Abstract
Rationale
Differential effects of δB and δC subtypes of Ca2+/calmodulin dependent protein kinase (CaMKII) on cardiomyocyte Ca2+ handling and survival have been suggested to result from their respective nuclear vs. cytosolic localizations. CaMKIIδ subtype localization and its relationship to enzyme activation and target phosphorylation has not, however, been systematically evaluated.
Objective
To determine whether CaMKIIδ subtypes are restricted to a particular subcellular location and assess the relationship of localization to enzyme activation and function.
Methods and Results
CaMKIIδ is highly expressed in mouse heart and cardiomyocytes and concentrated in sarcoplasmic reticulum (SR)/membrane and nuclear fractions. CaMKIIδB and δC subtypes differ by a nuclear localization sequence, but both are present in nuclear and SR/membrane fractions. Nonselective subtype distribution is also seen in mice overexpressing CaMKIIδB or δC, even in a CaMKIIδ null background. Fluorescently-tagged CaMKIIδB expressed in cardiomyocytes concentrates in nuclei whereas δC concentrates in cytosol but neither localization is exclusive. Mouse hearts exposed to phenylephrine (PE) show selective CaMKIIδ activation in the nuclear (vs. SR) compartment whereas caffeine selectively activates CaMKIIδ in SR (vs. nuclei), independent of subtype. Compartmentalized activation extends to functional differences in target phosphorylation at CaMKII sites: PE increases histone deacetylase 5 phosphorylation (Ser498) but not phospholamban (Thr17), while the converse holds for caffeine.
Conclusions
These studies demonstrate that CaMKIIδB and δC are not exclusively restricted to the nucleus and cytosol, and that spatial and functional specificity in CaMKIIδ activation is elicited by mobilization of different Ca2+ stores rather than by compartmentalized subtype localization.
Keywords: Ca2+/Calmodulin dependent protein kinase, nuclear localization, heart, splice variants, sarcoplasmic reticulum
Introduction
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serine/threonine kinase critical for Ca2+ signaling in cardiomyocytes. Our work and that of others has implicated CaMKII in the development of cardiac hypertrophy and heart failure (HF).1–7 The expression of CaMKII is elevated in animal HF models and human HF patients.1, 2 Transgenic overexpression of the predominant cardiac isoform, CaMKIIδ, elicits hypertrophy and HF, while genetic deletion or inhibition of CaMKIIδ prevents HF development. 3–7 Two splice variants, CaMKIIδB and δC, are known to be present in cardiac myocytes. 8 The CaMKIIδB and δC subtypes have been implicated in distinct cardiomyocyte functions2, but the exclusivity of their localization, potential selectivity in activation mechanisms, and relationship of localization and subtype to functional outcomes have not been well defined.
CaMKII is activated by Ca2+/calmodulin binding to the enzyme. The resultant conformational change favors subsequent autophosphorylation of the enzyme to a Ca2+-independent activated form. 9 Oxidation can also lead to CaMKII activation.10 Downstream targets phosphorylated by CaMKIIδ include proteins important for the modulation of Ca2+ handling such as phospholamban (PLN), ryanodine receptors (RyR2), voltage sensitive L-type Ca2+ channels, and the Nav1.5 Na+ channel subunit 1, 2, 11–14 CaMKIIδ can also regulate gene transcription, for example by phosphorylation of type II histone deacetylases (HDACs) which derepress myocyte enhancer factor-2 (MEF2),15–17 or through AP-1 18, 19 or GATA4.20
CaMKIIδB and δC subtypes differ only by the presence of an 11 amino acid NLS in CaMKIIδB. 8, 21 The Schulman laboratory established and we subsequently confirmed that heterologously expressed CaMKIIδB primarily localizes to the nucleus, whereas δC is found primarily in the cytosol.8, 21, 22 Accordingly, we postulated different functions of the two subtypes, with nuclear δB involved in hypertrophic gene regulation and cytosolic δC in the regulation of Ca2+ handling and ion channels. This was supported by early findings using isolated neonatal rat ventricular myocytes 23–30 and by the differential phenotypes that we observed in the CaMKIIδB and δC transgenic (TG) mice models.3, 4, 11, 22, 31 More specifically, CaMKIIδB TG mice primarily develop cardiac hypertrophy whereas hypertrophy in the δC TG mice rapidly transitions to HF characterized by severely disrupted cytosolic Ca2+handling. 3, 32 Subsequent work directly comparing the two lines showed that δB and δC both modulate MEF2 activity and gene expression, a result attributed to the ability of CaMKIIδ to phosphorylate HDAC in either the cytosol or the nucleus.32 In the studies presented here we more extensively investigate the localization of CaMKIIδB and δC subtypes in the mouse heart ventricle and isolated cardiomyocytes. We further test the hypothesis that enzyme location within the myocyte determines its activation by stimuli that mobilize distinct subcellular pools of Ca2+. The findings reported here demonstrate that CaMKII δB is indeed concentrated in the nucleus and CaMKII δC at the sarcoplasmic reticulum (SR), but that this localization is not exclusive, either for the endogenous or overexpressed enzyme. Additionally, we report that the nature of the stimulus and presumed site of localized Ca2+ release determines where CaMKII is activated and is indiscriminate with regard to enzyme subtype. Finally, we show that downstream target phosphorylation provides a functional readout of the consequences of activation of CaMKII at specific cellular locations.
