Abstract
Background and Aims
Morphological, molecular and biogeographical information bearing on early evolution of the sunflower alliance of families suggests that the clade containing the extant daisy family (Asteraceae) differentiated in South America during the Eocene, although palaeontological studies on this continent failed to reveal conclusive support for this hypothesis. Here we describe in detail Raiguenrayun cura gen. & sp. nov., an exceptionally well preserved capitulescence of Asteraceae recovered from Eocene deposits of northwestern Patagonia, Argentina.
Methods
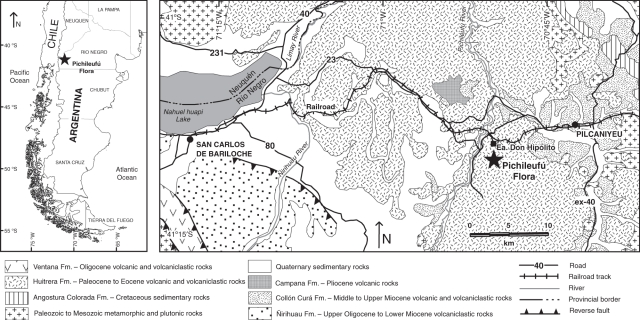
The fossil was collected from the 47·5 million-year-old Huitrera Formation at the Estancia Don Hipólito locality, Río Negro Province, Argentina.
Key Results
The arrangement of the capitula in a cymose capitulescence, the many-flowered capitula with multiseriate–imbricate involucral bracts and the pappus-like structures indicate a close morphological relationship with Asteraceae. Raiguenrayun cura and the associated pollen Mutisiapollis telleriae do not match exactly any living member of the family, and clearly represent extinct taxa. They share a mosaic of morphological features today recognized in taxa phylogenetically close to the root of Asteraceae, such as Stifftieae, Wunderlichioideae and Gochnatieae (Mutisioideae sensu lato) and Dicomeae and Oldenburgieae (Carduoideae), today endemic to or mainly distributed in South America and Africa, respectively.
Conclusions
This is the first fossil genus of Asteraceae based on an outstandingly preserved capitulescence that might represent the ancestor of Mutisioideae–Carduoideae. It might have evolved in southern South America some time during the early Palaeogene and subsequently entered Africa, before the biogeographical isolation of these continents became much more pronounced. The new fossil represents the first reliable point for calibration, favouring an earlier date to the split between Barnadesioideae and the rest of Asteraceae than previously thought, which can be traced back at least 47·5 million years. This is the oldest well dated member of Asteraceae and perhaps the earliest indirect evidence for bird pollination in the family.
Keywords: Compositae, capitulescence, fossil taxon, Raiguenrayun cura gen. & sp. nov., Eocene, Patagonia, southern South America
INTRODUCTION
Asteraceae are an evolutionarily successful family of angiosperms with >23 000 species, occupying all continents except Antarctica (Jeffrey, 2007). One of the most important features of Asteraceae is the presence of flowers tightly packed into a condensed inflorescence, known as the capitulum. It resembles a single large flower and therefore acts as a single ‘attraction unit’ to pollinators. This morphological specialization has been postulated as one of the major determinants of the evolutionary success of the family (Stebbins, 1967; Broholm et al., 2008). The evolution of floral display in the largely animal-pollinated Asteraceae is commonly attributed to pollinator-mediated selection (Lane, 1996): long corollas and high nectar secretion attract hummingbirds and sunbirds in some early branching groups [Barnadesiodeae, Mutisioideae and Carduoideae (Dicomeae)], whereas larger capitula or capitulescences attract solitary bees in more derived groups (Asteroideae).
The origin of Asteraceae has long been debated, with South America and the Palaeogene most frequently hypothesized as the place and time of their early evolution (DeVore and Stuessy, 1995; Stuessy et al., 1996). More recently, Lundberg (2009) postulated an ancestral Asteraceae ‘growing in what today is southern South America just north of Patagonia, sometime around middle or late Eocene’. Asteraceae underwent a worldwide explosive radiation during the Neogene (Graham, 1996; Barreda et al., 2010a), but their scarceness in Palaeogene ecosystems is remarkable: in modern grassland, wooded grassland and montane vegetation, this family is a dominant component. This raises the question of whether Asteraceae were truly rare in Palaeogene communities or whether our picture of these ecosystems is incomplete.
Recently, the study of a fossil specimen from the Middle Eocene [47·5 million years ago (Ma)] Huitrera Formation of Río Negro Province, northwestern Patagonia (Figs 1, 2) resulted in the recognition of the first capitulum of Asteraceae in the fossil record (Barreda et al., 2010b). This fossil displays several relevant features that we here present in detail for the first time; some may reveal past interactions with vertebrate pollinators. Furthermore, the age of the fossil-bearing strata suggests that the divergence between Asteraceae and its sister family Calyceraceae might have occurred far earlier than previously thought. In this contribution we formally describe Raiguenrayun cura gen. & sp. nov., the oldest well dated Asteraceae, and explore the evolutionary, ecological and biogeographical implications of this finding.
