Abstract
Marine organisms have yielded a variety of metabolites with neuropharmacological applications. Here we describe the isolation and pharmacological characterization of four novel, neurologically active purines 1–4, isolated from Haplosclerida sponges collected in the Republic of Palau. The structures were determined by analyses of spectral and X-ray data. Compound 1 induced convulsions upon intracerebroventricular injection into mice, with a CD50 value of 2.4 nmol/mouse. Purines 2–4 were active in mouse bioassays at higher doses. The seizurogenic activity of 1 was correlated with inhibition of neuronal GABAergic transmission, with only a modest impact on excitatory signaling, in electrophysiological recordings from hippocampal neurons. Despite having a purine template structure, the inhibitory activity of 1 was not prevented by a nonselective adenosine receptor antagonist. Thus, 1 represents a novel substituted purine that elicits convulsions through its actions on inhibitory neurotransmission. These 8-oxoisoguanine analogs comprise a new family of compounds closely related in structure to endogenous neurosignaling molecules and commonly used CNS stimulants.
Introduction
Purine derivatives play fundamental roles in metabolic systems, and structurally modified purines have diverse biological activities.1 Well-known examples include the plant-derived xanthine (2,6-oxopurine) derivatives caffeine and theophylline, which act as central nervous system stimulants with multiple biological targets. Their stimulant activity primarily results from antagonism of adenosine receptors; other pharmacological activities include inhibition of cyclic nucleotide phosphodiesterases and positive modulation of ryanodine receptors. At supra-physiological concentrations, the xanthines also inhibit GABAA a receptors and alter K+ channel activity.2 Numerous caffeine-inspired xanthine derivatives have been synthesized as drug candidates, in part because theophylline is used clinically as an antiasthma drug.2 Modified purines also occur widely in marine invertebrates;1 more than 20 such bases have been reported to date from a variety of organisms. The bioactivity of marine-derived purines is diverse and includes cytotoxicity,3 antimicrobial activity,4,5 enzyme inhibition,6,7 antiangiogenic activity,8 and alteration of neuronal signaling.9,10
Marine invertebrates continue to be a rich and relatively untapped source of molecules with novel neurological activities. In an ongoing series of studies, we have isolated new ligands for excitatory amino acid receptors from aqueous extracts of marine sponges using both in vitro and in vivo bioassays.11,12 Here we report the isolation, structure, and biological properties of a structurally related, novel set of purine derivatives that constitute the neuroactive principles from several Haplosclerida sponges collected in the Republic of Palau. This family of 8-oxoisoguanines includes a potent pro-convulsant molecule that reduces inhibitory neurotransmission. The intriguing behavioral and neurophysiological actions of these molecules suggest that they could serve as a yet-unexplored template for generation of additional pharmacological tools and neuromodulators.
Results
Isolation and Structural Determination
In screening for bioactivity in aqueous extracts from several Palauan sponges, including Cribrochalina olemda, Haliclona sp., and Amphimedon sp., all belonging to the order Haplosclerida, we found that intracerebroventricular (i.c.v.) injection of diluted aqueous fractions (1 mg/mL)11 elicited seizure like activity in mice. To isolate potential neuroactive compounds, an aqueous extract from one of the sponges, C. olemda, was further purified using a Sephadex LH-20 column. Fractions showing UV absorption (at 260 nm) were combined and concentrated in vacuo. Precipitate that formed upon evaporation was collected and separated by HPLC to give pure 1. NMR and high resolution FABMS data for 1 secured the molecular formula of C7H9-N5O2. In the1HNMR, only two singlets at δ 3.18 and 3.32were observed (Table 1), and these gave a cross peak at carbon resonances δ 28.4 and 31.2 in an HSQC, respectively. These resonances were assigned for methyl groups bound to nitrogen atoms on the basis of the chemical shifts in the NMR data in combination with 2JCH values of 146 Hz, which were observed as satellite peaks in an HMBC NMR data set. In the 13CNMR data, five aromatic resonances were observed between δ 93 and 154. UV spectrum gave two absorption maxima at λ 242 and 304 nm. Together, these data suggested the presence of a purine ring system in 1, while a lack of carbon-bound protons and the molecular formula suggested that C-2, C-6, and C-8 of the purine ring in 1 must be substituted with one nitrogen and two oxygen atoms. The N-methyl protons at each δ 3.32 and 3.18 showed HMBC cross peaks at two sets of carbons at δ 149.6 and 147.5 and δ 153.8 and 141.2, respectively (Table 1). These data revealed that both of the methyl groups must be substituted on the nitrogen atoms within the purine ring system. The carbon that resonated at δ 93.8 in 1 was assigned diagnostically to be C-5.13 Because no cross peaks between C-5 and any of the N-methyl signals were observed in the HMBC spectrum, the presence of a methyl group at N-7was precluded. Furthermore, each N-methyl proton correlated with two different set of aromatic carbons in the HMBC spectrum, indicating that the N-methyl groups were located far enough from each other that no common adjacent carbon atom was shared. Taken together, the position of the methyl group was assignable only to N-1 and N-9.
Table 1.
NMR Data for Compounds 1–4 and Compound 2 Reported from Pseudodistoma cereum (2P.s.)
| position | 1a | 2a | 2 (DMSO) | 2P.s. (DMSO)b | 3a | 3 (DMSO) | 4a |
|---|---|---|---|---|---|---|---|
| C-2 | 149.6a | 149.6a | 147.7 | 142.7 | 149.7b | 148.6 | 149.5 |
| C-4 | 141.2b | 141.9c | 143.0 | 147.9 | 143.8a | 143.5 | 143.6a |
| C-5 | 93.8 | 95.1 | 93.2 | 92.3 | 94.6 | 93.3 | 93.3 |
| C-6 | 147.5a | 146.8b | 143.9 | 151.1 | 145.2 | 144.8 | 146.0 |
| C-8 | 153.8b | 154.3 | 152.9 | 144.2 | 154.0c | 151.8 | 153.9a |
| N-1-Me | 31.2 (3.32)a | 32.2 (3.35)ab | 31.2 (3.36) | 30.7 (3.65) | |||
| N-3-Me | 33.1 (3.34)ac | 31.9 (3.34) | 29.6 (3.55) | 32.3 (3.56)ab | 31.6 (3.61) | ||
| N-9-Me | 28.4 (3.18)b | 31.1 (3.45)ac | 30.5 (3.50) | 28.5 (3.16)a | |||
| 6-NH2 | 8.47 | 8.25 | 8.28 | ||||
| N-7-H | 10.25 | 10.97 | 11.23 |
Data were taken in D2O with HCl (0.2 N). Each a–c denotes a spin system connected by HMBC.
Chemical shifts and assignment listed as they appear in ref 17, in that the chemical shifts for C6, C4, and N-methyl groups were assigned to be interchangeable.
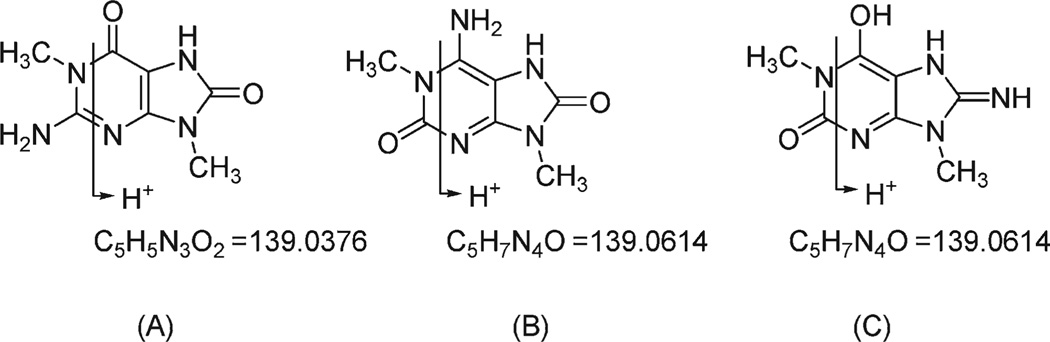
The data therefore supported the possible isomers 1,9-dimethyl-8-oxoguanine (A), 1,9-dimethyl-8-oxoisoguanine (B), or 1,9-dimethyl-8-aminoxanthine (C) as candidate structures of 1 (Scheme 1). While the 8-amino purine structure (C) was a formal possibility, it was considered least likely because of the rarity of this structure in natural products. To the best of our knowledge, saxitoxins are the only examples of such modified natural 8-aminopurines. The MALDI-TOF/MS/MS spectrum for 1 with a precursor ion at m/z 196 gave fragment ions, for example, at m/z 153, 139, 112, and 57. Although some of these product ions could be retro Diels–Alder type fragments often observed in purine analogues,14 for example, an ion at m/z 139, they are less diagnostic for distinguishing A from B or C. That is, loss of either C2H5N2 or C2H3NO from candidates A or B, respectively (Scheme 1), would result in ions too similar in m/z to differentiate in the mass spectroscopic analysis carried out in this study. Because of the remaining ambiguity, the structure of 1 was resolved by X-ray diffraction of a single crystal of 1 grown in hydrochloric acid (0.1 M) solution.
Scheme 1.
