Abstract
Background and Aims
Jatropha curcas is a drought-resistant tree whose seeds are a good source of oil that can be used for producing biodiesel. A successful crop establishment depends on a rapid and uniform germination of the seed. In this work we aimed to characterize the responses of J. curcas seeds to temperature and water availability, using thermal time and hydrotime analysis,
Methods
Thermal and hydrotime analysis was performed on germination data obtained from the incubation of seeds at different temperatures and at different water potentials.
Key Results
Base and optimum temperatures were 14·4 and 30 °C, respectively. Approximately 20 % of the seed population displayed absolute dormancy and part of it displayed relative dormancy which was progressively expressed in further fractions when incubation temperatures departed from 25 °C. The thermal time model, but not the hydrotime model, failed to describe adequately final germination percentages at temperatures other than 25 °C. The hydrotime constant, θH, was reduced when the incubation temperature was increased up to 30 °C, the base water potential for 50 % germination,Ψb(50), was less negative at 20 and 30 °C than at 25 °C, indicating either expression or induction of dormancy. At 20 °C this less negative Ψb(50) explained satisfactorily the germination curves obtained at all water potentials, while at 30 °C it had to be corrected towards even less negative values to match observed curves at water potentials below 0. Hence, Ψb(50) appeared to have been further displaced to less negative values as exposure to 30 °C was prolonged by osmoticum. These results suggest expression of dormancy at 20 °C and induction of secondary dormancy above 25 °C. This was confirmed by an experiment showing that inhibition of germination imposed by temperatures higher than 30 °C, but not that imposed at 20 °C, is a permanent effect.
Conclusions
This study revealed (a) the extremely narrow thermal range within which dormancy problems (either through expression or induction of dormancy) may not be encountered; and (b) the high sensitivity displayed by these seeds to water shortage. In addition, this work is the first one in which temperature effects on dormancy expression could be discriminated from those on dormancy induction using a hydrotime analysis.
Keywords: Jatropha curcas, Euphorbiaceae, seed germination, dormancy, thermal time, hydrotime, water potential
INTRODUCTION
Jatropha curcas (Euphorbiaceae) is a multipurpose drought-resistant shrub or tree, which is widely distributed in the wild or semi-cultivated areas in Central and South America, Africa, India and South East Asia (Cano-Asseleih et al., 1989). Jatropha curcas seeds are a good source of oil. The decorticated seeds contain 40–60 % oil (Sharma et al., 1997; Wink et al., 1997; Openshaw, 2000) which can be used to manufacture candles and soap, as a lubricant, in the cosmetics industry (Kumar and Sharma, 2008) and, most importantly, as a diesel substitute or biodiesel (Gubitz et al., 1999).
Jatropha curcas is well adapted to arid and semi-arid conditions and is often used for erosion control (Heller, 1996). Research conducted on jatropha seeds has been devoted to developing techniques for detoxifying and converting its oil into biodiesel, and to examining seed oil and some physical properties of jatropha fruit at various moisture levels (Haas and Mittelbach, 2000; Shah et al., 2004; Berchmans and Hirata, 2008; Garnayak et al., 2008; Pradhan et al., 2008). However, studies on germination and seedling establishment (which are regarded as critical stages in the plant life cycle) of this important species have not been conducted so far. Knowledge of the capacity of the species to complete this stage successfully is fundamental for crop production. Jatropha grows readily from seeds or cuttings; however, trees propagated by cuttings show a lower longevity and possess a lower drought and disease resistance than those propagated from seeds (Heller, 1996).
Under crop production, stand establishment determines plant density, uniformity and management options (Cheng and Bradford, 1999). In arid environments, the water needed for germination is available for only short periods and, consequently, successful crop establishment depends not only on rapid and uniform germination of the seed, but also on the ability of the seed to germinate under low water availability (Fischer and Turner, 1978; Windauer et al., 2007).
Temperature (T) and water potential (Ψ) are two primary environmental regulators of seed germination (Bewley and Black, 1994). All the attributes of the seed lot can be analysed through the thermal time model (θT; Garcia-Huidobro et.al., 1982) and the hydrotime model (θH; Gummerson et al., 1986).
