Abstract
Background
A plant is considered carnivorous if it receives any noticeable benefit from catching small animals. The morphological and physiological adaptations to carnivorous existence is most complex in plants, thanks to which carnivorous plants have been cited by Darwin as ‘the most wonderful plants in the world’. When considering the range of these adaptations, one realizes that the carnivory is a result of a multitude of different features.
Scope
This review discusses a selection of relevant articles, culled from a wide array of research topics on plant carnivory, and focuses in particular on physiological processes associated with active trapping and digestion of prey. Carnivory offers the plants special advantages in habitats where nutrient supply is scarce. Counterbalancing costs are the investments in synthesis and the maintenance of trapping organs and hydrolysing enzymes. With the progress in genetic, molecular and microscopic techniques, we are well on the way to a full appreciation of various aspects of plant carnivory.
Conclusions
Sufficiently complex to be of scientific interest and finite enough to allow conclusive appraisal, carnivorous plants can be viewed as unique models for the examination of rapid organ movements, plant excitability, enzyme secretion, nutrient absorption, food-web relationships, phylogenetic and intergeneric relationships or structural and mineral investment in carnivory.
Keywords: Carnivorous plants, model plants, traps, rapid organ movements, gland functioning, nutrient absorption, action potentials, plant excitability, plant indicators
INTRODUCTION
We are accustomed to thinking of plants as being immobile and harmless, and this may be a reason for our fascination with carnivorous plants and especially about those that move while trapping. Such interest began in Victorian England and spread with the popularization of Insectivorous Plants by Darwin (1875) (Chase et al., 2009). Leading to the emergence of the so-called carnivorous syndrome, a carnivorous lifestyle has resulted in significant adaptive and functional implications (Laakkonen et al., 2006). The most obvious manifestation of the syndrome is the emergence of traps. They originate from leaves that have become specialized in trapping, prey digestion and nutrient absorption, thereby decreasing their photosynthetic rates (to zero in case of the colourless traps of Genlisea and terrestrial Utricularia, Adamec, 2006). The compact anatomy of traps (reminiscent of roots), which is to restrict apoplastic conductivity (Pavlovič et al., 2007), serves the selective symplastic transport of nutrients gained from carnivory. Investments in the following cause considerable maintenance costs: attractants such as nectars and odours (Juniper et al., 1989; Moran, 1996; Bohn and Federle, 2004; Bennett and Ellison, 2009; Bhattarai and Horner, 2009; Jürgens et al., 2009); edible trichomes (Merbach et al., 2002); colourful projections (Schaefer and Ruxton, 2008) and UV patterns (Moran et al., 1999); resinous droplets (Voigt and Gorb, 2010) or slime that in Drosophyllum has a scent of honey, which may mimic nectar (Jürgens et al., 2009); glands excreting mucilage (Drosera, Pinguicula, Byblis) or a hydrophobic resin (Roridula) to catch prey (Juniper et al., 1989); glands excreting digestive enzymes – these digestive glands, with their attendant mechanisms for simultaneous enzyme secretion and nutrient absorption are an anatomical birthmark of the carnivorous syndrome (Lüttge, 1971; Benzing et al., 1976); exudation of organic compounds to support the microbial community associated with the traps (Sirová et al., 2009, 2010); and nutrient uptake machinery (An et al., 2001) required for functioning of each single trap (Knight, 1992; Adamec, 2006, 2010a; Pavlovič et al., 2007; Hájek and Adamec, 2010). Therefore, it is not surprising that the dual use of leaves for photosynthesis and nutrient uptake has deeply reduced the net photosynthetic rate of terrestrial carnivorous plants, leading ultimately to reduction of the relative growth rate (Ellison, 2006; Farnsworth and Ellison, 2008); giant carnivorous species are the exception rather than the rule: Triphyophyllum peltatum, Nepenthes rajah, N. edwardsi ana, N. ampullaria, N. rafflesiana and N. rafflesiana var. gigantea, N. palawanensis, N. attenboroughii (newly discovered by Robinson et al., 2009), Sarracenia leucophylla, Drosera gigantea and D. regia. For the reasons given above (i.e. high maintenance costs), some carnivorous plants are only carnivorous when favoured by environmental conditions, e.g. Pinguicula and pygmy Drosera spp. (Rice, 2007), or during periods of extended growth, when extra nutrient supply is needed (Triphyophyllum peltatum) (Bringmann et al., 2002). To assess the expenditures and gains of carnivory, an ecological cost–benefit model was created (Givnish et al., 1984). The model assumes that the cost of capturing animals is offset by the nutrient uptake in nutrient-poor environments. This is the case when the most photosynthetically productive leaves are supplied with macroelements gained from carnivory, otherwise being unable to conduct photosynthesis efficiently (Ellison, 2006; Farnsworth and Ellison, 2008; Ellison and Gotelli, 2009). Accordingly, as evaluated for non-carnivorous plants, the positive correlation between CO2 fixation and N-availability (foliar tissue N content) has long been known (Field and Mooney, 1986). Recently, the mineral cost of carnivory (i.e. the proportion of minerals contained in traps) has been defined and quantified in aquatic carnivorous species (Adamec, 2010b).
Because carnivory is largely a substitute for environmentally limited macroelements, carnivorous plants are able to cope with extreme habitats such as oligotrophic waters, dystrophic pools, peat bogs, fens, swamps, marshes, heaths, mountain slopes, dripping rocks, clayish sands, fire-impoverished soils and heavily leached areas (Juniper et al., 1989). The distribution pattern points, however, to the predominant co-occurrence of carnivorous plants with both sunny and wet habitats (Table 1), where neither light nor water are limiting factors (Givnish et al., 1984; Brewer et al., 2011). Under such conditions, these plants use their prey mostly as an alternative source of nitrogen, phosphorus and sulphur (Juniper et al., 1989; Butler and Ellison, 2007), but also as a source of ions, which though not necessarily environment-limited are easily taken up from prey bodies, e.g. potassium (Green et al., 1979; Karlsson, 1988; Płachno et al., 2009; Adamec et al., 2010a), magnesium (Adamec, 2002, 2010a; Płachno et al., 2009), manganese (Steinhauser et al., 2007) and even carbon (Fabian-Galan and Salageanu, 1968; Lüttge, 1983; Adamec, 1997; Rischer et al., 2002). The direct uptake of prey-derived organic carbon was shown to be of crucial importance when CO2 and light were limited (Adamec, 1997). There are over 700 carnivorous plant species recognized today (Table 2) and these species are constantly growing in number (Fleischmann et al., 2007, 2008; Cheek and Jebb, 2009; Fleischmann and Rivadavia, 2009; Lee et al., 2009; Robinson et al., 2009; Zamudio and Olvera, 2009; Suksathan and Parnell, 2010; Souza and Bove, 2011). On the other hand, the conservation status of some endemic carnivores growing in areas with intense human activity (e.g. Drosophyllum lusitanicum) present an alarming picture of ongoing declines (Gonçalves and Romano, 2005). In vitro propagation, especially in combination with conventional practices (seed banks or habitat conservation) could be a remedy for endangered species, providing an alternative to replenish wild stocks (Jang et al., 2003; Gonçalves and Romano, 2007; Grevenstuk et al., 2010)
Table 1.
Division of traps and distribution of habitats of carnivorous plants
| Trap | Genus/species | Common name | Natural habitat | Region |
|---|---|---|---|---|
| Pitfall | Sarracenia | pitcher plants | fens, swamps, coastal plains, grassy plains | north, east and south of North America |
| Darlingtonia californica | cobra lily | boggy areas and near streams in mountain | Sierra Nevada mountains in south Oregon and north California (elevation up to 2500 m) | |
| Heliamphora | sun pitchers | highland meadows | endemic to Guiana highlands | |
| Nepenthes | tropical pitcher plants | ‘Lowlanders’ – humid lowland forests; ‘Highlanders’ – tropical montane forests | subtropical regions of Asia (China, Singapore, India, Thailand, Vietnam, Cambodia, Philippines, Malay Peninsula), Australia, Seychelles and Madagascar | |
| Cephalotus follicularis | Albany pitcher, fly-catcher, moccasin plant | coastal plains | endemic to south-west Australia | |
| Brocchinia | bromeliads | B. tatei and B. micrantha – sunny, open areas with sandstone; others – epiphyte of unshaded trees | endemic to Guyana, Venezuela and Columbia (elevation 500–2900 m) | |
| Catopsis berteroniana | epiphyte of unshaded trees | southern USA, Latin America, Brazil | ||
| Eel-trap | Genlisea | corkscrew plants | wetlands up to 2500 m | endemic to Guiana highlands, Angola, Zambia, Tanzania, Madagascar |
| Sarracenia psittacina | parrot pitcher plant | wetter parts of boggy areas | south-eastern USA (Georgia, Florida) | |
| Fly-paper | Drosera | sundews | marshes, fens, wet stands, boggy shorelines | all continents but Antarctica |
| Drosophyllum lusitanicum | Portuguese dewy pine | slightly basic soils of narrow coastal or maritime regions | endemic to Portugal, southern Spain and northern Morocco | |
| Triphyophyllum peltatum | West African liana | tropical rain forests | Sierra Leone, Liberia, Ivory Coast | |
| Pinguicula | butterworts | highly humid, wet areas and montane regions (elevation up to 1900 m) | Europe, North America, northern Asia, West Africa, west coast of South America | |
| Byblis | rainbow plants | acid sands and desert areas | native to Australia and New Guinea | |
| Roridula gorgonias, Roridula dentata | flycatcher bushes or bug plants | fynbos area, costal mountain and slopes at elevation 900–1200 m | endemic to south Africa | |
| Snap-trap | Dionaea muscipula | Venus flytrap | bogs, swamps, wet savannahs | North and South Carolina (USA) |
| Aldrovanda vesiculosa | waterwheel plant | shallow and warm standing waters | central Europe, east Asia, Africa, Australia | |
| Suction-trap | Utricularia | bladderworts | all kinds of still waters and water films, wet soils, sands, rocks | all continents but Antarctica |
Table 2.