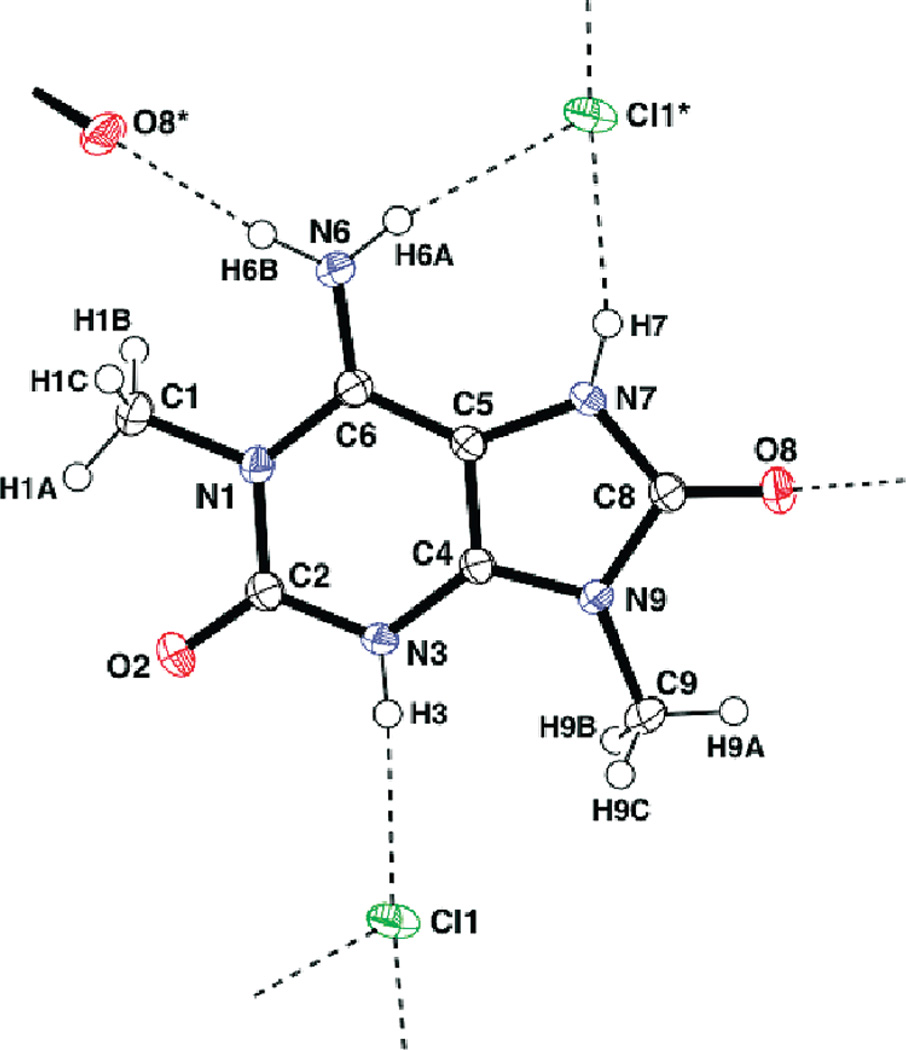
A crystal of compound 1 was obtained as chloride salt and its chemical structure was unambiguously determined as (B), as shown in Figure 1. The positions of four N-bound hydrogen atoms (H-3, H-6A, H-6B, and H-7) were clearly found in a difference Fourier map and refined satisfactorily. This protonation scheme was confirmed by examination of intermolecular hydrogen bonding within the crystal structure. Namely, the three hydrogen atoms of H-3, H-6A, and H-7 participate in hydrogen bonds with chloride anions (H-3 ⋯ Cl1 = 2.32 Å, N3–H3⋯Cl1 = 176°, H-6A⋯Cl1 = 2.40 Å, N-6–H-6A⋯Cl1 = 170°, H-7 ⋯Cl1 = 2.30 Å, and N-7–H-7⋯Cl1 = 161°). In addition, the other hydrogen atom (H-6B) bound to N-6 is hydrogen-bonded to the O-8 atom (H-6B⋯O-8 = 2.05 Å and N-6–H-6B⋯O-8 = 172°).
Figure 1.
ORTEP diagram of 1 with atom-labeling scheme and thermal ellipsoids drawn at the 50% probability level. Broken lines indicate intermolecular hydrogen bonds.
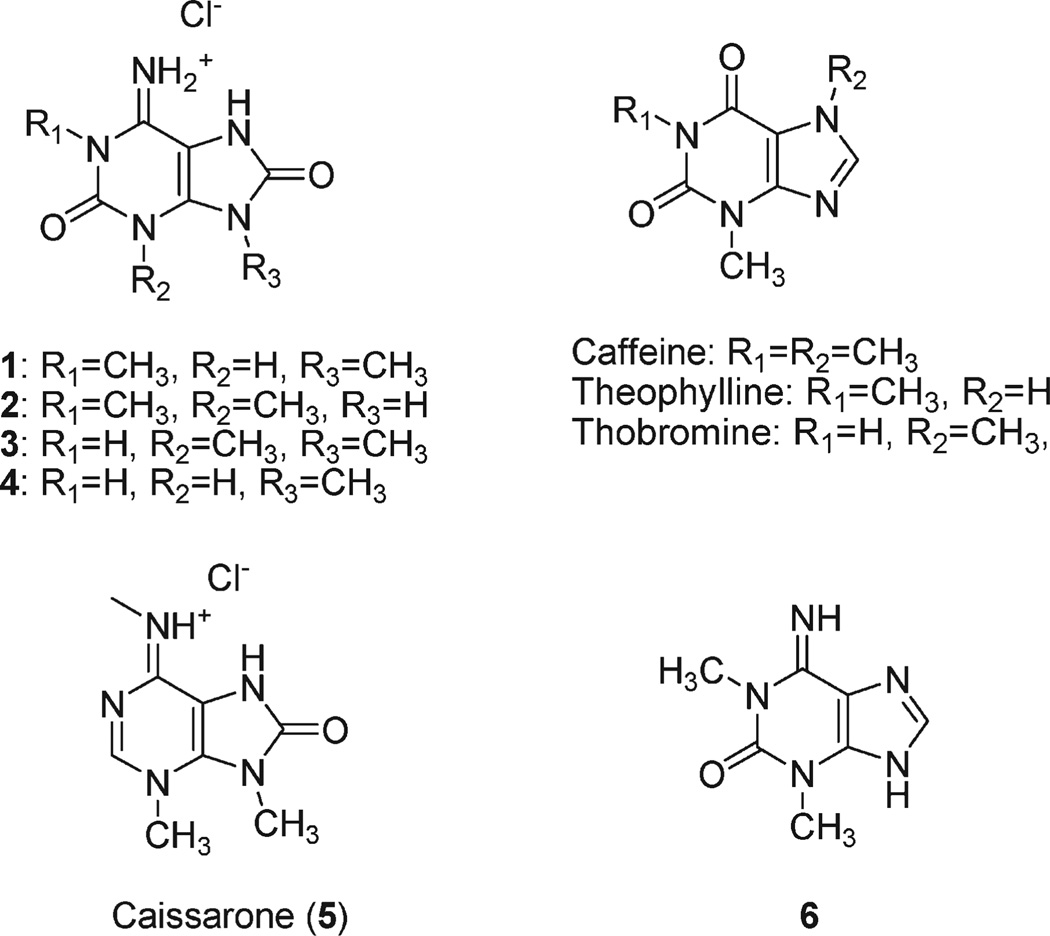
Examination of aqueous extracts from two other sponges, Haliclona sp. and Amphimedon sp., also resulted in isolation of 1 as a major convulsant constituent, suggesting that 1 might be a common metabolite in sponges of this geographical region. Further separation of the extract of Amphimedon afforded three other purines 2–4. The molecular formulas of 2 and 3 were established to be the same as that of 1 on the basis of HRFABMS and NMR data, implying that they were isomers. The carbon chemical shifts for the aromatic region of 2 and 3 (Table 1), as well as UV data, were very similar to that of 1, and it was reasonable to assume that both 2 and 3 possessed an 8-oxoisoguanine structure. On the other hand, the chemical shifts for the methyl groups varied between these molecules. These initial data suggested that both 2 and 3 were positional isomers of 1.
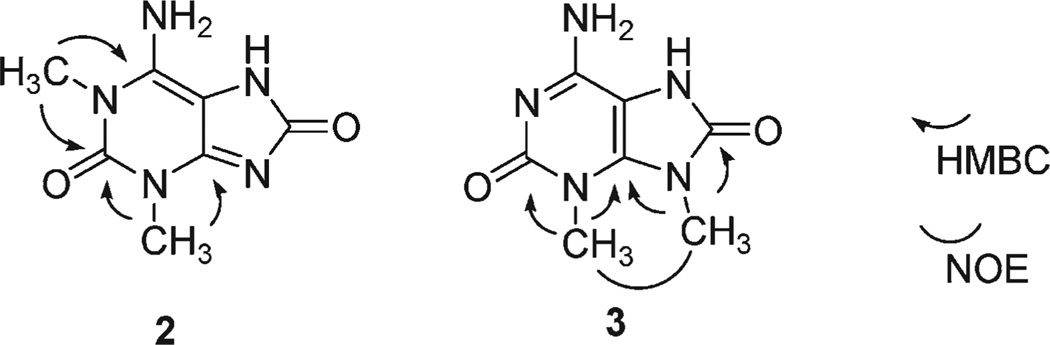
In an HMBC spectrum of 2, both methyl groups showed correlation peaks to a carbon resonating at δ 149.6. Two other carbons at δ 146.8 and 141.9 also were connected by HMBC to the methyl proton at δ 3.35 and 3.34, respectively. In the HMBC for 3, two methyl groups gave correlation peaks to a carbon signal at δ 143.8, and two other carbons at δ 149.7 and 154.0 were connected to each of the methyl protons at δ 3.56 and 3.45, respectively. These data yielded two candidates for the structures of 2 and 3, 1,3- or 3,9-dimethyl-8-oxoisoguanine. However, definitive structural assignment was again difficult because the chemical shifts for purine carbons, except for C-5, vary significantly depending on the substitution pattern.13 We, thus, measured NOE of 3 between the two methyl groups using both NOESY and difference NOE experiments. Because clear NOE was observed at δ 3.45 upon irradiation of the methyl signal at δ 3.56, and in the NOESY spectrum, the position of methyl groups in 3 was determined to be on N3 and N9. These results allowed unambiguous assignment of the chemical shift for C-4 for 3 to δ 143.8. The C-2 of compound 2, in turn, was assigned to the resonance at δ 149.6. Consequently, resonance at δ 154.3 of 2, which has no HMBC correlation from the methyl groups, was assigned for C-8. The data therefore resolve the structures of 2 and 3 as 1,3- and 3,9-dimethyl-8-oxoisoguanine, respectively (Scheme 2).
Scheme 2.
HMBC and NOE Correlations for 2 and 3
The molecular formula of 4 was deduced from HRFABMS and NMR data to be C6H7N5O2. The 13C NMR chemical shifts of 4 for aromatic carbons corresponded closely to those of 1, while only one methyl resonance was observed. These data suggested that 4 is a monomethyl derivative of 8-oxoisoguanine. In the HMBC spectrum, two correlation peaks were observed between the methyl group and resonances at both δ 153.9 and 143.6. These carbons were assignable to be C-8 and C-4, respectively, by analogy to δ13C of compounds 1–3. Thus, the methyl group of 4 was positioned on N-9. Finally, 13C NMR for the all the other aromatic carbons and UV data of 4, which were also very similar to those of 1–3, lead to the conclusion that 4 also has the 8-oxoisoguanine skeleton, thereby determining the structure of 4 to be 9-methyl-8-oxoisoguanine (Chart 1).
Chart 1.
In Vivo Neuroactivity of 8-Oxopurines
Intracerebroventricular injection of compounds 1–4 in ddY mice induced various behavioral effects, which were compared with those elicited by the structurally similar bioactive xanthines caffeine, theophylline, and theobromine (summarized in Table 2). Compound 1 induced convulsant behaviors in a dose-dependent manner. These violent episodes were brief in duration, lasting a maximum of 10 min without subsequent recurrence. A dose–response analysis (using a total of 20 mice, six doses) yielded a 50% maximal convulsant dose (CD50) of 2.4 nmol/mouse when behaviors were scored on a graded scale used previously to assess convulsant activities (see Experimental Section). At the higher doses (> 4.0 nmol/mouse), violent running, jumping, and whole body convulsion behavior was observed, similar to that induced typically by excitatory amino acids.11,15 Injection of 8 nmol or higher was lethal either immediately or after a brief period of violent hyperactivity in all mice (n=3). At lower doses (< 1 nmol/mouse), 1 induced motor suppression and catalepsy.
Table 2.