Germination responses to temperature of a seed lot can be characterized through three cardinal temperatures (Bewley and Black, 1994): a base temperature (Tb) below which germination of the seedlot does not proceed; an optimal temperature (To) at which the process occurs with highest speed; and a maximum temperature (Tm) over which the germination process does not proceed. In the sub-optimal range (i.e. between Tb and To) germination can be characterized through a thermal time (θT, °C h) [eqn. (1)] [i.e. the T in excess of Tb multiplied by the time (h) until reaching a certain percentage of germination (tg)]. The model assumes that this thermal time is different for each fraction g. On the other hand, Tb is assumed to be the same for the whole seed population. This model predicts that the germination rate for a given seed fraction or percentage g (GRg, or 1/tg) is a linear function of T above Tb, with a slope of 1/θT(g) and an intercept on the T axis of Tb.
| (1) |
This model allows the germination time course curve for a seed population to be characterized by the following probit equation:
| (2) |
Similarly, the hydrotime model describes seed germination responses to water potential (Ψ) [eqn. (2)]
| (3) |
where θH is the hydrotime (MPa h) the seeds require for germination, Ψ is the actual water potential of the germination medium (MPa), Ψb(g) is the theoretical threshold or base water potential that will just prevent germination of fraction g, and tg is the germination time (h) of the corresponding fraction g. The model assumes that Ψb varies among fractions of a seed population following a normal distribution with its mean, Ψb(50), and standard deviation, σΨb and θH is considered constant for a seed population (Bradford, 1990). This model predicts that the germination rate for a given seed fraction or percentage g (GRg, or 1/tg) is a linear function of Ψ above Ψb(g), with a slope of 1/θH(g) and an intercept on the Ψ axis of Ψb(g). Therefore, as Ψ is reduced towards Ψb the time to germination will be increased geometrically.
| (4) |
This model allows the germination time course curve for a seed population to be characterized by the following probit equation:
| (5) |
Nevertheless, the results coming from the thermal and hydrotime analysis should be carefully interpreted, placing them within a dormancy context when it applies. Indeed, while the thermal time model is not able to detect dormancy, the hydrotime model has this capability. For example, a displacement of Ψb(50) towards less negative values with changes in the incubation temperature could be regarded as expression of dormancy, or, alternatively, as dormancy reinforcement at this new incubation temperature (Batlla et al., 2009). Conversely, a displacement of Ψb(50) towards more negative values could be indicating a weaker dormancy expression or dormancy alleviation (Bradford, 1995). Dormancy reinforcement or induction into secondary dormancy implies a change in the physiology of the seed lot that narrows the width of the range of environmental conditions that permit germination. Dormancy expression, in contrast, does not imply any modification in the physiological state of the seed lot, but merely the absence of germination due to incubation in an environmental situation that is out of the range permissive for germination given by the dormancy status of the population (Benech-Arnold et al., 2000). In the last few decades, considerable effort has been directed towards modelling seed dormancy changes in seeds (Batlla and Benech-Arnold, 2007).
The objective of this work was to characterize the germination responses of J. curcas seeds to temperature and water availability, using thermal time and hydrotime analysis.
MATERIALS AND METHODS
Plant material
Seeds of Jatropha curcas L. were collected from a native stand at Mision Laishi, Formosa, Argentina (26°13′S, 37°60′W) and stored in bags at room temperature for 1 year until used in the experiment. Two different batches displaying a similar degree of dormancy (as evaluated through seed responses to temperature) were used for the experiments. One was used for the thermal time analysis and for the experiment to discriminate the effect of temperature on dormancy expression from that on dormancy induction. The other batch was used for hydrotime analysis.
Laboratory experiments
Thermal time analysis was performed on germination data resulting from the incubation of jatropha seeds in plastic boxes (12 × 15 × 5 cm ) with one layer of cotton wool and one layer of filter paper imbibed with 37 mL of distilled water (Ψa = 0 MPa) in four replications of 50 seeds at 15, 20, 25, 30 and 35 °C. Germination was recorded as protrusion of the radicle (2 mm) and was monitored daily over 20 d. The germinated seeds were removed.
Hydrotime analysis was performed on germination data resulting from the incubation of J. curcas seeds in Petri dishes in water (Ψa = 0 MPa) and PEG (polyethylene glycol 6000) solutions calibrated to obtain a similar range of Ψa [–0·3, –0·6 and –1·0 MPa according to Michel (1983)] at 20, 25 and 30 °C. The actual Ψa at all temperatures was measured using a vapour pressure osmometer (Wescor, Inc., Logan Model 5100C). Four replicates of 18 seeds were imbibed on blotters saturated with water and with each solution, and incubated in covered Petri dishes in the dark at each temperature. Seeds incubated on solutions containing PEG were transferred to fresh solutions after the first 24 h and every 2 d thereafter (Ni and Bradford, 1992) to maintain a constant water potential in the germination medium. Germination was recorded as protrusion of the radicle (2 mm) and was monitored daily over 20 d. The germinated seeds were removed.
To discriminate the effect of incubation temperature on dormancy expression from that on dormancy induction, 50 seeds were incubated in 12 × 15 × 5 cm plastic boxes with one layer of cotton wool and one layer of filter paper imbibed with 37 mL of distilled water in four replications, for 48 h at 20, 25, 30 and 35 °C. After this 48 h exposure period to each temperature, all the boxes were transferred to 25 °C (those seeds initially incubated at 25 °C were kept in the same incubator) and incubated for 18 d more. Another set (50 seeds in four replications incubated under the same conditions as described above) were incubated at 20, 25, 30 and 35 °C for 20 d. Germination was recorded as protrusion of the radicle (2 mm) and was monitored daily throughout the incubation period.