Phylogenetic position of carnivorous plants
| Division | Class | Order | Family | Genus/species | No. of species |
|---|---|---|---|---|---|
| Anthophyta (angiosperms) | Monocotyledones | Poales | Bromeliaceae | Brocchinia | 2 |
| Catopsis berteroniana | 1 | ||||
| Dicotyledones | Caryophyllales | Dioncophyllaceae | Triphyophyllum peltatum | 1 | |
| Droseraceae | Aldrovanda vesiculosa | 1 | |||
| Dionaea muscipula | 1 | ||||
| Drosera | >184 | ||||
| Drosophyllaceae | Drosophyllum lusitanicum | 1 | |||
| Nepenthaceae | Nepenthes | >110 | |||
| Oxalidales | Cephalotaceae | Cephalotus follicularis | 1 | ||
| Ericales | Roridulaceae | Roridula | 2 | ||
| Sarraceniaceae | Heliamphora | 18 | |||
| Sarracenia | 9 | ||||
| Darlingtonia californica | 1 | ||||
| Lamiales | Lentibulariaceae | Pinguicula | >96 | ||
| Genlisea | >22 | ||||
| Utricularia | >250 | ||||
| Byblidaceae | Byblis | >7 |
PASSIVE TRAPS
In passive traps there is no motion while trapping and enzyme secretion is constitutive, i.e. independent of the presence of a prey (Heslop-Harrison, 1975; Płachno et al., 2005a, b, 2006). In the presence of prey, however, the basal level of secretion increases (Gallie and Chang, 1997; McNally et al., 1988; Eilenberg et al., 2006). Moreover, the amount of enzymes released seems be correlated to the size of the prey (Darwin, 1875; Heslop-Harrison, 1975; Owen et al., 1999; An et al., 2002). In other words, the expression/secretion of digestive enzymes is regulated by a signal transduction mechanism. This lets the plant respond to the availability of food resources and thus adjust the cost–benefit ratio efficiently (Gallie and Chang, 1997). Nevertheless, passive traps can be viewed as the containers of digestive fluid: pitfalls (Sarracenia, Darlingtonia, Heliamphora, Cephalotus, Nepenthes), tanks (Brocchinia, Catopsis), vesicles (eel-traps of Genlisea) and fly-papers (Drosophyllum, Triphyophyllum, Byblis, Roridula, majority of Pinguicula spp.).
The pitfalls of dicots have the shape of pitchers (Fig. 1A, C, D, E), in which at least three distinctive zones can be recognized (Juniper et al., 1989). A rim of slick surface covered with nectaries and trichomes both lures and deceives; when wet, the rim is especially slippery (Bohn and Federle, 2004; Gorb and Gorb, 2006; Bennett and Ellison, 2009); moreover Sarracenia flava nectar contains coniine (an alkaloid anaesthetic to insects) to increase prey-capture efficiency. The waxy zone directly beneath the rim prevents escape; for this, its walls may be covered with waxy scales (Nepenthes), protruding aldehyde crystals (Sarracenia, Darlingtonia), cuticular folds (Nepenthes, Cephalotus, Heliamphora), downward-pointing hairs (Heliamphora, Sarracenia, Darlingtonia) or guard-cell-originating lunate cells (Nepenthes) (Juniper et al., 1989; Jaffe et al., 1992; Owen and Lennon, 1999; McPherson, 2009; Poppinga et al., 2010; Moran and Clarke, 2010). In Nepenthes, alkaloid fumes promote successful capture (Ratsirarson and Silander, 1996), while fluid viscosity increases its retentive properties (Giusto et al., 2008; Moran and Clarke, 2010; Bonhomme et al., 2011b). The lowest part of the pitcher, the digestive zone, harbours numerous digestive glands (Fig. 2A–D) or a glandular epithelium (Sarracenia). Nepenthes, Sarracenia and Cephalotus follicularis protect their enzymes (proteases, peptidases, phosphatases, esterases, chitinases, nucleases) from rainfall dilution by covering the pitchers with lids. As most Heliamphora species do not produce enzymes (Jaffe et al., 1992), its lid has reduced in size to become a small ‘nectar spoon’ while excess rainwater is drained off through a slit. Deprived of its own enzymes, too, Darlingtonia californica is unique in that it regulates the pitcher water level by pumping it up through its roots. As low pH promotes the action of proteolytic enzymes (Amagase, 1972; Heslop-Harrison, 1976) and the uptake of organic substances (Heslop-Harrison, 1976; Schulze et al., 1999), the pitcher fluid is highly acidic (An et al., 2001). Additionally, oxygen free radicals produced by the pitcher plants aid in the digestion of prey bodies (Chia et al., 2004).
Fig. 1.
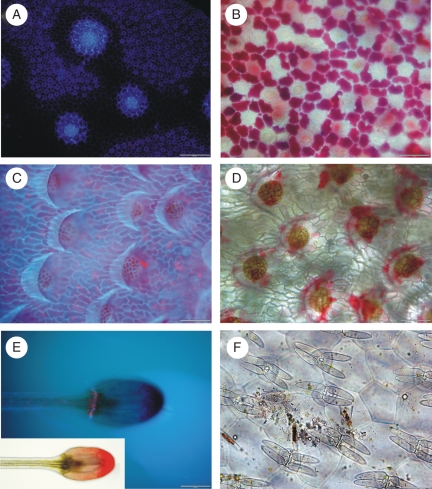
Diversity of pitfalls: (A) pitcher of an Albany pitcher plant Cephalotus follicularis; (B) Brocchinia reducta as an example of carnivorous bromeliads; (C) Nepenthes merrilliana; (D) Nepenthes hybrid ‘Miranda’; (E) North American pitcher plant Sarracenia purpurea.
Fig. 2.
Digestive glands of carnivorous plants. (A) Three large and numerous small digestive glands of the pitfall type of the Cephalotus follicularis trap – note strong auto-fluorescence of the apical part of the large glands under UV light; scale bar = 200 µm. (B) Numerous small glands from the pitcher of Cephalotus follicularis – note red anthocyanine in the epidermal cells, which surround glands; scale bar = 50 µm. (C,D) Digestive glands from the pitcher of Nepenthes ampullaria; scale bar = 100 µm. (E) Emergence of Drosera filiformis – note auto-fluorescence under UV light: blue – cutinized walls of barrier cells and red – chlorophyll; scale bar = 100 µm. Inset: the same emergence under light microscopy. (F) Quadrifid hairs from the trap of Utricularia volubilis; scale bar = 50 µm.
Although among Bromeliaceae (monocots), only Brocchinia reducta (Fig. 1B) has been shown to secrete phosphatase (Płachno and Jankun, 2005; this needs be confirmed with other methods) and neither B. hechtioides nor Catopsis berteroniana produces proteases, these three species are established plant carnivores. Depending on a food-web to acquire nutrients, these plants provide habitats for frogs, insects (e.g. ants), other carnivorous plants (e.g. Utricularia humboldtii) and bacteria (including nitrogen-fixing bacteria), themselves exploiting whatever is left over: faeces, animal or vegetable debris. Absorption of N-compounds is carried out by specialized trichomes (Benzing et al., 1976).
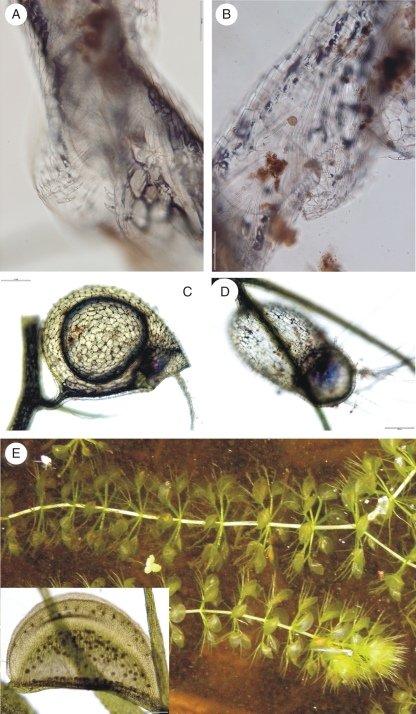
Small soil-borne organisms (bacteria, algae, nematodes, rotifers, annelids, crustaceans and mites) are found in the subterranean eel-traps of rootless Genlisea (Płachno et al., 2005a) (Fig. 3A, B). The Y-shaped eel-traps consist of two arms joined to a long (3–15 cm) thin (0·5–0·8 mm) neck, whose other end forms a digestive chamber known as a vesicle. Arranged along the arms and the long neck, the vesicle-pointing hairs prevent escape of prey. Digestion takes place in the vesicle, where there are numerous digestive glands, which in some species (Genlisea margaretae) are concentrated along vascular bundles (Płachno et al., 2007). Juniper et al. (1989) found that Genlisea's digestive glands morphologically resemble the sessile digestive glands of Pinguicula, which seems to be well corroborated by the common origin of both genera (Ellison and Gotelli, 2009). Although some organisms are chemotactically attracted in order to be digested by Genlisea (Barthlott et al., 1998), others grow inside the traps as symbionts or commensals, arguing for the prevalence of trophic microsystems in all genera equipped with passive traps (Studnička, 1996).
Fig. 3.
Subterranean and aquatic traps. (A,B) Part of the eel trap of Genlisea margaretae: (A) external view opening in the arm of the trap; (B) the same but from internal view; scale bars = 100 µm. (C,D) Bladders of Utricularia gibba; scale bars = 200 µm. (E) The waterwheel plant Aldrovanda vesiculosa in the Velký Londýn sand-pit near Třeboň (South Bohemia, Czech Republic); inset: magnification of the tropical clone Aldrovanda trap – dark points depict digestive glands; scale bar = 200 µm.
In general, trophic plant–commensal relationships are multi-level and complex, with the microbial component being of crucial importance (Barrera et al., 1989; Steinhauser et al., 2007; Butler et al., 2008; Peterson et al., 2008; Koopman et al., 2010; Adlassnig et al., 2011). The strategy of taking advantage of commensal organisms is especially critical for carnivores deprived of digestive enzymes such as bromeliads (Frank and O'Meara, 1984), Darlingtonia californica (Ellison and Farnsworth, 2005), the majority of Heliamphora spp. except H. tatei (Barrera et al., 1989; Jaffe et al., 1992; Adlassnig et al., 2011), Nepenthes ampullaria (Moran et al., 2003) and N. lowii (Clarke et al., 2009). Nepenthes lowii has a mutualistic relationship with treeshrews Tupaia montana and its pitchers act as faeces repositories for these small mammals native to tropical forests (Clarke et al., 2009, 2010). The small woolly bat Kerivoula hardwickii uses a different type of pitcher in Borneo, Nepenthes rafflesiana elongata, as a lavatory and home as well (Grafe et al., 2011). Nepenthes rajah utilizes faeces of both the diurnal Tupaia montana and the nocturnal rat Rattus baluensis (Greenwood et al., 2011; Wells et al., 2011). Additionally, Nepenthes rajah hosts mosquito larvae (Culex rajah, Toxorhynchites rajah), while N. bicalcarata shelters ants (Camponotus schmitzi). Although both latter named species secrete digestive enzymes, they benefit from their commensals in such a way that the crushing of ‘prey’ by larvae or ants speeds up the digestive breakdown of prey, which more than compensates for the partial loss of prey supply (Bonhomme et al., 2011a). Sarracenia purpurea, which is capable of producing some hydrolytic enzymes, also prefers mutual commensalism with bacteria, protozoa, algae and dipteran larvae (Atwater et al., 2006; Gebühr et al., 2006; Gray et al., 2006; Płachno et al., 2006; Peterson et al., 2008; Płachno and Wołowski, 2008; Buckley et al., 2010). Among ‘fly-papers’, Pinguicula vulgaris and P. lusitanica, which utilize nutrients from pollen-, seed- or canopy-leaching, need assistant microorganisms which support them by disposing of detritus (Darwin, 1875; Lavarack, 1979; Juniper et al., 1989; Moran et al., 2003). Byblis and Roridula both benefit from commensalism with Capsid bugs: Setocornis, Cyrtopeltis, Pameridea roridulae and Pameridea marlothii (Ellis and Midgley, 1996; Hartmeyer, 1998; Midgley and Stock, 1998; Anderson and Midgley, 2002, 2003, 2007). Thus, our understanding of carnivory may be broadened today by recognition of the fact that carnivorous plants are able to lure and capture a prey and then utilize (absorb) prey-derived compounds, regardless of whether these compounds are provided directly or by commensal or symbiotic organisms. In other words, the ability to efficiently absorb nutrients has become the real clincher of carnivory (Jolivet, 1998; Bringmann et al., 2001; Darnowski et al., 2007; Płachno et al., 2009), while the presence of commensal or symbiotic associations may be viewed as additional evidence (Hess et al., 2005). It can be argued that the production of enzymes by the plant itself, when commensals are already doing it, would be an unnecessary cost for the plant that is still able to absorb nutrients (Hartmeyer, 1998).