In Vivo Behavioral Activity Induced by 8-Oxoisoguanines
| compound | CD50 nmol/mouse | dose nmol/mouse | behaviors observed |
|---|---|---|---|
| 1 | 2.4 | > 4 | running-jumping, violent scratching, whole body convulsion |
| 1 | suppressiona | ||
| 2 | 54 | > 75 | whole body convulsion, tonic extension, tremor |
| 50-25 | suppressiona | ||
| 3 | 100 | suppressiona | |
| 4 | 18 | 30–110 | whole body convulsion, salivation, tang biting |
| 14 | stereotyped behaviors (squeaking, Straub tail), suppression | ||
| methylxanthines | 110 | no obvious effect |
Suppression includes loss of spontaneous motion, catalepsy, body rigidity.
Compound 2 also produced whole-body convulsions but the behavioral profile differed markedly from that induced by 1. Notably, a uniformly lethal dose (0.2 µmol/mouse, n = 3) produced violent whole-body convulsions, rather than the jumping–running type convulsions observed with 1. A lower dose of 2 (0.1 µmol/mouse) also caused death in some animals (2/5); the survivors (3/5) showed occasional tonic extension of forelimbs then weakness in their lower bodies. The lowest tested doses of 2 (25–50 nmol/mouse) elicited predominantly suppressive behaviors that included catalepsy and loss of spontaneous activity in all mice. The CD50 value of 2 was determined to be 54 nmol/mouse (19 mice, 6 doses), which was ~20-fold higher than 1. Administration of compound 3 induced a loss of voluntary movement and catalepsy as well at 0.1 µmol/mouse (n = 4). It was not possible to determine a CD50 for 3 because of the relatively low potency of the compound. Finally, compound 4 was lethally convulsant at higher doses (0.05–0.1 µmol/mouse) and the dose–response response (15 mice, 5 doses) gave a CD50 of 18 nmol/mouse. We noted, at the higher doses, salivation (2/5 mice at 110 nmol/mouse) and tongue biting (2/5 mice at 55 nmol/mouse) occurring in a subset of animals. Lower doses induced suppressive and stereotyped behaviors as well as salivation. Of note, the plant-derived methylxanthines caffeine, theophylline, and theobromine did not produce any detectable aberrant behavioral activities at the highest dose tested (0.11 µmol/mouse).
In Vitro Characterization of Neuroactivity
The excitatory amino-acid-like convulsant behavior elicited by compound 1 suggested it might be acting as a glutamate receptor agonist. To test this possibility, we used 1 to displace radioligands for the AMPA, kainate, and NMDA receptors after binding to rat brain membranes. However, no significant displacement of [3H]AMPA, [3H]kainate, or [3H]CGP39653 by compound 1 was observed at a concentration of 10 µM (data not shown). Thus, it is unlikely that the convulsant activity of this sponge purine arises from direct activation of excitatory amino acid receptors.
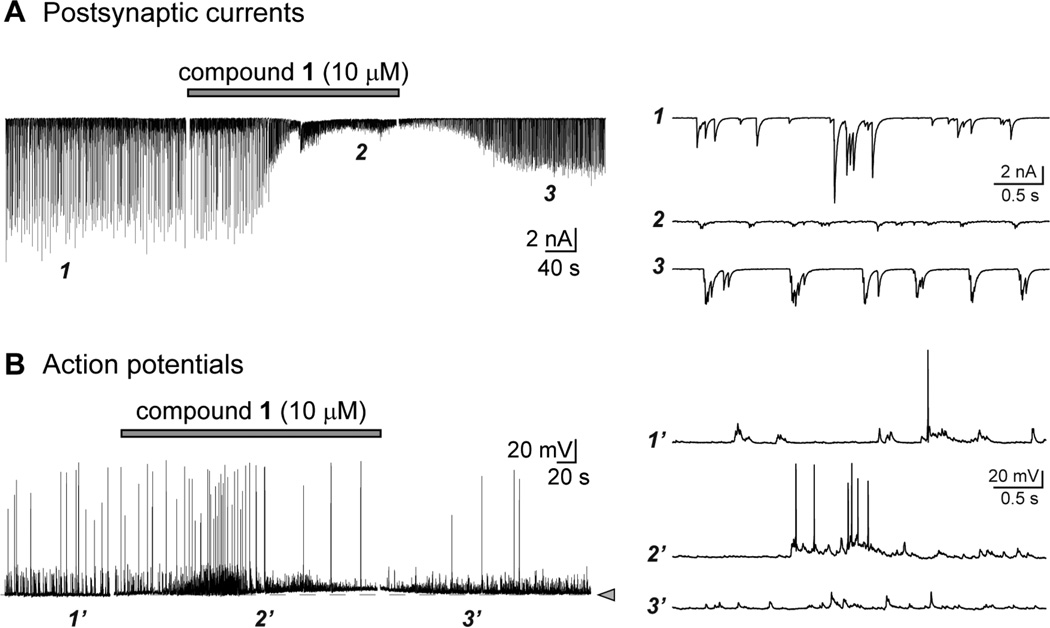
To determine if the pharmacological activity of the sponge 8-oxoisoguanines could be characterized using a neurophysiological-based approach, we first determined if they altered synaptic firing or neuronal excitability in networks of cultured rat hippocampal neurons. In voltage clamp recordings of postsynaptic currents (PSCs, both excitatory and inhibitory), application of compound 1 (1,9-dimethyl-8-oxoisoguanine, 30 µM) reliably elicited hyperexcitability, including high-frequency bursts of synaptic events and a variable amount of inward current in neurons (Figure 2A). The pattern of depolarizing bursts of compound synaptic events became more synchronized and rhythmic following application of 1 (compare traces before and after application at the expanded times scales shown in the right panel), which we noted was qualitatively similar to the effect of eliminating inhibitory transmission mediated by GABAA receptors in these cultures (data not shown). Compound 1 also impacted action potential firing to varying degrees in current clamp recordings from hippocampal neurons in culture. In some neurons, action potential firing was elevated and accompanied by a small depolarization or oscillation of the membrane voltage (e.g., Figure 2B), with the action potentials exhibiting a higher likelihood of occurring in bursts during application of 1 (see expanded traces on right). In others, action potential firing was depressed in the presence of the compound and rebounded with hyperactivity and seizure like discharges following removal of 1. While the cell-to-cell variability resulted in an overall absence of statistically significant changes in mean frequency, each neuron consistently responded with a marked alteration in intrinsic excitability in the presence of 1 (10 µM). While these recordings do not provide significant insight into the pharmacological activity of the marine toxin, taken together, they demonstrate that 1 alters the activity of functional neuronal networks to a detectable degree and therefore was efficacious in in vitro assays as well as the mouse in vivo behavioral tests.
Figure 2.
Compound 1 alters neuronal signaling. (A) Representative whole-cell voltage clamp recording of mixed excitatory and inhibitory postsynaptic currents (PSCs) recorded from cultured hippocampal neurons before, during, and after application of 1 (10 µM, gray bar, applied for 5 min). The purine analog reliably altered the frequency, amplitude, and bursts of PSCs in qualitative assessments. Expanded time scales shown in the traces on the right were taken from the trace at the indicated time points. (B) Representative whole-cell current clamp recording of action potentials and subthreshold depolarizations recorded from cultured hippocampal neurons before, during, and after application of 1 (10 µM, gray bar, applied for 5 min). The dotted line and arrowhead indicate the resting membrane potential for this cell, from which a small tonic depolarization is noted in this example. Compound 1 had variable effects on action potential firing. Expanded time scales shown in the traces on the right were taken from the trace at the indicated time points.
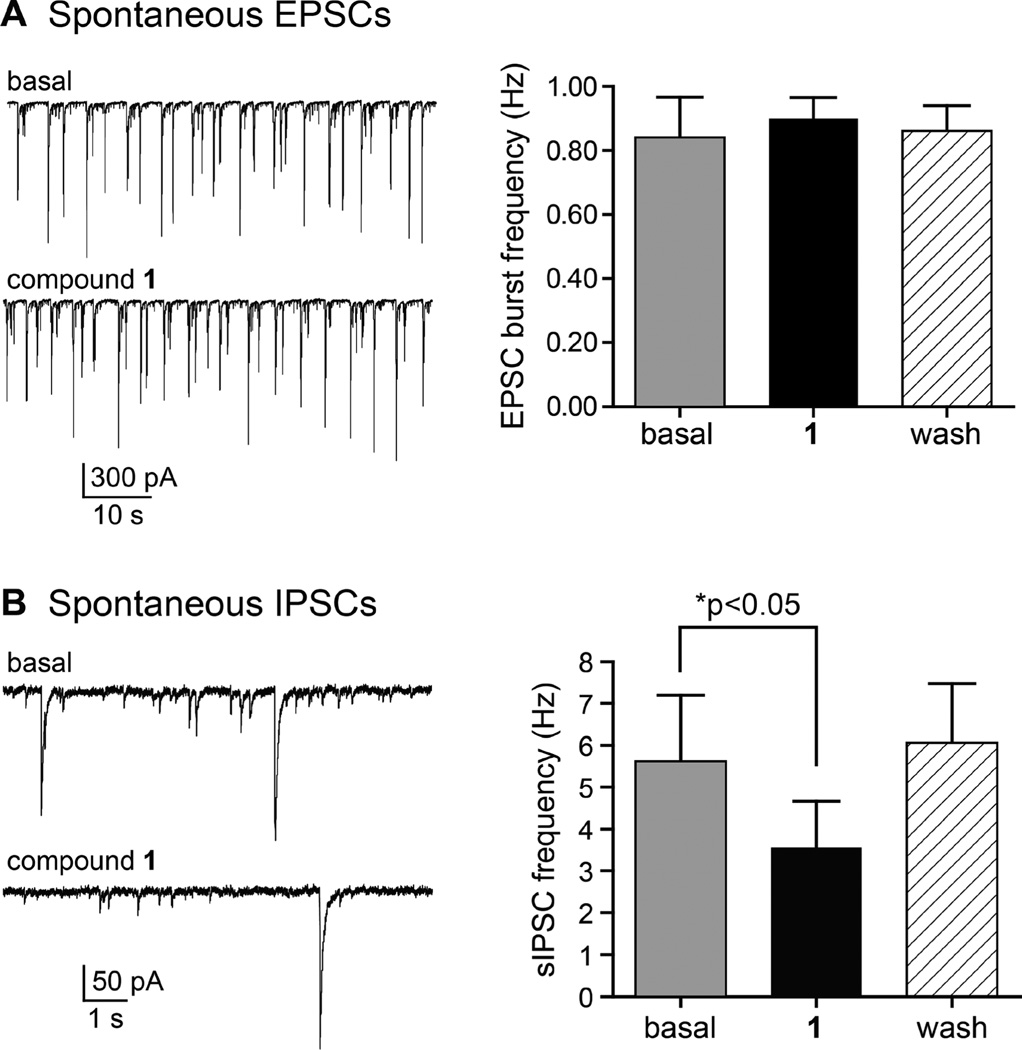
Given these promising initial results, we further defined the neuroactivity of 1 by recording pharmacologically isolated spontaneous (action potential-dependent) excitatory and inhibitory synaptic currents (sEPSCs and sIPSCs). In these highly interconnected cultured neurons, sEPSCs often occur as bursts of depolarizing currents comprised of multiple super-imposed synaptic currents, as shown in the compressed time scale in Figure 3A. Excitatory currents were isolated pharmacologically by inclusion of bicuculline (10 µM) and picrotoxin (50 µM) in the external solution to eliminate sIPSCs mediated by GABAA receptors. The frequency of sEPSC bursts was similar in the absence and presence of 1 (baseline, 0.84 ± 0.12 Hz; in 1, 0.90 ± 0.06 Hz; wash, 0.86 ± 0.08 Hz, n=4 recordings; p > 0.05, Figure 3A). Charge transferred during the burst events also was unchanged by application of 1 (data not shown).