Data analysis
The values of the thermal time model parameters Tb and θT were determined in two ways: (1) as described previously by García-Huidobro et al. (1982) and (2) through repeated probit regression analysis as described by Ellis et al. (1986). To determine the optimum temperature, germination rates at 30 and 35 °C were compared: if the former was significantly faster than the latter, the optimum temperature was assumed to be 30 °C.
The values of the parameters Ψb(30), θH, and σΨb were determined using repeated probit regression analysis as described previously by Bradford (1990, 1995).
Viability test
The tetrazolium test was carried out to determine the viability of individual and non-germinated seeds at the end of each germination test. The whole seed coats were removed and the embryos were placed in tetrazolium solution. After a 24 h staining period, seeds were rated as viable or non-viable.
RESULTS
Thermal time analysis
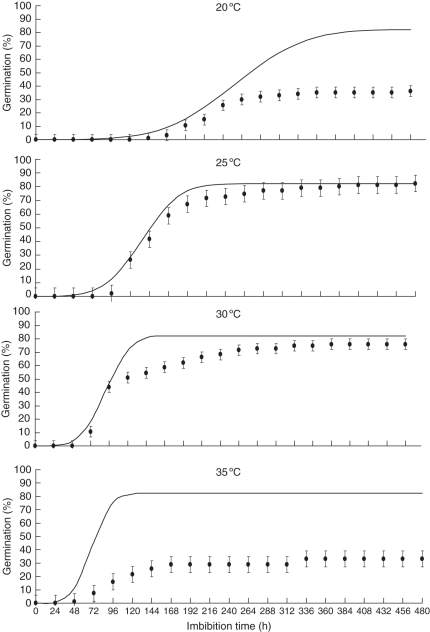
Increasing the temperature progressively shortened the time to germination for seeds incubated in water (Ψa = 0 MPa, Table 1) up to 30 °C. However, the maximum final germination percentage was only 82 % and was attained with seeds incubated at 25 °C. A tetrazolium test was performed on non-germinated seeds, and revealed that these seeds were viable, thus denoting absolute dormancy in a fraction of the population. Consequently, for thermal (and also hydric) analysis this 82 % was considered as the 100 % value. Below and above 25 °C, germination decreased progressively (Fig. 1). In spite of the reduction in final germination percentage, seeds incubated at 30 °C germinated faster than at any other temperature, including 25 °C (Fig. 1).
Table 1.
Time to germination of 30 % of the seed population, final percentage of germination (G) and germination rate of 30 % of the seed population at different incubation temperatures
| Temperature (°C) | Time(30) (h) | Final G (%) | GR(30) (d−1) |
|---|---|---|---|
| 15 | 0 | 0 | 0 |
| 20 | 264 | 36 | 0·0037 |
| 25 | 125 | 82 | 0·0080 |
| 30 | 104 | 75 | 0·0096 |
| 35 | 318 | 33 | 0·0031 |
Fig. 1.
Germination time courses of J. curcas seeds incubated at different constant temperatures (20, 25, 30 and 35 °C). The symbols are the actual data, and the lines are the time courses predicted by the thermal time model using the values shown in the text. Vertical bars indicate the s.e. (n = 50).
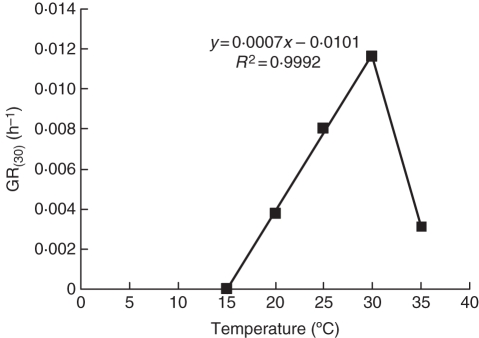
Base temperature (Tb) was determined by plotting the linear relationship between germination rate of the fraction g 30 % (GR30) (which was attained under all tested temperatures) and temperature, and extrapolating it until it intercepted the x-axis (Fig. 2). This Tb was calculated to be 14·4 °C. Optimum temperature (To) was determined by plotting the linear relationship between germination rate (GR30) and temperature and was found to be 30 °C, since GR30 was significantly lower at 35 °C than at 30 °C (Fig. 2). Thermal time (θT, degree-days or degree-hours in excess of the base temperature) required for completing germination of percentile 30 was calculated as the inverse of the slope of the relationship between GR30, and temperature and was determined to be 1428 °C h (Fig. 2). Repeated probit regression analysis yielded parameter values that were not very different from those obtained with the methodology of García-Huidobro et al. (1982).
