show mean ± SEM, respectively, of mf-LFP observed after a standard PP-LFS induction protocol. Asterisks represent significance of individual degrees of potentiation compared with baseline (*p < 0.05; **p < 0.01).
Varying the number of stimuli during the 1 Hz induction train also altered mf-EPSC potentiation. Our standard PP-LFS protocol consisted of pairs of stimuli separated by 40 ms during both 0.05 Hz basal stimulation frequency as well as the 1 Hz induction train. Single stimulation during the train rather than pairs failed to induce potentiation (96 ± 4%; n = 4; Fig. 2B), whereas addition of a third stimulus in each burst (with 40 ms between stimuli) produced a more robust potentiation of mf-EPSCs at 30 min (164 ± 20%; n = 8; p = 0.0096) (Fig. 2B).
The duration and frequency of the paired train stimulation were additional important determinants of the magnitude of mf-LFP at 30 min. A 0.5 min PP-LFS train did not elicit a significant degree of potentiation at 30 min (125 ± 15%; n = 6; Fig. 2C), but a 1 min train effectively potentiated currents (172 ± 26% after 30 min; n = 5; p = 0.05; Fig. 2C, bar graph). Paired train stimulation at 0.2 Hz instead of 1 Hz did not potentiate mf-EPSCs (97 ± 18%; n = 5) (Fig. 2D), whereas a train frequency of 0.5 Hz produced a 158 ± 27% increase in mf-EPSCs 30 min after tetanus (n = 5; p = 0.05; Fig. 2D). In both of these latter cases, the total number of stimuli was kept constant at 240, equivalent to the total in our standard 1 Hz protocol, which resulted in longer train durations. In summary, these data demonstrate that potentiation of mf-EPSCs is induced effectively by a PP-LFS paradigm of >1 min at frequencies of 0.5 Hz and higher.
Molecular mechanisms contributing to mf-LFP differ from those of mf-LTP
Given the putative presynaptic locus of expression of mf-LFP, we next investigated to what degree the molecular mechanisms engaged by PP-LFS overlapped with those underlying the classical presynaptic form of mf-LTP. A variety of receptors and channels have been implicated in the induction of mf-LTP, including kainate receptors, a subtype of ionotropic glutamate receptors (Bortolotto et al., 1999; Contractor et al., 2001; Lauri et al., 2001; Schmitz et al., 2003). Genetic ablation of GluK2 receptors reduces the threshold for mossy fiber LTP (Contractor et al., 2001; Schmitz et al., 2003; Breustedt and Schmitz, 2004). We therefore examined whether GluK2 kainate receptors also contribute to induction of mf-LFP by PP-LFS. mf-EPSCs recorded from CA3 neurons in hippocampal slices from GluK2−/− mice were potentiated by 137 ± 8% 30 min after 1 Hz PP-LFS (n = 7; p = 0.011 relative to control amplitudes) (Fig. 3A,C), which was equivalent to the potentiation in wild-type mice. Similar results were observed when mf-EPSCs from GluK2−/− mice were recorded in the presence of high extracellular divalent ion concentrations (4 mm Ca2+/4 mm Mg2+) (122 ± 11%; n = 9; Fig. 3C). We conclude from these data that kainate receptors containing the GluK2 subunit, a necessary constituent of both presynaptic and postsynaptic receptors (Mulle et al., 1998; Contractor et al., 2001), do not play a role in induction of mf-LFP.
Figure 3.

Induction of mf-LFP does not require kainate receptors or R-type Ca2+ channels. A, GluK2 kainate receptors do not mediate PP-LFS-induced potentiation of mf-EPSCs. Mean normalized mf-EPSC control amplitudes reveal that GluK2−/− mice still exhibit mf-LFP. Insets in this and other panels show representative mf-EPSCs during prestimulus control (a) and during the last 5 min of the recordings (b). Calibration: x-axis, 10 ms; y-axis, 100 pA. B, Administration of the R-type VDCC α1E (Cav2.3) antagonist SNX-482 (500 nm) depresses mf-EPSCs in the absence of tetanic stimulation. mf-EPSCs are potentiated from this attenuated level after PP-LFS, which was 188% of the mean EPSC amplitude in the basal recordings without tetanic stimulation (gray squares). Calibration: x-axis, 10 ms; y-axis, 100 pA. C, Bar graph comparing experiments with GluK2−/− mice under different divalent cation concentrations and selective inhibition of R-type VDCC α1E (Cav2.3) with SNX-482 after mf-LFP induction. Asterisks represent significance of individual degrees of potentiation compared with baseline amplitudes within each group (*p < 0.05; **p < 0.01). #In the presence of SNX-482, mean amplitudes of mf-EPSCs after PP-LFS are significantly higher (p < 0.05) relative to mean amplitudes when the train stimulation was omitted. The gray line and bar show mean ± SEM, respectively, of mf-LFP observed after a standard PP-LFS induction protocol.