Fig. 1.
Simplified geological map of north-western Río Negro Province showing the fossil locality (41°09′26·06′′S, 70°49′57·11′′W, WGS84). Modified from Giacosa et al. (2001).
Fig. 2.
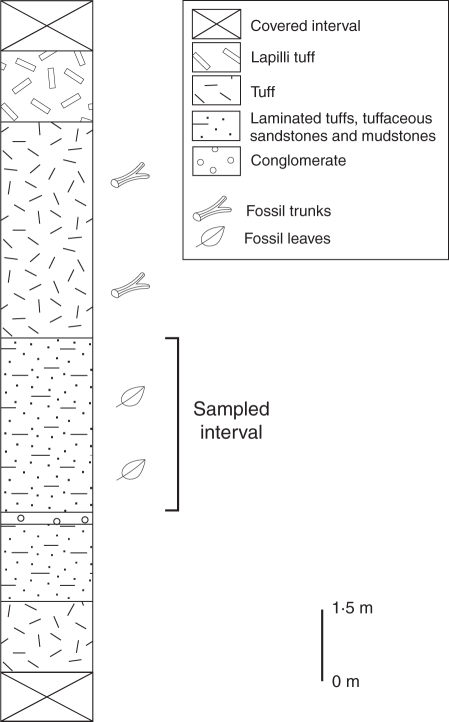
Stratigraphic section of the Huitrera Formation at the Río Pichileufú flora site, showing the location of the fossil-bearing levels.
MATERIALS AND METHODS
The fossil specimen reported here was collected by one of us (R.C.) from the Río Pichileufú fossil-bearing strata (Huitrera Formation), near the Estancia Don Hipólito locality (41°09′26·06′′S, 70°49′57·11′′W, WGS84), Río Negro Province, Argentina (Fig. 1). The Huitrera Formation corresponds to laminated tuffs and tuffaceous mudstones and sandstones (Fig. 2), interpreted as deposited in an ancient shallow lacustrine or swamp environment (Aragón and Romero, 1984). The age of the Río Pichileufú flora-bearing strata is well constrained (Middle Eocene 47·46 ± 0·05 Ma) based on 40Ar/39Ar dating of sanidine phenocrysts from stratigraphically related tuffs (Wilf et al., 2005).
The fossil capitulescence was examined under transmitted white light and fluorescent light. The fossil is housed in the palaeontological collection of the ‘Museo del Lago Gutiérrez Dr. Rosendo Pascual de Geología y Paleontología’ (San Carlos de Bariloche, Río Negro Province, Argentina): MLG 1156. Fluorescent photomicrographs were taken with a confocal Nikon microscope (objective PLAN UW 2 × /0·06) using a green filter. For non-microscope photographs a Nikon Coolpix 8000 camera was used. Fossil pollen was examined using a transmitted white light Leica microscope, and photomicrographs were taken with a Leica DFC 290 camera. Co-ordinates are referred to the England finder. Pollen terminology follows Punt et al. (2007).
The phylogenetic placement of the fossils (R. cura and Mutisiapollis telleriae) on the already available meta-tree (Funk et al., 2009) was determined on the basis of their morphological characters (Barreda et al., 2010b). We follow the terminology of Katinas et al. (2008), who considered tribes Onoserideae, Nassauvieae and Mutisieae within Mutisioideae sensu stricto and these, together with Stifftieae and Wunderlichioideae, within Mutisioideae sensu lato (s.l.) although Mutisioideae s.l. include a grade of around 12 clades according to the recent molecular phylogenetic analyses of Funk et al. (2009).
SYSTEMATICS
Genus
Raiguenrayun Barreda, Katinas, Passalia & Palazzesi gen. nov. (Figs 3, 4)
Fig. 3.
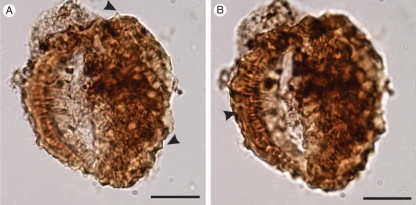
Raiguenrayun cura gen. and sp. nov., fossil Asteraceae from north-western Patagonia, southern South America. (A) General view of the cymose capitulescence-like fossil showing two long-pedunculate head-like inflorescences. (B) Detail of the central capitula. Arrowheads show involucral bracts. (C) Capitulescence under fluorescent light taken with a confocal microscope. Note the slender projection (bract?) in the peduncle of the lateral capitula (arrowhead). (D) Apical zone of the central capitula showing individual florets (arrowheads). (E) The same detail of D, but under fluorescent light. Note the shining slender projections (pappus) among florets. Scale bars = 1 cm.
Fig. 4.
Reconstruction of a hypothetical ancestral Asteraceae based on morphological features of both the fossil Raiguenrayun cura gen. & sp. nov. and the morphologically closest extant tribes.