Methods
Subcellular fractionation of mouse ventricle was performed by differential centrifugation using minor modifications of a published procedure.33 To compare expression levels in different subcellular compartments, equal portions (e.g. 50%, 100%) of each fraction were loaded onto SDS gels and analyzed by immunoblotting. Distribution of green fluorescent protein (GFP)-tagged CaMKII δB and δC in adult mouse ventricular myocytes (AMVM) was visualized by confocal microscopy following adenoviral overexpression. Isolated hearts were perfused in the Langendorff mode 34 and treated with vehicle, phenylephrine (PE) or caffeine. AMVMs were isolated from wild type and knockout as described.35 CaMKII activation was assessed by Western blotting using a P-CaMKII antibody from Affinity Bioreagents.4 All procedures were performed in accordance with NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Results are reported as averages ± SEM. Statistical significance was determined using ANOVA followed by the Tukey post hoc test. P<0.01 was considered statistically significant. For additional details regarding the methods used, see the Online Supplemental Material.
Results
Ventricular tissue and AMVMs isolated from adult wild type (WT) and CaMKIIδ knockout (δKO) mice were analyzed by Western blotting using a CaMKIIδ antibody that recognizes both CaMKIIδB and δC. Two bands were clearly evident in the WT and absent in the CaMKIIδKO mouse heart (Fig 1A). The difference in mobility of these bands is consistent with the inclusion of an 11 amino acid (2 kD) NLS in CaMKIIδB. 8, 21 Quantification of the individual bands indicates that CaMKIIδB is the more predominant splice variant, with approximately 60% of the total endogenous CaMKII migrating as the δB subtype, and just under 40% as δC (Fig 1B). To determine which subcellular compartments contain endogenous CaMKIIδ isolated left ventricle was fractionated into cytosolic (Cyto), mitochondrial (Mito), SR/membrane (SR/mem) and nuclear (Nuc) fractions. The purity of these fractions was verified using the markers Rho GDP-dissociation inhibitor (Rho-GDI), voltage-dependent anion channel (VDAC), sarcoplasmic reticulum Ca2+ ATPase (SERCA2a )and Lamin A/C respectively (Fig 2A). To compare CaMKII protein expression amongst these fractions, the entire volume of each fraction was loaded onto SDS gels. Immunoblotting for CaMKIIδ revealed that there were nearly equivalent amounts of CaMKIIδ in the SR/membrane and nuclear compartments of the cell (together accounting for approximately 75% of the total enzyme) while less than 20% was in the cytosolic fraction and a smaller percent was associated with the mitochondrial fraction (Fig 2B). The subtype composition of CaMKIIδ in each subcellular fraction was then analyzed by separately quantifying the individual δB and δC bands as seen in Fig 3A. This analysis revealed that both subtypes were present in every compartment examined (Fig 3B). Importantly, CaMKIIδB was detected not only in the nuclear compartment, but also in the SR/membrane compartment; conversely CaMKIIδC, while abundant in the SR/membrane, was also clearly present in the nuclear compartment (Fig 3B).
Figure 1. Expression of endogenous CaMKII δ in mouse ventricle and cardiomyocytes.

Ventricular tissue and adut mouse ventricular myocytes (AMVM) isolated from wild type (WT) and CaMKII δ knockout (δKO) mice, lysed and subjected to western blotting. (A) Representative blots of CaMKII δ expression in ventricular lysate and AMVM lysate demonstrating two bands absent in the δKO. (B) Quantitative analysis of the relative abundance of CaMKII δB and δC in WT ventricular tissue (n=8). * p<0.01
Figure 2. Subcellular distribution of endogenous CaMKII δ.
(A) Western blotting of fractionated mouse ventricle for cellular markers; Rho-GDI, cytosol; VDAC, mitochondria; SERCA 2a, SR; Lamin A/C, nucleus. Each lane represents a separate heart. (B) Quantitative analysis of the percent of total CaMKII δ in each subcellular compartment isolated from WT mouse ventricle. For the data shown in A and B, all fractions were suspended in the same volumes and equal portions loaded for western blotting. (n=6) * p < 0.01
Figure 3. Subcellular distribution of the endogenous CaMKII δB and δC isoforms.
