Abstract
A study was undertaken to evaluate the feasibility of synthesizing six sialic acid-PAMAM glycodendrimers using unprotected sialic acid in as few as 1-4 steps using two different reaction pathways, and to assess the sulfated derivatives for anti-HIV activity. The syntheses were accomplished through either the direct attachment of the sialic acid carboxyl group to amine-terminated PAMAM (a divergent-like approach) using BOP coupling, or by first reacting sialic acid with a polar bifunctional spacer molecule, attaching the sugar-linker to carboxy-terminated PAMAM (a convergent-like approach), and again using BOP-mediated coupling reactions. It was hypothesized that the latter approach would be the most successful method, as any steric congestion between the sialic acid and the PAMAM would be minimized using an intervening polar linker. However, the divergent-like synthesis proved to be the superior method, resulting in 11.4, 14, and 28% of the fully substituted generations 0, 1 and 2 sialic acid-PAMAM conjugates, respectively, as compared to 6.4% of only the generation -0.5 sialic acid-linker-PAMAM conjugate for the convergent-like method. Upon sulfation of the four glycodendrimers, binding capabilities to the recombinant HIV protein, gp120, were assessed using an ELISA assay. Compounds that showed promising binding characteristics were then further assessed for inhibition of HIV-1 infection using a well-characterized luciferase reporter gene neutralization assay. The generation 2 sulfated sialic acid-PAMAM glycodendrimer, sulfo-6, bearing 16 sialic acids with 11 sulfate groups incorporated at 4.03% sulfur content by weight, was found to inhibit all four HIV-1 strains tested in the low μM range.
Introduction
By the close of 2009, there were an estimated 33.3 million people worldwide infected with HIV. This included 2.6 million newly infected, and 1.8 million deaths.1 This is a three-fold increase compared to 1990.2 HAART (highly active anti-retroviral therapy) slows the progression of HIV to AIDS, and lengthens and improves the quality of life for those infected. There are now 4 million people worldwide on HAART, with 73% of those in Africa. However, three times as many people need treatment in resource-limited nations that are not receiving it.3,4
In addition to millions still needing HAART treatment, the utility of antiretroviral drugs is further limited by viral resistance and toxicity issues.5,6 Moreover, there still exists no safe, effective vaccine or exclusively prophylactic drug approved for preventing the acquisition of HIV. The introduction of potent and cost-effective therapies able to not only treat HIV, but also prevent the transmission of the virus is of the utmost importance. This is particularly critical in regions of the world such as sub-Saharan Africa, where 67% of the world's HIV infected individuals reside.2,7 Here, an estimated 80% of people infected are not aware of their own HIV status, and 90% of individuals do not know their partner's status.3
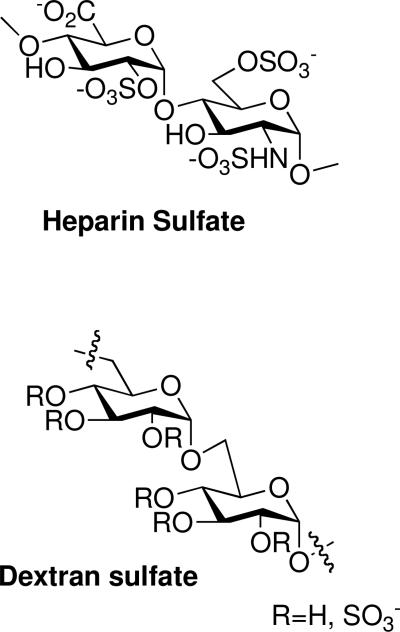
Sulfated polysaccharides have long been recognized as having potent anti-HIV activity. Several naturally occurring sulfated polysaccharides, such as heparin sulfate (HS) and dextran sulfate (DS), inhibit the binding of HIV to CD4 positive cells in vitro in the μg/mL or μM range (Figure 1).8-10 The inhibition of HIV by polyanionic polysaccharides has been actively studied. There are regions in the HIV surface glycoprotein gp120 containing multiple basic amino acids, namely the principle neutralizing domain (V3 loop, amino acids 303-338), the C-terminal region (amino acids 495-516), and a conserved region involved in chemokine coreceptor binding (discontinuous amino acids in regions 117-123, 207, 419-444).11-14 These regions have been shown to interact with polyanions such as HS and DS, which prevents binding to complementary antibodies.13,15 Surface plasmon resonance (SPR) studies revealed strong polyanion-gp120 binding with immobilized HS and monomeric gp120, yielding an affinity constant of 220 nM. These studies also determined that an average of 4.4 gp120 molecules bind each chain of heparin, indicating that higher avidity binding would be possible in a multivalent-sense.15-17 Mechanistically, it has been proposed that HIV binds to cell surfaces electrostatically between the polybasic V3 loop and host cell surface heparan sulfate proteoglycans (HSPG).18 Another group suggested that a sequential process occurs, whereby first HSPG binds through a high affinity, selective interaction with the V3 loop on gp120, followed by a second, lower affinity interaction between such polyanions and the conserved chemokine coreceptor region.15 It has also been noted that the binding between gp120 and polyanions occurs without disrupting the gp120-CD4 interaction, further strengthening the argument that the polybasic regions of gp120 bind to the chemokine coreceptors.11,15,18
Figure 1.
Structures of polyanionic polysaccharides possessing anti-HIV activity.
HS and DS, while having strong affinity to the basic regions of gp120, are also anticoagulants, making it difficult to achieve therapeutic anti-HIV levels of the drugs without compromising blood clotting time.19 Additionally, in Phase I/II clinical trials, DS was found to be poorly absorbed orally, and when given intravenously, resulted in toxic side effects such as reversible thrombocytopenia and alopecia and did not yield a therapeutic effect based on HIV marker levels such as p24.20,21 However, a later study of DS found good oral absorption of DS into the blood stream indicating that DS has therapeutic potential and merits further study.22
A wide variety of other polysaccharides isolated from native sources, and either naturally or synthetically sulfated, have been found to have in vitro anti-HIV activity. Included are polysaccharides isolated from bacteria such as E. coli K5, marine plants such as algae, marine invertebrate animals such as tunicates and sponges, and land plants such as lichen.23 These sulfated homo- and hetero-polysaccharides contain numerous simple monosaccharide building blocks such as glucuronic acid, N-acetylglucosamine, galactose, mannuronic acid and L-fucose, to name a few. Most of these polysaccharides have anti-HIV activities in the μg/mL range, all suffer from polydispersity of structure, and some having toxicity issues similar to DS and HS.23
Sulfated polysaccharides, while potent in vitro inhibitors of HIV, have not yet proven effective drug candidates for HIV due to their inherent polydispersity, as well as toxicity issues. We hypothesize that if several shorter chains of the sulfated sugars are anchored to a carrier molecule, anti-HIV activity can be maintained, while toxicity and polydispersity of structure can be decreased or eliminated. A carrier molecule that can be utilized in this fashion is a dendrimer.
Dendrimers are a unique class of multivalent polymers, first reported by Tomalia and coworkers in 1985.24 They are globular in shape and consist of a wide variety of architectures, with diverse sizes and chemical composition. Dendrimers are built in series with varying numbers of branches, which gives rise to a group of related molecules known as generations (G). With each branching reaction comes an increase in the number of branches, and subsequently, the next highest generation. Dendrimers can be synthesized in one of two ways, either divergently, or convergently. Divergent synthesis involves building the dendrimer from the core outward, while a convergent strategy entails first building blocks of the molecule separately, then attaching them to a minimal core structure in the final step. Dendrimers have been used in many medical applications ranging from drug delivery, to uses as immunodiagnostic reagents, MRI contrast reagents, gene delivery vectors, immunostimulation agents/adjuvants, or drug delivery vehicles.25-30 Dendrimer research is important due to the diversity of structures that can be devised and their utility in numerous biological applications. It is important to understand the toxicity, solubility, and other properties of dendrimers, to ensure that they can be safely used in the development of new therapeutics. PAMAM (poly(amido amine))-based dendrimers have been well evaluated both in vitro and in vivo. In the in vivo studies in mice and rats, it has been found that PAMAM is cytotoxic in a size-dependent fashion, with larger generations being more toxic.31 Other properties affecting toxicity are charge (anionic PAMAM is less toxic than cationic), and surface modification with other groups, such as PEG (polyethylene glycol) or sugars.32 PEGylation/glycosylation of PAMAM yields dendrimers with much lower toxicity profiles.31
When dendrimers are glycosylated, they are known as glycodendrimers, a class of compounds first reported by Roy in 1993.33 Glycodendrimers built on a variety of dendrimer scaffolds such as PAMAM have found numerous uses in medicine due to the multivalent effect. Monovalent carbohydrates typically have weak millimolar binding constants in protein-carbohydrate recognition.34 However, binding affinities achieved by the multivalent effect are orders of magnitude improved over a one molecule-one receptor binding event.35,36 In nature, the multivalent effect is observed in many interactions, for example, in the viral infection by influenza where the viral trimeric hemagglutinin protein recognizes multiple copies of the carbohydrate sialic acid on the host cell.37 A variety of sialic acid glycodendrimers were formulated as multivalent inhibitors of the binding between hemagglutinin and the host cell. The degree of inhibition achieved by these glycodendrimers was 32-50,000 fold higher than monovalent sialic acid.37 Glycodendrimers, therefore, present an alluring prospect for other medical applications because they increase the valency of a biologically active carbohydrate and improve the binding constants between the carbohydrate and the target protein.38,39
Scientists have begun to explore glycodendrimers as anti-HIV agents. Many of these are glycosphingolipid (GSL) mimics, as it is known that GSLs can be utilized by HIV-1 as alternate host cell receptors in the infection process.40 The first account by Schengrund reported the synthesis of sulfated galactosyl ceramide (SGalCer)-coated polypropyleneimine (PPI) dendrimers, directed at preventing infection in CD4-negative cells.41,42 In their studies, they determined that gp120 bound to their glycodendrimers in the nM range, as compared to the known polysaccharide HIV inhibitor DS, which bound in the pM range. Cytotoxicity of the glycodendrimers at up to 3 mg/mL was not observed. Since this initial report, there have been a few more examples of potential anti-HIV glycodendrimers. Two other GalCer-based dendrimers were reported by Castillon and coworkers and Blanzat, Turrin and coworkers.43,44 One had a polyglycerol (PG) dendrimer core, and had lower activity than the Schengrund glycodendrimers.42,43 The other GalCer glycodendrimers were based on a phosphonic acid core, and determined to have sub-μM IC50s in a cell-based HIV assay (IC50 is the concentration that reduces HIV infection by 50%). However, these suffered from high cytotoxicities.44,45 Another report was of a polylysine-sulfated cellobiose system.46,47 This glycodendrimer was found to have an EC50 (effective concentration for 50% effect) of 3.2 μg/mL, comparable to the NRTI (nucleoside reverse transcriptase inhibitor) ddC (2'-3'-dideoxycytidine), and was also determined to have low cytotoxicity. Additionally, a mannose-based PG glycodendrimer was reported that targeted the dendritic cell lectin DC-SIGN.48 The IC50 activity of this glycodendrimer was μM, similar to the others. Finally, Schengrund and coworkers recently reported two types of glycodendrimers based on the PPI core, terminating in either of the glycosphingolipid derived sugar headgroups, 3’-sialyllactose (GM3) or globotriose (Gb3).40 The GM3 and Gb3 glycodendrimers were assessed for anti-HIV-1 activity in T-cells and primary peripheral blood mononuclear cells (PMBCs), and yielded IC50s ranging from 0.1-7.4 μg/mL.40
While the above results are encouraging, the search for easy to make, cost-effective and potent new anti-HIV therapeutics remains a significant goal for the scientific/medical community in light of the high rate of infection continuing to this day, the longer life spans of infected individuals, and the therapeutic failures occurring due to drug toxicity and resistance. All of these factors contribute to fewer therapeutic strategies available to a patient the longer they have been infected. Additionally, if new anti-HIV therapeutics can be designed in such a way that they are simple and cost-effective to produce, it will be easier to implement therapy in resource limited regions of the world, where the bulk of HIV infections are occurring.
Along these lines, we endeavored to create an initial trial set of 6 compounds based on the commercially available dendrimer core, PAMAM (G=-0.5, 0, 0.5, 1, 1.5 and 2), and the unprotected sugar, sialic acid. This could be accomplished by one of two pathways, a one step divergent-like pathway, whereby sialic acid was directly coupled to PAMAM, or a 4 step convergent-like approach, where an intermediary linker was first reacted with sialic acid then the sugar-linker conjugate coupled to PAMAM). The first approach was anticipated to be the most difficult as the sialic acid carboxyl group is sterically congested and might not be accessible enough to the also sterically crowded multiple reaction centers in PAMAM. These potential pitfalls led us to develop the second pathway, where a long linear hydrophilic linker was first attached to sialic acid, followed by reaction of the sugar-linker to PAMAM. This was thought to be the best approach, as the steric consequences suffered by both reaction partners would be greatly minimized in the presence of the hydrophilic linker. Whichever pathway produced the desired glycodendrimer conjugates with the best yields would then be utilized in further syntheses.
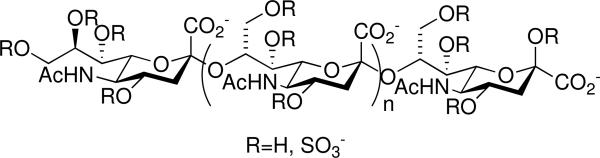
As for the individual sugar and dendrimer choices, there were many factors to consider. PAMAM was chosen primarily because of its availability and also because there was an abundance of data available on the cellular toxicities of PAMAM-based molecules (vide supra). Sialic acid was chosen for a few key reasons as well. First, it contains a carboxyl group attached to the anomeric carbon, making it easy to append it to either an amino-bearing linker or to an amino-terminated PAMAM using standard solution phase peptide coupling methodologies, all without having the sugar protected. Second, some preliminary anti-HIV data has been reported for a sialic acid-based polysaccharide, colominic acid, in the sulfated form. Colominic acid is an α-2→8-linked polymer of sialic acid (Figure 2). Yang and coworkers found EC50 values as low as 0.06 μg/mL for the larger molecular weight, more highly sulfated derivatives of colominic acid (MW ranging from 8-16 kDa, sulfation 8-12%) in MT-4 and C8166 cell lines infected with HIV in the presence of these molecules.19 Their assay control, DS, yielded EC50 values of 0.5 and 2.51 μg/mL in the same cell lines, respectively. Finally, by using the base sugar from a polymer with known anti-HIV activity, we would be able to quickly ascertain if utilizing PAMAM as a scaffold for the multivalent presentation of sulfated sugars to the target protein, gp120, was as/more effective in binding to gp120/inhibiting HIV infection than the known sulfated linear polysaccharide standard, DS.
Figure 2.
Sulfated colominic acid.
Once synthesized, all of the sulfated sialic acid-PAMAM glycodendrimers could be assessed for anti-HIV-1 activity by two separate, yet complementary assays. The first assay, a fast ELISA (enzyme-linked immunosorbent assay) developed in our lab, could be used to screen for the presence of gp120 binding. If activity was found, a second, more sensitive luciferase reporter gene assay could then be utilized to determine how well the sulfoglycodendrimers were able to inhibit HIV-1 infection of TZM-bl cells.
Experimental Procedures
General Materials and Methods
All nuclear magnetic resonance (NMR) spectroscopy was performed on a Bruker Avance III 500 MHz NMR spectrometer with either D2O or CDCl3 solvents purchased from Acros. To simplify the analysis of the 1H NMR spectra for compounds 1-6, the integrations were normalized for ¼ of total protons, the equivalent of 1 branch of the full glycodendrimer. For 13C analysis, 3-(trimethylsilyl) tetradeutero sodium propionate (TSP) from Wilmad was used as a zero point reference, and all spectra were proton decoupled. MALDI mass spectral analysis was conducted on a Kratos/Shimadzu Axima-CFR MALDI-TOF (University of the Pacific) and a Bruker Reflex III MALDI-TOF (The Ohio State University). High resolution ESI mass spectral analysis was conducted on an IonSpec Fourier Transform mass spectrometer (University of Arizona). Flash chromatography was performed using flash silica gel (32-63 μM) from Dynamic Adsorbents Inc. Dialysis purification was performed using Spectra/por® Biotech Cellulose Ester dialysis membrane from Spectrum Laboratories Inc. Reverse phase high pressure liquid chromatography (RP-HPLC) was conducted on a Hewlett Packard TI-series 1050 using a Grace Prevail C-18 5μ 250 × 10 mm column. Fast pace liquid chromatography (FPLC) was performed with a Pharmacia pump P-500 with a LC Controller LCC-500 Plus. The column used in conjunction with the FPLC was a Bio-Gel P-10 2.5 cm × 120 cm column.
The reagents used came from a variety of sources and were used without further purification: All poly amido(amine) (PAMAM) dendrimers were purchased from Aldrich. Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) was from Nova Biochem. N,N-diethylisopropyl amine (DIPEA) was purchased from Alfa Aesar. Trifluroacetic acid (TFA), triethylamine (TEA), Ammonium bicarbonate, and N,N-dimethylformamide (DMF) were from EMD. Di-tert-butyl dicarbonate ((Boc)2O) was purchased from Acros. Diaminotriethylene glycol was obtained from Huntsman. N-Acetylneuraminic acid (sialic acid) was purchased from Nacalai Tesque. 500 kDa dextran sulfate was purchased from Sigma-Aldrich.
Note on Characterization
All labels on protons in the 1H NMR data correspond to assignments given on the spectra. The spectra can be found in the Supporting Information section.
Nomenclature note
All glycodendrimers are named as follows: The number of end points, tetramer, octamer, or 16-mer, followed by glyco-. If a linker is present between PAMAM and sialic acid, the abbreviation DATEG (diamino triethylene glycol) appears. The name terminates in PAMAM dendrimer.
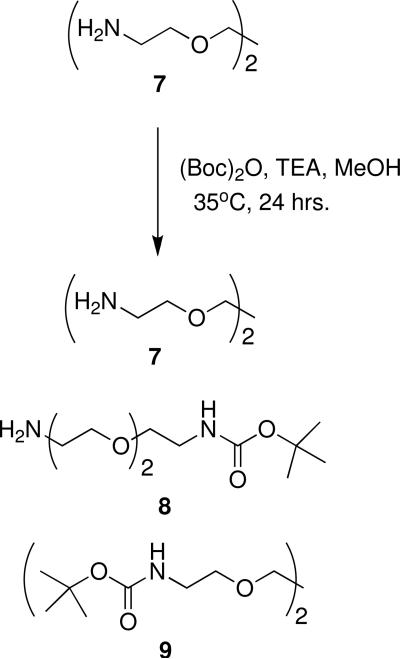
{2-[2-(2-Amino-ethoxy)-ethoxy]-ethyl}-carbamic acid tert-butyl ester (8)
Diamino triethyleneglycol 7 (5.0 g, 33.8 mmol) was weighed into a flame-dried 500 mL round-bottomed flask. Methanol (135 mL) was added, followed by TEA (340 mg, 3.4 mmol), then (Boc)2O (7.38 g, 33.8 mmol). The reaction was heated to 35°C and stirred overnight. The solvents were evaporated under reduced pressure and the crude residue was purified by flash chromatography using 1:1 methanol:chloroform, giving 8 as a viscous golden oil (4.25 g, 17.1 mmol, 51% yield). 1H NMR (D2O): δ 1.41 (s, 9H, HT), 2.79 (t, 2H, J=5.4 Hz, HS), 3.25 (t, 2H, J=5.4 Hz, HP), 3.57 (q, 4H, J= 5.0 Hz, 9.7 Hz, HQ), 3.66 (s, 4H, HR). 13C NMR (D2O, TSP internal std): δ 30.4, 42.6, 72.1, 72.2, 74.9, 161.
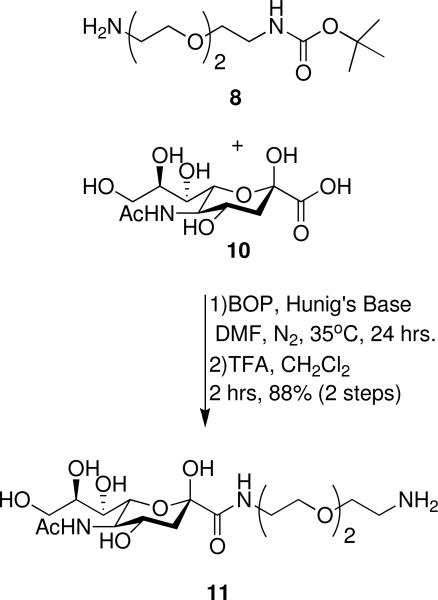
N-Acetylneuraminic acid {2-[2-(2-amino-ethoxy)-ethoxy]-ethyl}-amide (11)
Sialic acid (10, 100 mg, 0.32 mmol) was weighed into a flame-dried 25 mL round-bottomed flask, dissolved into DMF (2 mL) and placed under nitrogen. BOP (210 mg, 0.48 mmol) was added to the solution as a solid. In a separate flask, the linker 8 (87 mg, 0.35 mmol) was dissolved in DMF (1 mL). DIPEA (170 mg, 1.28 mmol) was then added. This mixture was next added to the sialic acid solution. The reaction was heated to 35°C and stirred for 24 hours. The solvent was evaporated under reduced pressure. The crude was dissolved in 1:2 dichloromethane:TFA (12 mL) and stirred for 2 hours before the solvents were evaporated under reduced pressure. The crude material was purified with RP-HPLC using a gradient of deionized water/0.1% trifluoroacetic acid and acetonitrile/0.1% trifluoroacetic acid giving 11, as a gummy white solid (155 mg, 0.35 mmol, 88% yield). 1H NMR (D2O): δ 1.67 (t, 1H, J=12.2 Hz, HB), 2.03 (s, 3H, HC), 2.31 (dd, 1H, J=4.7 Hz, 13 Hz, HA), 3.19 (t, 3H, J= 4.8 Hz, HP), 3.43 (m, 2H, HS), 3.55-3.73 (M, 11H, HG,H,J,Q,R) 3.82 (dd, 1H, J=2.6 Hz, 11.9 Hz, HE), 3.89 (t, 1H, J=10.3 Hz, HI), 4.05 (m, 2H, HD,F) 13C NMR (D2O, TSP internal std): δ 22.3, 39.0, 39.3, 39.8, 52.4, 63.4, 66.6, 67.0, 68.4, 68.9, 69.7, 69.8, 70.4, 70.7, 96.0, 173, 175. High resolution electrospray mass spectrometry (HR-ESI+: [M + H]+ (C17H34N3O10) calcd m/z = 440.2239. Found m/z = 440.2238.
General procedure for the synthesis of glyco-DATEG-PAMAM dendrimers
PAMAM G= -0.5, 0.5 or 1.5 (1 equiv) was weighed into a flame-dried 10 mL round-bottomed flask and dissolved in DMF (1 mL), and flushed with nitrogen. DIPEA (2.5 equiv/arm) was added, followed by BOP (1.2 equiv/arm). The sugar linker 11 (1.1 equiv/arm) was placed into a second 10 mL round-bottomed flask and dissolved in DMF (1 mL). The two solutions were mixed together, heated to 35°C, and stirred under nitrogen for 1, 7 and 14 days, respectively. The solvents were evaporated under reduced pressure and the crude product was dialyzed with 500 MWCO tubing in a 1L flask against nanopure water. The water was changed once every hour for four hours, and allowed to stir overnight at 4°C. The remaining crude material was then lyophilized. The dialyzed crude material was then purified using RP-HPLC with a linear gradient between water/0.1% trifluoroacetic acid and acetonitrile/0.1% trifluoroacetic acid. Fractions containing the product were collected, grouped, and further purified using a 2.5 × 120 cm Bio-Gel P-10 in 0.03 M ammonium bicarbonate. A 6.4% yield of only compound 1 was achieved by this method.
General procedure for the synthesis of glycoPAMAM dendrimers
Sialic acid (10, 1.1 equiv/arm) was weighed into a flame-dried 10 mL round-bottomed flask, flushed with nitrogen, then dissolved into DMF (4 mL). BOP (1.5 equiv/arm) was then added as a solid. PAMAM G= 0, 1, or 2 (20% wt. in methanol) (1 equiv) was next weighed into a separate 10 mL round-bottomed flask and the methanol was evaporated under reduced pressure. DMF (1.5-2.0 mL) and DIPEA (2.5 equiv/arm) were added to the flask with PAMAM. The PAMAM solution was then added to the sialic acid solution, heated to 35°C, and the reaction was stirred for 1, 7 or 14 days, respectively, under nitrogen. The crude reaction product was dialyzed with 500 MWCO tubing against nanopure water. The water was changed once an hour for four hours then left stirring overnight at 4°C. The crude material was then purified using RP-HPLC with a linear gradient between water/0.1% trifluoroacetic acid and acetonitrile/0.1% trifluoroacetic acid providing compounds 2, 4, and 6 in 11.4, 14, and 28% yields, respectively.
General Sulfation Procedure
Non-selective sulfation of the glycodendrimers was carried out according to the procedure of Kunou, et al.49 Briefly, the glycodendrimers were sulfated under a nitrogen atmosphere at 0°C, using a 3:1 ratio of SO3-Pyridine complex to hydroxyl groups per sugar unit. 10mg of each glycodendrimer and the appropriate mass of SO3-Pyrindine complex were each dissolved in 1.25mL of dry DMF under N2. The sulfating solution was added dropwise into the glycodendrimer solutions and the reaction mixtures were stirred for 1 hour at 0°C under N2. The reactions were terminated by the addition of 0.5mL iced-cold water and the pH of the reaction mixtures were adjusted to above 9 with 2M NaOH. The reaction mixtures were next added dropwise to 50mL of iced-cold acetone and left to precipitate at -20°C for 24 hours. The precipitates were collected by centrifugation, then decantation of the acetone. The precipitates were dissolved in small volumes of water and purified via dialysis (either 500 or 1000 MWCO tubing was used, Spectra/Por) against purified DI water at 4°C. The sulfated glycodendrimers were then obtained as fine white solids by lypohilization. Finally, the sulfated glycodendrimers were evaluated by Columbia Analytics in Tucson, AZ to determine the % sulfur for each. From this information, the approximate number of sulfate groups incorporated into each glycodendrimer was determined. For sulfo-1, 2, 4, and 6, the %S incorporated into each glycodendrimer was 11.22, 15.32, 2.02 and 4.03%, respectively.
General procedure for the competitive ELISA with biotinylated recombinant gp120
A competitive ELISA was developed to determine the inhibitive binding properties of the sulfated glycodendrimers against the HIV-1 monoclonal antibody peroxidase-labeled murine mAb1101-P gp120 HIV-I (Immunodiagnostics), to biotin-labeled rgp120 HIV-I IIIB (b-rgp120, Immunodiagnostics). Dextran sulfate (500 kDa) was used as a positive assay control, and buffer was used as a negative assay control. Competition between the b-rgp120 and the mAb was evidenced by a decrease in the overall absorbance at 450 nm. To run the assay, a 1:5000 dilution of b-rgp120 was prepared in PBS and 100μL of this dilution was transferred to the wells of Reacti-Bind NeutrAvidin coated strip plates (Thermo Scientific). The plates were incubated for 1 hour at 25°C while shaking at medium speed in a Jitterbug Plate Incubator Shaker (Boekel). The plates were washed three times with PBS containing 0.05% (v/v) Tween 20. 200μL of PBS containing 1% (w/v) BSA was added to each well to block non-specific binding sites and the plates were incubated for 1 hour at 25°C while shaking at medium speed. While blocking a 1:1000 dilution of the mAb 1101-P, and 0.2-400μg/mL dilutions of the sulfated glycodendrimers were prepared in PBS-Tween 20 containing 0.1% (w/v) BSA. The plates were washed three times, and then the wells were treated in duplicate with 50μL of the sulfated glycodendrimer dilutions plus 50μL of the mAb1101-P dilution and incubated for 1 hour at 25°C while shaking at medium speed. The plates were washed three times. The wells were treated with 100μL of SureBlue peroxidase substrate (KPL) and the kinetic reaction absorbances were read for 10 minutes at 655nm on a BioRad Model 550 plate reader. The kinetic reaction was stopped by the addition of 100μL of 1M HCl to each well. The endpoint absorbances were read at 450nm. The duplicate endpoint readings were averaged and plotted against the log of the concentration (g/L) in a non-linear regression analysis using GraphPad Prism 4 software. From this, IC50 (inhibitory concentration to achieve 50% of the effect, in this case 50% less binding between b-rgp120 and the mAb) values in μg/mL and R2 values were determined. A minimum of two assays were completed for each glycodendrimer or standard.
HIV Neutralization Luciferase Reporter Gene Assay
Inhibition of HIV infectivity by the sulfated glycodendrimers was assessed in a formally optimized, validated and GCLP (good clinical laboratory practice)-compliant infectivity reduction assay according to the method of Montefiori et al. and conducted at Duke University in the GCLP-certified Primate Central Immunology Laboratory.50,51 The solid glycodendrimer samples and dextran sulfate were prepared for analysis as follows: They were first dissolved in PBS and sterile filtered to give 1 mg/mL concentrations. The 1 mg/mL stock solutions were serially diluted 1:3 in concentrations ranging from 50-0.02 μg/mL. The HIV-1 strains utilized in the assay included Q23.17 (Clade A), MN.3 (Clade B), MW965.26 (Clade C) and TV1.21 (Clade C). The cells assayed for infection were TZM-bl cells expressing CXCR4, and engineered to express the human cell receptors CD4 and CCR5, in addition to the enzymes, firefly luciferase and Escherichia coli β-galactosidase. An additional positive control, HIVIG-C, a purified IgG pooled from HIV+ donors from South Africa, was prepared from a stock 12.5 mg/mL solution and used in the assays in 1:3 dilutions ranging from 625-0.29 μg/mL.
Results and Discussion
The purpose of this work was to examine the ease of synthesizing glycodendrimers of various generations using both convergent-like and divergent-like synthetic strategies, and to perform some preliminary assessments for potential HIV-1 activity. With the sole exception of the preparation of compound 8, no protecting group chemistry was used in this study. Because protecting group strategies require two additional steps per group, and result in lower overall yields, eliminating these steps saves time, and precludes the need for numerous purification steps. To achieve this, a synthetic route was devised where the reactions were conducted in a polar solvent to ease solubility issues, the reagents would not target the unprotected hydroxyls, and the chemistry utilized was efficient enough to allow for the desired conversion to the fully substituted products could be readily achieved. Whichever strategy proved superior would then allow for the creation of a variety of glycodendrimers of various sizes and sugar compositions more quickly. To test the convergent-like strategy for the synthesis of dendrimers, sialic acid was coupled to a hydrophilic linker, 8, which was then attached to PAMAM (poly(amido amine)), both utilizing amide linkages. As linker 8 was amine terminated, the half generations of carboxylic acid-terminated PAMAM (G= -0.5, 0.5, and 1.5) were employed in these cases. For the divergent-like strategy, the carboxyl group of sialic acid was coupled directly to the terminal amine of PAMAM G= 0, 1 and 2.
Glycodendrimer Synthesis
Generations -0.5, 0.5, and 1.5 were coupled to the sugar-linker complex through a convergent synthetic method. To achieve this, the linker (diaminotriethylene glycol, 7) was singly protected with di-tert-butyldicarbonate ((Boc)2O). As both ends of the linker are chemically equivalent, this reaction afforded three products: unreacted starting material (7), the mono-protected linker (8) and the di-protected linker (9). Mono-protected linker (8) was isolated by normal phase flash chromatography using 1:1 chloroform and methanol, giving the desired product (8) in a 51% yield (Scheme 1).
Scheme 1.
Monoprotection of diaminotriethylene glycol (8).
The mono protected linker (8) was next coupled to sialic acid (10) using benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) coupling conditions. After overnight reaction at 35°C, the Boc protecting group was removed in 1:2 dichloromethane/trifluroacetic acid (TFA) (Scheme 2).
Scheme 2.
Amide coupling of sialic acid to linker, followed by deprotection.
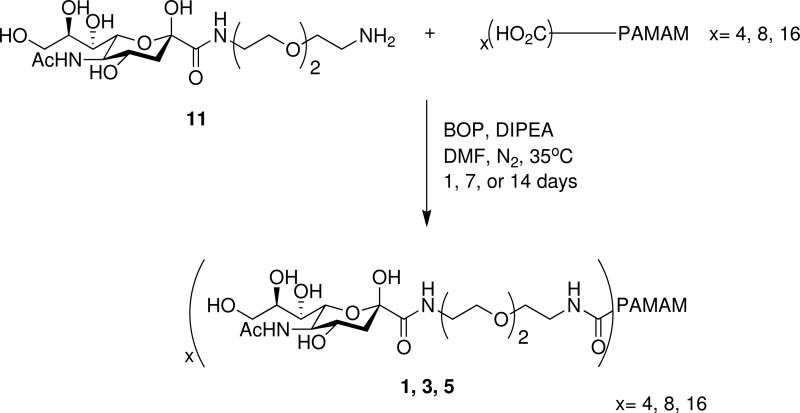
Compound 11 was purified by reverse phase high-pressure liquid chromatography (RP-HPLC), giving the desired product in 88% overall yield for two steps. Next, the sugar-linker (11) was attached to PAMAM generations -0.5, 0.5, or 1.5 via an amide coupling using BOP and DIPEA (N,N-diisopropylethylamine) in DMF at 35°C, under nitrogen for 1, 7 or 14 days, respectively, producing glycodendrimers 1 and 3 (Scheme 3).52 Glycodendrimer 5, unfortunately, was not isolated.
Scheme 3.
Amide coupling of sugar-linker PAMAM generations -0.5, 0.5, and 1.5.
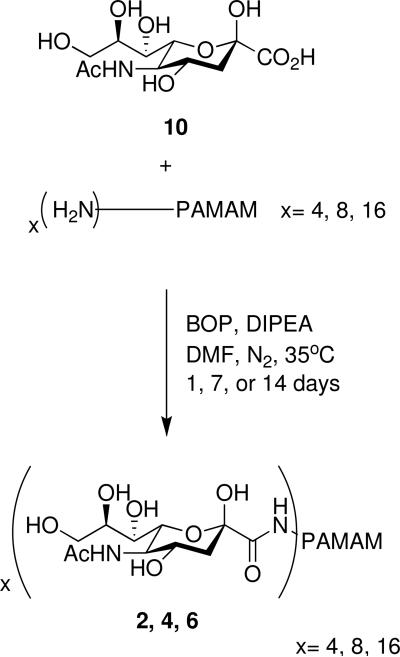
To make compounds 2, 4, and 6 using a divergent-like approach, sialic acid (10) was mixed with the appropriate PAMAM and allowed to react using BOP coupling conditions at 35°C under nitrogen gas for 1, 7 or 14 days. This produced glycodendrimers 2, 4, and 6 (Scheme 4).
Scheme 4.
Amide coupling of sialic acid (10) to PAMAM generations 0, 1, and 2.
Glycodendrimer Characterization
One of the primary goals of the research presented herein was to develop an efficient synthetic strategy to evaluate convergent and divergent-like synthetic methods to determine which method was simpler/more efficient for the synthesis of sialic acid-coupled glycodendrimers as that could serve as effective and potent anti-HIV-1 agents. An additional goal involved the minimization/elimination of the polydispersity inherent to the polysaccharides known to have anti-HIV activity. If successful, this would result in structurally well-defined glycodendrimers, and allow for a greater understanding of which features are the most critical for anti-HIV-1 activity. This was viewed as an important aspect of the study as many of the previous reports yielded incompletely substituted glycodendrimers (vide supra).40,41,43,46,47 In the current study, the divergent-like approach consisted of only a single step between sialic acid and the appropriate amine-terminated PAMAM via a BOP coupling (Scheme 4). This synthesis was anticipated to be difficult as the carboxyl group on sialic acid is sterically hindered. That, along with a large, bulky dendrimer, and the simultaneous occurrence of multiple reactions in the coupling step, was expected to present a great synthetic challenge and result in understandably low yields. The convergent-like synthesis, on the other hand, was expected to be much more facile as an intermediary polar primary amine-terminated linker was first attached to sialic acid in a 1:1 reaction, which was then coupled to carboxy-terminated PAMAM dendrimers (Scheme 3). This strategy was expected to be advantageous as the steric issues between sialic acid and PAMAM would be minimized by the presence of a long polar spacer molecule. It was thus anticipated that the coupling between the sialic acid-linker (11) and various PAMAM cores would result in greater yields than the divergent-like approach.
It was therefore a surprise to find that the divergent-like synthetic method, where sialic acid was directly coupled to PAMAM, produced the desired fully substituted products in higher yields than the convergent-like approach. For the divergent-like reactions, compounds 2, 4, and 6 were all isolated and completely characterized via 1H NMR, 13C NMR and MALDI-MS in modest yields of 11.4%, 14%, and 28%, respectively. The yields were likely low due to the steric issues previously mentioned.
For the convergent-like method, however, the addition of the diaminotriethylene glycol linker was expected to aid the reactions by reducing steric hindrance, yet resulted in minimal/no product isolation. Compound 1 was only isolated/characterized in 6.4% yield. For compound 3, the impurity of the sample and the low quantities produced precluded the ability to obtain confirmatory 1H or 13C data, only MALDI MS analysis was possible. The calculated molecular weight for 3 is 4462 Da, however, MALDI MS showed small peaks for (M+H) m/z=4467, (M-H2O) m/z=4448, and (M-2H2O) m/z=4428, along with impurity peaks (M-compd 11-H2O) m/z=4027 and (M-2 compd 11) m/z=3619. Compound 3 has more than 180 carbon atoms, so it is likely there are at least two 13C atoms adding weight beyond the calculated molecular mass. Compound 5 was neither isolated nor confirmed, even after multiple attempts at synthesizing and purifying this molecule. We hypothesize that the linker may have been too conformationally-flexible, allowing the linker to fold back on itself and participate in intramolecular hydrogen bonding with the polar face of the sialic acid. It is believed that this would result in reduced/absent reactivity of the sugar-linker to PAMAM.
These results clearly indicate that for our system, the divergent-like synthesis was the superior method as fully substituted sialic acid glycodendrimers resulted. Yield improvements for the divergent-like approach are currently being sought in the laboratory through substitution of the solvent and/or coupling reagent. It is thought that a better combination can be found for this system that will behave more favorably with the polarity/solubility issues of the reactants, and result in improved yields, all while maintaining the ability to use unprotected sugars.
Assessment of anti-HIV Activity
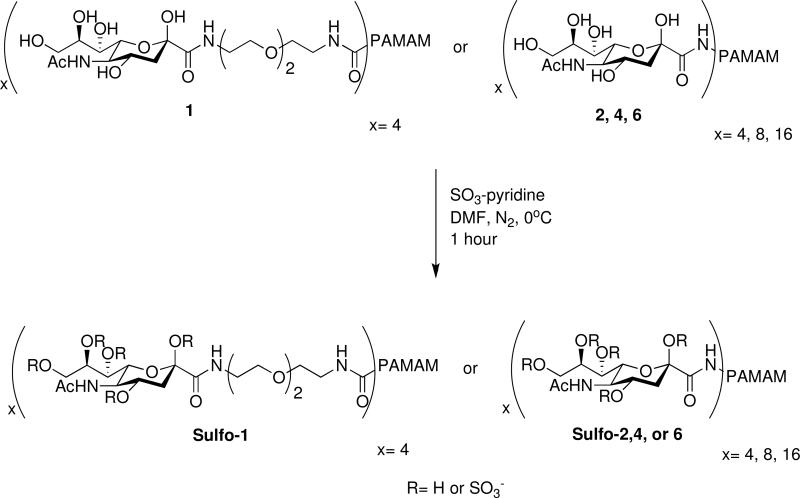
The second major goal for the work presented herein involved the evaluation of sulfated derivatives of the glycodendrimers for possible anti-HIV activity. Once all of the glycodendrimers (1, 2, 4 and 6) were successfully isolated and characterized, they were sulfated and purified according to the method of Kunou et al. (Scheme 5).49 As this method is not selective as to the location or number of sulfates incorporated on the sugar hydroxyl groups, and the molecules had been completely characterized after their initial synthesis, further NMR and mass spectral analysis was not conducted. Instead, elemental analysis for % sulfur (%S) was completed. Using the %S for each glycodendrimer, the average number of sulfate groups incorporated into each glycodendrimer was calculated. The %S for sulfated compounds sulfo-1, 2, 4 and 6 were 11.22, 15.32, 2.02 and 4.03%, respectively, which translated to an average number of sulfate groups incorporated of 10, 13, 2-3, and 11, respectively. The non-selective sulfation method was utilized, rather than a more selective method, as these require additional protection/deprotection steps to incorporate the sulfate group on a specific hydroxyl group. As this was a preliminary study to elucidate a synthetic path that would yield glycodendrimers with anti-HIV-1 activity in the fewest possible steps, it was decided that achieving a completely monomolecular compound at this stage of the project in terms of both the number of sugars and sulfate groups incorporated was of lesser importance than achieving a fully substituted glycodendrimer product. This will however continue to be a goal in future work.
Scheme 5.
Non-selective sulfation of glycodendrimer products.
To evaluate whether or not the sulfated glycodendrimers had the capability of binding to the target HIV-1 protein, gp120, a simple in-house competitive binding ELISA assay was developed. This assay was utilized as an initial screen to quickly assess whether or not the sulfated glycodendrimers had the ability to bind to b-rgp120 (biotinylated recombinant gp120) and thereby inhibit the binding between b-rgp120 and an HRP (horseradish peroxidase)-labeled monoclonal antibody specific to the V3 loop of gp120. Briefly, b-rgp120 was incubated with NeutrAvidin™-coated plates. NeutrAvidin™ is a proprietary biotin-binding protein capable of binding with high avidity to biotin-containing molecules and adhering them more/less permanently to the surface of the plate. This was done to ensure that gp120, with a high carbohydrate composition (~50% by weight), did not wash off of the plate. After blocking the open sites with bovine serum albumin (BSA), the competitive binding step was initiated where both the HRP-labeled monoclonal antibody specific to the V3-loop of gp120 and either the serially diluted sulfated glycodendrimer or assay controls were incubated with the anchored gp120. After the prescribed incubation period and rinsing, the HRP substrate was added and the reaction was monitored for 10 minutes at 655 nm, to observe linearity of the kinetics. The reaction was then stopped with the addition of HCl and the plate read again at 450 nm to ascertain the endpoint absorbances.
All of the sulfated glycodendrimers (sulfo-1, 2, 4, 6) were initially evaluated by the ELISA. Each compound was assayed in duplicate wells a minimum of two times, and all results were compared to the positive assay control, 500 kDa dextran sulfate (Figure 1), containing 17% sulfur (2.3 sulfates/sugar).53 Dextran sulfate consistently results in an average IC50 (inhibitory concentration for a 50% reduction in gp120 binding) of 1.6 nM with an R2 value of 0.99 for the non-linear regression fit for dose-response analysis (Table 1). Sulfo-1, 2, and 4 did not fit non-linear regression dose-response curves, evidenced by low R2 values (data not shown). For Sulfo-6, higher concentrations of this molecule led to a noticeable decline in the absorbance at 450 nm, indicating that some binding to gp120 was achieved, however a 50% inhibition of antibody binding to b-rgp120 was not achieved. It was hypothesized that lack of quantitative results by the ELISA may have had more to do with the lower sensitivity limits of the ELISA, rather than whether or not the compounds actually had anti-HIV activity, and we are in the process of optimizing the ELISA assay to improve its sensitivity. The decision was therefore made to evaluate the two higher order sulfated glycodendrimers (sulfo-4 and 6) directly for anti-HIV activity using the luciferase reporter gene assay because it is a more sensitive assay. Sulfo-1 and sulfo-2 were not evaluated further because we had recently determined that other sulfated glycodendrimers of similar size did not yield anti-HIV-1 activity in the luciferase assay (data not shown).
Table 1.
Summary of pertinent structural and HIV assay data for sulfated glycodendrimers and positive assay controls, dextran sulfate and antibody HIVIG-C.
| Compound | Appx. MW (g/mol) | % Sulfur | Avg. # Sulfates | ELISA IC50c | Q23.17 (Clade A) IC50d | MN.3 (Clade B) IC50d | MW965.26 (Clade C) IC50d | TV1.21 (Clade C) IC50d |
|---|---|---|---|---|---|---|---|---|
| Sulfo-1 | 2821 | 11.22 | 10 (2.5/sugar) | >71 μM | NA | NA | NA | NA |
| Sulfo-2 | 2707 | 15.32 | 13 (3.25/sugar) | >74 μM | NA | NA | NA | NA |
| Sulfo-4 | 3992 | 2.02 | 2 to 3 (0.25-0.38/sugar) | >50 μM | >12.5 μMb | >12.5 μMb | >12.5 μMb | >12.5 μMb |
| Sulfo-6 | 8777 | 4.03 | 11 (0.69/sugar) | >23 μMa | 5.1 μM | 2.4 μM | 1.6 μM | 4.0 μM |
| Dextran Sulfate | 500,000 | 17 | 2.3/sugar | 1.6 nM | 8.2 nM | 4.4 nM | 2.8 nM | 2.6 nM |
| HIVIG-C | 150,000 | NA | NA | NA | 98.7 nM | 35.3 nM | 8.7 nM | 0.34 μM |
NA=Not applicable/no assay was conducted.
Reduction in the absorbance at 450nm by Sulfo-6 was only observed at the highest compound concentrations tested and did not reach the cut-off level of a 50% reduction in absorbance at 450 nm.
Reduction in the luciferase signal by Sulfo-4 was only observed at the highest compound concentrations tested and did not reach the 50% neutralization cut-off.
Values are the sample concentration (μM) at which the absorbance at 450 nm was reduced by 50% compared to the control wells (no test sample). Sample concentrations ranged from 0.1-200 μg/mL in the assay wells.
Values are the sample concentration (μM) at which relative luminescence units (RLUs) were reduced 50% compared to virus control wells (no test sample). Sample concentrations ranged from 0.02-50 μg/mL for the sulfoglycodendrimers and DS, and 0.29-625 μg/mL for HIVIG-C.
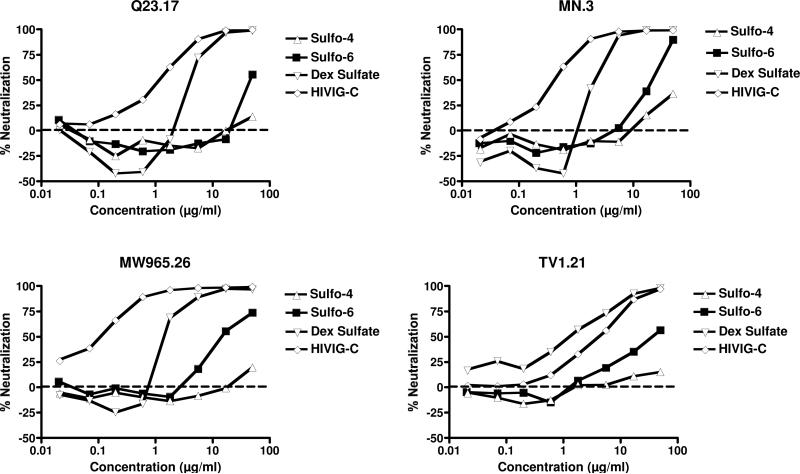
The luciferase reporter gene assay used in this analysis was first developed by Shaw and coworkers, then further optimized and validated by Montefiori and colleagues.50,51,54 This assay evaluates a single round of infection of a modified TZM-bl cell line, derived from HeLa. TZM-bl cells express the HIV-1 coreceptor, CXCR4, and have been engineered to express coreceptor CCR5 and receptor CD4, in addition to the integrated reporter genes for firefly luciferase and the β-galactosidase gene from Escherichia coli. The pseudoviruses utilized (Q23.17 (Clade A), MN.3 (Clade B), MW965.26 (Clade C) and TV1.21 (Clade C)) are infectious, but are not able to replicate to yield viable virions and are thus limited to one round of infection. Upon infection of the TZM-bl cells, the tat gene carried by the pseudovirus is expressed and the resultant Tat protein trans-activates the luciferase reporter gene. Development of the assay involves quantifying the luminescence resulting from the enzyme activity of luciferase, which is directly proportional to the number of infection events by the pseudoviruses. Addition of an agent with anti-HIV activity results in a decrease in the luminescence signal. Both sulfo-4 and 6 were analyzed in the TZM-bl luciferase reporter gene assay, in addition to the positive control, dextran sulfate. The IC50 values (Table 1) are the sample concentration at which the relative luminescence units were reduced by 50% compared to virus control wells (no test sample). HIV inhibitory activity was detected for Sulfo-6, with IC50 values for all viruses tested in the low micromolar range. Reduction in luciferase signal by Sulfo-4 was only observed at the highest compound concentrations tested and did not reach the 50% neutralization cut-off. The positive controls, dextran sulfate and HIVIG-C, were both potent inhibitors as expected, yielding nM inhibition in all four HIV-1 strains, except for TV1.21 for HIVIG-C, which was μM (Figure 3).
Figure 3.
HIV-1 luciferase reporter gene assay results for % neutralization of four different HIV-1 strains (Q23.17, MN.3, MW965.26 and TV1.21) in TZM-bl cells by glycodendrimers sulfo-4 and sulfo-6 with positive controls, dextran sulfate and HIVIG-C.
As with all in vitro assays, the results obtained are constrained by the system used. Experiments in other laboratories have shown that HIV neutralization assays in the TZM-bl cell system may be less sensitive than assays in other cells, such as T cell lines and PHA-stimulated PBMCs,40 and that viruses produced in 293T cells are more sensitive to neutralizing antibodies than the same virus produced in PBMCs.55 We have also observed that an assay based on the T cell line A3R5 can be 10- to 100-fold more sensitive in detection of neutralizing antibodies than the TZM-bl assay, but only for viruses with a tier 2 (less-sensitive) neutralization phenotype (unpublished data).56 The viruses assayed here for inhibition by sulfo-4 and sulfo-6 are all tier 1 (more neutralization sensitive) and are equally sensitive to neutralizing antibodies when assayed in TZM-bl and A3R5 cells. However, it is possible that neutralization by sulfated polysaccharides may behave differently in these two assay systems than neutralization by antibodies, and the results here may underestimate the potential of these two compounds to inhibit HIV in an optimal in vitro assay. Like Louder et al., we have also found that a virus produced in 293T cells is more sensitive to neutralization by antibodies than the same virus grown in activated human PBMCs. The viruses used herein were pseudoviruses produced in 293T cells. In humans, the infecting HIV would likely have been produced by lymphoid or monocytoid cells. If HIV inhibition by sulfated polysaccharides follows the trend observed with neutralization by antibodies, the results here may overestimate the inhibition of HIV by sulfo-4 and sulfo-6 in an infected individual. Clearly, further studies are needed to understand the true utility of these and similar compounds in protection from HIV infection in vivo, but the results presented here provide encouragement for continuing to evaluate this strategy.
The combined data from both the ELISA and luciferase reporter gene assay suggest a few conclusions regarding the potential of these molecules as anti-HIV molecules. First, the assays were found to be complementary to one another: the luciferase assay confirmed the anti-HIV activity of sulfo-6 that was suggested by the ELISA result. This supports our strategy for screening glycodendrimer compounds: the ELISA serves as a simple screen for whether or not a sulfated glycodendrimer has the ability to bind the target gp120 protein, while the luciferase assay measures how well the sulfated glycodendrimers prevent the infection of the TZM-bl cells. Second, the activity of sulfo-6 is superior to that of sulfo-4. Sulfo-4 contains 2.02% sulfur, 8 sugar residues and a MW of approximately 4000 Da. It cannot be determined from the data gathered whether the poor activity observed for sulfo-4 was due to the small size, the low %S, or as a result of both factors. Further study of these effects is required. Therefore, all future sulfoglycodendrimers envisioned will need to be equal/larger than sulfo-6, and have a minimum of 4% sulfur (by wt.). It is hypothesized that as the sulfoglycodendrimers increase in size/sulfur content, both assays will be better able to quantitate the HIV-1 activity, as the sulfoglycodendrimers will be more adept at binding to gp120 multivalently, and potentially serve as effective anti-HIV-1 agents. This is supported by reports in the literature illustrating a variety of (glyco)dendrimer-based molecules with anti-HIV activity (μM-nM), with molecular weights ranging from 12,400-24,581 Da.41, 43, 47, 57 As we have now reported a sulfoglycodendrimer (sulfo-6) with a MW of 8777 Da and 4.03% sulfur with μM activity across all HIV-1 strains evaluated, we are satisfied that the ELISA/luciferase assay combination will provide adequate sensitivity for future molecules evaluated, as these will all be larger in size and contain a minimum of 4% sulfate (by wt.). Finally, in taking the sialic acid monosaccharide, presenting it multivalently on PAMAM, and sulfating it, we were able to observe modest μM activity (IC50 values ranging from 1.6-5.1 μM). These values compared well to the EC50 value of 0.4 μM for an 8000 Da sulfated colominic acid derivative containing 6% sulfur, as reported by Yang et al.19 This indicates that the strategy of taking smaller pieces of a polysaccharide of known anti-HIV properties and presenting them in a multivalent sense on a dendrimer scaffold can yield structurally well-defined sulfoglycodendrimers with good anti-HIV activity. This approach may also result in derivatives with reduced or absent toxicity issues as compared to the sulfated polydisperse polysaccharides from which they are derived. This latter issue will be addressed in future studies.
Conclusions
In summary, an initial series of 6 sulfoglycodendrimers were sought, synthesized by either a divergent-like or convergent-like approach, as potential anti-HIV agents. The syntheses were planned with the fewest steps possible and no sugar protecting group chemistry, to find the fastest and most facile way to make sulfoglycodendrimers in the best yields possible. While it was initially believed that the 4 step convergent-like approach would be the best reaction sequence, as the steric issues of both sialic acid and PAMAM were minimized through the addition of an intermediary hydrophilic linker, only 1 was produced, and in poor yield. This contrasts with the 1 step divergent-like pathway, which at the outset was not expected to work well, given that both the sialic acid and PAMAM structures were sterically congested. Surprisingly, this path led to the production of all three desired glycodendrimers, 2, 4, and 6, in modest yields, and all in one step. Glycodendrimers 1, 2, 4 and 6 were all fully substituted with respect to the number of sugars attached to the reactive groups terminating the various generations of PAMAM, thereby eliminating the polydispersity of structure found in the parent polysaccharide colominic acid. While the chemical sulfation process was not selective, it was viewed to be of lesser importance at the current time than finding the appropriate synthetic method and determining the general minimum structural requirements necessary for these molecules to have anti-HIV-1 activity. This was achieved in sulfo-6. The synthesis of a completely monomolecular product in terms of both the number of sugars and sulfates incorporated remains a future goal.
In addition to finding an appropriate synthesis for the glycodendrimers, a simple binding screen and well-characterized confirmatory functional assay were utilized to rapidly determine their potential anti-HIV activity. Upon evaluation of the sulfoglycodendrimers, sulfo-1, 2, 4, and6, the in-house ELISA screening assay suggested that only sulfo-6 bound to b-rgp120. This was confirmed using the more sensitive luciferase reporter gene neutralization assay, which detected μM activity for sulfo-6. Thus, we have shown that a simple monosaccharide building block derived from a known anti-HIV sulfated polysaccharide can yield molecules possessing anti-HIV-1 activity when appended to a multivalent scaffold, even though they are smaller in size than the parent polysaccharide.
Supplementary Material
Acknowledgments
The authors are indebted to Dr. David Montefiori from Duke University for providing the expertise and resources for acquiring the HIV luciferase reporter gene assay results (supported by NIH contract AI30034). We would additionally like to thank Drs. Shiu-Lok Hu and Yun Li at Washington State University for the extensive conversations, guidance, and initial HIV luciferase reporter gene assay results (with partial support provided by NIH P51 grant RR000166). The authors would finally like to thank Careena Cary for running the sulfation reactions, and Drs. Andreas Franz from the University of the Pacific and Dr. Arpad Somogyi from the University of Arizona, for their assistance with the mass spectral analysis. Financial support for this work from NIH-AREA (1R15AI068444-01), Research Corporation Cottrell College Science Award (CC6610), and an NSF-MRI grant to fund the 500 MHz NMR (CHE MRI-0922676) are gratefully acknowledged.
Footnotes
Supporting Information Available. Full synthetic and characterization details of the sialic acid-PAMAM glycodendrimers and NMR spectra are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.UNAIDS UNAIDS. 2010:364. [Google Scholar]
- 2.UNAIDS UNAIDS. 2009:100. [Google Scholar]
- 3.Granich R, Crowley S, Vitoria M, Lo Y-R, Souteyrand Y, Dye C, Gilks C, Guerma T, De Cock KM, Williams B. Highly Active Antiretroviral Treatment for the Prevention of HIV Transmission. J. Int. AIDS Soc. 2010;13:1–8. doi: 10.1186/1758-2652-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broder S. The Development of Antiretroviral Therapy and its Impact on the HIV-1/AIDS Pandemic. Antiviral Res. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Este JA, Cihlar T. Current Status and Challenges of Antiretroviral Research and Therapy. Antiviral Res. 2010;85:25–33. doi: 10.1016/j.antiviral.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins T. Understanding and Managing the Adverse Effects of Antiretroviral Therapy. Antiviral Res. 2010;85:201–209. doi: 10.1016/j.antiviral.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Gupta U, Jain NK. Non-polymeric Nano-carriers in HIV/AIDS Drug Delivery and Targeting. Adv. Drug. Delivery Rev. 2010;62:478–490. doi: 10.1016/j.addr.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Ito M, Baba M, Sato A, Pauwels R, De Clerq E, Shigeta S. Inhibitory Effect of Dextran Sulfate and Heparin on the Replication of Human Immunodeficiency Virus (HIV) in vitro. Antiviral Res. 1987;7:361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuya H, Looney DJ, Kuno S, Ueno R, Wong-Staal F, Broder S. Dextran Sulfate Suppression of Viruses in the HIV Family: Inhibition of Virion Binding to CD4+ Cells. Science. 1988;240:646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- 10.Baba M, Pauwels R, Balzarini J, Arnout J, Desmyter J, De Clercq E. Mechanism of Inhibitory Effect of Dextran Sulfate and Heparin on Replication of Human Immunodeficiency Virus in vitro. Proc. Natl. Acad. Sci. USA. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callahan LN, Phelan M, Mallinson M, Norcross MA. Dextran Sulfate Blocks Antibody Binding to the Principal Neutralizing Domain of Human Immunodeficiency Virus Type 1 Without Interfering with Gp120-CD4 Interactions. J. Virol. 1991;65:1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batinic D, Robey FA. The V3 Region of the Envelope Glycoprotein of Human Immunodeficiency Virus Type 1 Binds Sulfated Polysaccharides and CD4-Derived Synthetic Peptides. J. Biol. Chem. 1992;267:6664–6671. [PubMed] [Google Scholar]
- 13.Meshcheryakova D, Andreev S, Taraswova S, Sidorova M, Vafina M, Kornilaeva G, Karamov E, Kaitov R. CD4-Derived Peptide and Sulfated Polysaccharides Have Similar Mechanisms of Anti-HIV Activity Based on Electrostatic Interactions with Positively Charged gp120 Fragments. Mol. Immunol. 1993;30:993–1001. doi: 10.1016/0161-5890(93)90124-t. [DOI] [PubMed] [Google Scholar]
- 14.Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. A Conserved HIV Gp120 Glycoprotein Structure Involved in Chemokine Receptor Binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 15.Moulard M, Lortat-Jacob H, Mondor I, Roca G, Wyatt R, Sodroski J, Zhao L, Olson W, Kwong PD, Sattentau QJ. Selective Interactions of Polyanions with Basic Surfaces on Human Immunodeficiency Virus Type 1 gp120. J. Virol. 2000;74:1948–1960. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertozzi CR, Kiessling LL. Chemical Glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 17.Kiessling LL, Gestwicki JE, Strong LE. Synthetic Multivalent Ligands in the Exploration of Cell-Surface Interactions. Curr. Opin. Chem. Biol. 2000;4:696–703. doi: 10.1016/s1367-5931(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 18.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib DC, Mostowski H, Norcross MA. Mediation of Human Immunodeficiency Virus Type 1 Binding by Interaction of Cell Surface Heparan Sulfate Proteoglycans with the V3 Region of Envelope gp120-gp41. J. Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang D-W, Ohta Y, Yamaguchi S, Tsukada Y, Haraguchi Y, Hoshino H, Amagai H, Kobayashi I. Sulfated Colominic Acid: An Antiviral Agent that Inhibits the Human Immunodeficiency Virus Type I in vitro. Antiviral Res. 1996;31:95–104. doi: 10.1016/0166-3542(96)00957-6. [DOI] [PubMed] [Google Scholar]
- 20.Lorentson KJ, Hendrix CW, Collins JM, Kornhauser DM, Petty BG, Klecker RW, Flexner C, Eckel RH, Lietman PS. Dextran Sulfate is Poorly Absorbed after Oral Administration. Ann. Intern. Med. 1989;111:561–566. doi: 10.7326/0003-4819-111-7-561. [DOI] [PubMed] [Google Scholar]
- 21.Flexner C, Barditch-Crovo PA, Kornhauser DM, Farzadegan H, Nerhood LJ, Chaisson RE, Bell KM, Lorentsen KJ, Hendrix CW, Petty BG, Lietman PS. Pharmacokinetics, Toxicity, and Activity of Intravenous Dextran Sulfate in Human Immunodeficiency Virus Infection. Antimicrob. Agents Chemother. 1991;35:2544–2550. doi: 10.1128/aac.35.12.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiebert LM, Wice SM, Jaques LB, Williams KE, Conly JM. Orally Administered Dextran Sulfate is Absorbed in HIV-Positive Individuals. J. Lab. Clin. Med. 1999;133:161–170. doi: 10.1016/s0022-2143(99)90009-4. [DOI] [PubMed] [Google Scholar]
- 23.McReynolds KD, Gervay-Hague J. Chemotherapeutic Interventions Targeting HIV Interactions with Host-Associated Carbohydrates. Chem. Rev. 2007;107:1533–1552. doi: 10.1021/cr0502652. [DOI] [PubMed] [Google Scholar]
- 24.Tomalia DA, Baker H, Dewald JR, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polymer J. (Tokyo) 1985;17:117–132. [Google Scholar]
- 25.Kono K, Kojima C, Hayashi N, Nishisaka E, Kiura K, Watarai S, Harada A. Preparation and Cytotoxic Activity of Poly(ethylene glycol)-Modified Poly(amidoamine) Dendrimers Bearing Adriamycin. Biomaterials. 2008;29:1664–1675. doi: 10.1016/j.biomaterials.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Fant K, Esbjorner EK, Jenkins A, Grossel MC, Lincoln P, Norden B. Effects of PEGylation and acetylation of PAMAM Dendrimers on DNA Binding, Cytotoxicity, and in vitro Transfection Efficiency. Mol. Pharmaceutics. 2010;17:1734–1746. doi: 10.1021/mp1001312. [DOI] [PubMed] [Google Scholar]
- 27.Medina SH, El-Sayed MEH. Dendrimers as Carriers for Delivery of Chemotherapeutic Agents. Chem. Rev. 2009;109:3141–3157. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 28.Rolland O, Turrin C-O, Caminade A-M, Majoral J-P. Dendrimers and Nanomedicine: Multivalency in Action. New J. Chem. 2009;33:1809–1824. [Google Scholar]
- 29.Heegaard PMH, Boas U, Sorensen NS. Dendrimers for Vaccine and Immunostimulatory Uses. A Review. Bioconj. Chem. 2010;21:405–418. doi: 10.1021/bc900290d. [DOI] [PubMed] [Google Scholar]
- 30.Tekade RK, Kumar PV, Jain NK. Dendrimers in Oncology: An Expanding Horizon. Chem. Rev. 2009;109:49–87. doi: 10.1021/cr068212n. [DOI] [PubMed] [Google Scholar]
- 31.Labieniec M, Watala C. PAMAM Dendrimers-Diverse Biomedical Applications. Facts and Unresolved Questions. Cent. Eur. J. Biol. 2009;4:434–451. [Google Scholar]
- 32.Jevprasesphant R, Penny J, Jalal R, Attwood D, MeKeown NB, D'Emanuele A. The Influence of Surface Modification on the Cytotoxicity of PAMAM Dendrimers. Int. J. Pharm. 2003;252:263–266. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 33.Roy R, Zanini D, Meunier SJ, Romanowska A. Solid-Phase Synthesis of Dendritic Sialoside Inhibitors of Influenza A Virus Haemagglutinin. J. Chem. Soc., Chem. Commun. 1993:1869–1872. [Google Scholar]
- 34.Dimick SM, Powell SC, McMahon SA, Moothoo DN, Naismith JH, Toone EJ. On the Meaning of Affinity: Cluster Glycoside Effects and Concanavalin A. J. Am. Chem. Soc. 1999;121:10286–10296. [Google Scholar]
- 35.Pohl NL, Kiessling LL. Scope of Multivalent Ligand Function. Lactose- Bearing Neoglycopolymers by Ring-Opening Metathesis Polymerization. Synthesis Special Issue. 1999:1515–1519. [Google Scholar]
- 36.Wang J-Q, Chen X, Zacharek S, Chen Y, Wang PG. Enhanced Inhibition of Human Anti-Gal Antibody Binding to Mammalian Cells by Synthetic α-Gal Epitope Polymers. J. Am. Chem. Soc. 1999;121:8174–8181. [Google Scholar]
- 37.Reuter JD, Myc A, Hayes MM, Gan Z, Roy R, Qin D, Yin R, Piehler LT, Esfand R, Tomalia DA, Baker JR. Inhibition of Viral Adhesion and Infection by Sialic-Acid-Conjugated Dendritic Polymers. Bioconj. Chem. 1999;10:271–278. doi: 10.1021/bc980099n. [DOI] [PubMed] [Google Scholar]
- 38.Woller EK, Cloninger MJ. The Lectin-Binding Properties of Six Generations of Mannose-Functionalized Dendrimers. Org. Lett. 2001;4:7–10. doi: 10.1021/ol016568+. [DOI] [PubMed] [Google Scholar]
- 39.Ashton PR, Hounsell EF, Jayaraman N, Nilsen TM, Spencer N, Stoddart JF, Young M. Synthesis and Biological Evaluation of α-D-Mannopyranoside-Containing Dendrimers. J. Org. Chem. 1998;63:3429–3437. [Google Scholar]
- 40.Borges AR, Wieczorek L, Johnson B, Benesi AJ, Brown BK, Kensinger RD, Krebs FC, Wigdahl B, Blumenthal R, Puri A, McCutchan FE, Birx DL, Polonis VR, Schengrund C-L. Multivalent Dendrimeric Compounds Containing Carbohydrates Expressed on Immune Cells Inhibit Infection by Primary Isolates of HIV-1. Virology. 2010;408:80–88. doi: 10.1016/j.virol.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kensinger RD, Yowler BC, Benesi AJ, Schengrund C-L. Synthesis of Novel, Multivalent Glycodendrimers as Ligands for HIV-1 gp120. Bioconj. Chem. 2004;15:349–358. doi: 10.1021/bc034156a. [DOI] [PubMed] [Google Scholar]
- 42.Kensinger RD, Catalone BJ, Krebs FC, Wigdahl B, Schengrund C-L. Novel Polysulfated Galactose-Derivatized Dendrimers as Binding Antagonists of Human Immunodeficiency Virus Type 1 Infection. Antimicrob. Agents Chemother. 2004;48:1614–1623. doi: 10.1128/AAC.48.5.1614-1623.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Serna JA, Boutureira O, Serra A, Matheu MI, Diaz Y, Castillon S. Synthesis of Hyperbranched β-Galceramide-Containing Dendritic Polymers that Bind HIV-1 rgp120. Eur. J. Org. Chem. 2010:2657–2660. [Google Scholar]
- 44.Perez-Anes A, Stefaniu C, Moog C, Majoral J-P, Blanzat M, Turrin C-O, Caminade A-M, Rico-Lattes I. Multivalent Catanionic GalCer Analogs Derived from First Generation Dendrimeric Phosphonic Acids. Bioorg. Med. Chem. 2010;18:242–248. doi: 10.1016/j.bmc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 45.Blanzat M, Turrin C-O, Aubertin A-M, Couturier-Vida C, Caminade A-M, Majoral J-P, Rico-Lattes I, Lattes A. Dendritic Catanionic Assemblies: In Vitro Anti-HIV Activity of Phosphorous-Containing Dendrimers Bearing Galβ1Cer Analogs. ChemBioChem. 2005;6:2207–2213. doi: 10.1002/cbic.200500203. [DOI] [PubMed] [Google Scholar]
- 46.Han S, Baigude H, Hattori K, Yoshida T, Uryu T. Synthesis of New Spherical and Hemispherical Oligosaccharides with Polylysine Core Scaffold. Carbohydr. Poly. 2007;68:26–34. [Google Scholar]
- 47.Han S, Yoshida D, Kanamoto T, Nakashima H, Uryu T, Yoshida T. Sulfated Oligosaccharide Cluster with Polylysine Core Scaffold as a New Anti-HIV Dendrimer. Carbohydr. Poly. 2010;80:1111–1115. [Google Scholar]
- 48.Tabarani G, Reina JJ, Ebel C, Vives C, Lortat-Jacobs H, Rojo J, Fieschi F. Mannose Hyperbranched Dendritic Polymers Interact with Clustered Organization of DC-SIGN and Inhibit gp120 Binding. FEBS Lett. 2006;580:2402–2408. doi: 10.1016/j.febslet.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 49.Konou M, Koizumi M, Shimizu K, Kawase M, Hatanaka K. Synthesis of Sulfated Colominic Acids and Their Interaction with Fibroblast Growth Factors. Biomacromolecules. 2000;1:451–458. doi: 10.1021/bm000011k. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human Immunodeficiency Virus Type 1 env Clones from Acute and Early Subtype B Infections for Standardized Assessments of Vaccine-Elicited Neutralizing Antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montefiori DC. In: Measuring HIV Neutralization in a Luciferase Reporter Gene Assay, in HIV Protocols. Second Edition. Prasad VR, Kalpana GV, editors. Humana Press; New York: 2009. pp. 395–404. [DOI] [PubMed] [Google Scholar]
- 52.Suhara Y, Kurihara M, Kittaka A, Ichikawa Y. Efficient synthesis of carbopeptoid oligomers: insight into mimicry of beta-peptide. Tetrahedron. 2006;62:8207–8217. [Google Scholar]
- 53.Sigma-Aldrich; St. Louis: 2001. [Google Scholar]
- 54.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody Neutralization and Escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 55.Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, Sanders-Buell E, Zolla-Pazner S, McCutchan FE, Roser JD, Gabuzda D, Lifson JD, Mascola JR. HIV-1 Envelope Pseudotyped Viral Vectors and Infectious Molecular Clones Expressing the Same Envelope Glycoprotein have a Similar Neutralization Phenotype, but Culture in Peripheral Blood Mononuclear Cells is Associated with Decreased Neutralization Sensitivity. Virology. 2005;339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. Tiered Categorization of a Diverse Panel of HIV-1 Env Pseudoviruses for Assessment of Neutralizing Antibodies. J. Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarthy TD, Karellas P, Henderson SA, Giannis M, O'Keefe DF, Heery G, Paull JRA, Matthews BR, Holan G. Dendrimers as Drugs: Discovery and Preclinical and Clinical Development of Dendrimer-Based Microbicides for HIV and STI Prevention. Mol. Pharm. 2005;2:312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.