ACTIVE TRAPS
Active traps move while trapping. To synchronize their movements action potentials (APs) are generated (see below). Adhesive fly-papers, and snap- and suction-traps may be counted as active traps. Adhesive traps (either active, Fig. 4A, C; or passive, Fig. 4B) are evenly covered with mucilage-producing glands which, when stalk-shaped, are designated tentacles (Fig. 2E). In active adhesive traps, the tentacles bend when stimulated. Drosera burmannii and D. glanduligera bend their tentacles quickly, i.e. in seconds (5–15 s) or even less (0·15 s), respectively (Gibson and Waller, 2009); it is to the latter we owe the term ‘snap-tentacle’ (Hartmeyer and Hartmeyer, 2005). Apart from tentacle bending, in most Drosera and some Pinguicula spp., the ‘stimulated’ leaf curves around the prey to maximize contact with it and extend the area of digestion/absorption around it (Legendre, 2002b). Although leaf movement takes on average a couple of hours, re-furling the unravelled position takes a whole day. An exception is D. capensis, which enwraps a prey in 30 min (http://en.wikipedia.org/wiki/Drosera_capensis).
Fig. 4.
Diversity of fly paper traps and numerous snap traps of the Venus flytrap Dionaea muscipula (D). (A) An example of the sundew Drosera – an active fly sticky trap type; note numerous emergences with mucilage droplets, which both attract and catch prey. (B) Portuguese sundew Drosophyllum lusitanicum – a passive fly sticky trap type. (C) Pinguicula gigantea from Mexico – an active fly sticky trap.
The tentacles of Drosera have direct connections with the vascular system, due to the increased water consumption during mucilage production (Williams and Pickard, 1974). On prey capture, mucilage production is intensified. First, formic acid initiates the digestive process of the insect body, and the excretion of digestive enzymes (proteases, phosphatases, peroxidases, nucleases, carbohydrases and amylases) then facilitates the break-down process (Heslop-Harrison and Knox, 1971; Marburger, 1979; Juniper et al., 1989). The chitin skeleton usually remains undigested, although chitinase activity may be present in the digestive exudation of Drosera (Matušiková et al., 2005). Chitinous remnants may also be disposed of by associated mites (Antor and García, 1995).
Dionaea muscipula with snap-traps (Fig. 4D) is the most splendid example of the prey capture among land plants (Darwin, 1875), while its closest descendent Aldrovanda vesiculosa (Fig. 3E), which displays a similar catching-technique, has adapted an aquatic lifestyle in dystrophic waters (Arber, 1920). Both species snap their bi-lobe-traps whenever the multicellular trigger hairs (20 in Aldrovanda, three in Dionaea) of epidermal origin are touched (Hodick and Sievers, 1986). In Aldrovanda, ten trigger hairs stand along the hinge (midrib), eight along the trap border line and two somewhere in the middle. In Dionaea, there are three in the centre. Around the rims of each trap there are four-armed glands in Aldrovanda and star-like sessile glands in Dionaea, which secrete sweet mucilage to attract prey and to seal the lobes hermetically during digestion (Juniper et al., 1989). The central zone of the lobes is richly covered with numerous sessile digestive glands both in Aldrovanda and in Dionaea. The two genera share a similar morphology of the digestive glands which, scattered over the inner epidermis, are without vascular connection (Hodick and Sievers, 1986). Digestion may take from one to several days, depending on the size of the prey. The secreted H+ ions speed up amino acid uptake (Rea and Whatley, 1983). When the trap eventually re-opens: (1) it is usually not capable of further functioning and starts to decay (Aldrovanda) – to prevent loss of valuable mineral nutrients, highly efficient re-utilization takes place in Aldrovanda; (2) it is ready to capture another insect (Dionaea), but the process may be less effective if the trigger hairs are partially damaged – the average trap of Dionaea is able to digest 2–4 prey items in its life span, reducing prey bodies to a husk of chitin (Darwin, 1875).
Suction-traps (bladders) are ranked among the most complex leaf structures ever to have been examined in plants (Juniper et al., 1989). They are found exclusively in Utricularia – all members of the genus are rootless, having bladders instead of roots (Fig. 3C, D). Charles Darwin thought that Utricularia traps were passive and it was Mary Treat who first called them active (Treat, 1876; Sanders, 2009/2010). The walls of the bladder are thin, consisting of two layers of cells. Various types of hairs cover the inside and outside of the bladder (Thurston and Seabury, 1975). The so-called trigger hairs (surrounding the entrance and the door of the bladder), button-like glands (absorbing or excreting salt depending on the developmental stage) and stalked trichomes (with mucilage-secretory functions) are found on the outer side (Lüttge, 1983). The quadrifids (four-armed trichomes; Fig. 2F) and the bifids (two-armed trichomes) – both names coined by Darwin (1875) – appear on the internal wall (Lüttge, 1983; Płachno and Jankun, 2004). Quadrifid trichomes play a substantial role in enzyme secretion/nutrient absorption as glands of this type are of a large surface, and thus provide an important means of increasing secretion/absorption area without extending the size of the trap (Fineran and Lee, 1975). Esterases, glucosidases, chitinase, aminopeptidases (proteases) and acid phosphatases have been identified in the trap fluid (Juniper et al., 1989; Sirová et al., 2003, 2009; Adamec et al., 2010b). The latter enzymes have also been found in the bifid hairs (Płachno et al., 2006). For most Utricularia species, phosphatases exhibit the highest activity, while the activities of other enzymes are usually lower by one or two orders of magnitude; very low or even zero activity was found for aminopeptidase (Sirová et al., 2003; Płachno et al., 2006; Adamec et al., 2010b). All studies show that phosphatase secretion is constitutive, independent of prey capture or N or P addition to the ambient culture water, but dependent on trap age. The fluid pH is usually 4·8–5·1 (but between 5·7 and 7·3 in U. foliosa) and seems to be regulated by the traps (Sirová et al., 2003).
The bifid hairs are indispensable for water removal (Sasago and Sibaoka, 1985a). Water moves from the bladder interior, crossing the bifid trichomes, bladder-wall cells and threshold cells (an additional layer of cells located directly below the entrance of a bladder) to reach the outer environment (Sasago and Sibaoka, 1985b). For this, the bifid arms inside as well as the button-like glands on the external side of the bladder are covered with an ‘open structure’ cuticle (cuticular gaps) (Lüttge, 1983; Płachno and Jankun, 2004). Along with the threshold cells, the bifid arms are presumed to function like a salt-excreting gland facilitating water extrusion (Sasago and Sibaoka, 1985a). More precisely, water moves passively after chlorides, while the energy-dependent transport of Cl− ions against an electrochemical gradient needs a continuous supply of ATP (Sydenham and Findlay, 1975). It was experimentally shown that the water outflow sufficient for the next trap firing lasts 20–30 min and requires energy from respiration only (Lloyd, 1929; Sasago and Sibaoka, 1985b). It was also hypothesized that Utricularia is able to reset the traps so quickly thanks to the irreversible mutation in cytochrome c oxidase in the respiratory chain (Jobson et al., 2004), which causes a 20 % reduction in the overall energy efficiency of the respiratory chain (Laakkonen et al., 2006). However, as the intact trap lumen is permanently anoxic, it is not clear which respiratory mechanism is used by the Utricularia trap to obtain the ATP energy for their exacting functions (Adamec, 2007).
Finally, when negative pressure is generated, elastic energy is stored (Skotheim and Mahadevan, 2005; Marmottant et al., 2009). Thereafter, the negative pressure equilibrium seems to ‘lean on’ the trigger hairs. Each trigger hair acts as a lever, breaking the seal and releasing the energy whenever something (living creature or strong current) disturbs it (Sydenham and Findlay, 1973). However, the existence of trigger hairs is not indispensable to bladder suction (Lloyd, 1942). This, in turn, is consistent with the recent finding that, after an extended lag period, the traps suck in water spontaneously without hair triggering (Marmottant et al., 2009; Adamec, 2011a; Vincent et al., 2011). During firing, the pressure difference between the inside and outside of the bladder decreases from –17 kPa to 0 (Sydenham and Findlay, 1973; Sasago and Sibaoka, 1985a, b).
Terrestrial bladderworts have tiny traps that mostly feed on protozoa and rotifers, while aquatic species can hold more substantial prey, such as crustacean zooplankton (e.g. water fleas), nematodes, mosquito larvae, insects, tadpoles and even small fish (Darwin, 1875). Still, for Utricularia living in nutrient-poor waters, algae constitute up to 80 % of their diet (Peroutka et al., 2008). Moreover, inside the traps, the living algae and microbial commensals make up trophic communities (food-webs) rather than submitting to predator–prey interaction, delivering hydrolytic enzymes in return for food supply (Richards, 2001; Sirová et al., 2003, 2009, 2010). To support those microbial commensal communities, aquatic Utricularia species secrete great amounts of organic substances into the trap fluid (Sirová et al., 2009, 2010).
The shunting of carbon into bladders results on the one hand in the decline of phototrophic organs in Utricularia macrorhiza (Knight, 1992). On the other hand, the same Utricularia is also in a position to decrease the number of traps when the macroelements are superfluous (Knight and Frost, 1991; Friday, 1992; Bern, 1997; Guisande et al., 2004; Kibriya and Jones, 2007; Adamec, 2008). The decrease in the number or biomass of trapping organs under nutrient excess resembles the behaviours of Drosera rotundifolia (Thorén et al., 2003), D. binata (Stewart and Nilsen, 1993), Triphyophyllum peltatum (Bringmann et al., 2002), Nepenthes talangensis (Pavlovič et al., 2010b) or Sarracenia (Ellison and Gotelli, 2002; Farnsworth and Ellison, 2008), while the decrease in the number of phototrophic leaves seems to resemble a dwelling strategy of Aldrovanda vesiculosa (Fabian-Galan and Salageanu, 1968). Thus, the adaptive capacity of aquatic Utricularia spp. in particular and of the majority of carnivorous plants in general is truly outstanding (Laakkonen et al., 2006).