Type species
Raiguenrayun cura Barreda, Katinas, Passalia & Palazzesi sp. nov. (Figs 3, 4)
Generic diagnosis
Loosely cymose capitulescence with two or three, long pedunculate capitula. Central capitulum (mature?) globose in shape, with multiseriate–imbricate involucral bracts (phyllaries), the basal-most series decurrent onto the long peduncle. Intermediate bracts widely oblong, with an apparent obtuse apex. Flowers numerous, large (approx. 1·5 cm long), with a developed ligule or lip. Slender projections like hairs present among flowers (pappus). Lateral capitulum (inmature?) oblong in shape.
Species
Raiguenrayun cura Barreda, Katinas, Passalia & Palazzesi sp. nov. (Figs 3, 4)
Specific diagnosis
As for the genus
Etymology
From the Aónikenk language used by native inhabitants of south-central Argentina. Raiguen-Rayún = flower, Curá = stone.
Holotype
Specimen MLG 1156 (Fig. 3)
Repository
‘Museo del Lago Gutiérrez Dr. Rosendo Pascual de Geología y Paleontología’ Villa Los Coihues, San Carlos de Bariloche, Río Negro Province, Argentina.
Type locality and stratigraphic position
The material was collected by one of us (R.C.) from the Río Pichileufú fossil-bearing strata (Huitrera Formation), near the Estancia Don Hipólito locality (41°09′26·06′′S, 70°49′57·11′′W, WGS84), about 60 km east of San Carlos de Bariloche, Río Negro Province, Argentina (Figs 1, 2). This specimen comes from volcanic and volcaniclastic beds interpreted as deposited in a shallow lacustrine environment that contain the outstanding Río Pichilefú flora with exceptionally preserved compressions of leaves, flowers, fruits and seeds (Berry, 1938; Aragón and Romero, 1984; Wilf et al., 2005).
Age
Middle Eocene – 47·5 million years – based on radiometric data (Wilf et al., 2005).
Description
The fossil inflorescence reported here consists of a loosely cymose capitulescence, laterally compressed. It comprised two, probably three, capitula sustained by equally elongated peduncles (2·7–3·0 cm long, 0·15–0·20 cm wide). The three peduncles depart from nearly the same ramification point, apparently without pubescence. A slender projection (bract?) is observed in the peduncle of the lateral head. At least one of the fossil capitula (the central one) appears to be fully mature at the time of preservation. It is globoid in shape (3·0 cm long, 2·9 cm wide) and possesses several series (six or eight) of imbricate protective bracts (phyllaries of an involucre), the basal-most series decurrent onto the long peduncle. Intermediate bracts are widely oblong (approx. 0·15–0·20 cm wide and 1 cm long), with an apparent obtuse apex, although this could be the result of preservation. Flowers (approx. 80) are relatively large (approx. 1·5 cm long), apparently homomorphic [no clear difference between inner (disc florets) and outer (ray florets) flowers was observed, probably due to preservation bias], with a developed ligule or lip. Although the apex of individual flowers can be identified with relative accuracy (especially in the central head), their morphological nature is not so evident: they may be interpreted as the lobes of an actinomorphic corolla, lips of a bilabiate corolla or fused petals of a ligulate corolla (Fig. 3). Slender projections like hairs (somewhat flattened capillary bristles) are present among flowers. They are interpreted as remains of the pappus. The lateral capitulum (immature?) is oblong in shape. A third peduncle is also observed, inferring a third head.
Comparisons and remarks
There is no similar fossil taxon in the literature. Fossil capitula of possible asteracean affinity have been described from the Cretaceous (Palaeanthus problematicus Newberry) and Oligocene–Miocene (Viguiera cronquistii Becker) of North America, and from the Oligocene of France (Hieracites nudatus Saporta and Hieracites stellatus Saporta), but none of these specimens possesses features that conclusively link them to Asteraceae.
Palaeanthus problematicus, originally described as an asteracean head (Newberry, 1895), was later suggested as a possible cycadophyte (Cronquist, 1977) or, at most, as an unclassified angiosperm fruit with many follicles (Crane and Dilcher, 1984). The Hieracites morphotaxon was split by de Saporta (1889) into two species and interpreted by Small (1919) as possible Asteraceae: H. stellatus (heads with a sub-biseriate involucre and a small receptacle) and H. nudatus (a mature receptacle with involucral bracts missing and achenes or florets densely packed). Despite the conviction of Small (1919) of the asteracean affinity of de Saporta's specimens, these are neither mentioned in fossil reviews (Cronquist, 1977; Crepet and Stuessy, 1978) nor accepted as Asteraceae (Martínez-Millán, 2010). Viguiera cronquistii is the megafossil most frequently attributed to Asteraceae. It was described by Becker (1969) who, following the observations of Cronquist (1977), remarked on some resemblances between the fossil and the extant genus Viguiera Kunth (Heliantheae). This fossil consists of a compressed head, completely covered by numerous helically arranged bracts, subtended by a short stalk (Becker, 1969) but, in contrast to R. cura, it lacks florets or floret-like structures. Although V. cronquistii was thought to be the first reliable megafossil of Asteraceae, Crepet and Stuessy (1978) re-examined the type specimen and concluded that this fossil ‘cannot be considered unequivocally to be the remains of a Compositae’.