Ventricular tissue was isolated from WT mice. (A) Representative western blot shows the relative distribution of CaMKII in the cytosolic, mitochondrial, SR/membrane and nuclear fractions. Equal portions of each fraction were loaded for western blotting. (B) Graphs show percent of total CaMKII δB or δC in each subcellular fraction from wild type mice. (n=6)
We have generated cardiac specific CaMKIIδB and δC TG mice and characterized these lines in numerous studies. 3, 4, 31, 32 We assumed that the phenotypic differences observed in these TG lines correlated with differential increases in expression of δB and δC in the nuclear vs. SR compartments respectively. To re-evaluate this assumption in light of our findings on the distribution of endogenous CaMKII subtypes, we isolated and fractionated ventricular tissue from CaMKIIδB and δC TG mice. As in the experiments above, the entire volume of each fraction was loaded onto SDS gels to compare CaMKII protein expression amongst these fractions (Online Fig I). The percent of the total CaMKIIδ transgene in each subcellular compartment was quantitated and the data from a series of experiments averaged and shown in Fig 4A. Remarkably, while CaMKIIδB TG mice show a high concentration of CaMKIIδB in cardiomyocyte nuclei based on immunofluorescence staining, 32 subcellular fractionation indicates that significant amounts of CaMKIIδB are also present outside of the nucleus in the cytosolic and SR/membrane fractions (Fig 4A). In the CaMKIIδC TG mice, immunostaining revealed relative exclusion of CaMKIIδC from the nucleus, 32 but whereas most CaMKIIδC is in the SR/membrane fraction, the CaMKIIδC subtype is clearly detectable in the nuclear fraction as well (Fig 4A). Thus distribution of the transgenes, like that of endogenous CaMKIIδ subtypes, is not exclusive.
Figure 4. Comparative subcellular distribution of CaMKII δB and δC isoforms in transgenic vs. transgenic in CaMKII δ null background.
Ventricular tissue isolated from δB TG, δCTG, δB/δKO andδC/δKO mice was harvested and fractionated into cytosolic, mitochondrial, SR/membrane and nuclear fractions and subjected to western blotting. The distribution of transgenically expressed CaMKII δB and δC is examined (A) in the wild type background or (B) in the CaMKII δ null background. (n=4)
CaMKIIδ is believed to exist as a multimer of 12 subunits. 9 Nuclear vs. cytosolic localization can be significantly affected by changing the expression ratio of δB and δC splice variants, consistent with heteromultimerization of these subtypes. 36,22, 37 Multimerization of transgenically expressed CaMKIIδC with endogenous CaMKIIδB could promote its localization to the nucleus, whereas multimerization of CaMKIIδB with endogenous CaMKIIδC could lead to its exclusion from the nuclear compartment. To test the hypothesis that the broad and relatively nonselective subcellular distribution of the CaMKII δB and δC subtypes results from their heteromultimerization we crossed the CaMKIIδB and δC TG mice with the CaMKIIδKO mice previously developed in our lab. Progeny from these crosses were shown to express only a single CaMKIIδ subtype (δB or δC) in the CaMKIIδ null background. Interestingly, the subcellular distribution of the CaMKIIδC and δB transgenes expressed in the CaMKIIδ null background (Fig 4B) was not appreciably different from that of the δB and δC transgenes expressed in the WT background (Fig 4A).
To examine the distribution of the δB and δC subtypes in a manner that does not require cell disruption and fractionation we infected AMVMs from δKO mice with GFP-tagged CaMKII δB and δC adenovirus. Myocytes infected with CaMKIIδB and visualized by confocal microscopy clearly showed accumulation of the overexpressed protein in the nuclear compartment, but a significant amount of CaMKIIδB was also seen outside the nucleus, distributed in a striated pattern corresponding to T-tubule organization (Fig 5A). Line scan quantification of fluorescent intensity of a 1 micron thick plane from several different cells showed the fluorescence intensity of CaMKIIδB in the nucleus to be 2.69 (±0.08) times higher than that of CaMKIIδB in the cytosol; expressed another way, CaMKII δB fluorescence intensity outside of the nuclear compartment is approximately one third that in the nucleus. Experiments were also carried out using GFP-tagged CaMKIIδC, and showed prominent localization coincident with the striated patterns of the cardiomyocyte (Fig 5B). Significant perinuclear CaMKIIδC staining was also observed. Line scan quantification of CaMKIIδC fluorescence intensity showed the nuclear δC signal to be 0.44 (±0.09) of that in the extranuclear compartment; expressed another way the fluorescence intensity of CaMKIIδC inside the nuclear compartment is approximately half of that in the cytosol.
Figure 5. GFP-tagged CaMKII δB and δC expressed in AMVMs isolated from δKO mice.
AMVMs isolated from δKO mice were infected with adenovirus expressing (A) GFP-tagged CaMKII δB or (B) GFP-tagged CaMKII δC. AMVMs were imaged using confocal microscopy, DAPI staining was used to identify the nuclei, and line scan quantification was used to measure fluorescence intensity and determine enzyme distribution. Scale bar is 15 microns (μm). Background measurements taken from non-infected AMVMs were averaged and subtracted from the fluorescence intensity measurements of the GFP-tagged enzyme.
Many of the functional effects of CaMKII in the myocardium, in particular those ascribed to CaMKIIδC, result from phosphorylation of SR targets involved in Ca2+ handling. Because the SR/membrane fraction obtained from the protocol used in the Figs 2–4 is heterogeneous (e.g. it includes sarcolemmal membranes) we optimized a sucrose density gradient separation protocol to isolate a more purified SR fraction. We looked at purity of the SR and nuclear fractions by immunoblotting using markers for cytosol, mitochondria, SR and nucleus and found the SR (Fig 6A) and nuclear (Fig 6B) fractions to contain some mitochondria (VDAC staining) but otherwise show little cross contamination. Immunoblots from the purified SR and nuclear fractions show two CaMKIIδ bands (Fig 6C,D). While the lower band, CaMKIIδC, is the more abundant in the SR (Fig 6C), and CaMKIIδB is predominant in the nuclear fraction (Fig 6D), the two subtypes are clearly not exclusively segregated to a specific compartment.