Pharmacological inhibition of R-type voltage-dependent calcium channels (VDCCs) or genetic ablation of the critical α1E (Cav2.3) channel subunit attenuated mf-LTP by trains of high-frequency stimuli (Breustedt et al., 2003). To determine whether R-type calcium channels contribute to mf-LFP, we performed PP-LFS stimulation in the presence of the peptide toxin SNX-482 (500 nm), a selective antagonist for the R-type α1E (Cav2.3) calcium channel subunit. An initial 5 min baseline was followed by bath application of SNX-482 for 12 min (10 min of baseline and 2 min during the PP-LFS); mf-EPSCs were then recorded for 30 min after train stimulation in the absence of the compound. As well, the effects of the compounds on basal transmission were assessed in parallel experiments that lacked only the 1 Hz train. Potentiation was measured between 25 and 30 min after the train (or the analogous time period in the control recordings). mf-EPSCs were unexpectedly reduced in amplitude by SNX-482 in the absence of a stimulus (52 ± 11% of control; n = 3; Fig. 3B, open circles, C), which differs from previous studies in which R-type channels did not contribute to basal release probability at this synapse (Breustedt et al., 2003; Dietrich et al., 2003). Nevertheless, PP-LFS stimulation potentiated EPSCs from this attenuated level to 98 ± 6% of control (predrug) amplitudes, which was 188% of the mean EPSC amplitude in the basal recordings that lacked tetanic stimulation (Fig. 3B, gray squares, C; n = 7; p < 0.05 relative to basal recordings with SNX-482). Thus, the potentiation induced by PP-LFS was not attenuated by SNX-482, and presynaptic R-type VDCCs do not seem to contribute to mf-LFP.
The role of postsynaptic calcium mobilization in high-frequency mf-LTP is controversial, with evidence both for and against its requirement for induction of plasticity (Williams and Johnston, 1989; Zalutsky and Nicoll, 1990; Yeckel et al., 1999; Mellor and Nicoll, 2001). We found that chelation of intracellular calcium with BAPTA (20 mm) contained within the recording pipette prevented intermediate potentiation of mossy fiber EPSCs (109 ± 11%; n = 7) (Fig. 4A). In addition, bath application of isradipine, a dihydropyridine L-type VDCC blocker, had no effect on basal mean mf-EPSC amplitudes at 5 μm (Fig. 4B, open circles; 103 ± 7% of control amplitudes; n = 3) but prevented induction of mf-LFP (Fig. 4B, gray squares; 112 ± 22% of control mean amplitudes; n = 7). These data therefore suggest that PP-LFS stimulation engages L-type VDCCs at a critical point in the induction of mf-LFP and that postsynaptic intracellular calcium entering from these channels (and potentially other sources) is required for patterned low-frequency potentiation of mossy fiber synapses.
Figure 4.
Postsynaptic calcium mobilization and L-type VDCCs are required for mf-LFP induction. A, Inclusion of BAPTA (20 mm) in the recording pipette occluded mf-LFP. Insets in A and B show representative mf-EPSCs during prestimulus control (a, black trace) and during the last 5 min of the recordings (b, gray trace). Calibration: x-axis, 10 ms; y-axis, 250 pA. B, Isradipine (5 μm), an L-type VDCC antagonist, has no effect on basal mean mf-EPSCs (open circles) but prevented induction of mf-LFP (gray squares). Inset calibration: x-axis, 10 ms; y-axis, 250 pA.