Figure 3.
Compound 1 reduces spontaneous inhibitory transmission in neuronal cultures. (A) Representative recordings of spontaneous excitatory postsynaptic currents (sEPSCs) recorded from cultured hippocampal neurons before and in the presence of 1 (10 µM, applied for 5 min). Spontaneous EPSC burst frequency was not altered by the purine analog, as shown in the graph on the right. (B) Representative recordings of spontaneous inhibitory postsynaptic currents (sIPSCs) recorded from cultured hippocampal neurons before and in the presence of 1 (10 µM, applied for 5 min). Spontaneous IPSC frequency was significantly reduced in the presence of 1 but returned to control levels following a 10 min wash-out period (p < 0.05, repeated measures ANOVA).
In contrast, inhibitory transmission was reduced by 1. Spontaneous IPSCs were isolated following bath application of CNQX (50 µM) and d-APV (50 µM) to preclude ionotropic glutamate receptor activation (Figure 3B). Individual spontaneous events occurred at a mean frequency of 5.6 ± 1.6 Hz under basal conditions; these were significantly reduced to 3.6 ± 1.1 Hz in the presence of 1 (10 µM) and returned to 6.0 ± 1.4 Hz after wash-out of the compound (n = 5; p < 0.05, repeated measures ANOVA). These data suggested that the seizurogenic properties of 1 could in part be attributed to a reduction in inhibitory transmission.
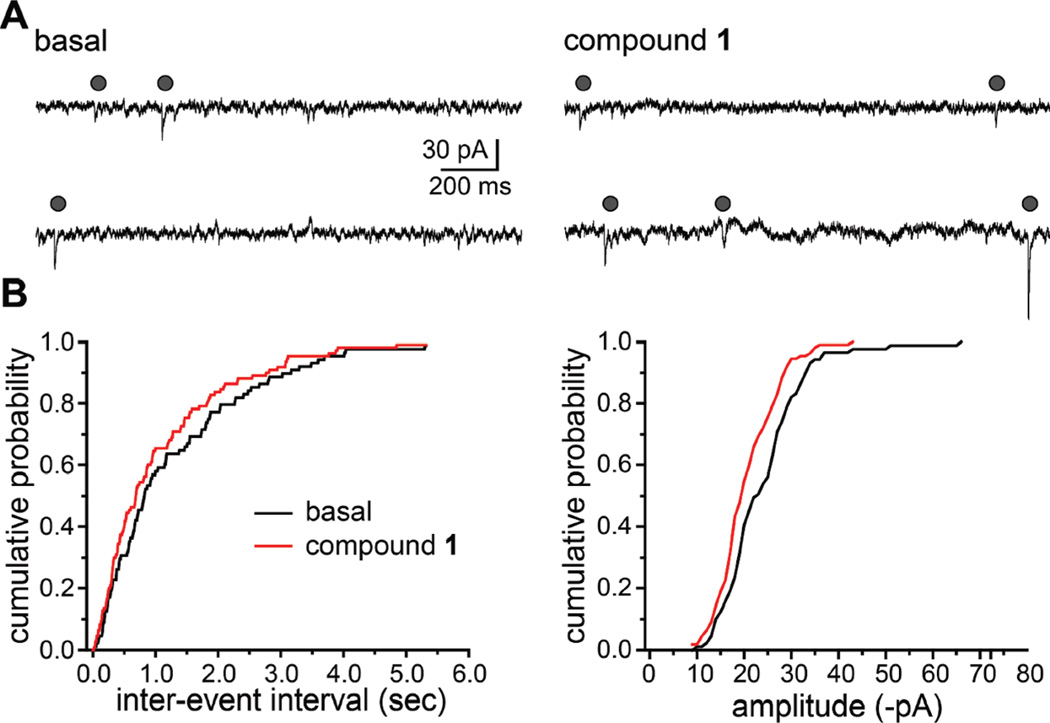
We next tested if action potential-independent synaptic events were similarly affected by 1. Miniature EPSCs (mEPSCs) were recorded in the presence of GABAA receptor antagonists and the sodium channel blocker tetrodotoxin (1 µM; examples shown in Figure 4A). Compound 1 increased the frequency of mEPSCs slightly, as illustrated in the representative cumulative probability histograms for the interevent interval in Figure 4B, but this did not reach statistical significance (basal frequency, 0.69 ± 0.21 Hz; frequency in 1, 0.95 ± 0.36 Hz; n = 4; p > 0.05). The mean amplitude of mEPSCs also were unaffected by the marine toxin, although individual cells showed slight variations as is apparent in the cumulative probability histogram in Figure 4B.
Figure 4.
Compound 1 does not alter action-potential independent excitatory transmission in neuronal cultures. (A) Representative recordings of action potential-independent excitatory postsynaptic currents (miniature EPSCs, mEPSCs) recorded from cultured hippocampal neurons before and in the presence of 1 (10 µM, applied for 5 min). Gray circles indicate mEPSCs recorded in the presence of 1 µM TTX. (B) Representative cumulative probability histograms of the interevent interval and amplitude of mEPSCs recorded from a single neuron. Black lines are control, and red lines are data in the presence of 1. Neither parameter was altered to a significant degree in the presence of the compound (p > 0.05, repeated measures ANOVA).
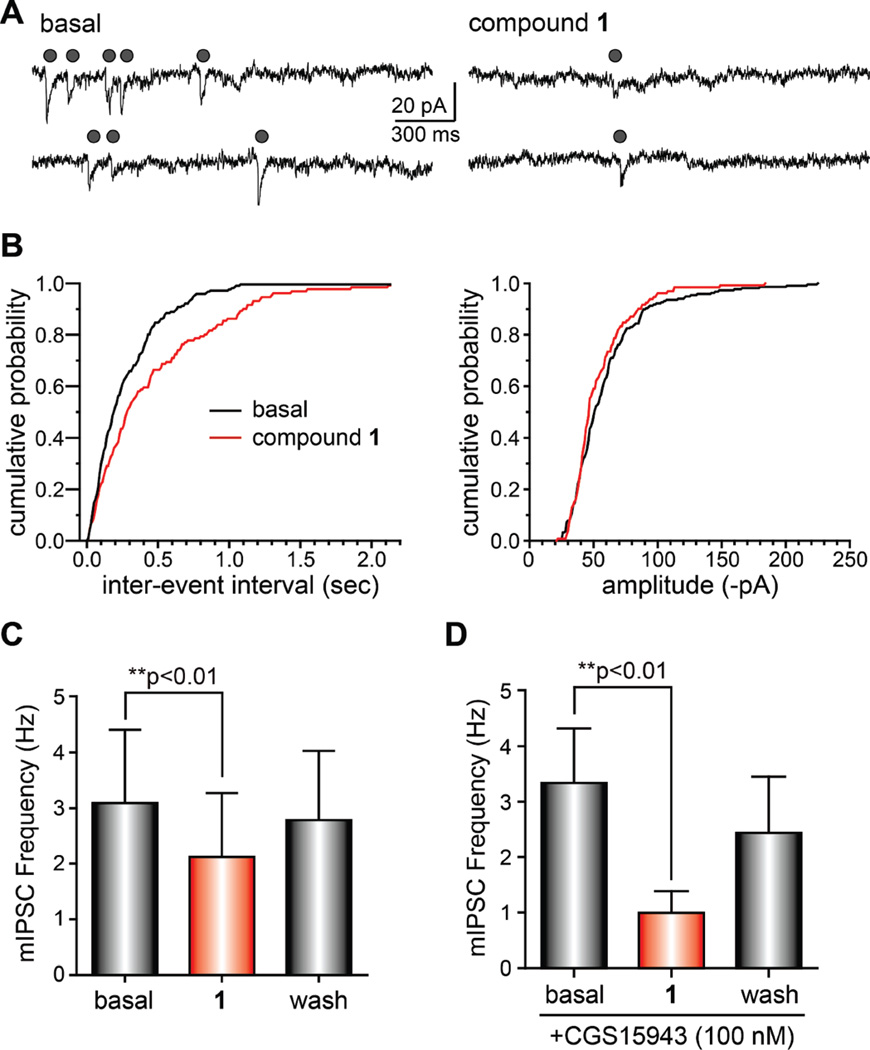
In contrast to mEPSCs, miniature IPSCs (mIPSCs) were reduced markedly by compound 1 (Figure 5A). Interevent intervals increased in the presence of the compound, whereas amplitudes of the mIPSCs were unchanged by 1 (representative cumulative probability histograms from a single recording are shown in Figure 5B). The mean frequency of mIPSCs was reduced by 1 in a reversible manner in each cell recorded (from a basal frequency of 3.1 ± 1.3 to 2.1 ± 1.2 Hz, a reduction of 32%; Figure 5C; n = 7; p < 0.01 in a repeated measures ANOVA). Mean mIPSC amplitudes were slightly reduced by compound 1, but this effect did not reach statistical significance. These data demonstrate that compound 1 primarily impacts GABAergic transmission, reducing inhibitory tone in the neuronal cultures.
Figure 5.
Compound 1 reduces action-potential independent inhibitory transmission in neuronal cultures. (A) Representative recordings of action potential-independent inhibitory postsynaptic currents (miniature IPSCs, mIPSCs) recorded from cultured hippocampal neurons before in and in the presence of 1 (10 µM, applied for 5 min). Gray circles indicate mIPSCs recorded in the presence of 1 µM TTX. (B) Representative cumulative probability histograms of the interevent interval and amplitude of mIPSCs recorded from a single neuron. Black lines are control and red lines are data in the presence of 1. Interevent intervals were significantly increased in the presence of the compound, whereas mIPSCs amplitudes were unaffected. (C) Graph showing the mean mIPSC frequency in control (basal), compound 1, and after wash-out of 1 (**p <0.01, repeated measures ANOVA). (D) Graph showing the mean mIPSC frequency in control (basal), compound 1, and after wash-out of 1 in parallel experiments performed in the presence of the nonselective adenosine receptor antagonist CGS15943 (100 nM). The effect of 1 on mIPSC frequency was not occluded by the presence of the antagonist (**p < 0.01, repeated measures ANOVA).