Botanical affinity
The above-described fossil is clearly a member of Asteraceae based on a combination of features diagnostic of the family (Barreda et al., 2010b). It superficially resembles capitula of several modern Asteraceae, showing a mosaic of morphological features of Mutisioideae s.l. (Fig. 5), Carduoideae and even Barnadesioideae, but it does not match exactly any of these subfamilies and represents an extinct taxon (stem representative) (Fig. 6). The characteristics of the fossil capitulum match well with the features attributed to a hypothetical ancestor of Asteraceae proposed by several authors (e.g. Bremer, 1994; Funk et al., 2009; Lundberg, 2009): inflorescence cyme-like; few heads per plant, heads indeterminate, each with many flowers; involucral bracts in several series, imbricate without hyaline or scabrous margins; florets mostly of one type, some differentiation in floral morphology in peripheral florets possible but without true rays; and pappus of capillary bristles.
Fig. 5.
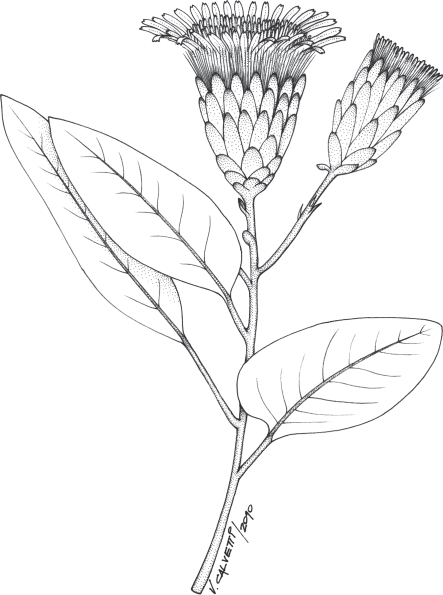
Extant member of an early branching lineage of Asteraceae (Mutisioideae), Cnicothamnus lorentzii Griseb., that superficially resembles the Patagonian fossil capitula.
Fig. 6.
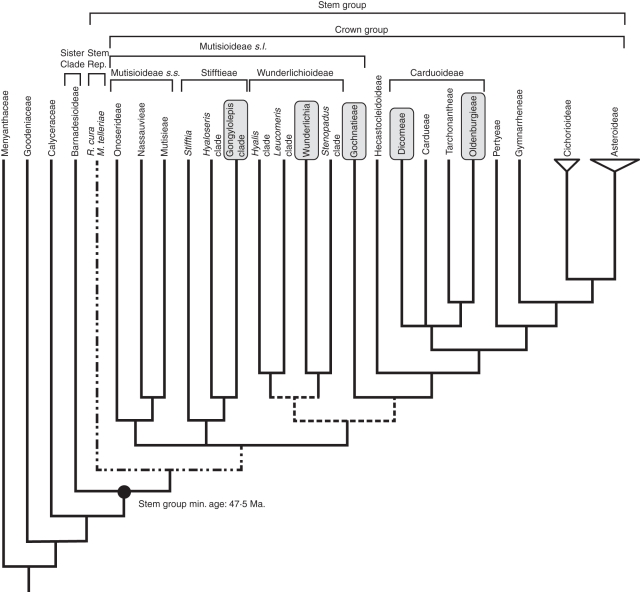
Modified meta-tree of Funk et al. (2009) with the inclusion of the fossils Raiguenrayun cura gen. & sp. nov. and Mutisiapollis telleriae (dashed-dotted lines). In grey: taxa that share more characters with R. cura and M. telleriae than any other extant member in Asteraceae. Some branches are not well supported as compared with the remainder of the tree (dashed lines; Funk et al., 2009). Crown group, stem group and stem representative definitions are based on Magallón (2004).
The associated pollen grains (Mutisiapollis telleriae Barreda and Palazzesi) were helpful in determining the accurate taxonomic position of the megafossil within Asteraceae (Barreda et al., 2010b). Mutisiapollis telleriae (Fig. 7) has morphological features currently found in early branching members of the family (Barreda et al., 2009; Tellería et al., 2010). In particular, the acaveate exine and the presence of well developed spines with an uneven arrangement characterize pollen of most members of genera of Stifftieae and Wunderlichioideae concentrated in the Guayana Highland, some Gochnatieae (Mutisioideae s.l.) and some members of Dicomeae and Oldenburgieae (Carduoideae) (Zao et al., 2006; Blackmore et al., 2010; Tellería et al., 2010) (Fig. 6). Pollen characters clearly exclude subfamily Barnadesioideae. The microechinate (or almost spineless) pollen grains of the early branching Barnadesioideae and the related families Calyceraceae and Goodeniaceae may indicate that the presence of well developed spines is an evolutionary novelty (apomorphy) within Mutisioideae and Carduoideae (with subsequent reduction in some species).
Fig. 7.