Bimodal signaling through mGlu1 receptors is engaged during PP-LFS to produce mf-LFP
The previous series of experiments demonstrated that mf-LFP and classical presynaptic mf-LTP seem to engage a different set of induction pathways. The patterned nature of PP-LFS and putative postsynaptic locus of induction led us next to examine group I mGlu receptors, because they are activated by repetitive mossy fiber stimulation (Kapur et al., 2001) and underlie some forms of plasticity induced by low-frequency induction paradigms in CA1 pyramidal neurons (Bellone et al., 2008; Lüscher and Huber, 2010). Furthermore, in the CA3 region, group I mGlu receptors are localized predominantly to pyramidal neurons (Lujan et al., 1996; Shigemoto et al., 1997; Ferraguti et al., 1998). Activation of mGlu1 or mGlu5 receptors was prevented in our experiments using the selective noncompetitive antagonists JNJ 16259685 (500 nm) or LY 367385 (100 μm) for mGlu1 receptors and MPEP (10 μm) for mGlu5 receptors. JNJ 16259685 eliminated potentiation of mf-EPSC at 30 min after tetanus (105.2 ± 13.7%; n = 7; p > 0.05) (Fig. 5A, gray squares, E), whereas basal synaptic strength was unaffected in recordings lacking the tetanic stimulation (97 ± 9%; n = 4; Fig. 5A, open circles, E). Occlusion of potentiation also was observed in the presence of another selective mGlu1 antagonist, LY 367385 (103 ± 9%; n = 5) (Fig. 5B, gray squares, E). Results from pharmacological experiments were additionally supported with comparative analyses of mGlu1 knock-out mice and their littermate wild-type mice; mf-LFP was absent in mGlu1−/− knock-out mice but was normal in recordings from mGlu1+/+ neurons (mGlu1+/+: 132 ± 8%, n = 5, p = 0.012; mGlu1−/−: 108 ± 19%, n = 5) (Fig. 5C,E). In contrast to mGlu1, mGlu5 receptors did not play a significant role in mf-LFP. Robust potentiation was induced by PP-LFS in the presence of the noncompetitive mGlu5 antagonist MPEP (156 ± 15%; n = 5; p = 0.019) (Fig. 5D, gray squares, E), which did not affect mf-EPSC amplitudes in the absence of a PP-LFS train (101 ± 4%; n = 4). Thus, activation of mGlu1, but not mGlu5, is required to effectively elicit low-frequency potentiation of mossy fiber EPSCs.
Figure 5.
mGlu1 metabotropic glutamate receptors are critical for mf-LFP induction. A, Inhibition of mGlu1 receptors with JNJ 16259685 (500 nm), a high-affinity noncompetitive antagonist, prevented mf-LFP (gray squares) induced by PP-LFS but has no effect on basal synaptic transmission (open circles). Insets in this and other panels show representative mf-EPSCs during prestimulus control (a, black traces) and during the last 5 min of the recordings (b, gray traces). Inset calibration: x-axis, 10 ms; y-axis, 100 pA. B, The mGlu1-selective antagonist LY 367385 (100 μm) also occluded potentiation of mf-EPSCs induced by PP-LFS (gray squares) and has no effect on basal synaptic transmission (open circles). Inset calibration: x-axis, 10 ms; y-axis, 100 pA. C, Mice lacking the mGlu1 gene do not exhibit mf-LFP. Inset calibration: x-axis, 10 ms; y-axis, 500 pA. D, Inhibition of mGlu5 receptors with MPEP (10 μm), a selective noncompetitive antagonist, had no effect on mf-LFP (gray squares) and did not affect basal synaptic strength during control experiments without the PP-LFS train (open circles). Inset calibration: x-axis, 10 ms; y-axis, 250 pA. E, The bar graph summarizes the effect of different mGluR antagonist application on mf-LFP. The gray line and bar show mean ± SEM, respectively, of mf-LFP observed after a standard PP-LFS induction protocol. Asterisks represent significance of individual degrees of potentiation compared with their baselines (*p < 0.05; **p < 0.01).
