Abstract
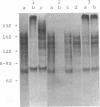
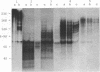
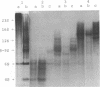
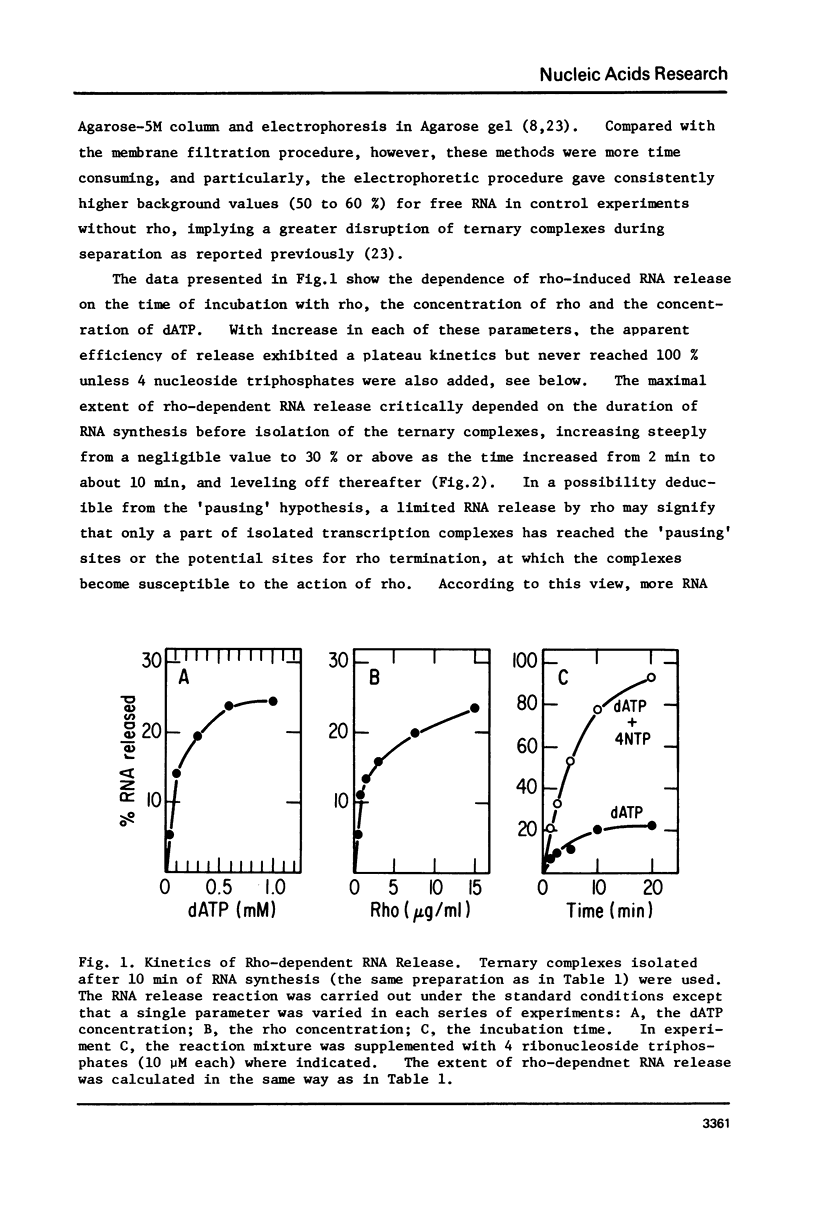
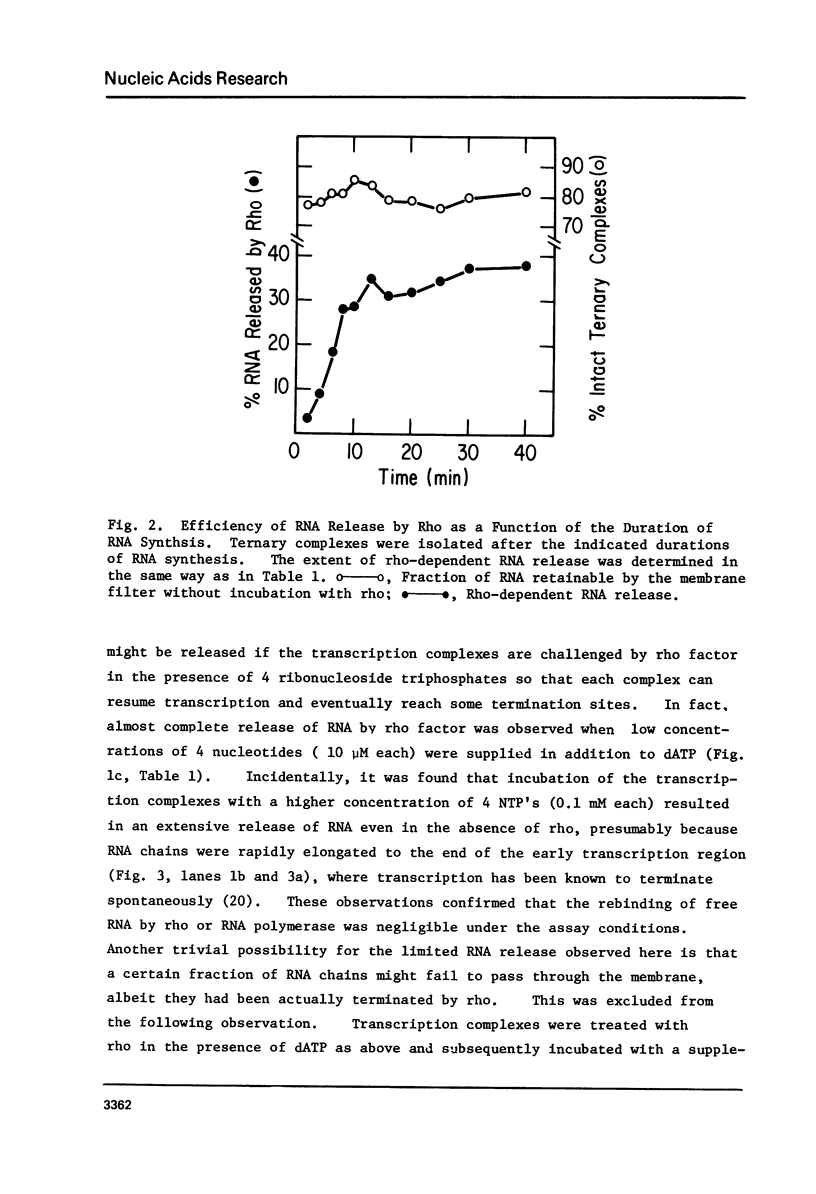
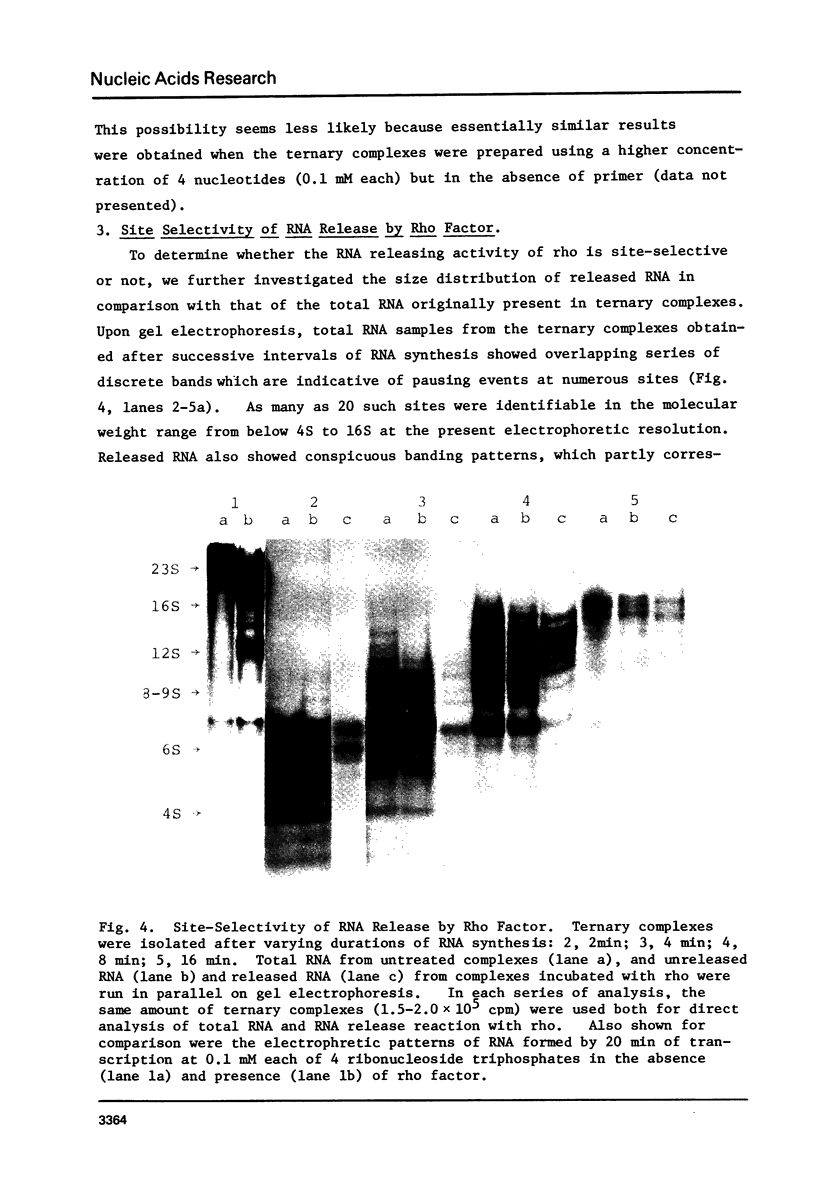
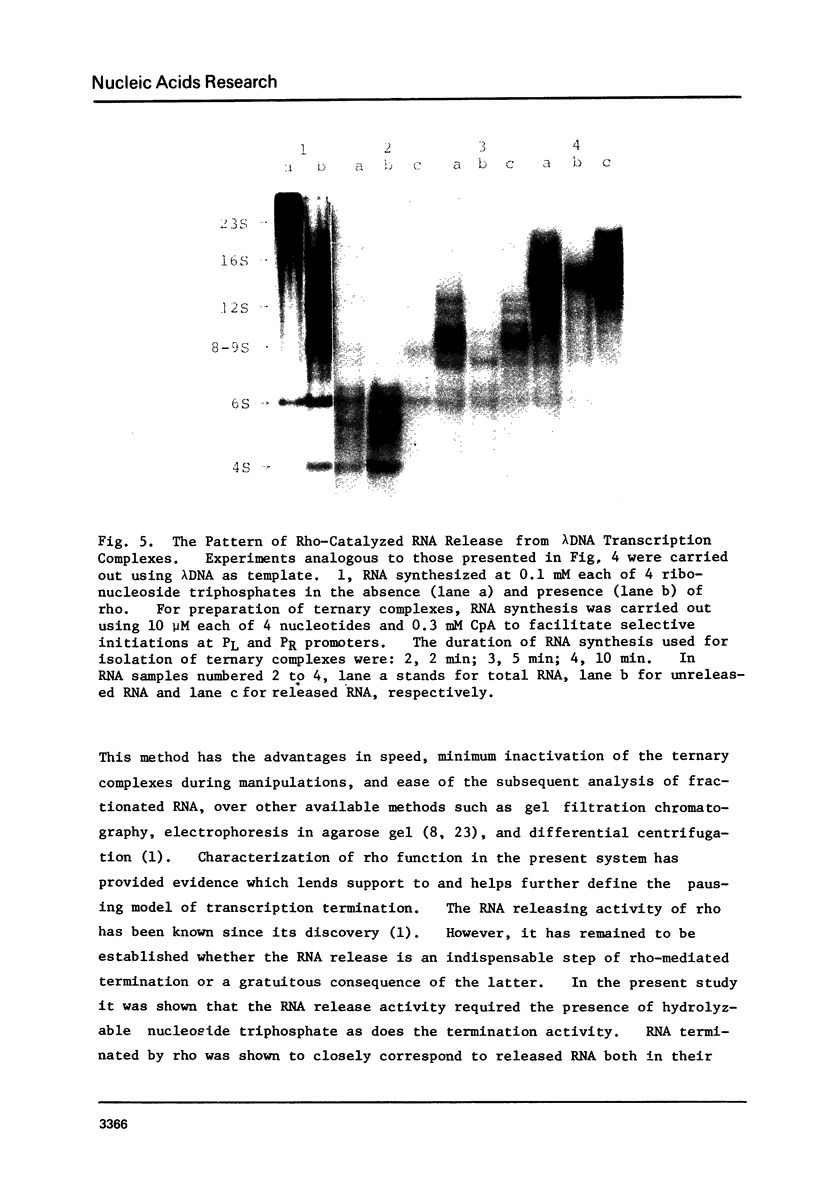
Protein factor rho catalyzes site-specific termination of transcription in a reaction requiring hydrolysis of nucleoside triphosphate with eventual release of RNA from RNA polymerase and DNA template. We have characterized the rho-catalyzed RNA release reaction using isolated transcription complexes. Transcription complexes containing T7 D111 DNA, RNA polymerase, and 3H-labeled nascent RNA were formed and isolated by gel filtration on an Agarose 5M column. When the ternary complexes were incubated with rho factor in the presence of ATP, or dATP, significant amounts of nascet RNA were released from the complexes as determined in a membrane filtration assay. Gel electrophoretic analysis of RNA has revealed that rho releases selected species of discrete-sized RNA from among those originally present in the ternary complexes. These results