Mutisiapollis telleriae, fossil pollen grain from the capitula-bearing sample. (A) General view, focus on well-developed spines (arrowhead). (B) Focus on exine structure (arrowhead). Specimen on slide MLG 1156: co-ordinates J51/J52. Scale bars = 10 µm.
DISCUSSION
New fossil discoveries from Patagonia, Argentina, have shed light on the emergence, early evolution and palaeobiology of Asteraceae. Raiguenrayun cura (capitulescence) and M. telleriae (pollen grains) do not match exactly any living member of the extant family and represent extinct taxa. Fossils show a mosaic of characters found in extant Stifftieae, Wunderlichioideae and Gochnatieae (Mutisioideae s.l.) and Dicomeae and Oldenburgieae (Carduoideae), tribes today endemic to or mainly distributed in South America and Africa, respectively (Ortiz, 2009; Ortiz et al., 2009a, b; Sancho and Freire, 2009). Some prevalent shared features in modern representatives of some African Dicomeae and Oldenburgieae and South American Wunderlichioideae and Gochnatieae (e.g. apiculate anther appendages) led Panero and Funk (2008) to suggest a more direct African and South American connection. This connection might have been even stronger further back in time than that seen today: the documentation of highly similar asteracean fossil pollen grains from the Eocene on both continents (Zavada and DeVilliers, 2000; Scott et al., 2006; Barreda et al., 2010b; Zavada and Lowrey, 2010) reinforces the proposed link between western Gondwana lands. Floral interchange between South America and Africa occurred during the Late Cretaceous and continued into the Palaeogene in spite of the increasing distance between these land masses. Even by the Eocene, when South America and Africa were approx. 2000 km apart, a strong floristic affinity prevailed (Jaramillo and Dilcher, 2001). The latest palaeogeographic reconstructions suggested the existence of a series of islands and shallow terrain in the South Atlantic during the mid-Cenozoic, particularly between 40 and 50 Ma (Bandoni de Oliveira et al., 2009). This palaeogeographic positive relief, which is underwater today, might have reduced considerably the distance of a possible biotic migration between South America and Africa. According to this scenario and previous biogeographical hypotheses, Asteraceae might have evolved in southern South America some time before the Eocene, and subsequently entered Africa, before the biogeographical isolation of these two continents became much more pronounced. In this context, the ancestor of Mutisioideae–Carduoideae might have occupied both continents during the Eocene, and ultimately diverged into two separate lineages when effective dispersal barriers had established. It remains unknown whether the current lack of pre-Eocene records of Asteraceae represents the true absence of the family, or whether our picture is incomplete due to the inevitable bias of the fossil record. The latter seems more likely in light of the facts that Mutisioideae–Carduoideae do not represent the earliest diverging branch of Asteraceae (a position occupied by Barnadesioideae) and that the appearance of Asteraceae in the current fossil record most probably documents the radiation of this lineage more than its time of origin. Previous divergence time estimates for the family (Kim et al., 2005) are somewhat discordant with the age of this Patagonian finding and previous African reports (Scott et al., 2006; Zavada and Lowrey, 2010). Such a discrepancy can most probably be attributed to the lack of the oldest fossil calibration points of Asteraceae in most of the published molecular-based estimate attempts. Raiguenrayun cura is a stem representative (Fig. 6) because it displays some but not all the synapomorphies of the crown group. The radiometric age of the fossil-bearing deposits (Huitrera Formation) is here used to date the stem group (sister clade/stem group split). The split between Barnadesioideae and the rest of Asteraceae can be traced back at least 47·5 million years. The early evolution of Asteraceae may have occurred earlier than previously thought, and we think that it might have diverged from the sister family Calyceraceae by the Palaeocene or even by the Cretaceous, although this hypothesis needs new empirical studies.