mGlu1 receptors are G-protein-coupled receptors that stimulate phospholipase C activity to generate diacylglycerol and IP3, leading to protein kinase C activation and mobilization of intracellular calcium as the principal downstream signaling pathways. More recent evidence suggests that additional non-G-protein-dependent signaling by mGlu-associated proteins, including β-arrestin, leads to activation of kinase cascades (Heuss et al., 1999; Benquet et al., 2002; Miyazaki et al., 2005). In CA3 pyramidal neurons, this noncanonical signaling pathway enhances NMDA receptor currents after activation of mGlu1 but not mGlu5 receptors (Benquet et al., 2002). To determine the respective contributions of conventional and non-G-protein-mediated signaling pathways to PP-LFS-induced mf-LFP, we inhibited either G-protein activation or tyrosine kinase activity. Irreversible inactivation of G-protein signaling by inclusion of GDPβS (2 mm) in the internal solution of the recording electrode reduced mf-LFP to 128 ± 12% (n = 8) (Fig. 6A,C); thus, mf-LFP was lower than that produced by standard 1 Hz PP-LFS (148 ± 13%; Fig. 1B) but not prevented completely. Conversely, bath application of genistein (30 μm), the broad-spectrum tyrosine kinase inhibitor, completely occluded mf-LFP (101 ± 10%; n = 7; p > 0.05) (Fig. 6B, gray squares, C) and had no effect on basal synaptic strength (95 ± 11% of control amplitudes; n = 3; data not shown). The inactive analog of genistein, genistin (30 μm), had no impact on mf-EPSC potentiation after PP-LFS (178 ± 25%; n = 5; p = 0.038) (Fig. 6B, open circles, C). These data confirm a central role of G-protein-independent signaling in low-frequency-induced potentiation of mf-EPSCs.
Figure 6.

Multiple downstream signaling pathways mediate mGlu1-dependent mf-LFP. A, Inactivation of G-protein signaling through inclusion of GDPβS (2 mm) in the recording pipette reduced potentiation compared with standard PP-LFS. Representative mf-EPSCs during prestimulus control (a, black) and during the last 5 min of the recordings (b, gray) are shown in A and B. Inset calibration: x-axis, 10 ms; y-axis, 100 pA. B, Application of the broad-spectrum tyrosine kinase inhibitor, genistein (30 μm), prevents induction of mf-LFP evoked by PP-LFS (gray squares), whereas the inactive analog of genistein, genistin (30 μm), has no effect on mf-EPSC amplitudes after PP-LFS (open circles). Inset calibration (from recording in genistein): x-axis, 10 ms; y-axis, 250 pA. C, The bar graph summarizes the effect of manipulating mGlu-dependent signaling pathways on mf-LFP. Statistically significant mf-LFP was observed when GDPβS was included in the recording pipette (*p < 0.05, paired t test). Mean amplitudes after PP-LFS in the presence of genistein were not significantly different from those of control, whereas PP-LFS elicited mf-LFP with genistin in the bath (**p < 0.01, paired t tests within each group). The gray line and bar show mean ± SEM, respectively, of mf-LFP observed after a standard PP-LFS induction protocol.
Induction of mf-LFP occurred postsynaptically, but paired-pulse and CV analyses were consistent with a presynaptic locus of expression of potentiation. A retrograde signaling pathway mediated by ephrins and Eph receptors was identified previously as one potential mechanism for LTP of mf-EPSCs after postsynaptic induction (Contractor et al., 2002), and Eph receptors are rich targets for tyrosine kinase phosphorylation. We therefore tested whether inhibition of trans-synaptic ephrin signaling occluded mf-LFP. In the presence of bath-applied soluble recombinant mouse EphB2 Fc chimera peptides (5 μg/ml), which block interactions between postsynaptic EphB2 receptors and their presynaptic ephrin ligands, PP-LFS did not elicit mf-LFP (108 ± 12%; n = 5) (data not shown). These results suggest that EphB2 receptors can initiate trans-synaptic signaling between their presynaptic ephrin ligands to regulate mossy fiber release during mf-LFP.
Discussion
Plasticity of excitatory transmission at the mossy fiber synapse is known to occur through diverse mechanisms to enhance signaling transiently, through rapid facilitation, and over extended periods of time through long-lasting increases in release probability. Here we describe a form of plasticity that is operative in the intermediate time domain between frequency facilitation and long-term potentiation. Low-frequency stimulation delivered in pairs with a frequency of 1 Hz potentiates mf-EPSCs for up to 1 h after induction. The degree of mf-LFP we observed was dependent on a variety of induction parameters that included the train stimulation frequency, the number of pulses and interstimuli intervals in each burst during the train, and the duration of the low-frequency train stimulation. Several independent experimental observations lead us to conclude that mf-LFP is primarily induced postsynaptically. mGlu1 receptor activation and divergent downstream signaling pathways, including noncanonical pathways via tyrosine kinase activity, play a central role in mf-LFP. We therefore propose that this form of synaptic plasticity is induced by subsaturating patterns of activation at the mossy fiber synapse as one mechanism of modulating synaptic gain over a prolonged temporal window while circumventing stable enhancement of release probability. Alternatively, mf-LFP may represent a temporally restricted form of metaplasticity in which granule cells prime CA3 neurons with low-frequency subthreshold input to alter synaptic sensitivity to subsequent short, high-frequency bursts typical of granule cell firing behavior that has been described in vivo (Jung and McNaughton, 1993; Henze et al., 2002).