Fig. 2.
Germination rate for 30 % [GR(30)] of the population in response to temperature. The line fitting the points between 15 and 30 °C was fitted using linear regression models and that joining the points at 30 and 35 °C was hand drawn.
The germination curves obtained experimentally under each temperature were compared against simulated curves constructed with the derived parameters and the thermal time model as presented in eqn (2) (see Introduction).
Seeds incubated at 15 °C reached a very low germination percentage after 20 d incubation (approx. 3 %), possibly because this temperature is too close to Tb and, consequently, thermal time accumulated by the end of the incubation period was not enough to permit the germination of most of the population (data not shown). The thermal time model fitted the germination data well when the seeds were incubated at 25 °C, but less satisfactorily for seeds incubated at 20, 30 and 35 °C, as can be seen from comparison between predicted and observed germination curves (Fig. 1) The main reason for this disagreement was the failure of the model to consider changes in the final germination percentage when seeds were incubated at 20, 30 and 35 °C. When the seeds were incubated at 20 °C, the model predicted that sufficient thermal time accumulated during the experimental period to permit the germination of all the percentiles, but the final germination percentage was only 36 %. The only explanation for this discrepancy is the expression of dormancy at temperatures lower than 25 °C for certain fractions of the population (in addition to those displaying absolute dormancy). Seeds incubated at 30 °C germinated very rapidly, and GR30 at this incubation temperature was the highest, thus determining the existence of an optimum temperature for germination at 30 °C. However, final germination was progressively lower at these incubation temperatures and, as in the case of 20 °C, the thermal time model did not describe this reduction. This fact denotes either expression of dormancy for certain fractions of the population, or induction of secondary dormancy during the incubation period. This should be possible to elucidate with a hydrotime analysis.
Hydrotime analysis
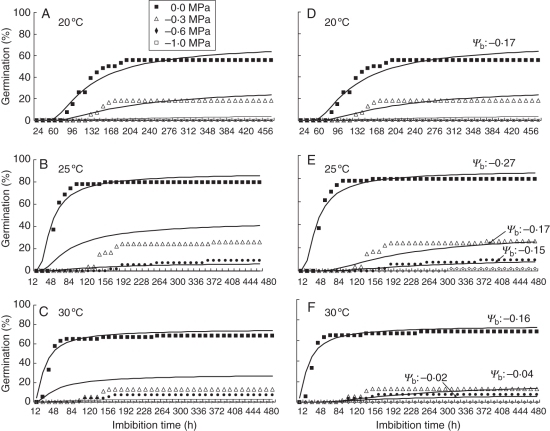
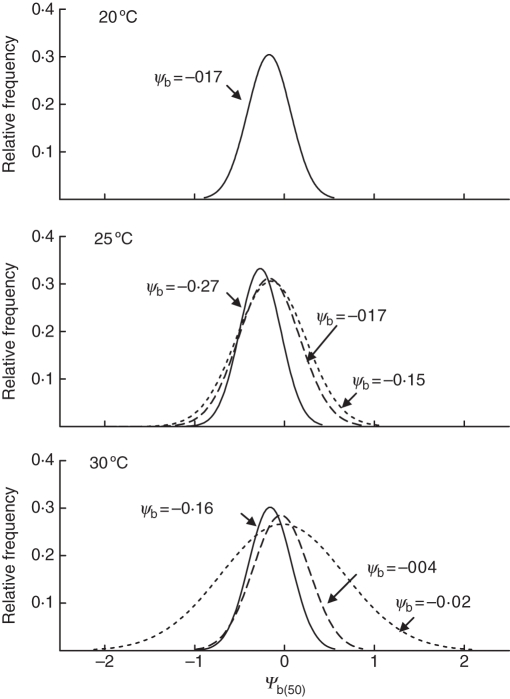
When seeds were placed to germinate at reduced Ψa in any constant T, a progressive delay in the germination dynamics took place relative to the time course in water, together with a lower final percentage of germination. When seeds were incubated at a water potential of –1·0 MPa, final germination was almost zero (Fig. 3). Using repeated probit regression analysis, the values of the hydrotime model parameters were determined for the seed lot. The θH constant was reduced when the incubation temperature was increased, whereas Ψb(50) was less negative at 20 and 30 °C than at 25 °C (Table 2; Fig. 4 solid line), again denoting either expression of dormancy for certain fractions of the population, or induction of secondary dormancy. The uniformity of germination, described by σΨb, did not change with incubation temperature (Table 2).
Fig. 3.
Germination time courses of J. curcas seeds at a range of water potentials,Ψ, as indicated, and incubated under different constant temperatures (20, 25 and 30 °C). The symbols are the actual data, and the lines are the time courses predicted by the hydrotime model using the values shown in Table 2 (A–C) or after correction of Ψb(50) (arrows showing the value after correction) and other hydrotime parameters (see Table 3) at 25 and 30 °C and at water potentials lower than 0 (E–F).
