Abstract
Background:
PROX1 is a specific target of the β-catenin/TCF pathway in the intestinal epithelium. It acts as a regulator of progression from a benign to a highly dysplastic phenotype in colorectal tumours. However, the clinical significance of PROX1 expression is not known.
Methods:
We studied the prognostic value of immunohistochemical expression of PROX1 in a series of 517 patients with colorectal cancer (CRC).
Results:
The majority of the tumour samples expressed PROX1 (91%, 471 out of 517). High PROX1 expression was associated with a poor grade of tumour differentiation (P<0.0001). In the subgroup of patients with colon cancer, high PROX1 expression was associated with unfavourable colorectal cancer-specific survival (CCSS) as compared with low PROX1 expression (CCSS 47% vs 62% P=0.045; RR 1.47). The association between high PROX1 and poor outcome was further strengthened in female colon cancer patients (CCSS 38% vs 63% P=0.007; RR 2.02). Nonetheless, in multivariate survival analysis PROX1 expression was not retained as an independent prognostic factor.
Conclusion:
High PROX1 expression is associated with a poor grade of tumour differentiation, and, in colon cancer patients, also with less favourable patient outcome. Our results strengthen the previous preclinical observations that PROX1 has a role in tumour progression in CRC.
Keywords: prospero-related homeobox gene, PROX1, colorectal cancer, expression, prognosis
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world (Parkin et al, 2005). Only a small fraction of CRCs occurs in dominantly inherited patterns. The two best-defined familial forms are familial adenomatous polyposis-related CRC and hereditary nonpolyposis colorectal cancer (reviewed in Kinzler and Vogelstein, 1996). Activation of the APC/β-catenin/TCF pathway is an initiating event of neoplasia in familial adenomatous polyposis patients. The adenomatous polyposis coli (APC) and β-catenin (CTNNB1) genes are two major components of the Wnt signalling pathway that are affected by mutations in CRC (Segditsas and Tomlinson, 2006). In normal cells, the APC protein binds to cytoplasmic β-catenin, targeting it for degradation. When the degradation is inhibited by Wnt signalling, β-catenin begins to accumulate in the nuclei of colorectal epithelial cells. Wnt signalling results in the formation of a complex containing β-catenin and T-cell factor (TCF). Familial adenomatous polyposis patients with APC mutation and blocked β-catenin degradation have an overactivated Wnt signalling pathway, which results in development of hundreds of intestinal polyps, and eventually CRC.
Loss of APC in the intestinal epithelium induces expansion of the progenitor cell population. The β-catenin/TCF pathway controls cancer cell proliferation and expression of progenitor cell-specific genes (Sansom et al, 2004). In humans, the progression from benign adenoma to malignant carcinoma takes several years and the cascade causing the malignant transformation is still unknown. Petrova et al (2008) have previously shown that transcription factor PROX1 is an intestinal specific target of the β-catenin/TCF pathway and has an essential role as a regulator of progression from a benign to a highly dysplastic phenotype in colorectal tumours.
PROX1 is an atypical homeodomain protein important for embryonic development of the lens, retina, liver, pancreas, and lymphatic vasculature, but little is known about PROX1 function in adult tissues (Oliver et al, 1993; Wigle and Oliver, 1999; Sosa-Pineda et al, 2002; Dyer et al, 2003). PROX1 is the mammalian homologue of the Drosophila homeobox protein Prospero, which acts as a brain tumour suppressor by inhibiting neuroblast self-renewal (Oliver et al, 1993; Betschinger et al, 2006). It has been suggested that PROX1 has a similar role in human cancer (Shimoda et al, 2006; Laerm et al, 2007). However, a recent publication shows that PROX1 expression is associated with a higher grade in astrocytic gliomas, the most common brain tumour type in adults (Elsir et al, 2010). Diverse results propose different roles for PROX1 in different cancer types. Moreover, the findings showing that PROX1 is overexpressed in the majority of CRCs and that it promotes neoplasia, tumour growth, and malignant progression suggest that PROX1 expression may be associated with the outcome of CRC patients (Petrova et al, 2008). In the present study, we investigated the clinical significance of PROX1 expression by immunohistochemistry in a large series of CRC patients.
Patients and methods
Patients
This study is based on a series of 643 consecutive patients who underwent surgery for histologically verified CRC at the Helsinki University Central Hospital in 1989–1998. The median follow-up time of the patients alive at the end of follow-up was 9 years (range 0.1–15.4). A tissue specimen suitable for evaluation of PROX1 expression by immunohistochemistry was available in 517 (80.4%) cases. Follow-up data, collected from the patient records and the files of the Finnish Cancer Registry and Statistics Finland, were available for all patients. The clinicopathological characteristics of the patients have been described previously in detail by Linder et al (2009), and are listed briefly in Table 1.
Table 1. Associations of PROX1 expression with clinicopathological variables.
| Variable | n | PROX1 low, n (%) | PROX1 high, n (%) | P-value n (%) a |
|---|---|---|---|---|
| Age | 0.4992 | |||
| <50 | 60 | 50 (83) | 10 (17) | |
| 50–64 | 156 | 117 (75) | 39 (25) | |
| 65–74 | 174 | 132 (76) | 42 (24) | |
| >75 | 127 | 93 (73) | 34 (27) | |
| Location | 0.5843 | |||
| Rectum | 226 | 174 (77) | 52 (23) | |
| Colon | 291 | 218 (75) | 73 (25) | |
| Site | 0.2069 | |||
| Right | 143 | 101 (71) | 42 (29) | |
| Left | 367 | 285 (78) | 82 (22) | |
| Transverse colon | 7 | 6 (86) | 1 (14) | |
| Gender | 0.8074 | |||
| Male | 282 | 215 (76) | 67 (24) | |
| Female | 235 | 177 (75) | 58 (25) | |
| Histological grade | <0.0001 | |||
| 1 | 15 | 15 (100) | 0 (0) | |
| 2 | 347 | 276 (80) | 71 (20) | |
| 3 | 134 | 85 (63) | 49 (37) | |
| 4 | 20 | 15 (75) | 5 (25) | |
| Dukes stage | 0.2975 | |||
| A | 72 | 215 (76) | 67 (24) | |
| B | 193 | 152 (79) | 41 (21) | |
| C | 129 | 92 (71) | 37 (29) | |
| D | 123 | 90 (73) | 33 (27) |
χ2 test.
Immunohistochemistry
PROX1 expression was assessed from tissue microarrays prepared as described in detail elsewhere (Linder et al, 2009). The tissue microarray blocks were cut into 4-μm-thick sections, fixed on slides, and dried for 12–24 h at 37 °C. The sections were then deparaffinised in xylene and rehydrated through graded alcohol series. For antigen retrieval, the sections were heated in the Pretreatment Module of the Autostainer 480 (LabVision UK Ltd, Newmarket, UK) in Tris-EDTA buffer (pH 9.0) for 20 min at 98 °C. The staining of the sections was performed in Autostainer 480. The tissue sections were then treated with 0.3% Dako REAL Peroxidase-Blocking Solution (Dako Denmark A/S, Glostrup, Denmark) for 30 min to block the endogenous peroxidases, followed by incubation with rabbit normal serum (Vectastain ABC Kit, Vector, Burlingame, CA, USA) diluted 1:50 in TNB blocking solution (0.1 M Tris-HCl (pH 7.5), 0.15 M NaCl, 0.5% Blocking reagent (supplied in kit); Renaissance TSA Biotin System; Perkin-Elmer, Boston, MA, USA) for 30 min. Goat anti-PROX1 antibody (R&D Systems, Minneapolis, MN, USA) was used to detect PROX1 expression. The antibody was diluted 1:2000 in TNB blocking solution and incubated with the samples overnight at +4 °C. The tissue sections were then incubated for 1 h with a biotinylated anti-goat secondary antibody (Vectastain ABC Kit), diluted 1:300 in TNB blocking solution, and treated for 30 min with Strepavidin-HRP Conjugate (Perkin-Elmer) diluted 1:1250 in TNB blocking reagent. Immunostaining was visualised with Dako REAL Diaminobenzidine Chromogen (10 min treatment). After each step in the staining procedure, the slides were washed with wash buffer (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, 0.04% Tween 20; pH 7.4). Finally, the slides were counterstained with Meyer's haematoxylin, washed in tap water for 10 min, and mounted in Aquamount (BDH, Poole, UK). Specificity of the PROX1 immunopositivity was confirmed by staining the same tissue without the primary antibody.
Scoring of PROX1 immunostaining
PROX1 expression was evaluated by two of the investigators (LCA and MS). Both the investigators were blinded to the clinicopathological data at the time of scoring. PROX1 staining in the cancer cell nuclei was scored as follows: 0=negative, no staining in cancer cells; 1=low, less than 25% of cancer cells stained positively for PROX1, intensity of staining was weak; 2=moderate, 25–50% of cancer cells were positive for PROX1; 3=strong, 50–75% of cancer cells were positive; 4=very strong, more than 75% of cancer cells were positive. If more than one tissue spot was available from the same patient, the highest score out of the parallel spots was selected for statistical analysis.
Statistical analyses
The association between PROX1 immunohistochemistry results and clinicopathological variables was assessed by using the χ2 test. Life tables were computed according to the Kaplan–Meier method. Colorectal cancer-specific survival (CCSS) was calculated from the date of the diagnosis to death from CRC. Patients who died from causes other than CRC (87 out of 517) were censored on the date of death. Survival between the groups was compared using the logrank test. Multivariate survival analyses were carried out using the Cox proportional hazards model, and a P-value of 0.05 was adopted as the limit for inclusion of a covariate. All P-values are two-tailed.
Results
PROX1 expression in CRC
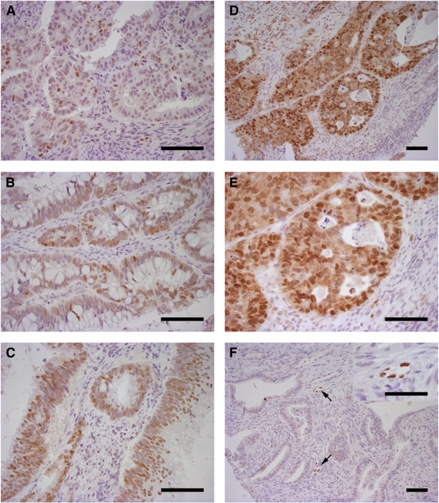
Normal epithelium was mostly negative for PROX1, but the expression could be observed in a few crypt and neuroendocrine cells, and in the nuclei of lymphatic vessel endothelium beneath the mucosa. The majority of the tumour samples showed some degree of PROX1 expression (91%, 471 out of 517). Low PROX1 expression was detected in 24% (122 out of 517) of the tumours, moderate in 43% (224 out of 517), strong in 20% (105 out of 517), and very strong expression in 4% (20 out of 517). Representative immunostaining results are shown in Figure 1.
Figure 1.
PROX1 expression in human colorectal cancer tissue microarray specimens. (A) Low PROX1 expression in colon cancer tissue; only few cancer cell nuclei are positive. (B) Moderate PROX1 expression in colon cancer tissue. (C) Strong PROX1 expression in rectal cancer tissue. (D and E) Very strong PROX1 expression in rectal cancer tissue. (F) Rectal cancer tissue negative for PROX1; however, adjacent lymphatic endothelial cells (arrow, insert) stained positively for PROX1. Scale bar=100 μm.
Association between PROX1 expression and clinicopathological parameters
High PROX1 expression was significantly more frequent in high-grade (grade 3–4) tumours when compared with low-grade (grade 1–2) tumours (P=0.0001; Table 1). None of the grade 1 tumours had high PROX1 expression. No statistically significant association was found between PROX1 and age at diagnosis, tumour location, tumour site, gender, or Dukes stage (Table 1).
Association of PROX1 expression with CCSS
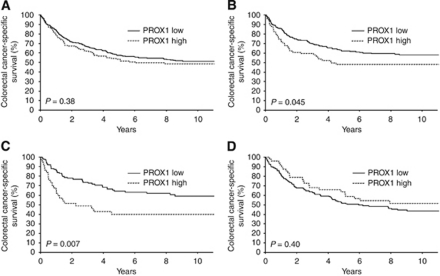
For further analyses we categorised the patients into two groups, PROX1 low (staining scores from 0 to 2) and PROX1 high (scores 3 and 4). In the entire patient series, high PROX1 expression was not significantly associated with CCSS (RR=1.14; P=0.38; Table 2, Figure 2A). The 5-year CCSS was 57% (95% confidence interval (CI), 52.1–62.5%) among patients with low PROX1 expression level, and 53% (95% CI, 43.6–62.1%) when the PROX1 expression was high. In the subgroup of patients with colon cancer, high PROX1 expression was associated with unfavourable survival (RR=1.47; P=0.045; Table 2, Figure 2B). The 5-year CCSS of the colon cancer patients with low PROX1 expression was 62% (95% CI, 55.2–68.9%), as compared with 47% for those with high staining intensity (95% CI, 35.3–59.0%). The 5-year CCSS among colon cancer patients with very high (score 4; n=11) PROX1 expression was only 24%, suggesting that increased tumour cell expression of PROX1 is associated with worse outcome of the colon cancer patients. Furthermore, the 5-year CCSS of female colon cancer patients with low PROX1 expression (score 0–2) was 63% (95% CI, 53.3–73.0%), compared with 38% when the expression of PROX1 was high (95% CI, 22.0–54.7% RR=2.02, P=0.007; Figure 2C), whereas no significant difference was detected among male colon cancer patients (data not shown). No significant association was detected between PROX1 and survival in rectal cancer patients. The 5-year CCSS for PROX1 low patients was 51% (95% CI, 43.6–59.4%) and that PROX1 high patients was 61% (95% CI, 47.1–75.9% RR=0.82, P=0.4; Table 2, Figure 2D).
Table 2. Five-year CCSS of 516 patients with colorectal cancer according to nuclear PROX1 expression.
| Factor | Score | n | 5-year CCSS (95% CI) | P-value a | RR |
|---|---|---|---|---|---|
| All tumours | |||||
| 0–2 | 392 | 57.3 (52.2–62.4) | |||
| 3–4 | 125 | 52.8 (43.8–61.8) | 0.3769 | 1.14 | |
| Age (years) | |||||
| <50 | 0–2 | 50 | 56.4 (42.3–70.5) | ||
| 3–4 | 10 | 80 (55.3–100.0) | 0.1188 | 0.335 | |
| 50–64 | 0–2 | 117 | 64.0 (55.4–72.6) | ||
| 3–4 | 39 | 56.0 (39.7–72.3) | 0.2426 | 1.381 | |
| 65–74 | 0–2 | 132 | 59.7 (51.1–68.3) | ||
| 3–4 | 42 | 45.2 (29.7–60.7) | 0.1074 | 1.472 | |
| >75 | 0–2 | 93 | 45.8 (35.2–56.4) | ||
| 3–4 | 34 | 50.7 (33.5–67.9) | 0.6167 | 0.869 | |
| Location | |||||
| Rectum | 0–2 | 174 | 51.5 (43.9–59.1) | ||
| 3–4 | 52 | 61.5 (47.4–75.6) | 0.4009 | 0.823 | |
| Colon | 0–2 | 218 | 62 (55.3–68.7) | ||
| 3–4 | 73 | 47.1 (35.5–58.7) | 0.0451 | 1.474 | |
| Site | |||||
| Right | 0–2 | 101 | 61.6 (51.8–71.4) | ||
| 3–4 | 42 | 44.4 (29.1–59.7) | 0.0705 | 1.602 | |
| Left | 0–2 | 285 | 56.0 (50.1–61.9) | ||
| 3–4 | 82 | 56.7 (45.5–67.9) | 0.9912 | 1.002 | |
| Transverse colon | 0–2 | 6 | 50.0 (10.0–90.0) | ||
| 3–4 | 1 | 100 (NA) | NA | NA | |
| Gender | |||||
| Male | 0–2 | 215 | 56.8 (49.9–63.7) | ||
| 3–4 | 67 | 61.7 (49.5–73.9) | 0.5679 | 0.885 | |
| Female | 0–2 | 177 | 57.9 (50.5–65.3) | ||
| 3–4 | 58 | 42.7 (29.8–55.6) | 0.0551 | 1.483 | |
| Dukes stage | |||||
| A | 0–2 | 58 | 83.9 (74.3–93.5) | ||
| 3–4 | 14 | 100.0 (100.0–100.0) | 0.4129 | 0.434 | |
| B | 0–2 | 152 | 78.1 (71.2–85.0) | ||
| 3–4 | 41 | 81.2 (68.7–93.7) | 0.2273 | 0.612 | |
| C | 0–2 | 92 | 55.1 (44.5–65.7) | ||
| 3–4 | 37 | 48.6 (31.9–65.3) | 0.2021 | 1.391 | |
| D | 0–2 | 90 | 7.1 (1.6–12.6) | ||
| 3–4 | 33 | 6.1 (0–14.3) | 0.8273 | 1.047 | |
| Histological grade | |||||
| 1–2 | 0–2 | 291 | 60.6 (54.9–66.3) | ||
| 3–4 | 71 | 61.8 (50.2–73.4) | 0.9745 | 0.994 | |
| 3–4 | 0–2 | 100 | 48.1 (37.9–58.3) | ||
| 3–4 | 54 | 40.6 (26.9–54.3) | 0.6056 | 1.125 | |
Abbreviations: CCSS=cancer-specific survival; RR=relative risk.
χ2 test.
Figure 2.
Disease-specific survival of 517 colorectal cancer patients according to PROX1 expression. (A) All colorectal cancer patients. ‘PROX1 low’, scores from 0 to 2, n=392; ‘PROX1 high’, scores 3 and 4, n=125. (B) Colon cancer patients. ‘PROX1 low’, n=218; ‘PROX1 high’, n=73. (C) Female colon cancer patients. ‘PROX1 low’, n=101; ‘PROX1 high’, n=36. (D) Rectal cancer patients. ‘PROX1 low’, n=174; ‘PROX1 high’, n=52.
Multivariate survival analysis
To adjust for established prognostic factors in colorectal cancer, PROX1 expression was entered into a Cox proportional hazards model together with Dukes stage, histological grade, age at diagnosis, tumour location, tumour site, and gender. In the multivariate survival analysis, PROX1 expression was not a significant prognostic factor (Table 3). Cox multivariate analysis was also performed for the subgroup of female colon cancer patients. However, PROX1 expression did not provide significant prognostic information in addition to the selected factors (data not shown).
Table 3. Cox multivariate regression of the association between PROX1 immunoreactivity and colorectal cancer-specific survival, adjusted for clinicopathological characteristics (n=516).
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
| PROX1 expression | |||
| Low | 1.00 | ||
| High | 0.908 | 0.672–1.227 | 0.5289 |
| Dukes stage | |||
| A | 1.00 | ||
| B | 1.712 | 0.882–3.323 | 0.1121 |
| C | 4.929 | 2.593–9.367 | <0.0001 |
| D | 29.213 | 15.386–55.467 | <0.0001 |
| Histological grade | |||
| 1–2 | 1.00 | ||
| 3–4 | 1.528 | 1.152–2.027 | 0.0033 |
| Age at diagnosis | |||
| <50 years | 1.00 | ||
| 50–64 years | 1.595 | 0.986–2.582 | 0.0571 |
| 65–74 years | 2.432 | 1.527–3.876 | 0.0002 |
| >75 years | 3.980 | 2.431–6.517 | <0.0001 |
| Tumour location | |||
| Colon | 1.00 | ||
| Rectum | 1.601 | 1.158–2.213 | 0.0044 |
| Site | |||
| Right | 1.00 | ||
| Left | 0.783 | 0.539–1.137 | 0.1989 |
| Transverse colon | 1.731 | 0.617–4.855 | 0.2970 |
| Gender | |||
| Male | 1.00 | ||
| Female | 0.975 | 0.752–1.265 | 0.8480 |
Abbreviations: CI=confidence interval; HR=hazard ratio.
Discussion
To explore the clinical significance of PROX1, we investigated expression of PROX1 by immunohistochemistry in tissue microarray specimens of 517 patients with CRC. The present results indicate that high PROX1 expression is associated with the high grade of tumour differentiation and less favourable prognosis in the subgroup of patients with colon cancer. Moreover, our data show that high PROX1 expression is associated with unfavourable outcomes among the subset of female colon cancer patients. These observations are in line with the hypothesis that PROX1 overexpression promotes the progression of CRC (Petrova et al, 2008).
We found nuclear PROX1 expression in 91% of the 517 CRC specimens, and 27% of these samples showed a high level of expression. Currently, tumour grade is an important clinical indicator of prognosis in CRC and our study revealed that high PROX1 expression was associated with high tumour grade, but not with other clinicopathological parameters. A distinct level of differentiation may indicate different biological behaviour of the cancer. In the present study, none of the grade 1 tumours had high PROX1 expression. The reason for the lack of PROX1 expression in highly differentiated tumours remains unknown, but this could be due to the lower Wnt pathway activation. Additional studies are needed to address the mechanism of PROX1 action.
Previous studies have shown that PROX1 acts as a nuclear transcription factor (Oliver et al, 1993; Rodriquez-Niedenführ et al, 2001). On the basis of these studies and the preclinical findings by Petrova et al (2008), we chose to evaluate PROX1 expression according to the staining in tumour cell nuclei. However, it is also possible that PROX1 is enriched and/or activated in the cytoplasm before translocation into the nucleus to perform its biological function, and thus cytoplasmic PROX1 staining may be detectable. Regulation of intracellular localisation is momentous for transcription factor action, and nuclear import can serve as a mechanism to regulate gene expression (reviewed in Yoneda, 2000). In addition, it has been shown that Prospero, the Drosophila counterpart of PROX1, is often found in the cytoplasm of proliferating and undifferentiated cells (Li and Vaessin, 2000).
The current study shows that PROX1 has prognostic value among colon cancer patients, whereas no difference was found in rectal cancer patients (Table 2, Figure 2). Among colon cancer patients the difference in survival was evident in females and high PROX1 expression was associated with worse outcome. It has been suggested that prognosis for right-sided colon cancers is different from that for left-sided colon cancers (reviewed in Distler and Holt, 1997). Various reasons for such difference could include environmental factors, genetic factors, and sex distribution (reviewed in Iacopetta, 2002). During embryonic development, the right colon arises from the midgut and the left colon from the hindgut. Analyses of genetic databases from normal colon and tumour specimens have revealed differences in gene expression between normal mucosa and colon carcinomas originating from the right and left colons (Glebov et al, 2003; Birkenkamp-Demtroder et al, 2005). It was recently shown in a large population-based study that right-sided colon cancers have a worse prognosis than left-sided cancers and that women are more likely to get right-sided colon cancer, although women did not have significant difference in mortality between left- and right-sided colon cancers (Meguin et al, 2008). In the present study, the proportion of right-sided vs left-sided tumours was similar in males and females, and we found no significant difference in PROX1 expression between right-sided and left-sided tumours (data not shown).
As compared with other cancer types, very few molecular prognostic markers have been reported in CRC. Molecular markers for tumour tissue would be important for clinical decision making, because targeted therapy is an important goal for improving the outcomes of patients with CRC. To date, the exact mechanism of PROX1 action in normal and diseased tissue is poorly understood. Thus, further studies regarding the molecular mechanisms that regulate PROX1 expression and the direct transcriptional target genes of PROX1 are needed. In summary, our results show that high nuclear PROX1 expression is associated with unfavourable outcome in colon cancer patients, and in particular among female colon cancer patients. In addition, these results confirm the previous preclinical observations suggesting that PROX1 has a role in tumour progression in CRC.
Acknowledgments
We thank Päivi Peltokangas and Tuire Koski for their excellent technical assistance. This study was funded by the Academy of Finland, the Finnish Cancer Society, Finska Läkaresällskapet, Medicinska understödsföreningen Liv och Hälsa, Sigrid Juselius Foundation, and Helsinki University Central Hospital Research Funds.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Betschinger J, Mechtler K, Knoblich JA (2006) Asymmetric segregation of the tumour suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124: 1241–1253 [DOI] [PubMed] [Google Scholar]
- Birkenkamp-Demtroder K, Olesen SH, Sørensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF (2005) Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut 54: 374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler P, Holt PR (1997) Are right- and left-sided colon neoplasms distinct tumours? Dig Dis 15: 302–311 [DOI] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G (2003) PROX1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 34: 53–58 [DOI] [PubMed] [Google Scholar]
- Elsir T, Eriksson A, Orrego A, Lindström MS, Nistér M (2010) Expression of PROX1 is a common feature of high-grade malignant astrocytic gliomas. J Neuropathol Exp Neurol 69: 129–138 [DOI] [PubMed] [Google Scholar]
- Glebov OK, Rodriguez LM, Nakahara K, Jenkins J, Cliatt J, Humbyrd CJ, DeNobile J, Soballe P, Simon R, Wright G, Lynch P, Patterson S, Lynch H, Gallinger S, Buchbinder A, Gordon G, Hawk E, Kirsch IR (2003) Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev 12: 755–762 [PubMed] [Google Scholar]
- Iacopetta B (2002) Are there two sides to colorectal cancer? Int J Cancer 101: 403–408 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Laerm A, Helmbold P, Goldberg M, Dammann R, Holzhausen HJ, Ballhausen WG (2007) Prospero-related homeobox 1 (PROX1) is frequently inactivated by genomic deletion and epigenetic silencing in carcinomas of the bilary system. J Hepatol 46: 89–97 [DOI] [PubMed] [Google Scholar]
- Li L, Vaessin H (2000) Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev 14: 147–151 [PMC free article] [PubMed] [Google Scholar]
- Linder N, Martelin E, Lundin M, Louhimo J, Nordling S, Haglund C, Lundin J (2009) Xanthine oxidoreductase—Clinical significance in colorectal cancer and in vitro expression of the protein in human colon cancer cells. Eur J Cancer 45: 648–655 [DOI] [PubMed] [Google Scholar]
- Meguin RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N (2008) Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 15: 2388–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P (1993) PROX1, a prospero-related homeobox gene expressed during mouse development. Mech Dev 44: 3–16 [DOI] [PubMed] [Google Scholar]
- Parkin D, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108 [DOI] [PubMed] [Google Scholar]
- Petrova TV, Nykänen A, Norrmén C, Ivanov KI, Andersson LC, Haglund C, Puolakkainen P, Wempe F, von Mechner H, Gradwohl G, Vanharanta S, Aaltonen LA, Saharinen J, Gentile M, Clarke A, Taipale J, Oliver G, Alitalo K (2008) Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 13: 407–419 [DOI] [PubMed] [Google Scholar]
- Rodriquez-Niedenführ M, Papoutsi M, Christ B, Nicolaides KH, von Kaisenberg CS, Tomarev SI, Wilting J (2001) Prox1 is a marker of ectodermal placodes, endodermal compartments, lymphatic endothelium and lymphangioblasts. Anat Embryol (Berl) 204: 399–406 [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18: 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segditsas S, Tomlinson I (2006) Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25: 7531–7537 [DOI] [PubMed] [Google Scholar]
- Shimoda M, Takahashi M, Yoshimoto T, Kono T, Ikai I, Kubo H (2006) A homeobox protein, PROX1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res 12: 6005–6011 [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Wigle JT, Oliver G (2002) Hepatocyte migration during liver development requires PROX1. Nat Genet 25: 254–255 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G (1999) PROX1 function is required for the development of the murine lymphatic system. Cell 98: 769–778 [DOI] [PubMed] [Google Scholar]
- Yoneda Y (2000) Nucleocytoplasmic protein traffic and its significance to cell function. Genes Cells 5: 777–787 [DOI] [PubMed] [Google Scholar]