The potentiation observed after paired low-frequency stimulation was surprising initially because at mossy fiber–CA3 pyramidal cell synapses, this stimulation paradigm is closely associated with either rapidly reversible short-term plasticity (Salin et al., 1996) or long-term depression with prolonged train durations (Kobayashi et al., 1996). In contrast, paired low-frequency stimulation of hippocampal CA1 and cortical–lateral amygdala synapses effectively elicits potentiation of EPSCs through divergent induction mechanisms that include mGlu5 receptors (Lanté et al., 2006), NMDA receptors (Huang and Kandel, 2006), or heptahelical receptors that stimulate protein kinase A activity (Huang and Kandel, 2007). PP-LFS engages a different set of signaling pathways at the mossy fiber synapses to effect mf-LFP, most notably requiring mGlu1 receptor activation rather than either NMDA or mGlu5 receptors. Group I mGlu receptors have been proposed to perform diverse functions in hippocampal long-term depression and potentiation, within both the CA1 and CA3 subfields, which have been studied and debated extensively (Bashir et al., 1993; Lu et al., 1997; Anwyl, 2009; Lüscher and Huber, 2010). At mossy fiber synapses specifically, there is evidence both for (Conquet et al., 1994) and against (Hsia et al., 1995) mGlu1 receptor contributions to HFS-induced mf-LTP based on studies from gene-targeted mice lacking this receptor isoform, and pharmacological inhibition of the mGlu1 receptor reduces mf-LTP in some studies (Yeckel et al., 1999; Contractor et al., 2001). Moreover, mGlu1 receptors can trigger bidirectional switches in plasticity, where HFS protocols that normally induce mf-LTP instead result in LTD, at synapses formed by mossy fibers on CA3 stratum lacunosum interneurons (Galvan et al., 2008). Granule cells are thought to fire at relatively low frequencies, with occasional short high-frequency bursts, in complex patterns dependent partially on spatial environment (Jung and McNaughton, 1993; Henze et al., 2002; Gundlfinger et al., 2010). Although the regularity of our patterned activation clearly does not reproduce irregular dynamics of physiological granule cell firing, the frequency domain and dependence on short bursts is similar, and therefore it is plausible that mGlu1-dependent pathways could be engaged to facilitate mossy fiber EPSP amplitudes, which are acutely sensitive to changes in instantaneous frequency in vivo (Henze et al., 2002; Gundlfinger et al., 2010). In CA3 pyramidal neurons, mGlu1 receptors may serve as a molecular substrate for processing low-frequency signals from the dentate gyrus into cellular responses that lead to intermediate or long-lasting alterations in mossy fiber output.
The CA3 region of the hippocampus has been linked to the short-term and rapid encoding of episodic information as a component of working memory (Kesner, 2007), pattern completion, and associative memory recall (Nakazawa et al., 2002). The physiological substrate for these behavioral learning tasks likely lays, in part, in the dynamic control of synaptic strength at the associational–commissural (A/C) excitatory synapses in CA3 pyramidal neurons (Nakazawa et al., 2002). mf-LFP and LTP potentially contribute to these processes by altering the efficacy of action potential firing driven by mossy fiber synaptic depolarization, which is strongly dependent on granule cell firing frequency (Henze et al., 2002). Back-propagating action potentials initiated by mossy fiber input contributes to heterosynaptic spike-timing plasticity in the autoassociative A/C network (Kobayashi and Poo, 2004) as well as homosynaptic potentiation of mossy fiber transmission (Astori et al., 2010). Induction of mf-LFP could provide an extended window of time during which granule cell firing would have a higher likelihood of initiating action potentials in CA3 pyramidal neurons.