The reduction in mIPSC frequency suggested that 1 reduced GABAergic transmission through activation of a presynaptic receptor negatively regulating vesicular release. The structural similarity to stimulatory xanthines, such as caffeine, prompted us to consider presynaptic adenosine receptors as a potential molecular target for 1.2 We therefore recorded mIPSCs in the presence of the nonselective adenosine receptor antagonist CGS15943 (100 nM). However, bath application of 1 reduced mIPSC frequency to 29.6% of control, which was equivalent to the reduction by 1 in the absence of CGS15943 (Figure 5C and D) (n = 7; p > 0.05 compared to mIPSC reduction in the presence of 1 alone). Activity on adenosine receptors therefore did not underlie the marked reduction of inhibitory transmission in primary cultures of hippocampal neurons; this was not surprising, given that most adenosine receptor agonists display anticonvulsant activity rather than the hyperactivity produced by high doses of 1. It remains possible that 1 interacts with adenosine receptors to affect different physiological systems in vivo.
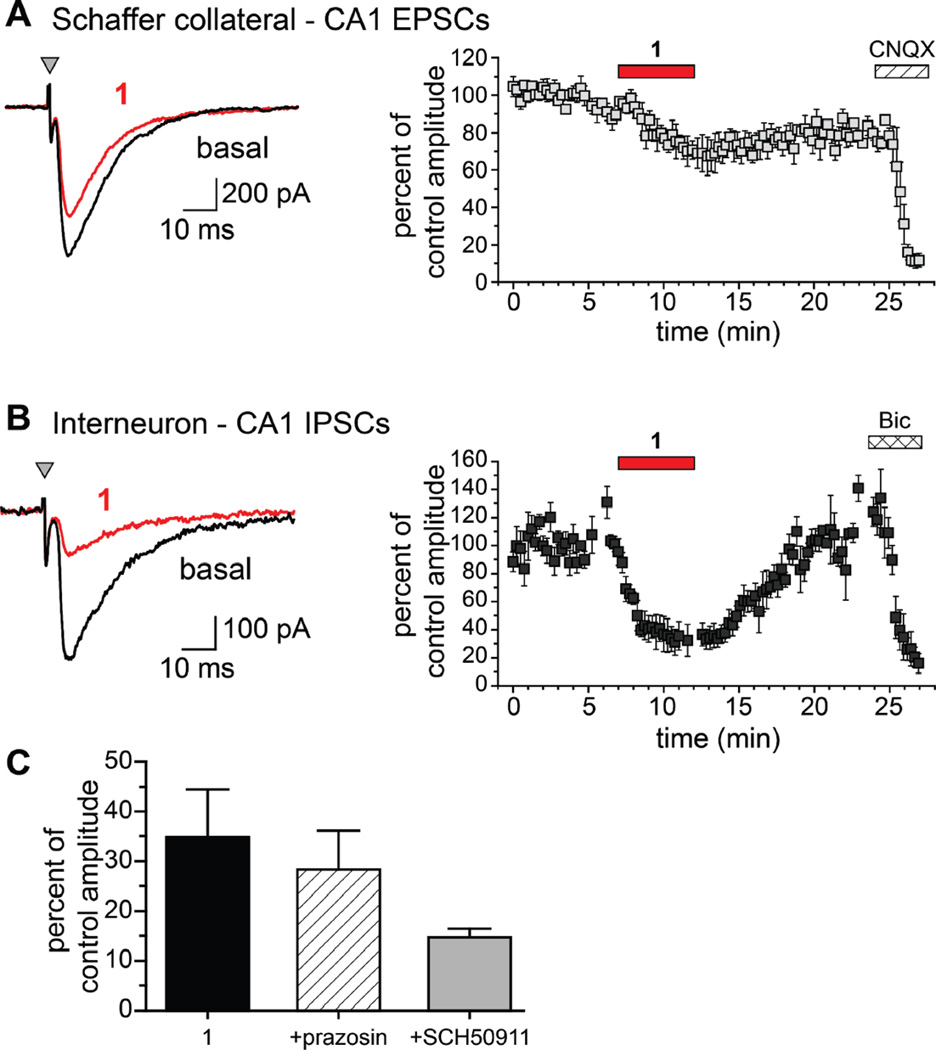
To determine if compound 1 had the same relative activity on excitatory and inhibitory neurotransmission in more intact physiological preparations, we recorded stimulation-evoked EPSCs and IPSCs, in the absence and presence of 1, from hippocampal CA1 neurons in acute brain slices prepared from juvenile mice (postnatal age 17–20 days). EPSCs in CA1 pyramidal neurons evoked by stimulation of Schaffer collateral afferents in the stratum radiatum were reduced modestly by 1 (30 µM) from a mean amplitude of 647 ± 109 pA to 502 ± 121 pA, an attenuation of 22% (n=3, p < 0.05; Figure 6A). The effect of the compound on inhibitory transmission was more profound. IPSCs elicited at an interval of 40 ms were markedly reduced by 1, from a mean control amplitude of 251 ± 59 pA to 72 ± 11 pA in the presence of the compound (71% reduction, n = 5; p < 0.05, paired Student’s t test; Figure 6B). While the paired-pulse ratio increased in the presence of 1 in a subset of recordings, the trend did not reach statistical significance (data not shown).
Figure 6.
Compound 1 reduces GABAA receptor mediated inhibitory neurotransmission to CA1 pyramidal neurons. (A) EPSCs from CA1 pyramidal neurons were evoked by monopolar stimulation of Schaffer collateral axons in mouse hippocampal slices. Left: Representative traces of stimulus-evoked CA1 neuron EPSCs from juvenile mouse hippocampal slices in the absence (black traces) and presence (red traces) of 30 µM 1. Right: Average traces for all experiments showing the modest effect of 1 after a 5 min application (red bar) on CA1 EPSCs. The nonselective AMPA/KAR receptor antagonist CNQX (50 µM) eliminated the EPSCs, confirming their identity. (B) IPSCs from CA1 pyramidal neurons were evoked by monopolar stimulation in the stratum radiatum in mouse hippocampal slices. Left: Representative traces showing that 1 (red trace) significantly reduced CA1 IPSC amplitudes when compared to basal (predrug) conditions. Right: Average traces for all experiments showing the significant reduction of IPSCs by 1 after a 5 min application period (red bar). After a washout period the GABAA antagonist bicuculline (10 µM) was added to confirm the IPSC recordings (n = 5; p < 0.05, paired Student’s t test). (C) Summary graph showing that compound 1 reduces IPSCs to a similar degree when coapplied with the nonselective α1 adrenoceptor antagonist prazosin (5 µM) or the selective GABAB receptor antagonist SCH50911 (20 µM).
As an additional means of determining the neuropharmacological target(s) of compound 1, the binding affinity of 1 to various neurologically relevant recombinant receptors were evaluated using radio ligands binding assays performed by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH-PDSP) conducted at the University of North Carolina at Chapel Hill. Of the 53 recombinant receptors tested (see Experimental Section for a complete list), 1 showed moderate affinity for α-adrenoceptors (KI values of 885, 758, and 497 nM for each α1A, α1B, and α1D, respectively) and weak affinity for the serotonin receptor 5-HT1E (2.9 µM), nicotinic receptor α3β2 (4.2 µM), and κ- and μ-opioid receptors (1.8 µM and 8.9 µM, respectively).
Based on the information derived from the PDSP screening, we determined if the inhibitory action of compound 1 on evoked IPSCs in recordings from CA1 pyramidal neurons was altered by prazosin (5 µM), a nonselective α1 antagonist. As shown in the graph in Figure 6C, compound 1 reduced IPSCs to an equivalent degree in the presence of prazosine, demonstrating that activation of α1-adrenoceptors did not underlie the toxin activity. We also tested if GABAB receptors represented the site of action of 1, because these auto-receptors are a prominent modulatory system controlling release of inhibitory neurotransmitter in the CNS. However, inhibition of GABAB receptors with SCH50911 (20 µM) did not mitigate the action of compound 1 on IPSC amplitudes (Figure 6C). Thus, we conclude that neither α-adrenoceptors nor GABAB receptors are the pharmacological targets of 1,9-dimethyl-8-oxoisoguanine relevant to its convulsant activity.
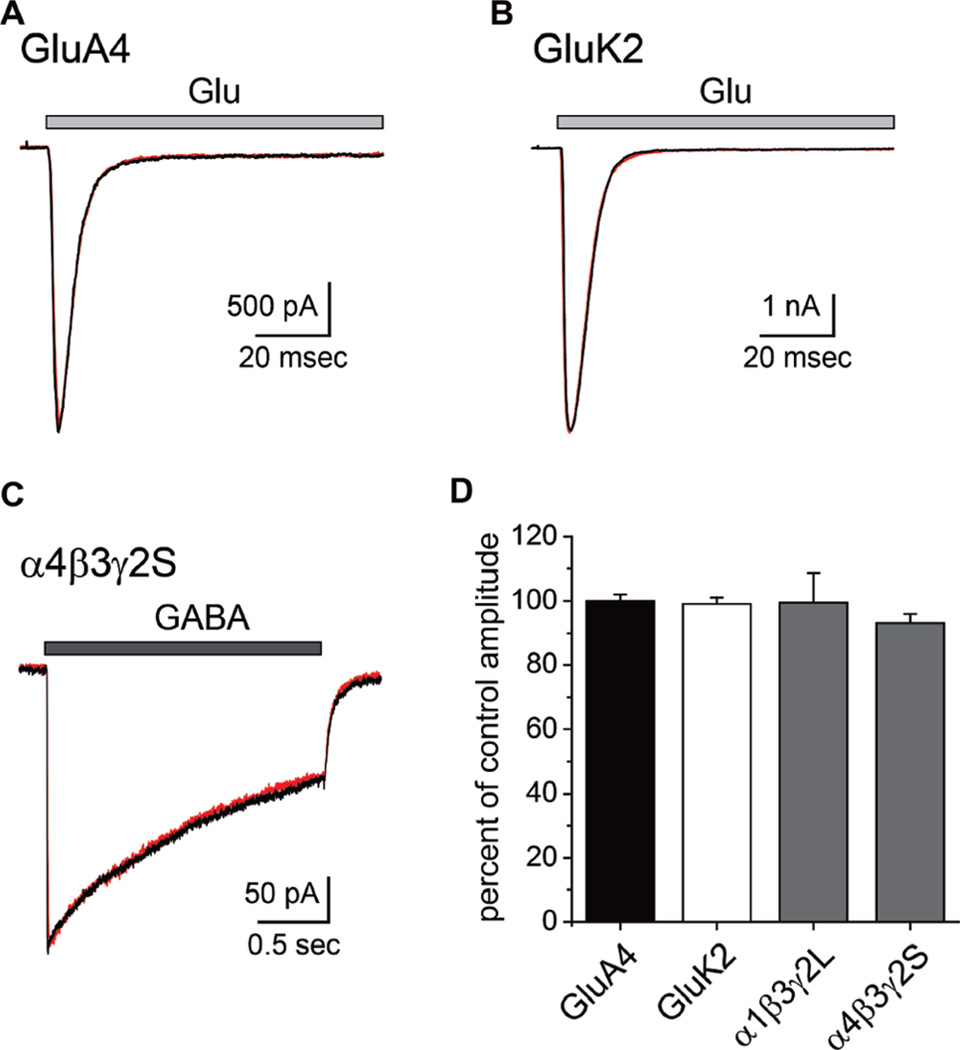
The reduction in AMPA receptor EPSCs and GABAA receptor IPSCs in hippocampal slice preparations could arise in part from direct antagonistic effects on the receptors themselves. As well, the structurally related xanthines have been characterized as competitive antagonists of GABAA receptors, albeit at nonphysiological concentrations (1–10 mM).16 To test these possibilities, we measured the effect of 1 on the amplitude of currents evoked from recombinant AMPA, kainate, or GABAA receptors transiently expressed in HEK293-T/17 cells (Figure 7). Currents elicited from either GluA4 AMPA or GluK2 kainate receptors by glutamate (10 mM, 100 ms application) were not reduced by preincubation with compound 1 (100 µM; 100 ± 2% and 99 ± 2%, n = 3 each, p > 0.05 relative to mean control amplitudes; Figure 7A, B, and D). Similarly, GABA-evoked currents from either α1β3γ2 L or α4β3γ2S GABAA receptors were not reduced by preincubation with 30 µM of 1 (99 ± 9 and 93 ± 3% of mean control amplitude, respectively, n = 5 each, p > 0.05; Figure 7C,D). In addition, 1 did not displace [3H]muscimol or [3H]flunitrazepam ligands for the orthosteric GABA binding site and allosteric benzodiazepine binding site, respectively, from rat brain membranes at concentrations of <10 µM. Thus, direct inhibition of receptor activation is unlikely to contribute to the neuroactive effects of 1.