ECOLOGICAL IMPLICATIONS
It appears that a significant majority of carnivorous plant species can survive without prey availability, especially under favourable conditions, e.g. if they are deprived of plant competitors and/or grow on fertilized soils (Bruzzese et al., 2010). Under such circumstances, the development of carnivory might even be partly blocked (Knight and Frost, 1991; Adamec, 1997; Ellison, 2006; Farnsworth and Ellison, 2008). In extreme cases, all metabolic investments in carnivory become invalid; for example, no pitchers are produced (Ellison and Gotelli, 2002) or no glandular leaves appear (Bringmann et al., 2002). However, under conditions of nutrient abundance, although they should benefit from a metabolic boost (Adamec, 1997, 2002; Hanslin and Karlsson, 1996), the majority of carnivores cannot compete with non-carnivorous plants in their natural habitats (Juniper et al., 1989; Schulze et al., 2001; Gaertner et al., 2010). Sacrificing photosynthesis and growth rate for the sake of the carnivorous syndrome, they cannot gain mass as quickly as non-carnivores. Their goal of survival, therefore, is to thrive in extreme habitats, where carnivory is the lesser of two evils (Chase et al., 2009).
The carnivorous habit may also be abandoned under conditions of low light, as is the case with Heliamphora (Jaffe et al., 1992), Pinguicula (Zamora et al., 1998), Utricularia (Bern, 1997) and Nepenthes (Pavlovič et al., 2007). This argues that investments in carnivory are not feasible under such circumstances. Alternatively, additional nutrient supply is indispensible only during increased demands such as fast growth (Jaffe et al., 1992), maturation (Bringmann et al., 2002) or reproduction (Darnowski et al., 2006). Irrespective of the reasons, the reported adaptations enable the plants to optimize the carnivory trade-off. The strategy of Cephalotus follicularis, Genlisea, Triphyophyllum peltatum, Utricularia and some Pinguicula of segregating into the photosynthetic and insectivorous leaves/organs serves the same function (Pavlovič et al., 2007). It can be concluded that such a variety of adaptive plasticity among carnivores is one of reasons for regarding them as ‘the real wonders’ like Darwin (1875) did. However, for Charles Darwin and his good friend Sir John Scott Burdon-Sanderson, fast movement while trapping and the attendant electrical responses were the biggest delight to discover (Burdon-Sanderson, 1873).
ELECTRICAL SIGNALLING IN CARNIVORES
The APs in the snap-traps of Dionaea muscipula and Aldrovanda vesiculosa are the fastest self-propagating electrical signals reported in plants to date (Trębacz et al., 2006; Fromm and Lautner, 2007). In Aldrovanda, they were shown to propagate at 80 mm s−1 (Iijima and Sibaoka, 1982), while average rates of AP transmission in non-carnivorous plants range from few up to 30 mm s−1 (Trębacz et al., 2006; Fromm and Lautner, 2007). In Dionaea, APs reach up to 250 mm s−1 in midrib-forward direction, and ‘only’ 60–170 mm s−1 when running towards the trap margins. Also, AP durations of 1 s in A. vesiculosa (Iijima and Sibaoka, 1981) and of 2 s in D. muscipula (Hodick and Sievers, 1988) are unique among plants. By comparison, APs in the closely related Drosera have an average duration of 10–20 s (Williams and Spanswick, 1976), whereas in lower plants a single AP can even last dozens of minutes (Koselski et al., 2008).
One AP for Aldrovanda vesiculosa and at least two for Dionaea muscipula are necessary to cause a trap to shut (Brown, 1916). Like a tightened spring, elastic energy, accumulated through an active process which is still imperfectly understood, is passively released within 100 ms to bring about a change in lobe position (Forterre et al., 2005). However, the trap is not yet completely closed and will reopen relatively quickly (within several hours), if not stimulated repeatedly (Lüttge, 1983). Incomplete closure produces gaps between interlocking tines, which gives a sufficiently small prey a chance to escape. This could be an adaptive trait of energy saving, because a small prey does not provide sufficient amounts of nutrients to benefit the plant (Pavlovič et al., 2010a). If the prey is too large to break out, it begins to scramble, touching the hairs repeatedly. The series of APs thus generated triggers a complete closure. Within 0·5–2 h the two lobes of the trap become tightly pressed to each other (Affolter and Olivo, 1975; Lichtner and Williams, 1977). This second closure step relies on a loss of turgor of the upper epidermis and adjacent mesophyll cells (Sibaoka, 1991) and simultaneous extension of the lower epidermis (Hodick and Sievers, 1989). The loss of turgor is associated with passive K+ release. The K+-channels that might be involved have already been identified in Dionaea (Iijima and Hagiwara, 1987). Accordingly, midrib-located K+ uptake is responsible for active trap re-opening in Aldrovanda (Iijima and Sibaoka, 1983). An entrapped animal stimulates digestive glands to produce enzymes either by triggering of successive APs or through urea excretion, or both (Lüttge, 1971, 1983; Robins and Juniper, 1980d). Needless to say, there are many more chemical substances other than urea which can stimulate gland exudation (e.g. amino acids, ammonium, methyl jasmonate, coronatin; Robins, 1976; Ślesak, 2002) and many of them also act as AP elicitors (Lüttge, 1983; Ueda et al., 2010). Once evoked, the AP spreads, without a fall of amplitude, through numerous plasmodesmata from one cell to another throughout the entire traps of Aldrovanda (Iijima and Sibaoka, 1982) and Dionaea (Hodick and Sievers, 1988). As almost all cells of the trap are electrically coupled, they exhibit comparable resting membrane potentials (from –80 to –160 mV, depending on experimental conditions), are equally excitable and their APs display similar amplitudes (about 130 mV, when the concentration of Ca2+ in the bath is around 1 mm) (Sibaoka, 1966; Iijima and Sibaoka, 1981; Hodick and Sievers, 1986, 1988). The AP amplitudes depend heavily on Ca2+-influx, which has been elegantly shown by observing the corresponding growth of their values with an increase in [Ca2+]ext (Iijima and Sibaoka, 1985; Hodick and Sievers, 1986, 1988; Sibaoka, 1991). The peak AP value increased by 26–28 mV with a tenfold increase in [Ca2+]ext. Inversely, Ca-ionophores or chemicals disturbing Ca-homeostasis hamper AP amplitudes, prolong repolarization (Fig. 5; Trębacz et al., 1996; Król et al., 2006) and slow down trap closure (Volkov et al., 2008). Apart from Ca2+, Cl− ions may also be considered part of the depolarization phase, which was indirectly revealed by the use of a Cl−-channel blocker (A9C) (Król et al., 2006).
Fig. 5.
Action potentials recorded in terminal gland cells after touching a trigger hair and in the presence of 10 mm CaCl2 (left) or of a calcium chelator [10 mm EGTA (right)] in the external solution. Note that in the absence of free calcium ions, not only is the amplitude of the action potential but also the membrane resting potential are drastically affected.
In Drosera, APs are generated in the head of tentacles in response to chemical (Na+, chitin or prey defecation) or mechanical (touch) stimulation (Williams and Pickard, 1974). As little weight as that of a human hair (0·822 mg) is sufficient to initiate the response; among various chemicals, NaCl, NH4Cl, urea, amino acids and phosphate are capable of triggering APs in this genus (Lüttge, 1983). The APs move down the stalk of the tentacle at 5 mm s−1, while upward propagation is twice as fast (Williams and Pickard, 1972a, b; Williams and Spanswick, 1976). The inner stalk cells (reminiscent of the phloem parenchyma) and the outer cells (epidermis), both electrically coupled by numerous plasmodesmata, are responsible for the rapid transmission of APs (Williams and Pickard, 1974; Williams and Spanswick, 1976). Two succeeding APs within 1 min are necessary for tentacle movement and just 1 min later the tentacle has completely curled (Williams and Pickard, 1972b).
It seems that the first AP facilitates the spread of another (Sibaoka, 1966; Fromm and Lautner, 2007). The requirement for two successive APs serves to protect the trap against any accidental and undesirable stimulation [e.g. by rain, temperature drops (Król et al., 2006) or light (Trębacz and Sievers, 1998)], and points to the interesting proposition that the plants (here, Dionaea and Drosera) may possess a kind of memory which allows them to take action only in response to the second AP (plant memory; Trewavas, 2005a, b; Baluška and Mancuso, 2007; Volkov et al., 2009a). As the membrane potential returns to its resting value directly after the passage of the AP, the resting potential cannot act as an ‘accumulator’ in the memory process. There is also no indication that the memory is associated in any way with a receptor potential (RP – membrane potential change that precedes AP but is too small to evoke it) (Jacobson, 1965). Instead, by analogy to animal nerve systems, a stepwise accumulation of bioactive substances during successive stimulations of the trap was suggested (Ueda and Nakamura, 2006; Ueda et al., 2010).
There is no consensus that APs are involved in movement of Utricularia bladders (Juniper et al., 1989). Although Diannelidis and Umrath (1953) reported that electrical stimulation causes the trap to ‘fire’, other researchers have been unable to repeat their results; even though single AP-like changes were noted, the traps did not fire (Sydenham and Findlay, 1973, 1975). Although recent findings indirectly support the mechanical concept of trap triggering (Joyeux et al., 2011; Vincent et al., 2011), it is tempting to speculate that APs must be evoked repeatedly – by analogy to snapping species (Brown, 1916) – to trigger trap movement.
A quite different role is played by APs in the protocarnivorous Stylidium (Darnowski et al., 2006, 2007), in that they are a part of its pollination strategy (Findlay, 1978; Findlay and Findlay, 1975, 1981, 1984; Findlay and Pallaghy, 1978). As they have little to do with its carnivorous lifestyle, we need not discuss them further at this point.