The signaling pathways engaged during the induction phase of mf-LFP have some commonality with two recently described postsynaptic forms of LTP that selectively enhance NMDA receptor function (Kwon and Castillo, 2008a; Rebola et al., 2008). These mechanistically distinct forms of LTP use slightly different signaling pathways to achieve a common aim: potentiation of NMDA receptor but not AMPA receptor EPSCs. Short bursts of high-frequency mossy fiber stimulation activate both mGlu5 receptors, which are postsynaptic in CA3 pyramidal cells (Lujan et al., 1996; Shigemoto et al., 1997), and NMDA receptors, leading to intracellular calcium mobilization and activation of protein kinase C, resulting in potentiation of NMDA receptor EPSCs via increased exocytosis of receptor-containing vesicles (Kwon and Castillo, 2008a). A similar experimental paradigm was found to engage A2A adenosine receptors, in addition to NMDA and mGlu5 receptors, and require Src family kinases to effect NMDA receptor EPSC potentiation at mossy fiber synapses (Rebola et al., 2008). We found that postsynaptic calcium and tyrosine kinase activation were crucial signaling components for induction of mf-LFP as well. Indeed, mf-LFP was dependent on the frequency of activation (and pairing during the low-frequency train), which likely produces a critical pattern of intracellular calcium transients that effectively engage downstream signaling enzymes yet to be fully elucidated. In contrast to NMDA receptor-selective postsynaptic mf-LTP, both AMPA and NMDA receptor EPSCs were potentiated in mf-LFP. mf-LFP also differed from postsynaptic LTP in that neither NMDA nor mGlu5 receptor activation was required for induction. Thus, these data imply that the precise timing and pattern of mossy fiber activation can lead to wholly distinct forms of plasticity through overlapping molecular mechanisms.
The signaling pathways that lead from activation of mGlu1 receptors to enhanced mossy fiber release probability are unusual in that crucial intermediary tyrosine kinase activation was only partially occluded by inhibition of G-proteins, suggesting that an unconventional route to kinase activation contributes to the mf-LFP induction mechanism. Heptahelical G-protein-coupled receptors are known to serve as scaffolds for signaling complexes independent of canonical G-protein interactions, mostly notably through association with arrestins, the multifunctional endocytic adapters and potent signal transducers. For example, mitogen-activated protein kinase cascades are initiated by arrestin association with β2 adrenergic and other receptors (Luttrell et al., 1999; Shenoy and Lefkowitz, 2005; Luttrell and Gesty-Palmer, 2010). Metabotropic glutamate receptors have a similar capacity to signal bimodally through both G-protein-dependent and -independent pathways (Kehoe, 1994; Heuss et al., 1999; Gee and Lacaille, 2004), including in CA3 pyramidal neurons (Gerber et al., 2007). After activation, mGlu receptors are desensitized by G-protein receptor kinases, which in turn enhances the binding of β-arrestins and initiates downstream tyrosine kinase cascades (Iacovelli et al., 2003; Emery et al., 2010). In CA3 pyramidal neurons, G-protein-independent mGlu receptor signaling occurs exclusively via mGlu1 (Benquet et al., 2002; Gerber et al., 2007). Both group I mGlu receptors can stimulate conventional G-protein-mediated activation of phospholipase Cβ, which leads to a reduction in slow afterhyperpolarization currents and an increase in NMDA receptor current amplitudes (Heuss et al., 1999; Benquet et al., 2002). However, NMDA receptor currents can also be enhanced through G-protein-independent, tyrosine kinase-dependent pathways initiated by mGlu1 activation (Benquet et al., 2002), which is also responsible for a slow inward current at mossy fiber synapses (Heuss et al., 1999). These findings parallel the selective role for mGlu1 in induction of mf-LFP in our experiments. Our observation that the tyrosine kinase inhibitor genistein completely occluded mf-LFP, whereas GDPβS only partially reduced potentiation, therefore implicates G-protein-independent pathways in this form of plasticity. Recruitment of arrestin to G-protein-coupled receptors is typically stimulated by agonist binding to the receptors and occurs after G-protein activation (Luttrell and Gesty-Palmer, 2010), and the competition between these two signaling systems after patterned mGlu1 activation might, in part, account for duration and frequency dependencies in the PP-LFS paradigm for induction of mf-LFP.
One putative pathway for retrograde signaling to effect changes in release probability involves Eph receptors and their cognate ephrin ligands on the presynaptic terminal (Contractor et al., 2002; Armstrong et al., 2006). Tyrosine kinases activated by mGlu1 receptors potentially could target sites found on EphB receptors and thus initiate retrograde signaling. As well, a direct interaction between EphRs and mGlu receptors has been proposed to mediate a form of long-term depression in the hippocampus (Calò et al., 2005; Piccinin et al., 2010). Thus, mf-LFP could be mediated via Eph receptor activation downstream of mGlu1 receptor activation, although clearly there are a number of questions remaining regarding the sequence of signaling events that lead to mf-LFP.