The early evolution and past ecology of Asteraceae are probably best understood within the context of their source assemblage. The middle Eocene Río Pichileufú flora ranks among the most diverse compression–impression floras ever found in the fossil record (Wilf et al., 2005). An equable climate with relatively warm and humid conditions may have supported highly diverse multilayered vegetation with canopy trees, vines, shrubs, ground cover and aquatic plants (Wilf et al., 2009); in this scenario the earliest interaction between ancestral Asteraceae and pollinators may have occurred. Currently, most Asteraceae are pollinated opportunistically by a wide variety of insects, but bird pollination has evolved in some lineages of the family (Lane, 1996). The main flower-visiting birds are hummingbirds (Trochilidae) in Barnadesioideae and Mutisioideae, and sunbirds (Nectariniidae) in Carduoideae (Lane, 1996; Anderberg et al., 2007). Funk et al. (1995) considered the ancestor of extant Asteraceae to be bird pollinated because Mutisioideae, Barnadesioideae and Carduoideae (Fig. 5) are the earliest branching lineages of Asteraceae. The capitula of the ancestral Asteraceae, according to previous reconstructions (Bremer, 1992) and our interpretations on the fossil inflorescences (Fig. 4), could have been appropriately shaped (elongated corollas) and sized (large capitulum) for bird visitation. Bird pollination appears to have evolved in the Palaeogene (Mayr, 2004, 2009; Louchart, 2008; Tambussi, 2011) and occurs in aseasonal tropical and sub-tropical regions where flowers and nectar are available all year to support nectarivorous birds (Cronk and Ojeda, 2008). This climatic context agrees with that inferred for the middle Eocene of northwestern Patagonia (Melendi et al., 2003; Wilf et al. 2005, 2009). However, the most important floral traits that are usually associated with bird pollination are virtually missing in the fossil record; many clues are not preserved at all (e.g. colour and nectar) and others only under the most extraordinary of circumstances (e.g. shape of the flower). However, because of ambiguity regarding the past pollination biology in Asteraceae, and the inherent bias of the fossil record, it is not possible for us to confirm whether or not R. cura was bird pollinated. Nevertheless, we can extrapolate our inductive inference into the unobservable past: if the closest living relatives of R. cura are today pollinated by hummingbirds (e.g. Gongylolepis) in South America or sunbirds (e.g. Dicoma) in South Africa, we can then infer that it might have been a bird-pollinated flower in the Eocene of Patagonia. Furthermore, families such as Proteaceae, Myrtaceae and Bombacaceae, with many of their current ornithophyllous members, were important elements of the Eocene ecosystems of northwestern Patagonia (Melendi et al., 2003; Wilf et al., 2005) coexisting with ancestral Asteraceae.
We have shown throughout this study the questions that can be answered as a result of the discovery of this fossil, but which questions remain unanswered? Much remains to be learned about the rapid radiation of Asteraceae since it is still unclear how its members rapidly colonized all habitats and became so incredibly diverse in a relatively short time interval (Stuessy, 2010). For example, this enormous family possesses one of the highest rates of diversification among all flowering plants, generating >23 000 species in a comparatively short period of time (Magallón and Sanderson, 2001). Such explosive radiation has been associated with innovations, particularly new traits (chemical and morphological) that made the reproductive process more efficient (Calabria et al., 2009). The capitulum existed in Patagonia 47·5 Ma, far earlier than the ecological dominance of the family at least 20 million years later.
ACKNOWLEDGEMENTS
The helpful comments of two reviewers (V. Funk and anonymous) and the Editor (M. Fay) are sincerely appreciated. We thank Pablo Do Campo, Mauricio Bonifacino and Victor Hugo Calvetti for assistance with microscopy, photography and drawing. This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0342, 5604, 2340), Agencia Nacional de Promoción Científica y Tecnológica (PICT 01977), Argentina and the National Science Foundation (NSF DEB 0919071).
LITERATURE CITED
- Anderberg AA, Baldwin BG, Bayer RG, et al. Compositae. In: Kadereit JW, Jeffrey C, editors. The families and genera of vascular plants. VIII. Flowering plants, eudicots, asterales. Berlin: Springer; 2007. pp. 61–588. [Google Scholar]
- Aragón E, Romero EJ. Geología, paleoambientes y paleobotánica de yacimientos terciarios del occidente de Río Negro, Neuquén y Chubut. 9° Congreso Geológico Argentino. 1984;1:475–507. [Google Scholar]
- Bandoni de Oliveira F, Cassola Molina E, Marroig G. Paleogegraphy of the South Atlantic: a route for primates and rodents into the New World? In: Garber PA, Estrada A, Bicca-Marques CJ, Heymann EW, Strier KB, editors. South American primates, developments in primatology: progress and prospects. New York: Springer Science; 2009. pp. 55–68. [Google Scholar]