Table 2.
Estimated population hydrotime parameters for Jatropha curcas seeds under various incubation temperatures
| Temperature (°C) | Ψb(50) (MPa) | σΨb (MPa) | θH (MPa h) | R2 |
|---|---|---|---|---|
| 20 | –0·17 | 0·24 | 35 | 0·94 |
| 25 | –0·27 | 0·23 | 14 | 0·95 |
| 30 | –0·16 | 0·24 | 7 | 0·91 |
Fig. 4.
Normal distribution showing the relative frequencies of Ψb(50) values within the population at each incubation temperature. The solid line shows the Ψb(50) after incubation at each temperature as estimated by the hydrotime model to maximize fitness between observed and simulated curves for the germination course in distilled water and at all water potentials for the case of 20 °C. Dashed and dotted lines show the normal distribution of Ψb(50) after manual correction to maximize fitness between observed and simulated curves for germination courses of seeds incubated at –0·3 and – 0·6 Mpa, respectively, at 25 and 30 °C.
The model gave a good description of germination time courses at all water potentials when seeds were incubated at 20 °C. In contrast, at 25 and 30 °C, the hydrotime model closely matched actual jatropha seed germination time courses in water, but progressively departed from observed values at lower (more negative) water potentials of the incubation medium. Not only did the model predict a faster germination than was observed, but it also overestimated final germination percentages, as can be seen from comparison between predicted and observed germination curves (Fig. 3B, C). Only when Ψb(50) values were manually corrected (i.e. they were estimated to be less negative than those used to describe germination time courses in pure water at both 25 and 30 °C – Fig. 4, dotted line) did the model make a good description of germination time courses and final germination percentages of seeds incubated at water potentials other than zero, at 25 and 30 °C (Fig. 3E, F). This might suggest that delayed germination due to incubation at negative water potentials and, consequently, longer exposure as ungerminated seeds to 25 and 30 °C, could have displaced, somehow, Ψb(50) to less negative values. Indeed, exposure as ungerminated seeds to 25 and 30 °C must have been longer during incubation at –0·6 MPa than at –0·3 MPa. In consequence, a further displacement of Ψb(50) to less negative values was required to improve the agreement between the simulated and observed germination time courses of seeds incubated under –0·6 MPa (Fig. 3, Table 3). In addition to corrections to Ψb(50), the values of the θH constant and of σΨb needed to be adjusted (i.e. increased) to obtain a better match between simulated and observed germination time courses in seeds incubated at water potentials lower than zero and at 25 and 30 °C (Table 3).
Table 3.
Population hydrotime parameters manually modified to maximize the agreement between predicted and observed germination time courses as shown in Fig. 3D–F for Jatropha curcas seeds incubated under various temperatures and water potentials
|
Ψb(50) (MPa) |
σΨb (MPa) |
θH (MPa h) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 0 MPa | –0·3 MPa | –0·6 MPa | 0 MPa | –0·3 MPa | –0·6 MPa | 0 MPa | –0·3 MPa | –0·6 MPa |
| 20 | –0·17 | –0·17 | –0·17 | 0·24 | 0·24 | 0·24 | 35 | 35 | 35 |
| 25 | –0·27 | –0·17 | –0·15 | 0·23 | 0·35 | 0·40 | 14 | 50 | 55 |
| 30 | –0·16 | –0·04 | –0·02 | 0·24 | 0·31 | 0·70 | 7 | 40 | 60 |
Note that for Ψ = 0 MPa at all temperatures, and for Ψ = –0·3 and –0·6 MPa at 20 °C, the values of the parameters remained unmodified with respect to those displayed in Table 2.
Expression or induction of dormancy in relation to the incubation temperature
To discriminate the effect of incubation temperature on dormancy expression from that on dormancy induction, we incubated seeds at different temperatures (20, 25, 30 and 35 °C) either for for 20 d, or for 48 h followed by transfer to 25 °C for the remainder of the 20 d.
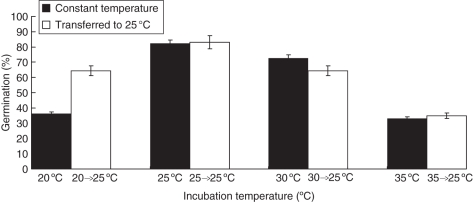
Incubation at 25 °C resulted in the highest germination percentage but, again, a fraction (approx. 20 %) displayed absolute dormancy (Fig. 5). Below and above 25 °C, germination decreased to a different extent depending on the incubation temperature (Fig. 5), with the lowest germinations at 20 and 35 °C. Inhibition of germination imposed by incubation at 20 °C for 48 h could be almost totally overcome by transferring the seeds to 25 °C, while seeds transferred to 25 °C after 48 h incubation at 35 °C did not germinate further when transferred to 25 °C (Fig. 5). Something similar occurred for seeds transferred from 30 °C, though this was less evident due to the lower inhibition of germination at this temperature; incubation at 25 °C did not increase germination further (Fig. 4).
Fig. 5.
Final germination percentages (%) of seeds incubated at different constant temperatures (20, 25, 30 and 35 °C) for 20 d, and seeds incubated at 20, 25, 30 and 35 °C for 48 h and then transferred to 25 °C for 18 d. Vertical bars indicate s.e. (n = 50).
DISCUSSION
The results obtained with this work revealed that (1) approx. 20 % of the population displayed absolute dormancy since these seeds did not germinate at any of the tested temperatures; this dormancy was confirmed through the tetrazolium viability test; (2) part of the population displayed relative dormancy which was progressively expressed in further fractions when incubation temperatures departed from 25 °C towards lower values; and (3) temperatures above 25 °C (i.e. 30 and 35 °C) again allowed the expression of dormancy or, alternatively, induced secondary dormancy.
It is well known that the thermal time model is not able to consider dormancy expression as an effect of incubation temperature (Batlla and Benech-Arnold, 2003). Indeed, the thermal time model is based on the assumption that the only effect of temperature is on the germination rate. Consequently, the thermal time analysis performed in this work failed to describe adequately final germination percentages at temperatures other than 25 °C. Nevertheless, the thermal time analysis allowed parameters to be obtained whose values should be taken into account if the aim is to put this species under production conditions. First, Tb was estimated to be 14·4 °C; this Tb can be regarded as quite high and precludes the sowing of these seeds in soils where the temperature does not considerably exceed this value. Secondly, GR30 was found to be the highest at 30 °C, demonstrating that maximum germination velocity would be attained at soil temperatures approaching this value. However, our analysis revealed that the final germination percentage decreases sharply at temperatures higher than 30 °C. Although, as in the case of temperatures below 25 °C, dormancy expression at 30–35 °C cannot be ruled out, it is more likely that these temperatures increase the rate of induction of secondary dormancy, in spite of the fact that germination is most rapid at 30 °C. Indeed, in a recent paper, Batlla et al. (2009) observed that seed germination at a given temperature could be the outcome of two different forces: the effect of temperature on germination per se, and the effect of temperature on seed dormancy status. In the case of the present work, this was suggested by the hydrotime analysis performed.
The hydrotime analysis yielded results indicating that (1) the θH constant was reduced when incubation temperature was increased up to 30 °C; this is consistent with the notion that maximum germination rates are displayed with an incubation temperature of 30 °C (see thermal time analysis); and (2) Ψb(50) were less negative at 20 and 30 °C than at 25 °C; this is consistent with the lower germination percentages observed at 20 and 30 °C compared with 25 °C. According to the hydrotime theory, a displacement of ψb(50) towards less negative values might result in some fractions of the population (i.e. Ψb is normally distributed within the population) having a Ψb larger than zero, which is a functional definition of dormancy (Bradford, 1995). In other words, our results show that at 20 °C, and possibly also at 30 °C, dormancy is expressed in certain fractions of the population. At 20 °C a single and less negative Ψb(50) than at 25 °C allowed the model to describe with reasonable accuracy the germination time courses at all water potentials. However, at 30 °C it was necessary to correct the Ψb (towards even less negative values) value with respect to that used to describe adequately the germination time course in distilled water, to enable the model to give a good description of germination time courses at water potentials other than zero. This suggests that Ψb(50) was further displaced to less negative values as exposure to 30 °C was prolonged by the presence of osmoticum in the incubation medium. Moreover, when incubation was performed under –0·6 MPa, the Ψb(50) values needed to be corrected towards even less negative values to obtain a better match between simulated and observed time courses. Other studies have also reported variation in this parameter throughout seed incubation (Dahal and Bradford, 1990, 1994; Battaglia, 1993).
Prolonged exposure to low water potentials (i.e. incubation under –0·3 and –0·6 MPa at 25 and 30 °C) also increased the values of the θH constant and of σΨb. A similar effect was reported by Dahal and Bradford (1994) in tomato seeds, ascribing the effect to physiological changes produced by prolonged exposure to osmoticum. However, the displacement of Ψb(50) towards less negative values during incubation could be considered as induction of secondary dormancy by high incubation temperatures (i.e. 30–35 °C). In other words, the longer the exposure to 30–35 °C, the higher the number of fractions within the population that are induced into secondary dormancy. It should be noted that the induction of secondary dormancy (i.e. due to exposure at 30–35 °C) is substantially different, as a physiological process, from the expression of dormancy (i.e. as in the case of incubation at 20 °C or lower temperatures). The former modifies the dormancy status of the population while the latter does not (Benech-Arnold et al., 2000). The results obtained with an independent experiment confirmed that the inhibition of germination by high incubation temperatures (i.e. 30 and 35 °C) is a permanent effect, since it cannot be removed by incubation at temperatures that do not permit expression of dormancy (i.e. 25 °C). In contrast, inhibition of germination when incubation is performed at 20 °C is not permanent since it can be overcome by incubation at 25 °C.