In summary, our data demonstrate that paired stimulation in a low-frequency domain elicits a state of synaptic potentiation at mossy fiber synapses. This process requires mGlu1 receptor activation and at least, in part, is transduced by unconventional postsynaptic signaling pathways within CA3 pyramidal neurons. Low-frequency potentiation at the mossy fiber synapse may serve as a mechanism for temporarily strengthening the impact of granule cell input on associative processing of information in the CA3 recurrent network.
Footnotes
This work was supported by NINDS Grant R01 NS044322 (G.T.S.). We thank Dr. Anis Contractor (Northwestern University Feinberg School of Medicine, Chicago, IL) for valuable feedback. mGlu1−/− mice were a generous gift from Dr. Kimberly Huber (University of Texas Southwestern Medical Center, Dallas, TX).
The authors declare no competing financial interests.
References
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Armstrong JN, Saganich MJ, Xu NJ, Henkemeyer M, Heinemann SF, Contractor A. B-ephrin reverse signaling is required for NMDA-independent long-term potentiation of mossy fibers in the hippocampus. J Neurosci. 2006;26:3474–3481. doi: 10.1523/JNEUROSCI.4338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori S, Pawlak V, Köhr G. Spike-timing-dependent plasticity in hippocampal CA3 neurons. J Physiol. 2010;588:4475–4488. doi: 10.1113/jphysiol.2010.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Frotscher M, Jonas P. Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Pflugers Arch. 2006;453:361–372. doi: 10.1007/s00424-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Breustedt J, Schmitz D. Assessing the role of GLUK5 and GLUK6 at hippocampal Mossy fiber synapses. J Neurosci. 2004;24:10093–10098. doi: 10.1523/JNEUROSCI.3078-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breustedt J, Vogt KE, Miller RJ, Nicoll RA, Schmitz D. Alpha1E-containing Ca2+ channels are involved in synaptic plasticity. Proc Natl Acad Sci U S A. 2003;100:12450–12455. doi: 10.1073/pnas.2035117100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò L, Bruno V, Spinsanti P, Molinari G, Korkhov V, Esposito Z, Patanè M, Melchiorri D, Freissmuth M, Nicoletti F. Interactions between ephrin-B and metabotropic glutamate 1 receptors in brain tissue and cultured neurons. J Neurosci. 2005;25:2245–2254. doi: 10.1523/JNEUROSCI.4956-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Condé F, Collingridge GL, Crépel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Heinemann SF. Kainate receptors are involved in short and long term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29:209–216. doi: 10.1016/s0896-6273(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Dietrich D, Kirschstein T, Kukley M, Pereverzev A, von der Brelie C, Schneider T, Beck H. Functional specialization of presynaptic Cav2.3 Ca2+ channels. Neuron. 2003;39:483–496. doi: 10.1016/s0896-6273(03)00430-6. [DOI] [PubMed] [Google Scholar]
- Emery AC, Pshenichkin S, Takoudjou GR, Grajkowska E, Wolfe BB, Wroblewski JT. The protective signaling of metabotropic glutamate receptor 1 is mediated by sustained, beta-arrestin-1-dependent ERK phosphorylation. J Biol Chem. 2010;285:26041–26048. doi: 10.1074/jbc.M110.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knopfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol. 1998;400:391–407. [PubMed] [Google Scholar]
- Galván EJ, Calixto E, Barrionuevo G. Bidirectional Hebbian plasticity at hippocampal mossy fiber synapses on CA3 interneurons. J Neurosci. 2008;28:14042–14055. doi: 10.1523/JNEUROSCI.4848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee CE, Lacaille JC. Group I metabotropic glutamate receptor actions in oriens/alveus interneurons of rat hippocampal CA1 region. Brain Res. 2004;1000:92–101. doi: 10.1016/j.brainres.2003.11.046. [DOI] [PubMed] [Google Scholar]