- Barreda VD, Palazzesi L, Marenssi S. Palynological record of the Paleogene Río Leona Formation (southernmost South America): stratigraphical and paleoenvironmental implications. Review of Palaeobotany and Palynology. 2009;154:22–33. [Google Scholar]
- Barreda VD, Palazzesi L, Tellería MC, Katinas L, Crisci J. Fossil pollen indicates an explosive radiation of basal asteracean lineages and allied families during Oligocene and Miocene times in the Southern Hemisphere. Review of Palaeobotany and Palynology. 2010a;160:102–110. [Google Scholar]
- Barreda VD, Palazzesi L, Tellería MC, et al. Eocene Patagonia fossils of the daisy family. Science. 2010b;329:1621. doi: 10.1126/science.1193108. [DOI] [PubMed] [Google Scholar]
- Becker HF. Fossil plants of the Tertiary Beaverhead Basin in southwestern Montana. Palaeontographica. 1969;127:1–142. [Google Scholar]
- Berry EW. Tertiary flora from the Río Pichileufú, Argentina. Geological Society of America, Special Paper. 1938;12:1–149. [Google Scholar]
- Blackmore S, Wortley AH, Skvarla JJ, Gabarayeva NI, Rowley JR. Developmental origins and structural diversity in pollen walls of Compositae. Plant Systematics and Evolution. 2010;284:17–32. [Google Scholar]
- Bremer K. Ancestral areas: a cladistic reinterpretation of the center of origin concept. Systematic Biology. 1992;41:436–445. [Google Scholar]
- Bremer K. Asteraceae: cladistics and classification. Portland, OR: Timber Press; 1994. [Google Scholar]
- Broholm SK, Tähtiharju S, Laitinen RAE, Albert VA, Teeri TH, Elomaa P. A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proceedings of the National Academy of Sciences, USA. 2008;105:9117–9122. doi: 10.1073/pnas.0801359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria L, Emerenciano VP, Scotti MT, Mabry TJ. Secondary chemistry of Compositae. In: Funk VA, Susanna A, Stuessy TF, Bayer R, editors. Systematics, evolution and biogeography of the Compositae. Vienna: IAPT; 2009. pp. 73–88. [Google Scholar]
- Crane PR, Dilcher DL. Lesqueria: an early angiosperm fruiting axis from the mid-Cretaceous. Annals of the Missouri Botanical Garden. 1984;71:384–402. [Google Scholar]
- Crepet WL, Stuessy TF. A reinvestigation of the fossil Viguiera conquistii (Compositae) Brittonia. 1978;30:483–491. [Google Scholar]
- Cronk Q, Ojeda I. Bird-pollinated flowers in an evolutionary molecular context. Journal of Experimental Botany. 2008;59:715–727. doi: 10.1093/jxb/ern009. [DOI] [PubMed] [Google Scholar]
- Cronquist A. The Compositae revisited. Brittonia. 1977;29:137–153. [Google Scholar]
- DeVore M, Stuessy TF. The place and time of origin of the Asteraceae, with additional comments on the Calyceraceae and Goodeniaceae. In: Hind DJN, Jeffrey C, Pope GV, editors. Advances in Compositae systematics. Kew: Royal Botanic Gardens; 1995. pp. 23–40. [Google Scholar]
- Funk VA, Robinson H, McKee GS, Pruski JF. Neotropical montane Compositae with an emphasis on the Andes. In: Churchill SP, Balsev H, Forero E, Lutyen JL, editors. Biodiversity and conservation of neotropical montane forests. Bronx, NY: New York Botanical Garden; 1995. pp. 451–471. [Google Scholar]
- Funk VA, Susanna A, Stuessy T, Bayer R. Systematics, evolution, and biogeography of the Compositae. Vienna: IAPT; 2009. [Google Scholar]
- Giacosa R, Heredia N, Zubia M, et al. Hoja geológica 4172-IV, San Carlos de Bariloche, Provincias de Río Negro y Neuquén. 2001 Servicio Geológico Minero Argentino, Boletín 279. [Google Scholar]
- Graham A. A contribution to the geologic history of the Compositae. In: Hind DJN, editor. Compositae: systematics. Kew: Royal Botanic Gardens; 1996. pp. 123–140. [Google Scholar]
- Jaramillo CA, Dilcher DL. Middle Paleogene palynology of Central Colombia, South America: a study of pollen and spores from tropical latitudes. Palaeontographica. 2001;258:87–213. [Google Scholar]
- Jeffrey C. Compositae: Introduction with key to tribes. In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer Verlag; 2007. pp. 61–87. [Google Scholar]
- Katinas L, Pruski J, Sancho G, Tellería MC. The subfamily Mutisioideae (Asteraceae) Botanical Review. 2008;74:469–716. [Google Scholar]
- Kim KJ, Choi KS, Jansen RK. Two chloroplast DNA inversions originated simultaneously during the early evolution of the sunflower family (Asteraceae) Molecular Biology and Evolution. 2005;22:1783–1792. doi: 10.1093/molbev/msi174. [DOI] [PubMed] [Google Scholar]
- Lane MA. Pollination biology of Compositae. In: Caligari PDS, Hind DJN, editors. Compositae: biology and utilization. Kew: Royal Botanic Gardens; 1996. pp. 61–80. [Google Scholar]
- Louchart A, Tourment N, Carrier J, Roux T, Mourer-Chauviré C. Hummingbird with modern feathering: an exceptionally well-preserved Oligocene fossil from southern France. Naturwissenschaften. 2008;95:171–175. doi: 10.1007/s00114-007-0309-0. [DOI] [PubMed] [Google Scholar]