Since 30 °C was found to be an optimum for seed germination (i.e. GR30 was maximum at this temperature), it can be assumed that some seeds within the population germinated so quickly that they escaped from the dormancy induction effect of this temperature. This example confirms the observation of Batlla et al. (2009) that seed germination at a given temperature is the outcome of two different forces. Indeed, incubation at 30 °C determined the fastest germination velocity but, on the other hand, these temperatures induced secondary dormancy very efficiently when germination was slowed down through osmoticum. As a result of this, the effect on dormancy induction prevailed over that on seed germination. We are not aware of any other work in which temperature effects on dormancy expression during incubation have been discriminated from those on induction of secondary dormancy using hydrotime analysis as we have done here. Even so, the hydrotime model has been effective for elucidating the nature of other seed responses to temperature; for example, Alvarado and Bradford (2002) used it to explain cardinal temperatures for seed germination in true potato seeds, and Huarte and Benech-Arnold (2005) used it for investigating the effect of fluctuating temperatures on termination of dormancy. Correction of Ψb(50) values was also necessary at 25 °C to allow the model a good description of germination time courses at water potentials other than zero. This suggests that part of the population was also induced into secondary dormancy at 25 °C when germination was delayed by osmoticum. However, since germination percentages at 25 °C in water were higher than at any other temperature, it might be concluded that 25 °C is less effective than 30 °C in inducing secondary dormancy, and 30 °C is less effective than 35 °C. Consequently, at these temperatures, seeds require a longer exposure time to be induced into secondary dormancy (e.g. such as that resulting from a low water potential in the incubation medium). From a crop production standpoint, the Ψb(50) values (–0·27 MPa at 25 °C) determined by the analysis, even at temperatures that do not allow dormancy expression or induce secondary dormancy very effectively (i.e. 25 or 30 °C), suggest that seeds from this species are sensitive to water restrictions. This characteristic requires improvement by breeders if this species is to be domesticated as a crop for semi-arid regions.
In summary, this work allowed a wide characterization of J. curcas seed responses to temperature and water availability. Our results revealed severe limitations for seed germination and stand establishment that need to be considered during domestication. The most important of these limitations is the extremely narrow thermal range within which dormancy problems (either through expression or induction of dormancy) may not be encountered. The other important limitation is related to the high sensitivity displayed by these seeds to water shortage: this factor could not only prevent germination by itself, but it could also delay germination sufficiently to permit induction of secondary dormancy by prolonged exposure to temperatures that elicit this process. On the other hand, our work is the first in which incubation temperature effects on dormancy expression could be discriminated from those on dormancy induction using a hydrotime analysis.
ACKNOWLEDGEMENTS
This work was supported by the Universidad de Buenos Aires (UBACyT G012). We would like to thank the anonymous reviewers for their constructive comments on the first version of this manuscript.
LITERATURE CITED
- Alvarado V, Bradford KJ. A hydrothermal time model explains the cardinal temperatures for seed germination. Plant, Cell and Environment. 2002;25:1061–1069. [Google Scholar]
- Battaglia M. Seed germination physiology of Eucalyptus delegatensis R.T. Baker in Tasmania. Australian Journal of Botany. 1993;41:119–136. [Google Scholar]
- Batlla D, Benech-Arnold RL. A quantitative analysis of dormancy loss dynamics in Polygonum aviculare L. seeds: development of a thermal time model based on changes in seed population thermal parameters. Seed Science Research. 2003;13:55–68. [Google Scholar]
- Batlla D, Benech-Arnold RL. Predicting changes in dormancy level in weed seed soil banks: implications for weed management. Crop Protecction. 2007;26:189–197. [Google Scholar]
- Batlla D, Grundy A, Dent KC, Clay HA, Finch-Savage WE. A quantitative analysis of temperature-dependent dormancy changes in Polygonum aviculare seeds. Seed Science Research. 2009;49:428–438. [Google Scholar]
- Benech-Arnold RL, Sánchez RA, Forcella F, Kruk BC, Ghersa CM. Environmental control of dormancy in weed seed soil banks. Field Crops Research. 2000;67:105–122. [Google Scholar]
- Berchmans HJ, Hirata S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresource Technology. 2008;99:1716–1721. doi: 10.1016/j.biortech.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. Plenum Press: New York; 1994. [Google Scholar]
- Bradford KJ. A water relations analysis of the seed germination rates. Plant Physiology. 1990;94:840–849. doi: 10.1104/pp.94.2.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ. Water relations in seed germination. In: Kigel J, Galili G, editors. Seed development and germination. Marcel Dekker: New York; 1995. pp. 351–395. [Google Scholar]
- Cano-Asseleih LM, Plumbly RA, Hylands PJ. Purification and partial characterization of the hemagglutinin from seeds of Jatropha curcas. Journal of Food Biochemistry. 1989;13:1–20. [Google Scholar]
- Cheng Z, Bradford KJ. Hydrothermal time analysis of tomato seed germination responses to priming treatments. Journal of Experimental Botany. 1999;50:89–99. [Google Scholar]
- Dahal P, Bradford KJ. Effects of priming and endosperm integrity on germination rates of tomato genotypes. II. Germination at reduced water potential. Journal of Experimental Botany. 1990;41:1441–1453. [Google Scholar]
- Dahal P, Bradford KJ. Hydrothermal time analysis of tomato seed germination at suboptimal temperature and reduced water potential. Seed Science Research. 1994;4:71–80. [Google Scholar]
- Ellis RH, Covell S, Roberts E, Summerfield R. The influence of temperature on seed and germination rate in grain of legumes. II. Intraspecific variation in chickpea at constant temperatures. Journal of Experimental Botany. 1986;37:119–126. [Google Scholar]
- Fischer RA, Turner NC. Plant productivity in the arid and semiarid zones. Annual Review of Plant Physiology. 1978;29:277–317. [Google Scholar]
- Garcia-Huidobro J, Montheith JL, Squire GR. Time, temperature and germination of pearl millet (Pennisetum typhoides S. H.). I. Constant temperature. Journal of Experimental Botany. 1982;33:288–296. [Google Scholar]
- Garnayak DK, Pradhan RC, Naik SN, Bhatnagar N. Moisture-dependent physical properties of Jatropha seed (Jatropha curcas L.) Industrial Crops and Products. 2008;27:123–129. [Google Scholar]
- Gummerson RJ. The effect of constant temperature and osmotic potentials on the germination of sugar beet. Journal of Experimental Botany. 1986;37:729–741. [Google Scholar]
- Gubitz GM, Mittelbach M, Trabi M. Exploitation of the tropical oil seed plant Jatropha curcas L. Bioresource Technology. 1999;67:73–82. [Google Scholar]
- Haas W, Mittelbach M. Detoxification experiments with the seed oil from Jatropha curcas L. Industrial Crops and Products. 2000;12:111–118. [Google Scholar]
- Heller J. Physic nut. Jatropha curcas L. promoting the conservation and use of underutilized and neglected crops. Rome: International Plant Genetic Resources Institute; 1996. [Google Scholar]
- Huarte H, Benech-Arnold RL. Fluctuating temperatures reduces mean base water potential for seed germination in several non-cultivated species. Seed Science Research. 2005;15:89–97. [Google Scholar]
- Kumar A, Sharma S. An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Industrial Crops and Products. 2008;28:1–10. [Google Scholar]
- Michel BE. Evaluation of the water potential of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiology. 1983;72:66–70. doi: 10.1104/pp.72.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni BR, Bradford KJ. Quantitative models characterizing seed germination responses to abscisic acid and osmoticum. Plant Physiology. 1992;101:607–617. doi: 10.1104/pp.98.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw K. A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass and Bioenergy. 2000;19:1–15. [Google Scholar]
- Pradhan RC, Naik SN, Bhatnagar N, Vijay VK. Moisture-dependent physical properties of jatropha fruit. Industrial Crops and Products. 2008;27:123–129. [Google Scholar]
- Shah S, Sharma A, Gupta MN. Extraction of oil from Jatropha curcas L. seed kernels by enzyme assisted three phase partitioning. Industrial Crops and Products. 2004;20:275–279. [Google Scholar]
- Sharma GD, Gupta SN, Khabiruddin M. Cultivation of Jatropha curcas as a future source of hydrocarbon and other industrial products. In: Gubitz GM, Mittelbach M, Trabi M, editors. Biofuels and industrial products from Jatropha curcas. Graz: DBV; 1997. pp. 19–21. [Google Scholar]
- Wink M, Koschmieder C, Sauerwein M, Sporer F. Phorbol esters of J. curcas—biological activities and potential applications. In: Gubitz GM, Mittelbach M, Trabi M, editors. Biofuels and industrial products from Jatropha curcas. Graz: DBV; 1997. pp. 160–166. [Google Scholar]
- Windauer LB, Altuna A, Benech-Arnold RL. Hydrotime analysis of Lesquerella fendleri seed germination responses to priming treatments. Industrial Crops and Products. 2007;25:70–74. [Google Scholar]