Figure 6. Distribution of CaMKII δB and CaMKII δC in purified SR and nuclei.

SR and nuclear fractions were isolated from WT ventricular tissue. Representative western blots showing purity of (A) SR preparation or (B) nuclear preparation. Representative western blot and quantification of δB vs. δC in (C) SR or (D) nuclear fractions. (n=4)
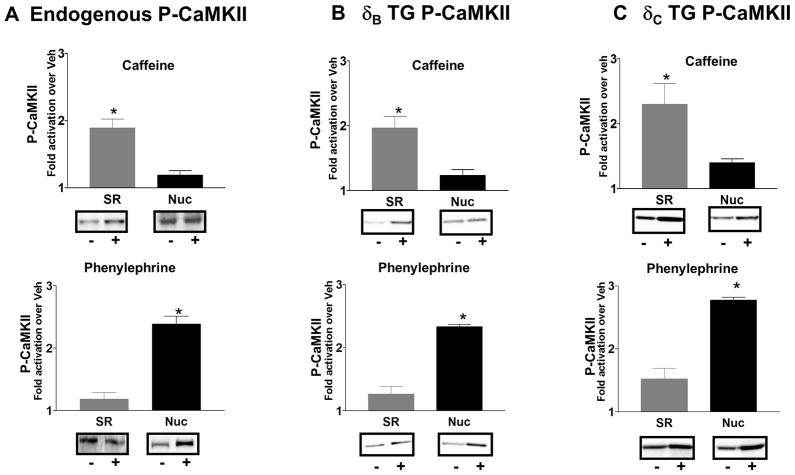
The finding that CaMKII δB and δC subtypes co-localize in the same subcellular compartment suggested that they could also be activated in parallel. To determine whether this is the case, we isolated hearts from WT mice, perfused them in the Langendorff mode and then either treated them with a bolus injection of 10mmol/L caffeine (to release SR Ca2+) or perfused them for 15 minutes with 100nmol/L PE (to increase nuclear Ca2+ levels). 17, 38 Hearts were then fractionated to obtain purified SR and nuclei (as described in Fig 6) and analyzed by Western blot analysis. Phosphorylation of CaMKII at Thr286, the site of enzyme autophosphorylation, was used as a read-out for CaMKII activation. Perfusion with caffeine increased P-CaMKII in the SR fraction but not in the nuclear fraction (Fig 7A). In contrast, PE treatment increased P-CaMKII levels in the nuclear fraction but not in the SR fraction (Fig 7A). Since the resolution of the P-CaMKII antibody is not adequate to individually distinguish the endogenously expressed phosphorylated CaMKIIδB and CaMKIIδC subtypes we repeated these experiments using the δB and δC TG mice (in the CaMKIIδ null background). The data shown in Figure 7B and C demonstrate that caffeine significantly increases phosphorylation of both CaMKIIδB and CaMKIIδC in the SR (but not in the nuclear fraction). In contrast PE treatment elicits 2–3 fold increases in phosphorylation of both CaMKIIδB and CaMKIIδC in the nuclear fraction but not in the SR. Thus caffeine selectively activates whichever CaMKIIδ subtype is located at the SR but not that located in the nucleus, whereas PE selectively activates whichever CaMKII subtype is localized to the nucleus.
Figure 7. Caffeine activates CaMKII at the SR and phenylephrine activates CaMKII at the nucleus.
SR or nuclei were isolated from ventricular tissue of WT, CaMKII δB or CaMKII δC TG mice in the CaMKII δ null background following bolus injection of 10mmol/L caffeine or 15 minutes of perfusion with 100nmol/L PE. P-CaMKII levels were measured by western blotting using an antibody directed against the CaMKII autophosphorylation site, (Thr286). Data are shown for (A) WT (endogenous CaMKII), (B) δB TG in the δKO background and (C) δC TG in the δKO background. * p< 0.01 for SR vs. Nuc
The experimental findings cited above suggest that CaMKIIδB or CaMKIIδC can be activated by the same agonists and could subserve similar functions. We examined functional consequences of compartmentalized CaMKII activation by measuring the phosphorylation of established CaMKII phosphorylation sites on two CaMKII targets, PLN localized to the SR and HDAC5, largely localized to the nucleus. Perfused hearts were treated with PE or caffeine using the same protocol used to examine CaMKII activation and homogenized for analysis of CaMKII substrate phosphorylation. In WT mice (Fig 8) or CaMKIIδB TG (Online Fig II), treatment with caffeine lead to significant increases in phosphorylation of PLN at Thr17 while treatment with PE did not lead to PLN phosphorylation (Fig 8A). Conversely treatment with PE increased phosphorylation of HDAC5 at Ser498 while caffeine did not (Fig 8B). Concomitant perfusion with KN-93, a CaMKII inhibitor, prevented caffeine induced phosphorylation of PLN Thr17 and PE induced phosphorylation of HDAC5 at Ser498 (Online Fig III). Additionally, we observed no increase in phosphorylation of these substrates at their putative CaMKII phosphorylation sites in CaMKIIδKO mice treated with caffeine or PE (Fig IV). These data demonstrate that PLN Thr17 and HDAC5 Ser498 are CaMKII phosphorylation sites and that there is specificity in the effects of caffeine and PE on CaMKII mediated phosphorylation of these substrates.