SECRETION AND ABSORPTION
Darwin (1875) was the first to show that carnivorous plants secrete their digestive fluid in response to nitrogenous substances. Among these substances, uric acid (the principal constituent of insect faeces) and glutamine (the major amino acid of insect haemolymph) turned out to be the most effective elicitors of secretion (Robins, 1976). In Dionaea, repetitive electrical or mechanical stimulation evokes enzyme secretion, too (K. Trębacz and E. Król, unpubl. res.). Thus, APs can also be considered as elicitors of secretion. Once stimulated, the gland cells undergo a cycle of ultrastructural changes generally divided into two phases, the secretory and the resorptive (Heslop-Harrison and Heslop-Harrison, 1981). During the secretory phase, cell wall erodes, plasmalemma becomes less invaginated and vacuoles shrink (pointing to their involvement in enzyme storage and release), but de novo protein synthesis also takes place (Robins and Juniper, 1980b; McNally et al., 1988). In general, mucilage-secreting cells discharge the Golgi-originated bodies [Drosera (Outenreath and Dauwalder, 1982a)], while the exocytosis of digestive enzymes involves a membrane fusion with storage vesicles of endoplasmic reticulum (ER) origin [Pinguicula (Vassilyev and Muravnik, 1988a, b), Genlisea (Płachno et al., 2007), Drosera (Heslop-Harrison, 1976), Dionaea (Robins and Juniper, 1980c)]. A split between mucilage- and enzyme-secreting glands has been reported for Lamiales (Pinguicula, Utricularia, Byblis) and for some Droseraceae sensu lato [Drosophyllum (Heslop-Harrison and Knox, 1971; Juniper et al., 1989; Legendre, 2002a, Płachno et al., 2006)]. On the other hand, there are reports of glands secreting polysaccharides and proteolytic enzymes at the same time, for example Pinguicula (Lüttge, 1971) and Dionaea (Robins and Juniper, 1980c). Although Pinguicula's sessile glands were presumed to undergo holocrine secretion (Heslop-Harrison, 1975, 1976; Heslop-Harrison and Heslop-Harrison, 1980, 1981), other reports clearly showed that these cells display a granulocyte mode of secretion (Vassilyev and Muravnik, 1988a, b), as in the case of the other carnivores. By analogy to enzyme secretion, absorption of nutrients is either constitutive or needs to be triggered (Rea and Whatley, 1983). Absorption of nutrients is executed by all gland types (Outenreath and Dauwalder, 1982b) as well as through any available apertures in the protecting cuticle (Juniper et al., 1989; Anderson, 2005). Within any one gland, these two processes may be temporally (Heslop-Harrison and Heslop-Harrison, 1981) or developmentally (Owen et al., 1999) separated. In Dionaea, secretion precedes absorption only slightly, and during several-day-long digestion, both processes happen at the same time (Robins and Juniper, 1980e). In Nepenthes, too, both processes coincide after the first successful capture, and a bi-directional transport of various compounds takes place (Owen et al., 1999). Because for Pinguicula each sessile gland is used only once, the absorption starts only when the secretion ends. The absorbed substances migrate through the apoplast and symplast of the terminal cells (Owen et al., 1999). In the endodermal layer, they must enter the symplast, which is an active and selective process. The previously secreted H+ ions now play a direct role in the symport uptake of nitrogenous compounds (Rea and Whatley, 1983). Among these (ammonium, amino acids, peptides, uric acid) ammonium is preferred, and ammonium transporters have long been shown to be highly expressed in the glands (Schulze et al., 1999). The absorbed material is stored in the small vacuoles, which persist in an increased number through the whole absorption phase (Robins and Juniper, 1980e). At the end of the phase, the cell walls are rebuilt and the glands return to their state prior to stimulation.
GLAND MORPHOLOGY
The secretory (slime and digestive) glands may be described as stalk- [Drosera (Fig. 2E), Drosophyllum, Triphyophyllum, Pinguicula, Byblis, Roridula, Utricularia (Fig. 2F)], sessile- [Cephalotus (Fig. 2A, B), Drosophyllum, Drosera, Dionaea, Aldrovanda, Pinguicula, Genlisea and Utricularia] or sunken-glands [Nepenthes (Fig. 2C, D), Brochinia, Catopsis), (Juniper et al., 1989). The main role of digestive glands is to secrete digestive fluids and to absorb nutrients. The special pitcher epithelium has developed as an alternative in Sarracenia to perform both these functions (Hepburn et al., 1920; Joel and Heide-Jørgensen, 1985; Gallie and Chang, 1997). Nevertheless, in Sarracenia purpurea only a small amount of the fluid is produced by the epithelium and most of the enzymes come from the pitcher inhabitants (Hepburn and St. John, 1927; Hepburn et al., 1927; Plummer and Jackson, 1963); in another member of this family, Darlingtonia, the fluid is rarely secreted (Treat, 1875) or is produced in very small amounts (Adlassnig et al., 2011; our pers. obs.).
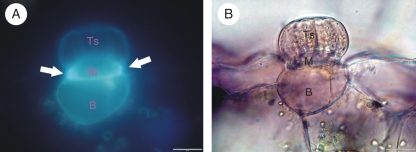
The stalk glands may resemble tentacles, trichomes, arms, emergences, projections, papillae, bristles or hairs. As for the sessile or sunken glands, their anatomical structures are less distinctive as they look like pinheads more or less embedded into the epidermis (Owen and Lennon, 1999). In all the types, however, at least three functional compartments are always present: terminal (secretory), middle (endodermal) and basal (reservoir) cells (Fig. 6). The terminals of secretory cells make up the outermost layer of cells, forming a visible swelling. Within a gland, the number of secretory layers as well the number of secretory cells in each layer varies: one (rarely) to four (typically) and up to eight secretory cells forming one-layered heads in Utricularia and Genlisea, respectively; two layers of secretory cells in Dionaea and Aldrovanda; and more than two layers in the large glands of Nepenthes. The cuticular coat covering the outermost cells is extremely thin and discontinuous, enabling secretion as well as absorption (Williams and Pickard, 1974; Joel et al., 1983). The presence of cuticular material is, nevertheless, indispensable for the protection of the inside of the traps from self-digestion and microbial infection. A markedly protruding plasmalemma is a distinct feature of terminal cells. Thanks to numerous cell-wall ingrowths, the plasma membrane of terminal cells is deeply invaginated. A labyrinthine structure of the plasmalemma considerably extends the surface which faces the outer environment. Some secretory cells are further characterized by abundant pit-fields with numerous plasmodesmata [Nepenthes (Owen et al., 1999)], which facilitate fast symplastic connection between terminal and middle cells [Genlisea (Płachno et al., 2007)]. The plasmodesmata as well as the labyrinthine ingrowths are laid down non-randomly, indicating that their distribution and orientation are precisely controlled (Robins and Juniper, 1980a). The plasmalemma is assisted by many mitochondria, extended ER and Golgi vesicles, all of which are reminiscent of transfer cells (Offler et al., 2003). The large vacuole of terminal cells (Płachno et al., 2007) usually houses storage substances which may be used as energy supplies during intensive secretion (Robins and Juniper, 1980b). It may also serve as a reservoir for digestive enzymes (Schwab et al., 1969) and osmotically active salts (Heslop-Harrison and Heslop-Harrison, 1981). Like plasmalemma morphology, the dynamic status of the vacuoles during secretion/absorption cycles is carefully monitored (Robins and Juniper, 1980a; Robins and Juniper, 1980e).
Fig. 6.
Digestive gland anatomy. (A,B) Section through the Pinguicula digestive gland: TS, terminal (secretory) cells; M, middle (stalk) cell with Casparian-like lateral wall (arrows), auto-fluorescence under UV light; B, basal cell; scale bars = 20 µm.
In Utricularia, bifids and quadrifids (Fig. 2F) are unique examples of terminal cells, in which a highly specialized regional separation of function occurs (Fineran and Lee, 1975; Płachno and Jankun, 2004). The arms execute absorption and secretion in the upper section, while the bottom regions (stalks) discharge supportive and conductive functions typical of the middle cells (Fineran and Lee, 1975). In the glandular organs of the other carnivores, the secretory cells lie over the highly specialized endodermal middle cells. In these endodermal cells, heavily suberinized cell walls create a barrier for apoplastic transport (and also for external solutions) in such a way that the hydrophobic suberin-like endodermal deposits form a continuity with epidermal cuticle (Robins and Juniper, 1980a; Owen and Lennon, 1999). The middle cells are simply ‘a bottleneck’ for apoplastic–symplastic exchange. They play a pivotal role in nutrient uptake as they prevent uncontrolled leakage between the apoplast and symplast (Fineran and Lee, 1975).
Numerous plasmodesmata between the middle and basal cells are of vital importance. Their distribution within the gland is not random, but shows a strong polarity, increasing toward the inside [Dionaea (Robins and Juniper, 1980a)]. Storing K+ and Cl− in their central vacuole, the basal cells are sometimes called reservoir cells [Pinguicula (Heslop-Harrison and Heslop-Harrison, 1980)]. The high K+ content in basal cells points to their osmoregulatory functions linked to the transfer of different solutes into and out of the traps [Nepenthes (Osunkoya et al., 2007)]. Beside the large vacuole, the other cytoplasmic features reflecting the activities of basal cells include abundant ribosomes, rudimentary plastids and numerous electron-dense vesicles in close proximity to the nucleus and plasmalemma. The basal layer is either in direct contact with the conductive vessels (Nepenthes, Pinguicula, Drosera) or separated from plant vasculature by 2–3 layers of mesophyll or parenchymal cells (Dionaea, Aldrovanda, Genlisea).
The activity of digestive glands varies depending on trap type and conditions. In pitfalls, eel-traps, suction-traps and flypapers of Pinguicula, Drosophyllum and Drosera they are characterized by a low constitutive expression of hydrolysing enzymes, intensified after prey capture (McNally et al., 1988). The terrestrial Dionaea and aquatic Aldrovanda are two ‘extreme’ examples, where secretion of digestive enzymes commences only after induction (Juniper et al., 1989).
CONCLUSIONS
Despite being of little economic importance, carnivorous plants have long held a fascination, being among the most popular plants in cultivation. They still draw the attention of many scientists as convenient model plants for such topics as: fast movements (Joyeux et al., 2011), excitability (Król et al., 2006; Volkov et al., 2009a, b, 2011), negative excitability–photosynthesis coupling (Pavlovič et al., 2011), enzyme secretion (Vassilyev, 2005; Rottloff et al., 2009), nutrient absorption (Płachno et al., 2009), heavy metal phytotoxicity (Moody and Green, 2010), food–web relationships (Butler et al., 2008; Mouquet et al., 2008; Sirová et al., 2009, 2010, 2011; Gotelli et al., 2011), plasticity and genetic radiation (Greilhuber et al., 2006; Albert et al., 2010), phylogenetic and intergeneric relationships (Rogers et al., 2010; Rahman, 2010), trade-off assessments (Pavlovič et al., 2010a), and structural and mineral investment in carnivory (Guisande et al., 2004, 2007; Adamec, 2008, 2009, 2010b). Carnivorous species (namely Utricularia) can nowadays be used as plant indicators for qualifying the degree of surface water eutrophication (Jennings and Rohr, 2011); such an approach meets the requirements of the Water Framework Directive of the European Commission 2000 (Kopeć et al., 2008). Sarracenia spp. are suggested to be the best indicators of threats from agriculture, over-collection and invasive species, while Drosera spp. are particularly sensitive to agriculture (Jennings and Rohr, 2011). The biological appliance of carnivore secondary metabolites has also gained in popularity (Gonçalves et al., 2008; Krolicka et al., 2008; Eilenberg et al., 2010; Putalun et al., 2010; Mithöfer, 2011). The more that is learnt about the different taxa, the clearer it becomes that carnivory is far more common than previously thought. Because morphological and physiological adaptations to carnivorous lifestyles are quite complex (the concurrence of such abilities as prey attraction and capture, digestion, absorption, enzyme secretion and nutrient re-utilization), there are a considerable number of plants carrying on ‘subtle’ forms of carnivory (Chase et al., 2009; Shaw and Shackleton, 2011). Thus, plant carnivory still requires a great deal of study, in terms of both its basic features and its co-evolution with non-carnivorous traits.