- Gerber U, Gee CE, Benquet P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Gundlfinger A, Breustedt J, Sullivan D, Schmitz D. Natural spike trains trigger short- and long-lasting dynamics at hippocampal mossy fiber synapses in rodents. PLoS One. 2010;5:e9961. doi: 10.1371/journal.pone.0009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Heuss C, Scanziani M, Gähwiler BH, Gerber U. G-protein-independent signaling mediated by metabotropic glutamate receptors. Nat Neurosci. 1999;2:1070–1077. doi: 10.1038/15996. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Salin PA, Castillo PE, Aiba A, Abeliovich A, Tonegawa S, Nicoll RA. Evidence against a role for metabotropic glutamate receptors in mossy fiber LTP: the use of mutant mice and pharmacological antagonists. Neuropharmacology. 1995;34:1567–1572. doi: 10.1016/0028-3908(95)00115-m. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Age-related enhancement of a protein synthesis-dependent late phase of LTP induced by low frequency paired-pulse stimulation in hippocampus. Learn Mem. 2006;13:298–306. doi: 10.1101/lm.166906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Low-frequency stimulation induces a pathway-specific late phase of LTP in the amygdala that is mediated by PKA and dependent on protein synthesis. Learn Mem. 2007;14:497–503. doi: 10.1101/lm.593407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggiò S, Sallese M, Porcellini A, Nicoletti F, De Blasi A. Role of G protein-coupled receptor kinase 4 and beta-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem. 2003;278:12433–12442. doi: 10.1074/jbc.M203992200. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kapur A, Yeckel M, Johnston D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience. 2001;107:59–69. doi: 10.1016/s0306-4522(01)00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Glutamate activates a K+ conductance increase in Aplysia neurons that appears to be independent of G proteins. Neuron. 1994;13:691–702. doi: 10.1016/0896-6273(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Poo MM. Spike train timing-dependent associative modification of hippocampal CA3 recurrent synapses by mossy fibers. Neuron. 2004;41:445–454. doi: 10.1016/s0896-6273(03)00873-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Manabe T, Takahashi T. Presynaptic long-term depression at the hippocampal mossy fiber-CA3 synapse. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008a;57:108–120. doi: 10.1016/j.neuron.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Castillo PE. Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron. 2008b;60:1082–1094. doi: 10.1016/j.neuron.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanté F, de Jésus Ferreira MC, Guiramand J, Récasens M, Vignes M. Low-frequency stimulation induces a new form of LTP, metabotropic glutamate (mGlu5) receptor- and PKA-dependent, in the CA1 area of the rat hippocampus. Hippocampus. 2006;16:345–360. doi: 10.1002/hipo.20146. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A critical role of a facilitatory presynaptic kainate receptor in mossy fiber LTP. Neuron. 2001;32:697–709. doi: 10.1016/s0896-6273(01)00511-6. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Mellor J, Nicoll R. Hippocampal mossy fiber LTP is independent of postsynaptic calcium. Nat Neurosci. 2001;4:125–126. doi: 10.1038/83941. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Ishizuka T, Yawo H. Synapse-to-synapse variation of calcium channel subtype contributions in large mossy fiber terminals of mouse hippocampus. Neuroscience. 2005;136:1003–1014. doi: 10.1016/j.neuroscience.2005.08.049. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Piccinin S, Cinque C, Calò L, Molinaro G, Battaglia G, Maggi L, Nicoletti F, Melchiorri D, Eusebi F, Massey PV, Bashir ZI. Interaction between Ephrins and mGlu5 metabotropic glutamate receptors in the induction of long-term synaptic depression in the hippocampus. J Neurosci. 2010;30:2835–2843. doi: 10.1523/JNEUROSCI.4834-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Rebola N, Carta M, Lanore F, Blanchet C, Mulle C. NMDA receptor-dependent metaplasticity at hippocampal mossy fiber synapses. Nat Neurosci. 2011;14:691–693. doi: 10.1038/nn.2809. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Breustedt J, Nicoll RA. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci. 2003;6:1058–1063. doi: 10.1038/nn1116. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor- mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D. Long-term potentiation of hippocampal mossy fiber synapses is blocked by postsynaptic injection of calcium chelators. Neuron. 1989;3:583–588. doi: 10.1016/0896-6273(89)90268-7. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Greenwood AC, Kairiss EW, Brown TH. Quantal mechanism of long-term potentiation in hippocampal mossy-fiber synapses. J Neurophysiol. 1994;71:2552–2556. doi: 10.1152/jn.1994.71.6.2552. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]