- Lundberg J. Asteraceae and relationships within Asterales. In: Funk VA, Susanna A, Stuessy TF, Bayer R, editors. Systematics, evolution, and biogeography of the Compositae. Vienna: IAPT; 2009. pp. 157–169. [Google Scholar]
- Magallón SA. Dating lineages: molecular and paleontological approaches to the temporal framework of clades. International Journal of Plant Science. 2004;165:7–21. [Google Scholar]
- Magallón S, Sanderson MJ. Absolute diversification rates in angiosperm clades. Evolution. 2001;55:1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Martínez Millán M. Fossil record and age of the Asteridae. Botanical Review. 2010;76:83–135. [Google Scholar]
- Mayr G. Old world fossil record of modern-type hummingbirds. Science. 2004;304:861–862. doi: 10.1126/science.1096856. [DOI] [PubMed] [Google Scholar]
- Mayr G. Paleogene fossil birds. Berlin: Springer-Verlag; 2009. [Google Scholar]
- Melendi DL, Scafati LH, Volkheimer W. Palynostratigraphy of the Paleogene Huitrera Formation in N-W Patagonia, Argentina. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2003;228:205–273. [Google Scholar]
- Newberry JS. The flora of the Amboy Clays. Monograph of the United States Geological Survey. 1895;26:1–26. [Google Scholar]
- Ortiz S. Oldenburgieae (Carduoideae) In: Funk VA, Susanna A, Stuessy TF, Bayer R, editors. Systematics, evolution and biogeography of the Compositae. Vienna: IAPT; 2009. pp. 287–291. [Google Scholar]
- Ortiz S, Carbajal R, Serrano M, Coutinho AXP. Dicomeae (Carduoideae) In: Funk VA, Susanna A, Stuessy TF, Bayer R, editors. Systematics, evolution and biogeography of the Compositae. Vienna: IAPT; 2009a. pp. 267–278. [Google Scholar]
- Ortiz S, Bonifacino JM, Crisci JV, et al. The basal grade of the Compositae: Mutisieae (sensu Cabrera) and Carduoideae. In: Funk VA, Susanna A, Stuessy TF, Bayer R, editors. Systematics, evolution and biogeography of the Compositae. Vienna: IAPT; 2009b. pp. 193–213. [Google Scholar]
- Panero JL, Funk VA. The value of sampling anomalous taxa in phylogenetic studies: major clades of the Asteraceae revealed. Molecular Phylogenetics and Evolution. 2008;47:457–782. doi: 10.1016/j.ympev.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Punt W, Hoen PP, Blackmore S, Nilsson S, Le Thomas A. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology. 2007;143:1–81. [Google Scholar]
- Sancho G, Freire SE. Gochnatieae (Gochnatioideae) and Hyalideae (Wunderlichioideae p.p.) In: Funk VA, Susanna A, Stuessy TF, Bayer R, editors. Systematics, evolution and biogeography of the Compositae. Vienna: IAPT; 2009. pp. 249–260. [Google Scholar]
- de Saporta G. Dernières adjonctions à la flore fossile 'Aix-en-Provence. Descriptions des espèces. 2° Partie. Annales des Sciences Naturelles Botanique. 1889;10:1–192. [Google Scholar]
- Scott L, Cadman A, McMillan I. Early history of Cainozoic Asteraceae along the Southern African west coast. Review of Palaeobotany and Palynology. 2006;142:47–52. [Google Scholar]
- Small J. The origin and development of the Compositae. London: New Phytologist; 1919. [Google Scholar]
- Stebbins GL. Adaptive radiation and trends of evolution in higher plants. In: Dobzhansky T, Hecht MK, Steere WC, editors. Evolutionary biology. New York: Appleton Century Crofts; 1967. pp. 101–142. [Google Scholar]
- Stuessy TF. The rise of sunflowers. Science. 2010;329:1605–1606. doi: 10.1126/science.1195336. [DOI] [PubMed] [Google Scholar]
- Stuessy TF, Sang T, DeVore ML. Phylogeny and biogeography of the subfamily Barnadesioideae with implications for early evolution of Compositae. In: Hind DNN, Beentje H, editors. Compositae: systematics. Kew: Royal Botanical Garden; 1996. pp. 463–490. [Google Scholar]
- Tambussi CP. Palaeoenvironmental and faunal inferences based on the avian fossil record of Patagonia and Pampa: what works and what does not. Biological Journal of the Linnean Society. 2011;103:458–474. [Google Scholar]
- Tellería MC, Barreda VD, Palazzesi L, Katinas L. Echinate fossil pollen of Asteraceae from the Late Oligocene of Patagonia: an assessment on its botanical affinity. Plant Systematics and Evolution. 2010;285:75–81. [Google Scholar]
- Wilf P, Johnson KR, Cúneo NR, Smith ME, Singer BS, Gandolfo MA. Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. American Naturalist. 2005;165:634–650. doi: 10.1086/430055. [DOI] [PubMed] [Google Scholar]
- Wilf P, Little SA, Iglesias A, et al. Papuacedrus (Cupressaceae) in Eocene Patagonia: a new fossil link to Australasian rainforests. American Journal of Botany. 2009;96:2031–2047. doi: 10.3732/ajb.0900085. [DOI] [PubMed] [Google Scholar]
- Zao Z, Skvarla JJ, Jansen RK. Mutisieae (Asteraceae) pollen ultrastructure atlas. Lundellia. 2006;9:51–76. [Google Scholar]
- Zavada MS, De Villiers SE. Pollen for the Asteraceae from the Paleocene–Eocene of South Africa. Grana. 2000;39:39–45. [Google Scholar]
- Zavada MS, Lowrey TK. Comparative pollen morphology of Brachylena, Tarchananthus and two species of Tubulifloridites (Asteraceae) from the Eocene, Knysna Lignite of South Africa. Review of Palaeobotany and Palynology. 2010;162:183–192. [Google Scholar]