With the use of state-of-the-art approaches such as large-scale analysis of genes (genomic) and their products (transcriptomic, proteomic, metabolomic) we have just begun the journey to a full appreciation of the carnivorous syndrome. For example, by the means of forward genetics based on a recognizable phenotypic characteristic, one can search for genes involved in a trap movement. With the use of reverse genetics it may be possible to unmask channels involved in excitation-triggered trapping or AP spread; one can think of creating a plant line with altered activity of an ion channel by transgenic methods (over-expressor, knock-out, antisense or RNA interference). Additionally, certain expression systems afford one the opportunity of working out the channel functions in detail (Geiger et al., 2009). Another approach would be to focus on nutrient transporters, following their gene expression patterns which vary under distinct nutritive stresses. One could also utilize proteome profiling or metabolic fingerprinting to trace cellular processes under nutrient limits. The very recent study by Gotelli et al. (2011) suggests the use of proteomic signatures of Sarracenia purpurea inhabitants for monitoring pitcher ecosystem biomass, health and state. As an entire aquatic food web is harboured within the traps of this carnivorous plant, the S. purpurea pitcher ecosystem can be used to model macro ecosystems such as lakes or dystrophic waters (Gotelli and Ellison, 2006; Gotelli et al., 2011). The biggest challenge, however, is to combine all of these approaches to obtain a more complete picture of carnivorous plants. Finally, any substantial progress made in our understanding of carnivores must surely lead to better apprehension of plant biology as a whole.
Note added in proof
The authors encourage the reader to refer to the last chapter of the recently published book All Flesh is Grass. Plant–Animal Interrelationships (Adamec, 2011b; Rice, 2011).
ACKNOWLEDGEMENTS
We cordially thank the referees, whose helpful and valuable comments have greatly improved the manuscript, the director of the Botanic Garden of the Jagiellonian University Prof. Bogdan Zemanek for permission to use plants from the Garden collections, and the gardener Lucyna Kurleto. Financial support by the Polish Ministry of Education to K.T. (grant no. N301 464534) and to B.J.P. (grant nos. N304 002536 and N304 220135) is gratefully acknowledged. B.J.P. acknowledges the support of an award from the Foundation for Polish Sciences (Start Programme). L.A. was partially supported by the Research Programme of the Academy of Sciences of the Czech Republic, AV0Z60050516.
LITERATURE CITED
- Adamec L. Mineral nutrition of carnivorous plants: a review. Botanical Review. 1997;63:273–299. [Google Scholar]
- Adamec L. Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist. 2002;155:89–100. doi: 10.1046/j.1469-8137.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- Adamec L. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biology. 2006;8:765–769. doi: 10.1055/s-2006-924540. [DOI] [PubMed] [Google Scholar]
- Adamec L. Oxygen concentrations inside the traps of the carnivorous plants Utricularia and Genlisea (Lentibulariaceae) Annals of Botany. 2007;100:849–856. doi: 10.1093/aob/mcm182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec L. Mineral nutrient relations in the aquatic carnivorous plant Utricularia australis and its involvement in carnivory. Fundamentals in Applied Limnology. 2008;171:175–183. [Google Scholar]
- Adamec L. Photosynthetic CO2 affinity of the aquatic carnivorous plant Utricularia australis (Lentibulariaceae) and its investment in carnivory. Ecological Research. 2009;24:327–333. [Google Scholar]
- Adamec L. Dark respiration of leaves and traps of terrestrial carnivorous plants: are there greater energetic costs in traps? Central European Journal of Biology. 2010a;5:121–124. [Google Scholar]
- Adamec L. Mineral cost of carnivory in aquatic carnivorous plants. Flora. 2010b;205:618–621. [Google Scholar]
- Adamec L. The comparison of mechanically stimulated and spontaneous firings in traps of aquatic carnivorous Utricularia species. Aquatic Botany. 2011a;94:44–49. [Google Scholar]
- Adamec L. Part 7: Carnivorous plants: ecophysiological look at plant carnivory. Why are plants carnivorous? In. In: Seckbach J, Dubinski Z, editors. All flesh is grass. Plant-animal interrelationships. Cellular origin, life in extreme habitats and astrobiology. Dordrecht: Springer Science + Business Media B. V; 2011b. pp. 457–492. [Google Scholar]
- Adamec L, Sirová D, Vrba J. Contrasting growth effects of prey capture in two aquatic carnivorous plant species. Fundamentals in Applied Limnology. 2010a;176:153–160. [Google Scholar]
- Adamec L, Sirová D, Vrba J, Rejmánková E. Enzyme production in the traps of aquatic Utricularia spiecies. Biologia. 2010b;65:273–278. [Google Scholar]
- Adlassnig W, Peroutka M, Lendl T. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Annals of Botany. 2011;107:181–194. doi: 10.1093/aob/mcq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter JM, Olivo RF. Action potentials in Venus's-flytraps: long term observations following the capture of prey. American Midland Naturalist. 1975;93:443–445. [Google Scholar]
- Albert VA, Jobson RW, Michael TP, Taylor DJ. The carnivorous bladderwort (Utricularia, Lentibulariaceae): a system inflates. Journal of Experimental Botany. 2010;61:5–9. doi: 10.1093/jxb/erp349. [DOI] [PubMed] [Google Scholar]
- Amagase S. Digestive enzymes in insectivorous plants. III. Acid proteases in the genus Nepenthes and Drosera peltata. Journal of Biochemistry. 1972;72:73–81. doi: 10.1093/oxfordjournals.jbchem.a129899. [DOI] [PubMed] [Google Scholar]
- An CI, Fukusaki E, Kobayashi A. Plasma-membrane H+-ATPases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta. 2001;212:547–555. doi: 10.1007/s004250000455. [DOI] [PubMed] [Google Scholar]
- An CI, Fukusaki E, Kobayashi A. Aspartic proteinases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta. 2002;214:661–667. doi: 10.1007/s004250100665. [DOI] [PubMed] [Google Scholar]
- Anderson B. Adaptations to foliar absorption of faeces: a pathway in plant carnivory. Annals of Botany. 2005;95:757–761. doi: 10.1093/aob/mci082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Midgley JJ. It takes two to tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula. Oecologia. 2002;132:369–373. doi: 10.1007/s00442-002-0998-1. [DOI] [PubMed] [Google Scholar]
- Anderson B, Midgley JJ. Digestive mutualism, an alternate pathway in plant carnivory. Oikos. 2003;102:221–224. [Google Scholar]
- Anderson B, Midgley JJ. Density-dependent outcomes in a digestive mutualism between carnivorous Roridula plants and their associated hemipterans. Oecologia. 2007;152:115–120. doi: 10.1007/s00442-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Antor RJ, García MB. A new mite-plant association: mites living amidst the adhesive traps of a carnivorous plant. Oecologia. 1995;101:51–54. doi: 10.1007/BF00328899. [DOI] [PubMed] [Google Scholar]
- Arber A. Water plants: a study of aquatic angiosperms. Cambridge: Cambridge University Press; 1920. [Google Scholar]
- Atwater DZ, Butler JL, Ellison AM. Spatial distribution and impacts of moth herbivory on Northern pitcher plants. Northeast Naturalist. 2006;13:43–56. [Google Scholar]
- Baluška F, Mancuso S. Plant neurobiology as a paradigm shift not only in the plant sciences. Plant Signaling & Behavior. 2007;2:205–207. doi: 10.4161/psb.2.4.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Fish D, Machado-Allison CE. Ecological patterns of aquatic insects communities in two Heliamphora pitcher-plant species of the Venezuelan highlands. Ecotropicos. 1989;2:31–44. [Google Scholar]
- Barthlott W, Porembski S, Fischer E, Gemmel B. First protozoa-trapping plant found. Nature. 1998;392:447. [Google Scholar]
- Bennett KF, Ellison AM. Nectar, not colour, may lure insects to their death. Biology Letters. 2009;5:469–472. doi: 10.1098/rsbl.2009.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing DH, Henderson K, Kessel B, Sulak J. The absorptive capacities of bromeliad trichomes. American Journal of Botany. 1976;63:1009–1014. [Google Scholar]
- Bern AL. Studies on nitrogen and phosphorus uptake by the carnivorous bladderwort Utricularia foliosa L. in South Florida wetlands. 1997. MSc thesis, Florida International University, Miami. [Google Scholar]
- Bhattarai GP, Horner JD. The importance of pitcher size in prey capture in the carnivorous plant, Sarracenia alata Wood (Sarraceniaceae) American Midland Naturalist. 2009;161:264–272. [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme V, Gounand I, Alaux C, Jousselin E, Barthelemy D, Gaume L. The plant-ant Camponotus schmitzi helps its carnivorous host-plant Nepenthes bicalcarata to catch its prey. Journal of Tropical Ecology. 2011a;27:15–24. [Google Scholar]
- Bonhomme V, Pelloux-Prayer H, Jousselin E, Forterre Y, Labat JJ, Gaume L. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytologist. 2011b;191:545–554. doi: 10.1111/j.1469-8137.2011.03696.x. [DOI] [PubMed] [Google Scholar]
- Brewer JS, Baker DJ, Nero AS, Patterson AL, Roberts RS, Turner LM. Carnivory in plants as a beneficial trait in wetlands. Aquatic Botany. 2011;94:62–70. [Google Scholar]
- Bringmann GM, Wenzel M, Bringmann HP, Schlauer J. Uptake of the amino acid alanine by digestive leaves: proof of carnivory in the tropical liana Triphyophyllum pelatatum (Dioncophyllaceae) Carnivorous Plant Newsletter. 2001;30:15–21. [Google Scholar]
- Bringmann G, Rischer H, Schlauer J, et al. The tropical liana Triphyophyllum pelatatum (Dioncophyllaceae): formation of carnivorous organs is only a facultative prerequisite for shoot elongation. Carnivorous Plant Newsletter. 2002;31:44–52. [Google Scholar]
- Brown WH. The mechanism of movement and the duration of the effect of stimulation in the leaves of Dionaea. American Journal of Botany. 1916;3:68–90. [Google Scholar]