Figure 7.
Compound 1 does not antagonize recombinant AMPA, kainate, or GABAA receptors. (A) Representative traces showing currents evoked by glutamate from recombinant GluA4 receptors in the absence (black) and presence (red) of 1 (100 µM). Glutamate (10 mM) was fast-applied for 100 ms. (B) Analogous traces showing the lack of effect of 1 on glutamate-evoked currents from GluK2 kainate receptors. (C) Representative traces showing that the amplitude of currents gated by GABAA receptors composed of α4β3γ2S subunits were unaffected by the presence of 1 (30 µM). GABA (1 mM) was applied for 2 s in these experiments. All recordings were in voltage-clamp mode at a command potential of −70 mV. (D) Summary graph showing the mean ± sem for current amplitudes in the presence of 1 expressed as a percentage of the control amplitudes in the absence of the purine.
In summary, these data provide a physiological basis for the convulsant phenotype; while compound 1 clearly impacts both excitatory and inhibitory neurotransmission, the latter is much more sensitive, and consequently, a profound reduction in inhibitory tone is the predominating neurological pharmacological effect.
Discussion
We report here the isolation of three new and one known 8-oxoisoguanine derivatives. Despite their structural simplicity, rigorous structure determination of these purines, including assignment of heterofunctionality on the purine ring, was not trivial using spectral analyses alone. X-ray structural information for 1, isolation of a series of positional isomers, and NOE information for 3 offered a unique opportunity to assign all the 1H and 13C NMR data through careful data comparisons.
Although marine organisms are a prolific source of modified purines, 8-oxo derivatives are relatively rare. Compounds 1, 3, and 4 have not been described in the literature, while 2 was identified from the New Zealand tunicate Pseudodistoma cereum.17 The NMR data reported previously (taken in DMSO), however, were found to differ substantially from those of 2 and, in fact, were rather closer to that of 3 (Table 1). In the structural determination of the tunicate-derived compound, the authors assigned C-5, N-7, and NH2 rigorously on the basis of 13C NMR and 1H–15N gHMBC NMR data. However, assignments of the N-methyl groups at N-1 and N-3 were less clear because the authors did not examine the possibility of alternative methyl substitutions at N-3 and N-9. These could reside in the correlation pattern observed in the NMR data, given that C-5 is the only carbon atom whose chemical shift was assigned unambiguously.17 In the present study, isolation of a unique set of methylated 8-oxoisoguanines yielded rational assignment of 13C NMR chemical shifts for each purine carbon atom. Thus, it is possible that the structure of 3, rather than 2, is better suited for the ascidian purine isolated previously.17
A large number of purine-based molecules have been isolated from natural sources, but few beyond the well-known plant-derived xanthines have been examined rigorously for neuroactivity. One such compound, the sea anemone-derived caissarone (5;Chart 1),18 shares some structural elements with the novel purines isolated in our study, as does 1,3-dimethylisoguanine (6).9 Both compounds elicited twitch responses from electrically stimulated guinea pig ileum-myenteric plexus that competed with adenosine receptor agonists such as adenosine, the A1 selective agonist R-phenylisopropyladenosine (R-PIA) and the A2 selective agonist 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA). Thus, the molecular target of 5 was deduced to be adenosine receptors, although the potency was much lower than that of theophylline.10 Compound 6 exhibited similar but weaker activity compared to 5.
The new marine sponge-derived 8-oxoisoguanines were shown to exhibit various in vivo activities in mice upon icv injection. Compounds 1, 2, and 4 were generally convulsant, but their behavioral profiles were divergent (Table 2), and at subconvulsant doses, these compounds displayed rather suppressant behaviors. Compound 3 did not induce convulsant behavior at the highest dose tested. These complex behavioral profiles observed for the purines suggest multifaceted in vivo mechanisms of action. None of these in vivo actions was reproduced by injection of caffeine, theophylline, or theobromine at 110 nmol/mouse, which was consistent with earlier results showing that the seizurogenic thresholds of methylxanthines are quite high. For example, the cerebrospinal concentration of theophylline required to induce seizures in rats is about 1 mM.19 Thus, compounds 1, 3, and 4 are more potent convulsants as compared to themethylxanthines, suggesting that both themethyl substitution pattern and the heteroatom substitution on the purine ring play crucial roles in producing this diversity of in vivo biological activity.
Compound 1 proved to have an in vitro physiological neuroactivity consistent with its convulsant actions in vivo. Both action potential-dependent and -independent inhibitory transmission mediated by GABAA receptors was reduced by micromolar concentrations of 1. This effect was particularly evident in our analysis of evoked IPSCs in hippocampal slices from mouse brain, in which 30 µM of the purine profoundly depressed IPSC amplitudes in CA1 pyramidal neurons. Compound 1 also caused a marked reduction in mIPSC frequency, but not amplitude, in cultured hippocampal neurons. While the target of 1 could not be definitively identified in the present study, these results suggest that appropriate candidates are one or more presynaptic autoreceptors that preferentially modulate inhibitory rather than excitatory transmission. Based on the physiological activity in the hippocampal slice preparations, two obvious possibilities were adenosine orGABAB receptors, but the physiological and behavioral effects of 1, a moderately potent convulsant, are not consistent with agonism at either of these targets, activation of which generally dampens, rather than enhances, CNS excitability.20–22 Physiological analysis confirmed this supposition, in that antagonists for neither adenosine nor GABAB receptors occluded the inhibitory effect of 1 on IPSCs.
While we cannot yet exclude postsynaptic contributions, our initial studies with recombinant GABAA receptors suggest that pharmacological antagonism of the receptors was unlikely to explain the marked effect on inhibitory transmission. Similarly, direct effects on ionotropic glutamate receptors probably do not account for the modest inhibitory effect of 1 on EPSC amplitudes in hippocampal CA1 pyramidal neurons. It remains possible that higher concentrations of the isoguanines could act on GABAA receptors, analogous to the effect of millimolar concentrations of theophylline on recombinant α1β2γ2 GABAA receptor-mediated currents.16
There are clear similarities between the activity of 1 and the complex neuropharmacological activity of caffeine. High concentrations of caffeine (1–30 mM) have been shown to modulate IPSCs through both [Ca2+]i-dependent23,24 and-independent25 mechanisms. Ca2+ release from ryanodine sensitive calcium store, a well-characterized pharmacological action of caffeine, accounts for the [Ca2+]i-dependent process. The [Ca2+]i-independent action of caffeine, however, was not affected by ryanodine.25 Application of adenosine did not affect the phenomenon and neither did forskolin attenuate the action of caffeine, eliminating a role for adenosine receptors and cyclic nucleotide phosphodiesterases, which are important targets of the stimulant.25These observations parallel our data, which also suggests that adenosine receptors do not mediate the actions of 1. We have not formally ruled out inhibition of phosphodiesterases as a contributing factor, but we note that the pharmacological action of 1 is inconsistent with the known effects of elevating cAMP on GABAergic transmission in the hippocampus.26 It therefore remains to be determined if the weak effects of caffeine and more potent activity of 1,9-dimethyl-8-oxoisoguanine on hippocampal inhibitory transmission share a common pharmacological basis.
Screening by the PDSP served as an alternate means of discovering the pharmacological target of 1. In a series of target receptor screening assays carried out by the PDSP, 1 showed moderate (micromolar) binding affinity toward the α-adrenoceptors α1A, α1B, and α1D that was 400–1900 times lower than that of the classic antagonist prazosin, a nonselective α1 receptor antagonist. Compound 1 did not bind to any α2 or β-adrenergic receptors in these screens, and thus appears to be selective for the α1 subtype. It is of interest that the molecule possessed this receptor affinity because it represents the first bioactive purine, including methylxanthines, identified as an adrenergic receptor ligand. It will be of interest to test the biological relevance of this binding data, even in view of our results that suggest these binding properties do not directly account for the in vivo phenotype of 1 observed in the present study. The binding data, though preliminary, is supportive of a pleiotropic molecular basis for the complex behavioral effects of the purine. This broad spectrum but weak binding affinity is not very surprising for the small purine like 1; structurally similar xanthines such as caffeine and theophylline are known to have a variety of pharmacological targets.2
Taken together, the newly discovered purines 1–4 are simple but biologically significant entities, and 1 may modulate presynaptic receptors to alter inhibitory transmission. The oxoisoguanine template scaffold may serve as starting point for drug discovery given the relevance of purine and purine-derived molecules to treatment of a variety of diseases. For example, synthetic caffeine analogues have been extensively studied as therapeutic agents for neurological diseases such as Alzheimer’s and Parkinson’s disease and pain, as well as non-neurological diseases that include cancer and diabetes.2 New and unexpected biological targets for substituted purines continue to be discovered; most recently, methylxanthine drugs, including theophylline, were found to inhibit family 18 Chitinase, which could represent a therapeutically approachable target for asthma treatment.27 Most synthetic analogues developed to date utilized the 2,6-oxopurine core as a template and therefore do not possess an isoguanine backbone. Our data suggests, however, that expansions of functional modification in the purine ring, including polar atoms at C2, 6, and 8, as well as various substitutions at the nitrogen atoms within the purine ring could lead to discovery of molecules with novel neuropharmacological profiles.
Significance
Our study demonstrates that (i) simple purines with structural similarity to caffeine and other methylxanthines display novel pharmacological profiles, including modulation of inhibitory transmission in the mammalian CNS. (ii) Neuronal targets of the purines are likely broad rather than specific to a single molecular entity, and the biological activity of the purines can be “fine tuned” by simple alkyl or heteroatom substitution on the purine ring. (iii) Close structural similarity to molecules that are used routinely by humans, as well in the clinic, suggest that these molecules represent potential new templates for drug discovery in that they might act on a different set of molecular targets with therapeutic benefit.
Experimental Section
Chemistry. General
1H and 13C NMR data were measured at 500 and 125 MHz, respectively. Chemical shifts are reported in ppm with HDO peak at δH 4.67 (at 300 K), and methanol-d4 at δC 49.0 as a reference for 1H and 13C data, respectively. MALDI-TOFMS and -TOFMS/MS were obtained on Applied Biosystems AB 4700 spectrometer using gentisic acid or α-cyano-4-hydroxycinnamic acid as matrixes. FABMS and HIRESFABMS data were measured on JEOL JMS-SX102A using glycerol as matrix. UV data were measured on JASCO V630 spectrophotometer in water or HCl (4 × 10−4 M). IR data (KBr) were measured on JASCO FTIR 4200. High performance liquid chromatography (HPLC) was performed using COSMOSIL (C18, Naklarai Tasque) with multiwavelength detector (JASCO MD910) at a flow rate of 1 mL/min for 10 × 250mmcolumn or 4 mL/min for 25 × 200 mm column, (MeOH–H2O 1:9, 0.1% trifluoroacetic acid). The purity of compounds 1–4 were assessed to be >95% by HPLC analysis.
Sponge Samples
Samples of C. olemda, Haliclona sp., and Amphimedon sp. were collected in Republic of Palau in 2006 and 2007. An ethanol-preserved sample of each animal was identified by Dr. John Hooper at Queensland museum, Australia.
Isolation
A sample of C. olemda (100 g) was squeezed gently in water and the aqueous extract was dialyzed against water using cellulose tubing. The outer solution was concentrated in vacuo, and the resulted residue (5 g) was chromatographed on a Sephadex LH20 column with water. The purine fractions with absorption at 260 nm were pooled and concentrated to give a solid (12 mg). Fine crystalline precipitations were collected, redissolved in 1 M HCl. A colorless plate crystal of 1 was obtained by slow evaporation from the hydrochloric acid solution (0.1 M):UV(H2O) λmax (log ε) 211 (4.31), 242 sh (3.71), 304 (4.05) nm. IR (KBr) νmax 3100, 1710, 1677, 1630, 1580, 1508, 1388, 1339, 1001 cm−1. HRFABMS m/z: Calcd for C7H10N5O2, 196.0835 (M + H)+; found, 196.0781.
A sample of Amphimedon sp. (700 g) was extracted and dialyzed as above to give the outer residue (28 g). A part of the residue (7.3 g) was flash-chromatographed on C18 reversed-phase column with successive water, 25%, and then 50% methanolic water as eluents. The purine fraction was eluated with 25% MeOH (430 mg). The precipitation was collected by centrifugation (40 mg), dissolved in 1 M HCl, and then separated and purified by HPLC to give compound 1 (TR=25.6 min, 18.7 mg); 2 (TR = 22.6 min, 12.9 mg), UV(H2O; log ε) 202 (4.21), 221 (4.12 sh), 311 (4.19). IR (KBr) ν 3158, 1701, 1656, 1546, 1204, 1146 cm−1. HRFABMS found 196.0838; 3 (TR = 17.1 min, 2.12 mg). UV (HCl) λmax (log ε) 214 (4.51), 239 sh (3.93), 305 (4.21) nm. IR (KBr) ν 3379, 3156, 2783, 1757, 1688, 1612, 1553, 1515, 1428, 1397, 1345, 1253, 1105, 988 cm−1. HRFABMS: Found 196.0828; and 4 (TR = 19.6 min. 3.28 mg). UV (HCl) λmax (log ε) 212 (4.33), 236 sh (3.87), 310 (4.13) nm. IR (KBr) ν 3376, 3154, 3043, 2907, 1769, 1699, 1613, 1587, 1472, 1403, 1331, 1260, 1040 cm−1. HRFABMS m/z: Calcd for C6H8N5O2 (M + H)+, 182.0678; found, 182.0686.
X-ray Crystal Structure Analysis of 1
Crystal data: Empirical formula = C7H10N5O2 · Cl,Mr=231.65,T=193 K, crystal system= monoclinic, space group = P21/c, unit cell dimensions: a = 9.2154(3) Å, b = 15.7485(5) Å, c = 6.8189(2) Å, β = 104.581(2)° V = 957.75(5) Å 3, Z = 4, Dcalc = 1.607 mg·m−3, λ = 1.54178 Å, µ(Cu Kα) = 3.487 mm−1, F000 = 480, crystal size = 0.50 × 0.25 × 0.10 mm3, θ range for data collection: θ = 4.96–68.23°, index ranges = −10 ≤ h ≤ 11, −18 ≤ k ≤ 18, −8 ≤ l ≤ 8, reflections collected = 11789, independent reflections = 1742 [Rint = 0.0393], completeness to θ = 68.23°, 99.8%, absorption correction = numerical correction, maximum and minimum transmission = 0.706 and 0.366, respectively. Refinement method: full-matrix least-squares on F2, data/restraints/parameters = 1742/0/155, S = 1.075, final R indices [I > 2σ(I)]: R1 = 0.0284, wR2 = 0.0758; Rindices (all data):R1 = 0.0295,wR2 = 0.0764, largest difference peak and hole = 0.289 and −0.197 e ·Å−3.
Behavioral Assays
The mouse behavioral assays were performed under approval by the Ethical Committee of Experimental Animal Care at Hokkaido University. The effects of purines 1–4 and caffeine, theophylline, and theobromine on mice behavior were assessed as described earlier.11 Briefly, an aqueous solution of sample (20 µL) was injected intracerebroventricularly inmale ddY mice of 3–4 weeks (Japan SLC Inc., Hamamatsu), as described. Because the highest concentration of 3 and 4 (1 mg/mL) was not completely soluble even in hot water, forming fine precipitation, the sonicated emulsion was directly transferred and then diluted to the concentration used. At least three mice were used at each dose. Behaviors were observed for 60 min and were graded in order of severity as follows: death 7, whole body convulsion 6, loss of balance 5, violent running–jumping 4, stereotype behaviors 3, loss of voluntary movement 2, sedating 1, and no effect 0. Data were analyzed by using a computer software (GraphPad Prism, Graph-Pad Software, CA).
Receptor Binding Profiles
Receptor binding profiles and Ki determinations were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program [Contract # HHSN-271-2008-00025-C (NIMH PDSP)]. The NIMH PDSP is directed by Bryan L. Roth MD, Ph.D. at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda, MD. The recombinant or native receptors tested thus far for primary binding assay include serotonin receptors (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT5A, 5-HT6, and 5-HT7), nicotinic receptors (α2β2, α2β4, α3β2, α3β4, α4β2, and α4β4), adrenergic receptors (α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β3), the benzodiazepine receptor (BZP rat brain site), Ca2+ channel, dopamine receptors (D1, D2, D3, D4, D5), dopamine transporter (DAT), prostaglandin receptors (EP3, EP4), GABAA (rat brain muscimol binding site), histamine receptors (H1, H2, H3, H4), muscarinic receptors (M1, M2, M3, M4, M5), opioid receptors (μ, κ, δ), biogenic amine transporters (NET, SERT), and sigma receptors (σ1, σ2). For experimental details, refer to the PDSP web site http://pdsp.med.unc.edu/.
Receptor binding studies for ionotropic glutamate receptor sites were performed according to the method previously reported.11,28–30 Briefly, a synaptic membrane was prepared from Sprague–Dawley rats (200–220 g). Labeled [3H]kainic acid, [3H]AMPA, and [3H]CGP39653 were used as the ligands for the kainic acid, AMPA, or NMDA binding site. The conditions for each binding assay were as follows (ligand, ligand concentration, incubation temperature, incubation time, buffer): [3H]KA, 1 nM, 4 °C, 1 h, 100 mM Tris-HCl (pH 7.1); [3H]AMPA, 5 nM, 4 °C, 1 h, 50 mM Tris-HCl (pH 7.6) containing 100 mM KSCN; [3H]CGP39653, 2 nM, 4 °C, 1 h, 5 mM Tris-HCl (pH 7.7).
Electrophysiology. Cultured Neurons
Standard patch-clamp techniques were used to record from primary neurons prepared from embryonic (E18) rat hippocampi. Whole-cell voltage or current clamp recordings were carried out after 17–28 days in vitro. Neurons were either voltage-clamped at −70 mV or current-clamped to resting membrane potential (~60 mV) in whole-cell mode. The extracellular solution contained (in mM) 140 NaCl, 10 glucose, 10 Cs-HEPES, 3 KCl, 2 CaCl2, and 1 MgCl2 (pH 7.3). The internal solution for voltage clamp recordings contained (in mM) 95 CsF, 25 CsCl, 10 Cs-HEPES, 10 EGTA, 2 NaCl, 2 Mg-ATP, 10 QX-314, 5 TEA, and 5 4-AP (pH 7.3) and the internal solution for current clamp recordings contained (in mM) 120 KMeSO4, 5 KCl, 5 NaCl, 1 MgCl2, 11 Na-HEPES, 10 phosphocreatine, 4 Na-ATP, and 0.3 Na-GTP (pH 7.0). Borosilicate patch electrodes were pulled and fire polished to 3–5MΩ resistance. Compounds were bath-applied. All recordings were carried out with an Axon Axopatch 200B or Multiclamp 700A using pClamp software (Molecular Devices, Sunnyvale, CA). Analysis was performed off-line using Clampfit or MiniAnal software (Synaptosoft, Decatur, GA).
Recombinant Recordings in HEK 293-T/17 Cells
Recombinant plasmid DNAs containing GluK2 and GluA4cDNAs were contributed by Drs. Stephen Heinemann (The Salk Institute, La Jolla, CA) and Peter Seeburg (Max-Planck Institute, Heidelberg, Germany), respectively. GABAA receptor cDNAs were kindly donated by Drs. Stephen Moss (Tufts University, Boston, MA) and Toshio Narahashi (Northwestern University, Chicago, IL). Receptor and eGFP cDNAs were transiently expressed in HEK293-T/17 cells (CRL-11268) following transfection using Mirus Trans-IT reagent (Mirus Bio Corporation, Madison, WI). Whole-cell recordings were conducted 1–3 days following transfection using protocols and solutions similar to those utilized in cultured neuron voltage clamp experiments. Two to three days post-transfection, eGFP-expressing cells were lifted from the coverslip and voltage clamped in whole-cell mode. Lifted cells were maintained in a laminar stream of extracellular solution from a triple-barreled flow pipe, which was rapidly translated using a piezoceramic bimorph for fast application of glutamate or GABA. Glutamate was applied at a concentration of 10 mM for 100 ms. GABA was applied at either 1 mM (α4β3γ2S) or 100 mM (α1β3 γ2 L).
Acute Slice Preparations
Hippocampal CA1 pyramidal neurons were voltage-clamped in brain slices (350 µm) prepared from 129SvEv mice aged P16–P21. Hippocampal slices were prepared in a sucrose slicing solution containing (in mM) 85 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose, 75 sucrose, 0.5 CaCl2, 4 MgCl2, 0.5 Na ascorbate, 10 µM DL-APV, and 100 µM kynurenic acid (pH 7.3). After slicing, the solution was exchanged with an oxygenated incubation solution containing (in mM) 125 NaCl, 2.4 KCl, 1.2 NaH2PO4, 25 NaHCO3, 25 glucose, 1 CaCl2, 2 MgCl2, 0.5 Na ascorbate, 10 µM DL-APV, and 100 µM kynurenic acid (pH 7.3). Recordings in voltage clamp mode used CsF/CsCl internal solution and carbogenated external recording solution containing (inmM) 120 NaCl, 2.4 KCl, 2MgCl2, 1 CaCl2, 25 NaHCO3, 1.2 NaH2PO4, and 25 glucose (pH 7.3). For EPSC recordings, 10 µM bicuculline methiodide, 50 µM PTX, and 50µM D-APV were added to the solution. For IPSCs, 50 µM D-APV and 50 µM CNQX were added to the external solution. Hippocampal CA1 pyramidal cells were voltage clamped at −70 mV and stimulated with a monopolar electrode in the stratum radiatum.
Acknowledgment
We thank Dr. Eri Fukushi and Mr. Kenji Watanabe at GC-MS and NMR Laboratory, Graduate School of Agriculture, Hokkaido University for NMR and HRFABMS measurements, respectively. We also are grateful to Dr. Bill Marszalec at Northwestern University Feinberg School of Medicine for preparation of cultured hippocampal neurons. We thank Eri Yoshida, Yoichi Ishizuka, and Koichi Doi at Kitasato University School of Fisheries Sciences for initial isolation work. This work was financially supported by the Naito Foundation and a Grant-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science and Technology, Japan (22380114 to R.S.), Japan Science and Technology agency to R.S., and NIH R01 NS44322 to G.T.S. We are grateful to Dr. J. Hooper at the Queensland Museum for identification of the sponges. We also would like to thank the NIMH Psychoactive Drug Screening Program (PDSP) for target screening.
Footnotes
GABAA, γ-aminobutyric acid A receptor; CD, convulsant dose; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, NMDA, N-methyl-d-aspartic acid; PSC, postsynaptic current; IPSC, inhibitory postsynaptic current; EPSC, excitatory postsynaptic current; R-PIA, R-phenylisopropyladenosine; CPCA, 5′-(N-cyclopropyl)-carboxamido-adenosine; HEK, human embryonic kidney; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; D-APV, D-2-amino-5-phosphonovaleric acid; CNS, central nervous system; TEA, tetraethylammonium; 4-AP, 4-aminopyridine; eGFP, enhanced green fluorescence protein.
References
- 1.Rosemeyer H. The chemodiversity of purine as a constituent of natural products. Chem. Biodiversity. 2004;1:361–401. doi: 10.1002/cbdv.200490033. [DOI] [PubMed] [Google Scholar]
- 2.Daly JW. Caffeine analogs: biomedical impact. Cell. Mol. Life Sci. 2007;64:2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell SS, Whitehill AB, Trapido-Rosenthal HG, Ireland CM. Isolation and characterization of 1,3-dimethylisoguanine from the Bermudian sponge Amphimedon viridis. J. Nat. Prod. 1997;60:727–728. doi: 10.1021/np970015j. [DOI] [PubMed] [Google Scholar]
- 4.Moon B, Baker BJ, McClintock JB. Purine and nucleoside metabolites from the Antarctic sponge Isodictya erinacea. J. Nat. Prod. 1998;61:116–118. doi: 10.1021/np970358h. [DOI] [PubMed] [Google Scholar]
- 5.Cafieri F, Fattorusso E, Mangoni A, Taglialatelascafati O. Longamide and 3,7-dimethylisoguanine, two novel alkaloids from the marine sponge Agelas longissima. Tetrahedron Lett. 1995;36:7893–7896. [Google Scholar]
- 6.Killday KB, Yarwood D, Sills MA, Murphy PT, Hooper JN, Wright AE. Microxine, a new cdc2 kinase inhibitor from the Australian marine sponge Microxina species. J. Nat. Prod. 2001;64:525–526. doi: 10.1021/np000546z. [DOI] [PubMed] [Google Scholar]
- 7.Yagi H, Matsunaga S, Fusetani N. Isolation of 1-methylherbipoline, a purine base, from a marine sponge, Jaspis sp. J. Nat. Prod. 1994;57:837–838. [Google Scholar]
- 8.Jeong SJ, Inagaki M, Higuchi R, Miyamoto T, Ono M, Kuwano M, Van Soest RW. 1,3-Dimethylisoguaninium, an antiangiogenic purine analog from the sponge Amphimedon paraviridis. Chem. Pharm. Bull. 2003;51:731–733. doi: 10.1248/cpb.51.731. [DOI] [PubMed] [Google Scholar]
- 9.Chehade CC, Dias RL, Berlinck RG, Ferreira AG, Costa LV, Rangel M, Malpezzi EL, de Freitas JC, Hajdu E. 1,3-Dimethylisoguanine, a new purine from the marine sponge Amphimedon viridis. J. Nat. Prod. 1997;60:729–731. doi: 10.1021/np970021f. [DOI] [PubMed] [Google Scholar]
- 10.Cooper RA, de Freitas JC, Porreca F, Eisenhour CM, Lukas R, Huxtable RJ. The sea anemone purine, caissarone: adenosine receptor antagonism. Toxicon. 1995;33:1025–1031. doi: 10.1016/0041-0101(95)00047-p. [DOI] [PubMed] [Google Scholar]
- 11.Sakai R, Swanson GT, Shimamoto K, Green T, Contractor A, Ghetti A, Tamura-Horikawa Y, Oiwa C, Kamiya H. Pharmacological properties of the potent epileptogenic amino acid dysiherbaine, a novel glutamate receptor agonist isolated from the marine sponge Dysidea herbacea. J. Pharmacol. Exp. Ther. 2001;296:650–658. [PubMed] [Google Scholar]
- 12.Sakai R, Matsubara H, Shimamoto K, Jimbo M, Kamiya H, Namikoshi M. Isolations of N-methyl-d-aspartic acid-type glutamate receptor ligands from Micronesian sponges. J. Nat. Prod. 2003;66:784–787. doi: 10.1021/np020590+. [DOI] [PubMed] [Google Scholar]
- 13.Marek R, Sklenar V. NMR studies of purines. Annu. Rep. NMR Spectrosc. 2005;54:201–242. [Google Scholar]
- 14.Tasdemir D, Mangalindan GC, Concepcion GP, Harper MK, Ireland CM. 3,7-Dimethylguanine, a new purine from a Philippine sponge Zyzzya fuliginosa. Chem. Pharm. Bull. 2001;49:1628–1630. doi: 10.1248/cpb.49.1628. [DOI] [PubMed] [Google Scholar]
- 15.Chiamulera C, Costa S, Valerio E, Reggiani A. Domoic acid toxicity in rats and mice after intracerebroventricular administration: comparison with excitatory amino acid agonists. Pharmacol. Toxicol. 1992;70:115–120. doi: 10.1111/j.1600-0773.1992.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 16.Sugimoto T, Sugimoto M, Uchida I, Mashimo T, Okada S. Inhibitory effect of theophylline on recombinant GABA(A) receptor. Neuroreport. 2001;12:489–493. doi: 10.1097/00001756-200103050-00013. [DOI] [PubMed] [Google Scholar]
- 17.Appleton DR, Page MJ, Lambert G, Copp BR. 1,3-Dimethyl-8-oxoisoguanine, a new purine from the New Zealand ascidian Pseudodistoma cereum. Nat. Prod. Res. 2004;18:39–42. doi: 10.1080/1057563031000122077. [DOI] [PubMed] [Google Scholar]
- 18.Zelnik R, Haraguchi M, Matida AK, Lavie D, Frolow F, Weis AL. X-Ray molecular structure of caissarone, a novel purine derivative from the sea-anemone Bunodosoma caissarum Correa 1964. J. Chem. Soc., Perkin Trans. 1. 1986:2051–2053. [Google Scholar]
- 19.Ramzan IM, Levy G. Kinetics of drug action in disease states. XVI. Pharmacodynamics of theophylline-induced seizures in rats. J. Pharmacol. Exp. Ther. 1986;236:708–713. [PubMed] [Google Scholar]
- 20.Dragunow M, Goddard GV. Adenosine modulation of amygdala kindling. Exp. Neurol. 1984;84:654–665. doi: 10.1016/0014-4886(84)90212-7. [DOI] [PubMed] [Google Scholar]
- 21.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ault B, Nadler JV. Anticonvulsant-like actions of baclofen in the rat hippocampal slice. Br. J. Pharmacol. 1983;78:701–708. doi: 10.1111/j.1476-5381.1983.tb09423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Koninck Y, Mody I. The effects of raising intracellular calcium on synaptic GABAA receptor channels. Neuropharmacology. 1996;35:1365–1374. doi: 10.1016/s0028-3908(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 24.Akopian A, Gabriel R, Witkovsky P. Calcium released from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J. Neurophysiol. 1998;80:1105–1115. doi: 10.1152/jn.1998.80.3.1105. [DOI] [PubMed] [Google Scholar]
- 25.Taketo M, Matsuda H, Yoshioka T. Calcium-independent inhibition of GABA(A) current by caffeine in hippocampal slices. Brain Res. 2004;1016:229–239. doi: 10.1016/j.brainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Capogna M, Gahwiler BH, Thompson SM. Presynaptic enhancement of inhibitory synaptic transmission by protein kinases A and C in the rat hippocampus in vitro. J. Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao FV, Andersen OA, Vora KA, Demartino JA, van Aalten DM. Methylxanthine drugs are Chitinase inhibitors: investigation of inhibition and binding modes. Chem. Biol. 2005;12:973–980. doi: 10.1016/j.chembiol.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Sills MA, Fagg G, Pozza M, Angst C, Brundish DE, Hurt SD, Wilusz EJ, Williams M. [3H]CGP 39653: a new N-methyl-d-aspartate antagonist radioligand with low nanomolar affinity in rat brain. Eur. J. Pharmacol. 1991;192:19–24. doi: 10.1016/0014-2999(91)90063-v. [DOI] [PubMed] [Google Scholar]
- 29.Murphy DE, Snowhill EW, Williams M. Characterization of quisqualate recognition sites in rat brain tissue using dl-[3H]α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) and a filtration assay. Neurochem. Res. 1987;12:775–781. doi: 10.1007/BF00971514. [DOI] [PubMed] [Google Scholar]
- 30.London ED, Coyle JT. Specific binding of [3H]kainic acid to receptor sites in rat brain. Mol. Pharmacol. 1979;15:492–505. [PubMed] [Google Scholar]