show that rho essentially acts to release RNA from those ternary complexes which have come to pause, and that this reaction proceeds in a discrete step separately from the pausing of RNA synthesis. Under the conditions used, the extent of RNA release widely varied at individual pausing sites and thus the action of rho exhibited certain site-selectivity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chelm B. K., Geiduschek E. P. Gel electrophoretic separation of transcription complexes: an assay for RNA polymerase selectivity and a method for promoter mapping. Nucleic Acids Res. 1979 Dec 11;7(7):1851–1867. doi: 10.1093/nar/7.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Horaist M. Existence and possible roles of transcriptional barriers in T7 DNA early region as shown by electron microscopy. Nature. 1975 Jul 24;256(5515):288–292. doi: 10.1038/256288a0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Platt T. The attenuator of the tryptophan operon in E.coli: rho-mediated release of RNA polymerase from a transcription termination complex in vitro. Nucleic Acids Res. 1978 Dec;5(12):4613–4623. [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B. ATPase activity required for termination of transcription by the Escherichia coli protein factor rho. J Biol Chem. 1976 Apr 25;251(8):2520–2524. [PubMed] [Google Scholar]
- Hurwitz J., Yarbrough L., Wickner S. Utilization of deoxynucleoside triphosphates by DNA-dependent RNA polymerase of E. coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):628–635. doi: 10.1016/0006-291x(72)90394-4. [DOI] [PubMed] [Google Scholar]
- Küpper H., Sekiya T., Rosenberg M., Egan J., Landy A. A rho-dependent termination site in the gene coding for tyrosine tRNA su3 of Escherichia coli. Nature. 1978 Mar 30;272(5652):423–428. doi: 10.1038/272423a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee F., Squires C. L., Squires C., Yanofsky C. Termination of transcription in vitro in the Escherichia coli tryptophan operon leader region. J Mol Biol. 1976 May 15;103(2):383–393. doi: 10.1016/0022-2836(76)90318-1. [DOI] [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Chamberlin M. J. Kinetic analysis of ribonucleic acid chain initiation by Escherichia coli Ribonucleic acid polymerase bound to DNA. J Biol Chem. 1975 Dec 10;250(23):9112–9120. [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Weissman S., deCrombrugghe B. Termination of transcription in bacteriophage lambda. Heterogeneous, 3'-terminal oligo-adenylate additions and the effects of rho factor. J Biol Chem. 1975 Jun 25;250(12):4755–4764. [PubMed] [Google Scholar]
- Shigesada K., Imai M. Studies on the altered rho factor in nitA mutants of Escherichia coli defective in transcription termination. II. Purification and molecular properties of the mutant rho. J Mol Biol. 1978 Apr 25;120(4):467–486. doi: 10.1016/0022-2836(78)90349-2. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. An expanded transcriptional map of T7 bacteriophage. Reading of minor T7 promoter sites in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1977 Jun 5;112(4):577–601. doi: 10.1016/s0022-2836(77)80165-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Platt T. Transcription termination: nucleotide sequence at 3' end of tryptophan operon in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5442–5446. doi: 10.1073/pnas.75.11.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]