- Bruzzese B, Bowler R, Massicotte H, Fredeen A. Photosynthetic light response in three carnivorous plant species: Drosera rotundifolia, D. capensis and Sarracenia leucophylla. Photosynthetica. 2010;48:103–109. [Google Scholar]
- Buckley HL, Miller TE, Ellison AM, Gotelli NJ. Local- to continental-scale variation in the richness and composition of an aquatic food web. Global Ecology and Biogeography. 2010;19:711–723. [Google Scholar]
- Burdon-Sanderson J. Note on the electrical phenomena which accompany irritation of the leaf of Dionaea muscipula (Venus flytrap) Proceedings of the Royal Society of London. 1873;21:495–496. [Google Scholar]
- Butler JL, Ellison AM. Nitrogen cycling dynamics in the carnivorous northern pitcher plant, Sarracenia purpurea. Functional Ecology. 2007;21:835–843. [Google Scholar]
- Butler JL, Gotelli NJ, Ellison AM. Linking the brown and green: nutrient transformation and fate in the Sarracenia microsystem. Ecology. 2008;89:898–904. doi: 10.1890/07-1314.1. [DOI] [PubMed] [Google Scholar]
- Chase MW, Christenhusz MJM, Sanders D, Fay MF. Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Botanical Journal of the Linnean Society. 2009;161:329–356. [Google Scholar]
- Cheek M, Jebb M. Nepenthes group montanae (Nepenthaceae) in Indo-China, with N. thai and N. bokor described as new. Kew Bulletin. 2009;64:319–325. [Google Scholar]
- Chia TF, Aung HH, Osipov A, N., Goh NK, Chia LS. Carnivorous pitcher plant uses free radicals in the digestion of prey. Redox Report. 2004;9:255–261. doi: 10.1179/135100004225006029. [DOI] [PubMed] [Google Scholar]
- Clarke CM, Bauer U, Lee CiC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biology Letters. 2009;5:632–635. doi: 10.1098/rsbl.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C, Moran JA, Chin L. Mutualism between tree shrews and pitcher plants: perspectives and avenues for future research. Plant Signaling & Behavior. 2010;5:1187–1189. doi: 10.4161/psb.5.10.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnowski DW, Carroll DM, Płachno B, Kabanoff E, Cinnamon E. Evidence of protocarnivory in triggerplants (Stylidium spp.; Stylidiaceae) Plant Biology. 2006;8:805–12. doi: 10.1055/s-2006-924472. [DOI] [PubMed] [Google Scholar]
- Darnowski DW, Moberly S, Płachno B. Triggerplants (Stylidium spp.; Stylidiaceae): a previously unrecognized genus of carnivorous plants. Proceedings of the Botany and Plant Biology Joint Congress. 2007 Botanical Society of America. Available at http://www.2007.botanyconference.org/engine/search/index.php?func=detail&aid=18 . [Google Scholar]
- Darwin C. Insectivorous plants. London: Murray; 1875. [Google Scholar]
- Diannelidis T, Umrath K. Aktionsströme der Blasen von Utricularia vulgaris. Protoplasma. 1953;42:58–62. [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Schuster S, Movtchan A, Zilberstein A. Isolation and characterization of chitinase genes from pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany. 2006;57:2775–2784. doi: 10.1093/jxb/erl048. [DOI] [PubMed] [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Rahamim Y, et al. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany. 2010;61:911–922. doi: 10.1093/jxb/erp359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AG, Midgley JJ. A new plant–animal mutualism involving a plant with sticky leaves and a resident hemipteran insect. Oecologia. 1996;106:478–481. doi: 10.1007/BF00329705. [DOI] [PubMed] [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology. 2006;8:740–747. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Farnsworth EJ. The cost of carnivory for Darlingtonia californica (Sarraceniaceae): evidence from relationships among leaf traits. American Journal of Botany. 2005;92:1085–1093. doi: 10.3732/ajb.92.7.1085. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpurea. Proceedings of the National Academy of Sciences USA. 2002;99:4409–4412. doi: 10.1073/pnas.022057199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Energetics, the evolution of carnivorous plants – Darwin's ‘most wonderful plants in the world. Journal of Experimental Botany. 2009;60:19–42. doi: 10.1093/jxb/ern179. [DOI] [PubMed] [Google Scholar]
- Fabian-Galan G, Salageanu N. Consideration on the nutrition of certain carnivorous plants (Drosera capensis and Aldrovanda vesiculosa) Review Roumain de Biologie, Série de Botanica. 1968;13:275–280. [Google Scholar]
- Farnsworth EJ, Ellison AM. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. Journal of Ecology. 2008;96:213–221. [Google Scholar]
- Field C, Mooney H. The photosynthesis – nitrogen relationship in wild plants. In: Givnish TJ, editor. On the economy of plant form and function. Cambridge: Cambridge University Press; 1986. pp. 25–55. [Google Scholar]
- Findlay GP. Movement of the column of Stylidium crassifolium as a function of temperature. Functional Plant Biology. 1978;5:477–484. [Google Scholar]
- Findlay GP, Findlay N. Anatomy and movement of the column in Stylidium. Functional Plant Biology. 1975;2:597–621. [Google Scholar]
- Findlay GP, Findlay N. Respiration-dependent movements of the column of Stylidium graminifolium. Functional Plant Biology. 1981;8:45–56. [Google Scholar]
- Findlay GP, Pallaghy CK. Potassium chloride in the motor tissue of Stylidium. Functional Plant Biology. 1978;5:219–229. [Google Scholar]
- Findlay N, Findlay GP. Movement of potassium ions in the motor tissue of Stylidium. Functional Plant Biology. 1984;11:451–457. [Google Scholar]
- Fineran BA, Lee MSL. Organization of quadrifid and bifid hairs in the trap of Utricularia monanthos. Protoplasma. 1975;84:43–70. [Google Scholar]
- Fleischmann A, Wistuba A, McPherson S. Drosera solaris (Droseraceae), a new sundew from the Guayana Highlands. Willdenowia. 2007;37:551–555. [Google Scholar]
- Fleischmann A, Gibson R, Rivadavia F. Drosera ericgreenii (Droseraceae), a new species from the fynbos of South Africa. Bothalia. 2008;38:141–159. [Google Scholar]
- Fleischmann A, Rivadavia F. Utricularia rostrata (Lentibulariaceae), a new species from the Chapada Diamantina, Brazil. Kew Bulletin. 2009;64:155–159. [Google Scholar]
- Forterre Y, Skotheim JM, Dumais J, Mahadevan L. How the Venus flytrap snaps. Nature. 2005;433:421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- Frank JH, O'Meara GF. The bromeliad Catopsis berteroniana traps terrestrial arthropods but harbours Wyeomyia larvae. Florida Entomologist. 1984;67:418–424. [Google Scholar]
- Friday LE. Measuring investment in carnivory: Seasonal and individual variation in trap number and biomass in Utricularia vulgaris L. New Phytologist. 1992;121:439–445. doi: 10.1111/j.1469-8137.1992.tb02944.x. [DOI] [PubMed] [Google Scholar]
- Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environment. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- Gaertner M, Konold W, Richardson DM. Successional changes on a former tank range in eastern Germany: Does increase of the native grass species Molinia caerulea cause decline of less competitive Drosera species? Journal of Nature Conservation. 2010;18:63–74. [Google Scholar]
- Gallie DR, Chang SC. Signal transduction in the carnivorous plant Sarracenia purpurea. Regulation of secretory hydrolase expression during development and in response to resources. Plant Physiology. 1997;115:1461–1471. doi: 10.1104/pp.115.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebühr C, Pohlon E, Schmidt AR, Küsel K. Development of microalgae communities in the phytotelmata of allochthonous populations of Sarrcenia purpurea (Sarraceniaceae) Plant Biology. 2006;8:849–860. doi: 10.1055/s-2006-924474. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proceedings of the National Academy of Sciences USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TC, Waller DM. Evolving Darwin's ‘most wonderful’ plant: ecological steps to a snap-trap. New Phytologist. 2009;183:575–587. doi: 10.1111/j.1469-8137.2009.02935.x. [DOI] [PubMed] [Google Scholar]
- Giusto BD, Grosbois V, Fargeas E, Marshall DJ, Gaume L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. Journal of Bioscience. 2008;33:121–136. doi: 10.1007/s12038-008-0028-5. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist. 1984;124:479–497. [Google Scholar]
- Gonçalves S, Romano A. Micropropagation of Drosophyllum lusitanicum (Dewy pine), an endangered West Mediterranean endemic insectivorous plant. Biodiversity Conservation. 2005;14:1071–1081. [Google Scholar]
- Gonçalves S, Romano A. In vitro minimum growth for conservation of Drosophyllum lusitanicum. Biol Plant. 2007;51:795–798. [Google Scholar]
- Gonçalves S, Gonçalves MA, Ameixa O, Nogueira JMF, Romano A. Insecticidal activity of leaf extracts from Drosophyllum lusitanicum against Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) Journal of Horticultural Science Biotechnology. 2008;83:653–657. [Google Scholar]
- Gorb EV, Gorb SN. Physicochemical properties of functional surfaces in pitchers of the carnivorous plant Nepenthes alata Blanco (Nepenthaceae) Plant Biology. 2006;8:841–848. doi: 10.1055/s-2006-923929. [DOI] [PubMed] [Google Scholar]
- Gotelli NJ, Ellison AM. Food-web models predict species abundances in response to habitat change. PLoS Biology. 2006;4:e324. doi: 10.1371/journal.pbio.0040324. http://dx.doi.org/10.1371/journal.pbio.0040324 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli NJ, Smith AM, Ellison AM, Ballif BA. Proteomic characterization of the major arthropod associates of the carnivorous pitcher plant Sarracenia purpurea. Proteomics. 2011;11:2354–2358. doi: 10.1002/pmic.201000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe TU, Schöner CR, Kerth G, Junaidi A, Schöner MG. A novel resource–service mutualism between bats and pitcher plants. Biology Letters. 2011;7:436–439. doi: 10.1098/rsbl.2010.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S, Miller T, Mouquet N, Daufresne T. Nutrient limitation in detritus-based microcosms in Sarracenia purpurea. Hydrobiologia. 2006;573:173–181. [Google Scholar]
- Green S, Green TL, Heslop-Harrison Y. Seasonal heterotrophylly and leaf gland features in Triphyophyllum (Dioncophyllaceae), a new carnivorous plant genus. Botanical Journal of the Linnean Society. 1979;78:99–116. [Google Scholar]
- Greenwood M, Clarke C, Lee CC, Gunsalam A, Clarke RH. A unique resource mutualism between the giant Bornean pitcher plant, Nepenthes rajah, and members of a small mammal community. PLoS One. 2011;6:e21114. doi: 10.1371/journal.pone.0021114. http://dx.doi.org/10.1371/journal.pone.0021114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Borsch T, Muller K, Worberg A, Porembski S, Barthlott W. Smallest angiosperm genomes found in lentibulariaceae, with chromosomes of bacterial size. Plant Biology. 2006;8:770–777. doi: 10.1055/s-2006-924101. [DOI] [PubMed] [Google Scholar]
- Grevenstuk T, Coelho N, Gonçalves S, Romano A. In vitro propagation of Drosera intermedia in a single step. Biol Plant. 2010;54:391–394. [Google Scholar]
- Guisande C, Aranguren N, Andrade-Sossa C, et al. Relative balance of the cost and benefit associated with carnivory in the tropical Utricularia foliosa. Aquatic Botany. 2004;80:271–282. [Google Scholar]
- Guisande C, Granado-Lorencio C, Andrade-Sossa C, Duque SR. Bladderworts. Functional Plant Science Biotechnology. 2007;1:58–66. [Google Scholar]
- Hájek T, Adamec L. Photosynthesis and dark respiration of leaves of terrestrial carnivorous plants. Biologia. 2010;65:69–74. [Google Scholar]
- Hanslin HM, Karlsson PS. Nitrogen uptake from prey and substrate as affected by prey capture level and plant reproductive status in four carnivorous plant species. Oecologia. 1996;106:370–375. doi: 10.1007/BF00334564. [DOI] [PubMed] [Google Scholar]
- Hartmeyer I, Hartmeyer S. Drosera glanduligera: Der Sonnentau mit ‘Schnapp-Tentakeln. Taublatt. 2005;2:34–38. [Google Scholar]
- Hartmeyer S. Carnivory in Byblis revised II: The phenomenon of symbiosis on insect trapping plants. Carnivorous Plant Newsletter. 1998;27:110–113. [Google Scholar]
- Hepburn JS, St., John EQ. A bacteriological study of the pitcher liquor of the Sarraceniaceae. Transactions of the Wagner Free Institute of Sciences Philadelphia. 1927;11:75–83. [Google Scholar]
- Hepburn JS, Jones FM, St., John E. The absorption of nutrients and allied phenomena in the pitchers of the Sarraceniaceae. Journal of the Franklin Institute. 1920;189:147–184. [Google Scholar]
- Hepburn JS, St., John EQ, Jones FM. The biochemistry of the American pitcher plants. Transactions of the Wagner Free Institute of Sciences Philadelphia. 1927;11:1–74. [Google Scholar]
- Heslop-Harrison Y. Enzyme release in carnivorous plants. Frontiers in Biology. 1975;43:525–578. [PubMed] [Google Scholar]
- Heslop-Harrison Y. Enzyme secretion and digest uptake in carnivorous plants. In: Sunderland N, editor. Perspectives in experimantal biology 2: Botany. Oxford: Pergamon Press; 1976. pp. 463–476. [Google Scholar]
- Heslop-Harrison Y, Heslop-Harrison J. Chloride ion movement and enzyme secretion from the digestive glands of Pinguicula. Annals of Botany. 1980;45:729–731. [Google Scholar]
- Heslop-Harrison Y, Heslop-Harrison J. The digestive glands of Pinguicula: structure and cytochemistry. Annals of Botany. 1981;47:293–319. [Google Scholar]
- Heslop-Harrison Y, Knox RB. A cytochemical study of the leaf-gland enzymes of insectivorous plants of the genus Pinguicula. Planta. 1971;96:183–211. doi: 10.1007/BF00387439. [DOI] [PubMed] [Google Scholar]
- Hess S, Frahm JP, Theisen I. Evidence of zoophagy in a second liverwort species, Pleurozia purpurea. Bryologist. 2005;108:212–218. [Google Scholar]
- Hodick D, Sievers A. The influence of Ca2+ on the action potential in mesophyll cells of Dionaea muscipula Ellis. Protoplasma. 1986;133:83–84. [Google Scholar]
- Hodick D, Sievers A. The action potential of Dionaea muscipula Ellis. Planta. 1988;174:8–18. doi: 10.1007/BF00394867. [DOI] [PubMed] [Google Scholar]
- Hodick D, Sievers A. On the mechanism of trap closure of Venus flytrap (Dionaea muscipula Ellis) Planta. 1989;179:32–42. doi: 10.1007/BF00395768. [DOI] [PubMed] [Google Scholar]
- Iijima T, Hagiwara S. Voltage-dependent K channels in protoplasts of trap-lobe cells of Dionaea muscipula. Journal of Membrane Biology. 1987;100:73–81. doi: 10.1007/BF02209142. [DOI] [PubMed] [Google Scholar]
- Iijima T, Sibaoka T. Action potentials in the trap-lobes of Aldrovanda vesiculosa. Plant Cell Physiology. 1981;22:1595–1601. [Google Scholar]
- Iijima T, Sibaoka T. Propagation of action potentials over the trap-lobes of Aldrovanda vesiculosa. Plant Cell Physiology. 1982;23:679–688. [Google Scholar]
- Iijima T, Sibaoka T. Movements of K+ during shutting and opening of the trap-lobes in Aldrovanda vesiculosa. Plant Cell Physiology. 1983;24:51–60. [Google Scholar]
- Iijima T, Sibaoka T. Membrane potentials in excitable cells of Aldrovanda vesiculosa trap-lobes. Plant Cell Physiology. 1985;26:1–13. [Google Scholar]
- Jacobson SL. Receptor response in Venus's fly-trap. Journal of General Physiology. 1965;49:117–129. doi: 10.1085/jgp.49.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe K, MicheIangeli F, Gonzalez JM, Miras B, Ruiz MC. Carnivory in pitcher plants of the genus Heliamphora (Sarraceniaceae) New Phytologist. 1992;122:733–744. [Google Scholar]
- Jang G-W, Kim K-S, Park R-D. Micropropagation of Venus fly trap by shoot culture. Plant Cell Tissue Organ Culture. 2003;72:95–98. [Google Scholar]
- Jennings DE, Rohr JR. A review of the conservation threats to carnivorous plants. Biological Conservation. 2011;144:1356–1363. [Google Scholar]
- Jobson RW, Nielsen R, Laakkonen L, Wikstrom M, Albert VA. Adaptive evolution of cytochrome c oxidase: infrastructure for a carnivorous plant radiation. Proceedings of the National Academy of Sciences USA. 2004;101:18064–18068. doi: 10.1073/pnas.0408092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel DM, Heide-Jørgensen HS. Ultrastructure and development of the pitcher epithelium of Sarracenia. Israel Journal of Botany. 1985;34:331–349. [Google Scholar]
- Joel DM, Rea PA, Juniper BE. The cuticle of Dionaea muscipula Ellis (Venus's Flytrap) in relation to stimulation, secretion and absorption. Protoplasma. 1983;114:44–51. [Google Scholar]
- Jolivet P. Interrelationship between insects and plants. London: CRC Press; 1998. [Google Scholar]
- Joyeux M, Vincent O, Marmottant P. Mechanical model of the ultrafast underwater trap of Utricularia. Physics Review E. 2011;83:021911. doi: 10.1103/PhysRevE.83.021911. http://dx.doi.org/10.1103/PhysRevE.83.021911 . [DOI] [PubMed] [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Jürgens A, El-Sayed AM, Suckling DM. Do carnivorous plants use volatiles for attracting prey insects? Functional Ecology. 2009;23:875–887. [Google Scholar]
- Karlsson PS. Seasonal patterns of nitrogen, phosphorus and potassium utilization by three Pinguicula species. Functional Ecology. 1988;2:203–209. [Google Scholar]
- Kibriya S, Jones JI. Nutrient availability and the carnivorous habit in Utricularia vulgaris. Freshwater Biology. 2007;52:500–509. [Google Scholar]
- Knight SE. Costs of carnivory in the common bladderwort, Utricularia macrorhiza. Oecologia. 1992;89:348–355. doi: 10.1007/BF00317412. [DOI] [PubMed] [Google Scholar]
- Knight SE, Frost TM. Bladder control in Utricularia macrorhiza: lake-specific variation in plant investments in carnivory. Ecology. 1991;72:728–734. [Google Scholar]
- Koopman MM, Fuselier DM, Hird S, Carstens BC. The carnivorous pale pitcher plant harbors diverse, distinct, and time-dependent bacterial communities. Applied Environmental Microbiology. 2010;76:1851–1860. doi: 10.1128/AEM.02440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeć D, Dałkowski R, Urbaniak P. Using macrophytes as trophic state indicators in upland river waters: a case study of the Czarna Maleniecka River. International Journal of Oceanography and Hydrobiology. 2008;39:1–8. [Google Scholar]
- Koselski M, Trębacz K, Dziubińska H, Król E. Light- and dark-induced action potentials in Physcomitrella patens. Plant Signal Behaviour. 2008;3:13–18. doi: 10.4161/psb.3.1.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolicka A, Szpitter A, Gilgenast E, Romanik G, Kaminski M, Lojkowska E. Stimulation of antibacterial naphthoquinones and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzyme and Microbial Technology. 2008;42:216–221. [Google Scholar]
- Król E, Dziubińska H, Stolarz M, Trębacz K. Effects of ion channel inhibitors on cold- and electrically-induced action potentials in Dionaea muscipula. Biologia Plantarum. 2006;50:411–416. [Google Scholar]
- Laakkonen L, Jobson RW, Albert VA. A new model for the evolution of carnivory in the bladderwort plant (Utricularia): Adaptive changes in cytochrome c oxidase (COX) provide respiratory power. Plant Biology. 2006;8:758–764. doi: 10.1055/s-2006-924459. [DOI] [PubMed] [Google Scholar]
- Lavarack PS. Rainforest Drosera of north Queensland. Carnivorous Plant Newsletter. 1979;8:61–62. [Google Scholar]
- Lee CC, McPherson S, Bourke G, Mansur M. Nepenthes pitopangii (Nepenthaceae), a new specie from central Sulawesi, Indonesia. Gardens' Bulletin Singapore. 2009;61:95–100. [Google Scholar]
- Legendre L. The genus Pinguicula L (Lentibulariaceae): an overview. Acta Botanica Gallica. 2002a;141:77–95. [Google Scholar]
- Legendre L. The genus Pinguicula L. (Lentibulariaceae): an overview. Acta Botanica Gallica. 2002b;141:77–95. [Google Scholar]
- Lichtner FT, Williams SE. Prey capture and factors controlling trap narrowing in Dionaea (Droseraceae) American Journal of Botany. 1977;64:881–886. [Google Scholar]
- Lloyd FE. The mechanism of the water tight door of the Utricularia trap. Plant Physiology. 1929;4:87–102. doi: 10.1104/pp.4.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd FE. The carnivorous plants. New York: The Ronald Press Company; 1942. [Google Scholar]
- Lüttge U. Structure and function of plant glands. