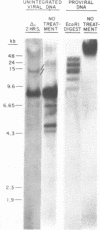
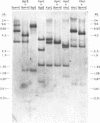
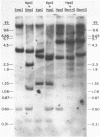
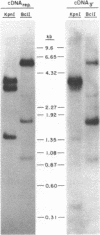
Abstract
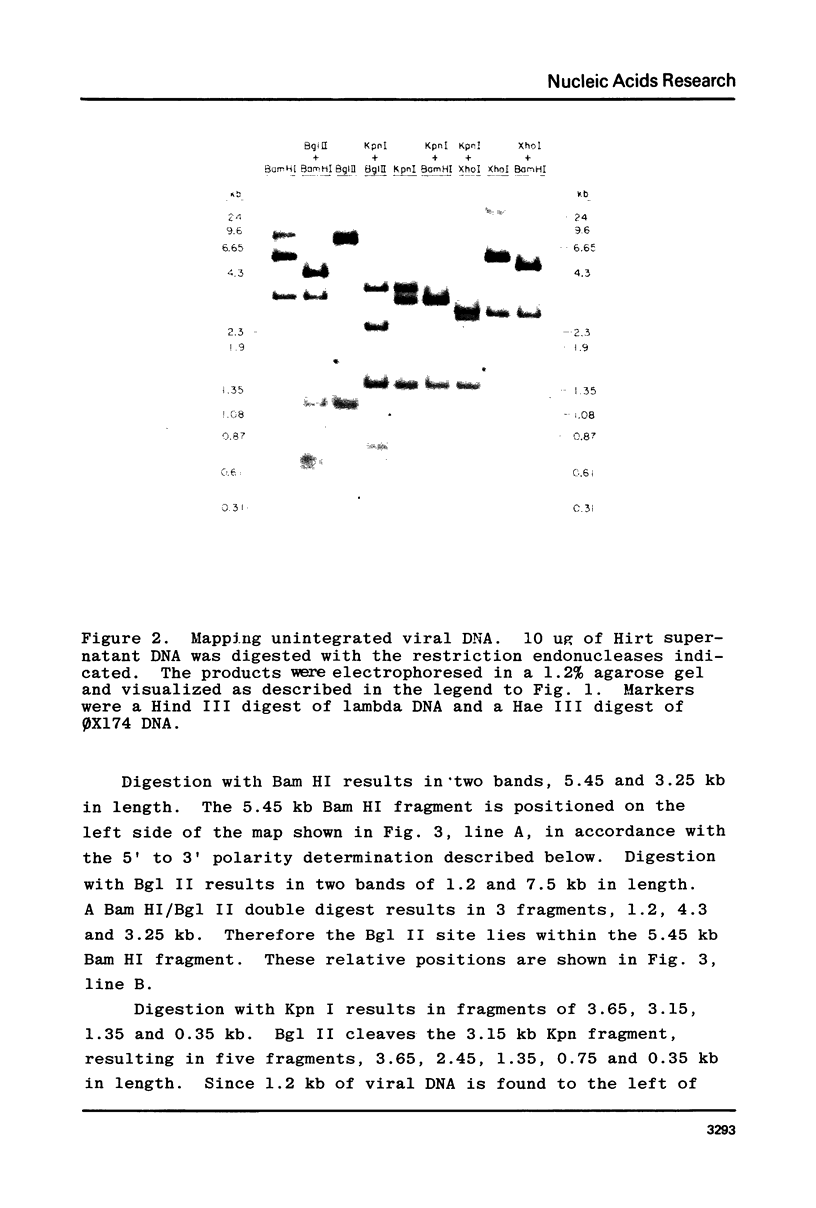
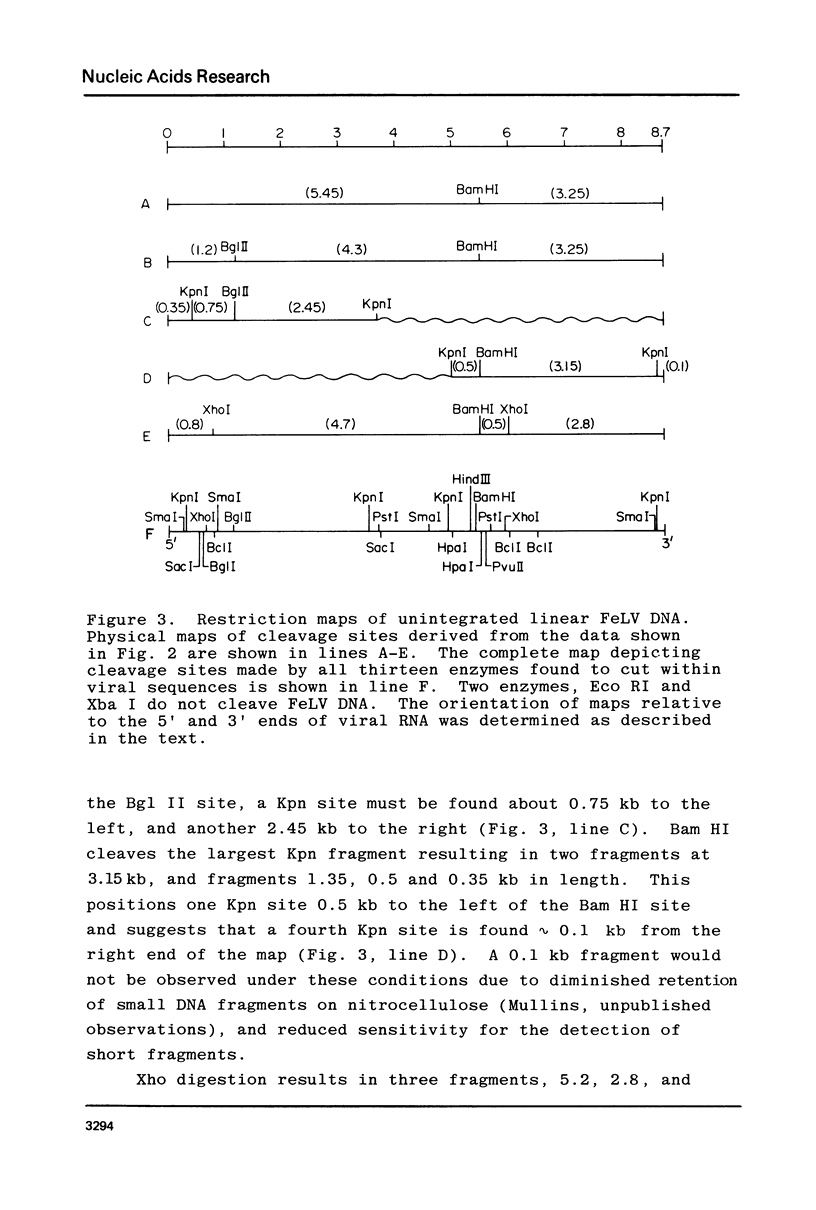
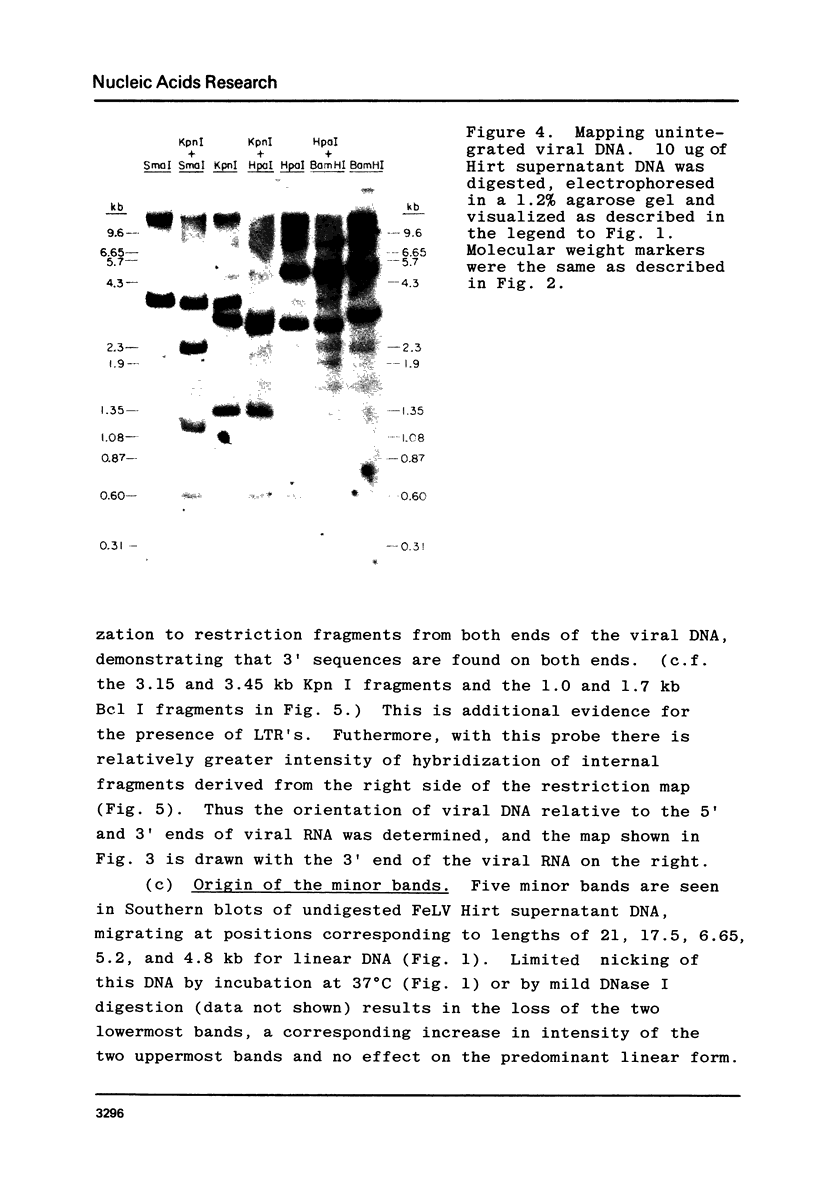
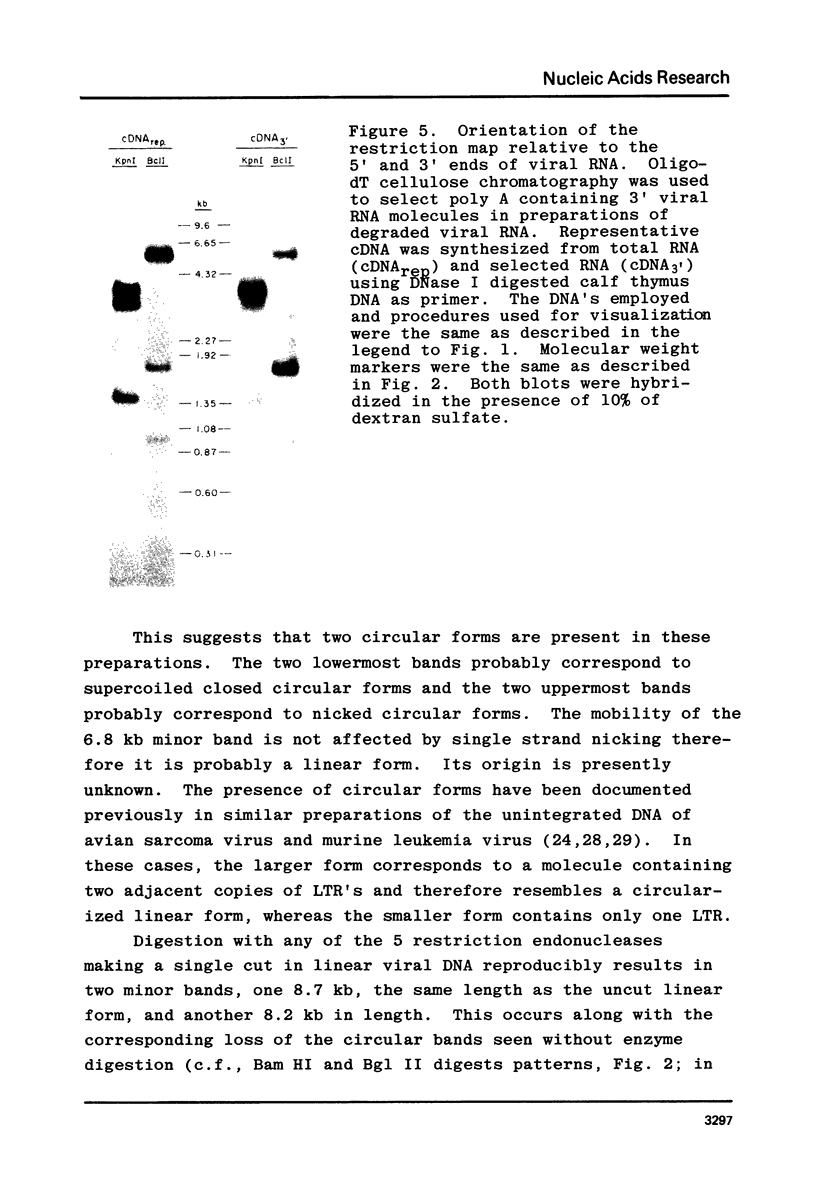
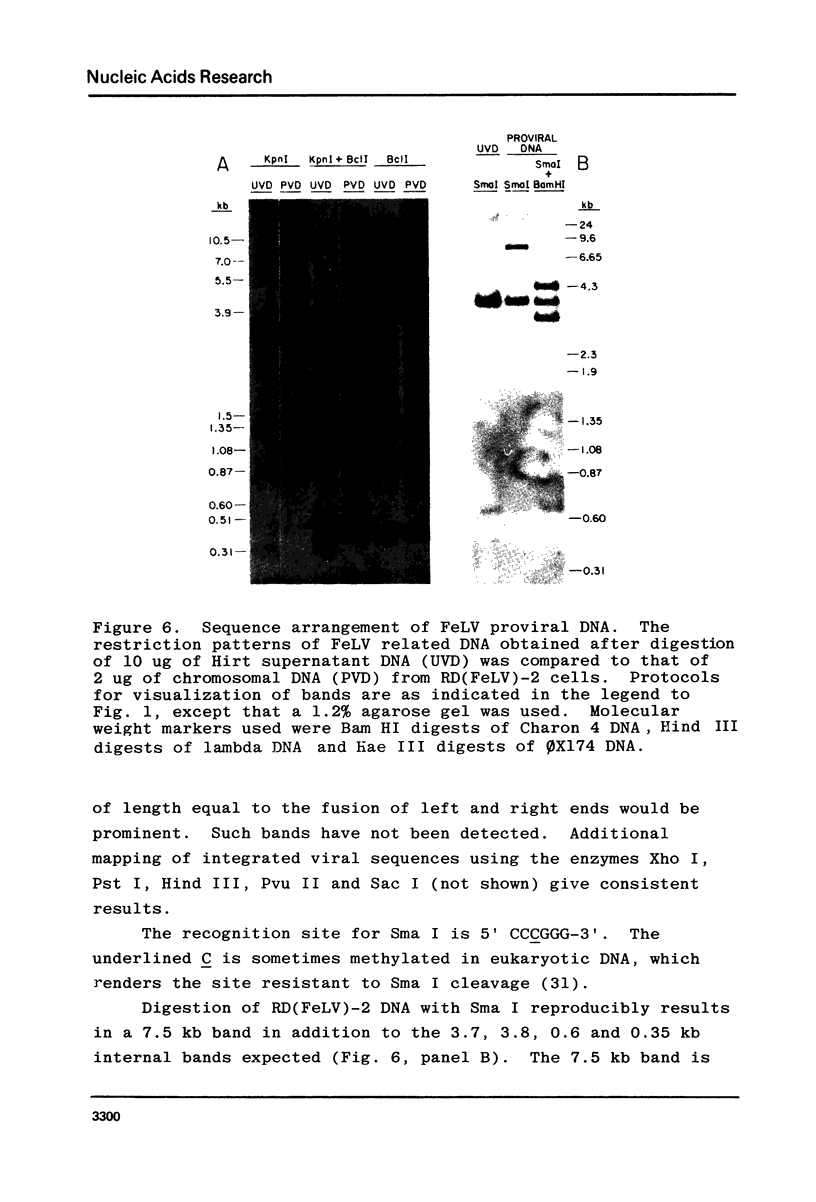
A restriction site map has been deduced of unintegrated and integrated FeLV viral DNA found in human RD cells after experimental infection with the Gardner-Arnstein strain of FeLV. Restriction fragments were ordered by single and double enzyme digests followed by Southern transfer (1) and hybridization with 32P-labeled viral cDNA probes. The restriction map was oriented with respect to the 5' and 3' ends of viral RNA by using a 3' specific hybridization probe. The major form of unintegrated viral DNA found was a 8.7 kb linear DNA molecule bearing a 450 bp direct long terminal redundancy (LTR) derived from both 5' and 3' viral RNA sequences. Minor, circular forms, 8.7 kb and 8.2 kb in length were also detected, the larger one probably containing two adjacent copies of the LTR and the smaller one containing one comtaining one copy of the LTR. Integrated copies of FeLV are colinear with the unintegrated linear form and contain the KpnI and SmaI sites found in each LTR.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azocar J., Essex M. Susceptibility of human cell lines to feline leukemia virus and feline sarcoma virus. J Natl Cancer Inst. 1979 Nov;63(5):1179–1184. [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Dorn C. R., Taylor D. O., Schneider R., Hibbard H. H., Klauber M. R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968 Feb;40(2):307–318. [PubMed] [Google Scholar]
- Essex M. Immunity to leukemia, lymphoma, and fibrosarcoma in cats: a case for immunosurveillance. Contemp Top Immunobiol. 1977;6:71–106. doi: 10.1007/978-1-4684-3051-6_2. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Essex M., Hardy W. D., Jr Excretion of feline leukaemia virus by naturally infected pet cats. Nature. 1977 Sep 15;269(5625):252–254. doi: 10.1038/269252a0. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Essex M. Leukemia and lymphoma: infrequent manifestations of common viral infections? A review. J Infect Dis. 1978 Dec;138(6):916–923. doi: 10.1093/infdis/138.6.916. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Gautier F., Bünemann H., Grotjahn L. Analysis of calf-thymus satellite DNA: evidence for specific methylation of cytosine in C-G sequences. Eur J Biochem. 1977 Oct 17;80(1):175–183. doi: 10.1111/j.1432-1033.1977.tb11869.x. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Old L. J., Hess P. W., Essex M., Cotter S. Horizontal transmission of feline leukaemia virus. Nature. 1973 Aug 3;244(5414):266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Olsen R. G., Hardy W. D., Jr, Schaller J. P. Horizontal transmission of feline leukemia virus under experimental conditions. J Natl Cancer Inst. 1977 Feb;58(2):443–444. doi: 10.1093/jnci/58.2.443. [DOI] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARRETT W. F., CRAWFORD E. M., MARTIN W. B., DAVIE F. A VIRUS-LIKE PARTICLE ASSOCIATED WITH LEUKEMIA (LYMPHOSARCOMA). Nature. 1964 May 9;202:567–569. doi: 10.1038/202567a0. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D., Crighton G. W. Replication of cat leukemia virus in cell cultures. Nature. 1968 Aug 3;219(5153):521–522. doi: 10.1038/219521a0. [DOI] [PubMed] [Google Scholar]
- Jarrett W., Jarrett O., Mackey L., Laird H., Hardy W., Jr, Essex M. Horizontal transmission of leukemia virus and leukemia in the cat. J Natl Cancer Inst. 1973 Sep;51(3):833–841. doi: 10.1093/jnci/51.3.833. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rasheed S., Rongey R. W., Hardy W. D., Jr, Gilden R. V. RD-114 virus compared with feline and murine type-C viruses released from RD cells. Nat New Biol. 1973 Mar 21;242(116):75–78. doi: 10.1038/newbio242075a0. [DOI] [PubMed] [Google Scholar]
- Nicolson M. O., Hariri F., Krempin H. M., McAllister R. M., Gilden R. V. Infectious proviral DNA in human cells infected with transformation-defective type C viruses. Virology. 1976 Apr;70(2):301–312. doi: 10.1016/0042-6822(76)90273-7. [DOI] [PubMed] [Google Scholar]
- Okabe H., DuBuy J., Gilden R. V., Gardner M. B. A portion of the feline leukaemia virus genome is not endogenous in cat cells. Int J Cancer. 1978 Jul 15;22(1):70–78. doi: 10.1002/ijc.2910220114. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Sarma P. S., Log T., Jain D., Hill P. R., Huebner R. J. Differential host range of viruses of feline leukemia-sarcoma complex. Virology. 1975 Apr;64(2):438–446. doi: 10.1016/0042-6822(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Singer J., Roberts-Ems J., Riggs A. D. Methylation of mouse liver DNA studied by means of the restriction enzymes msp I and hpa II. Science. 1979 Mar 9;203(4384):1019–1021. doi: 10.1126/science.424726. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura F. K., Weinberg R. A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979 Feb;16(2):323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]