Figure 8. Caffeine preferentially increases phospholamban phosphorylation and PE preferentially increases HDAC5 phosphorylation in mouse heart.
Hearts were isolated from mice perfused with 10mmol/L caffeine or 100nmol/L phenylephrine. Ventricular homogenate was subjected to western blotting for A PLN phosphorylation at the CaMKII phosphorylation site, threonine-17 and B. HDAC5 phosphorylation using an antibody for the CaMKII specific epitope. Quantitatated data are from n=5 * p<0.01
Discussion
CaMKIIδB and δC subtypes, which differ only by the inclusion of a nuclear localization sequence, are present in the mouse heart ventricle at similar protein levels (Fig 1). Seminal papers from the Schulman laboratory describing these two splice variants, 8, 21 along with our early studies in which we expressed CaMKIIδB and δC in neonatal rat ventricular myocytes (NRVMs), 22 supported the notion that CaMKIIδB would be localized to and signal in the nucleus whereas δC would localize to and signal outside of the nucleus. These conclusions were based on studies in which CaMKIIδ was heterologously expressed in COS cells or NRVMs. 22, 39, 40
Subsequently we generated CaMKIIδB or δC TG mice and examined the HA-tagged protein by immunostaining of myocytes isolated from these mice. 3, 4, 32 Our findings were consistent with the predominant localization of CaMKIIδB in the nucleus and δC in the cytosol.3, 4, 32 The concept that nuclear and cytosolic splice variants/subtypes subserved different functions was supported by the distinct phenotypes that we observed in the CaMKIIδB and δC TG mice. We recognized that the pathological changes seen in these mouse models could be exaggerated by overexpression, but reasoned that this approach emphasized the compartment specific effects of the two subtypes: nuclear effects on gene expression leading to hypertrophy in the CaMKIIδB TG mice, and effects on SR protein phosphorylation and Ca2+ handling leading to HF development in the CaMKIIδC TGs.
The more extensive analysis presented in the current manuscript was motivated by our observation that endogenous CaMKIIδ is found in both the SR/membrane and nuclear compartments isolated from mouse ventricle and that there are two CaMKIIδ immunoreactive bands in both of these compartments (Fig 3A and 6). That these bands are absent in CaMKIIδ knockout mouse hearts (Fig 1A) indicates that they are both CaMKIIδ gene products, while the fact that they differ in mobility by approximately 2 kD suggests that they represent δC and the 11 amino acid larger NLS containing δB.21 The ability of various CaMKII isoforms and subtypes to form heteromultimers 9, 36 provides a feasible explanation for the appearance of either subtype in the nucleus (or SR) independent of whether it possesses an NLS. Remarkably, however, the expression of CaMKIIδB in the absence of CaMKIIδC did not restrict its localization to the nucleus nor was δC confined to the cytosolic/SR compartment when expressed in the absence of δB (Fig 4B). Thus heteromultimers of CaMKIIδB and δC appear unlikely to account for the indiscriminate distribution of these subtypes. We cannot rule out the possibility that other minor cardiac CaMKII isoforms, including CaMKIIγ and β, heteromultimerize with CaMKIIδ and contribute to its appearance in unexpected locations, although this seems quantitatively unlikely. Regardless of the molecular mechanism, the conclusion from our subcellular fractionation experiments is that CaMKIIδB is not restricted to the nuclear compartment, and δC is not excluded from the nuclear compartment.
Since subcellular fractionation does not yield complete separation of organelles and also can disrupt normal structure we extended our studies using confocal microscopy of intact AMVMs infected with GFP-tagged CaMKIIδB or δC. Data obtained by confocal imaging shows extensive accumulation of GFP-tagged CaMKIIδB in the nucleus, consistent with what we reported previously.32 Notably however, quantitative analysis confirmed that δB is not wholly restricted to the nucleus; indeed δB fluorescence intensity outside of the nuclear compartment was approximately one third that in the nucleus. The intensity of the nuclear staining is indeed striking but this reflects, in part, the fact that the enzyme is concentrated in a very small compartment. The distribution of GFP-tagged CaMKIIδC appeared largely consistent with the earlier studies from our lab suggesting that δC is excluded from the nucleus. However, quantitative analysis showed that the fluorescence intensity inside the nuclear compartment was not zero, but was approximately half of that in the cytosol. Of additional note, our assessment of CaMKIIδC nuclear fluorescence intensity does not include what appears to be a prominent pool of GFP–tagged perinuclear CaMKIIδC; whether this represents mitochondria, SR or other cellular organelles in confluence with the nucleus, this compartment of CaMKIIδC would likely be included in our nuclear fractionation and thus contribute to higher estimates for the proportion of nuclear CaMKIIδC in the fractionation experiments. Finally it should be noted that the insoluble fraction discarded in the low speed spin of the fractionation protocol would contain some of the total cellular CaMKIIδ, thus the percent of total calculated for each fraction is somewhat inflated. Regardless of the limitations inherent in the use of either the adenoviral overexpression or subcellular fractionation experiments, and independent of judgement as to which approach give the most valid estimate of CaMKIIδB and δC in each compartment, all of the preparations and approaches utilized here lead us to the same conclusions: the CaMKIIδB and CaMKIIδC isoforms are not restricted to specific subcellular locations.
The finding that both CaMKII subtypes are present throughout the cell raised the question of whether localization or subtype would determine when and how the enzyme was activated. We used interventions expected to mobilize Ca2+ from distinct cellular locations to examine CaMKIIδ activation in WT mouse hearts. This was supplemented with studies using hearts from the subtype-specific transgenics to facilitate analysis of the activation of individual subtypes. Our findings clearly demonstrated that PE increases phosphorylation of CaMKIIδB or δC in the nuclear compartment with little change in activation of either subtype at the SR; conversely caffeine activates both CaMKIIδB and δC in the SR, with little change in activation of either subtype in the nuclear compartment (Fig 7). Several published studies have highlighted the importance of localized Ca2+ stores and subsequent compartmentalized signaling within the cardiomyocyte.41–43 In cardiomyocytes, the majority of inositol trisphosphate receptors (IP3R2) are located on the nuclear envelope and our previous work demonstrated that endothelin-1 and PE increase Ca2+ release from nuclear IP3 sensitive stores.17, 38 Thus we believe that the selectivity of PE for inducing nuclear CaMKII activation reflects Ca2+ mobilization through IP3 sensitive stores in or around the nucleus, although other similarly localized signaling pathways cannot be ruled out. Treatment with caffeine would instead be expected to cause a large [Ca]i increase in the cleft region as a result of SR Ca2+ mobilization, consistent with CaMKII activation at the SR. Thus the studies presented here demonstrate for the first time that there is compartmentalized activation of CaMKIIδ, with the cellular compartment determined by the stimulus and presumed site of Ca2+ release, and notably independent of subtype.
The functional relevance of compartmentalized CaMKIIδ activation was demonstrated by studies in which we examined substrate phosphorylation. Phosphorylation of the SR target, phospholamban at its well documented CaMKII-specific phosphorylation site, 5, 44, 45 was confirmed here (Online Figs III, IV) and shown to be selectively increased following addition of caffeine (Fig 8). Phosphorylation of the nuclear transcriptional regulator HDAC5 at a site indicated by previous studies and in Online Figs III and IV to be a CaMKII phosphorylation site 17, 46, 47 was selectively increased following PE treatment (Fig 8). Agonist selectivity in substrate phosphorylation was demonstrated in studies using both WT (Fig 8) and CaMKIIδB TG (Online Fig II) mice. The high basal level of PLN and HDAC phosphorylation seen in the CaMKIIδC TG heart (Online Fig II) precluded detection of further agonist induced increases, although it does indicate that both of these substrates are in vivo targets for CaMKIIδC. Notably our previous analysis of the CaMKII δC TG mouse heart demonstrated increased PLN and RyR2 phosphorylation associated with dysfunctional Ca2+ handling and heart failure phenotype. 3, 32 The reason that we did not see RyR2 and PLN phosphorylation and Ca2+ handling changes in the CaMKIIδB TG mice 32 may be that the level of CaMKII transgene expression is lower in the SR of the CaMKII δB TG mice than in the SR of CaMKII δC TG mice (Online Figure V). We do find, however, that both PLN and RyR2 are highly phosphorylated in neonatal rat cardiomyocytes following adenoviral expression of equal levels of either CaMKII δB or CaMKII δC (data not shown), supporting the notion that either subtype can phosphorylate these SR targets.
In conclusion, we demonstrate for the first time that CaMKIIδB and δC subtypes are not exclusively localized. We also present evidence that both subtypes can be activated at the same cellular locations and that the activation is stimulus and location dependent rather than subtype dependent. Phosphorylation of different CaMKIIδ substrates is also dependent upon the nature of the stimulus. The evidence for nonselective CaMKIIδ subtype localization is particularly interesting and challenging with regard to understanding mechanisms by which CaMKIIδB could subserve a protective role, and δC a more deleterious role in cardiomyocyte survival and heart disease.12, 48, 49
Supplementary Material
Novelty and Significance.
What is known?
Ca2+/CaM kinase II regulates cardiac Ca2+ handling and plays a critical role in adverse cardiac remodeling in response to pressure overload, catecholamines and ischemic stress.
CaMKII delta (CaMKIIδ) is the predominant cardiac isoform and is present as two major splice variants (subtypes): CaMKIIδB which contains a nuclear localization sequence and CaMKIIδC which does not.
Based on cellular or transgenic overexpression, the two subtypes are differentially localized and accordingly serve different functions: CaMKIIδB regulates gene expression and cell survival whereas CaMKIIδC regulates Ca2+ handling and cell death.
What new information does the article provide?
The two CaMKIIδ subtypes are not as exclusively localized as previously believed: the SR compartment contains considerable amounts of CaMKIIδB and the nuclear compartment contains significant amounts of CaMKIIδC.
Two Ca2+ mobilizing agonists, caffeine and phenylephrine, differentially activate CaMKIIδ in accordance with enzyme localization (caffeine in SR, phenylephrine in nucleus) and increase substrate phosphorylation (phospholamban and HDAC-5), independent of CaMKIIδ subtype
Specificity in CaMKIIδ signaling results from compartmentalized rather than subtype specific activation.
This study was designed to test the concept that CaMKIIδB and CaMKIIδC, the two predominant cardiac splice variants (subtypes) of CaMKII, serve different functions due to their distinct localizations. Surprisingly, subcellular fractionation studies revealed that all fractions examined, including mitochondria, SR/membrane and nucleus contained a mixture of the two subtypes. Using hearts from mice in which only one of the two subtypes was expressed (δB or δC transgenic mice in a CaMKIIδ knockout background), we show that this is not a result of heteromultimerization of the subtypes. We then asked whether the subtypes, if not distinctly localized, were differentially regulated. Two agonists that mobilized Ca2+, caffeine and phenylephrine, were shown to activate both CaMKIIδB and δC. Strikingly, regardless of subtype, caffeine activated CaMKIIδ in the SR compartment and increased phosphorylation of the SR CaMKII substrate, phospholamban, whereas phenylephrine only activated CaMKIIδ in the nucleus and increased phosphorylation of the nuclear CaMKII target, HDAC-5. These findings question the accepted notion of strict “nuclear” vs. “cytoplasmic” isoforms of CaMKIIδ, while demonstrating that there is compartmentalized activation of CaMKIIδ and its functional targets in cardiomyocytes.
Acknowledgments
The authors thank Katherine Huang for assistance in adult mouse ventricular myocyte isolation and other technical assistance, Melissa Ridout for exceptional animal care and breeding, and Ruchi Patel for providing the GFP-tagged CaMKIIδ constructs.
Sources of Funding
This work was supported by NIH grant P01-HL080101 to JHB and DMB. SM and CBBG were supported in part by the UCSD Graduate Training Program in Cellular and Molecular Pharmacology through an institutional training grant from the National Institute of General Medical Sciences, T32 GM007752.
Nonstandard Abbreviations and Acronyms
- AMVM
adult mouse ventricular myocyte
- CaMKII
Ca2+/Calmodulin dependent protein kinase II
- ET-1
endothelin-1
- GFP
green fluorescent protein
- HDAC
histone deacetylase
- HF
heart failure
- KO
knock out
- MEF2
myocyte enhancer factor 2
- NLS
nuclear localization sequence
- PE
phenylephrine
- PLN
phospholamban
- Rho-GDI
Rho GDP-dissociation inhibitor
- RyR2
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- TG
transgenic
- VDAC
voltage-dependent anion channel
- WT
wild type
Footnotes
Disclosures
None.
Reference List
- 1.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda ) 2008 June;23:151–9. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 2.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004 August 15;63(3):476–86. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–9. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, Belke DD, Dillmann WH, Rogers TB, Schulman H, Ross J, Jr, Brown JH. The cardiac-specific nuclear δB isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J Biol Chem. 2002;277:1261–7. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 5.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller BJ. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009 May;119(5):1230–40. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005 April;11(4):409–17. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 7.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009 February 17;106(7):2342–7. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edman CF, Schulman H. Identification and characterization of delta B-CaM kinase and delta C-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim Biophys Acta. 1994 March 10;1221(1):89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 9.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002 June 15;364(Pt 3):593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, ykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008 May 2;133(3):462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006 December;116(12):3127–38. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng W, Zhang Y, Zheng M, Cheng H, Zhu W, Cao CM, Xiao RP. Cardioprotection by CaMKII-{delta}B is mediated by phosphorylation of heat shock factor 1 and subsequent expression of inducible heat shock protein 70. Circ Res. 2009 November 12; doi: 10.1161/CIRCRESAHA.109.210914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O'Rourke B, Akar FG, Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010 February 1;85(3):454–63. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009 September;54(3):180–7. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci USA. 2000;97:14400–5. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003 August 15;17(16):1937–56. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006 March;116(3):675–82. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani SK, Egan EA, Addy BK, Grimm M, Kasiganesan H, Thiyagarajan T, Renaud L, Brown JH, Kern CB, Menick DR. beta-adrenergic receptor stimulated Ncx1 upregulation is mediated via a CaMKII/AP-1 signaling pathway in adult cardiomyocytes. J Mol Cell Cardiol. 2009 November 27;48(2):342–51. doi: 10.1016/j.yjmcc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional integration of TLR2 and TLR4 signaling at the NCoR derepression checkpoint. Mol Cell. 2009 July 10;35(1):48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu YM, Shioda N, Yamamoto Y, Han F, Fukunaga K. Transcriptional upregulation of calcineurin Abeta by endothelin-1 is partially mediated by calcium/calmodulin-dependent protein kinase IIdelta3 in rat cardiomyocytes. Biochim Biophys Acta. 2010 May;1799(5–6):429–41. doi: 10.1016/j.bbagrm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan M, Edman CF, Schulman H. Alternative splicing introduces a nuclear localization signal that targets multifunctional CaM kinase to the nucleus. J Cell Biol. 1994;126:839–52. doi: 10.1083/jcb.126.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez MT, Zhao X, Schulman H, Brown JH. The nuclear δB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J Biol Chem. 1997;272:31203–8. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- 23.Adams JW, Sah VP, Henderson SA, Brown JH. Tyrosine kinase and Jun NH2-terminal kinase mediate hypertrophic responses to prostaglandin F2α in cultured neonatal rat ventricular myocytes. Circ Res. 1998;83:167–78. doi: 10.1161/01.res.83.2.167. [DOI] [PubMed] [Google Scholar]
- 24.Adams JW, Migita DS, Yu MK, Young R, Hellickson MS, Castro-Vargas FE, Domingo JD, Lee PH, Bui JS, Henderson SA. Prostaglandin F2α stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J Biol Chem. 1996;271:1179–86. doi: 10.1074/jbc.271.2.1179. [DOI] [PubMed] [Google Scholar]
- 25.Simpson P, McGrath A, Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982;51:787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- 26.Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an α1-adrenergic response. J Clin Invest. 1983;72:732–8. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shubeita HE, McDonough PM, Harris AN, Knowlton KU, Glembotski CC, Brown JH, Chien KR. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes: a paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990;265:20555–62. [PubMed] [Google Scholar]
- 28.Ito H, Hirata Y, Adachi S, Tanaka M, Tsujino M, Koike A, Nogami A, Murumo F, Hiroe M. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J Clin Invest. 1993;92:398–403. doi: 10.1172/JCI116579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadoshima J-I, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–84. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 30.Knowlton KU, Michel MC, Itani M, Shubeita HE, Ishihara K, Brown JH, Chien KR. The α1a-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem. 1993;268:15374–80. [PubMed] [Google Scholar]
- 31.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–11. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007 November 30;282(48):35078–87. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 33.Whittaker R, Glassy MS, Gude N, Sussman MA, Gottlieb RA, Glembotski CC. Kinetics of the translocation and phosphorylation of alphaB-crystallin in mouse heart mitochondria during ex vivo ischemia. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1633–H1642. doi: 10.1152/ajpheart.01227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, Castillo R, Glembotski CC, Sussman MA, Newton AC, Brown JH. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ Res. 2010 August 20;107(4):476–84. doi: 10.1161/CIRCRESAHA.109.215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y-Y, Wang S-Q, Zhu W-Z, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao R-P. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 36.Lantsman K, Tombes RM. CaMK-II oligomerization potential determined using CFP/YFP FRET. Biochim Biophys Acta. 2005 October 30;1746(1):45–54. doi: 10.1016/j.bbamcr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Hagemann D, Xiao RP. Dual site phospholamban phosphorylation and its physiological relevance in the heart. Trends Cardiovasc Med. 2002 February;12(2):51–6. doi: 10.1016/s1050-1738(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 38.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, Bers DM, Mignery GA. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J Biol Chem. 2006 January 6;281(1):608–16. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 39.Sei CA, Irons CE, Sprenkle AB, McDonough PM, Brown JH, Glembotski CC. α-Adrenergic stimulation of atrial natriuretic factor expression in cardiac myocytes requires calcium influx, protein kinase C and calmodulin-regulated pathways. J Biol Chem. 1991;266:15910–6. [PubMed] [Google Scholar]
- 40.Passier R, Zeng h, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek PA, Richardson JA, Grant SR, Olson EN. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008 August;45(2):128–47. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge MJ. Inositol trisphosphate and calcium signaling mechanisms. Biochim Biophys Acta. 2009 June;1793(6):933–40. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell. 2009 February 27;33(4):472–82. doi: 10.1016/j.molcel.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998 October;78(4):921–47. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 45.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996 December;79(6):1059–63. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 46.Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN. Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol. 2008 May;28(10):3437–45. doi: 10.1128/MCB.01611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM. Ca2+/calmodulin-dependent protein kinase IIdelta and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ Res. 2008 March 28;102(6):695–702. doi: 10.1161/CIRCRESAHA.107.169755. [DOI] [PubMed] [Google Scholar]
- 48.Little GH, Saw A, Bai Y, Dow J, Marjoram P, Simkhovich B, Leeka J, Kedes L, Kloner RA, Poizat C. Critical role of nuclear calcium/calmodulin-dependent protein kinase IIdeltaB in cardiomyocyte survival in cardiomyopathy. J Biol Chem. 2009 September 11;284(37):24857–68. doi: 10.1074/jbc.M109.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J Biol Chem. 2007 April 6;282(14):10833–9. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.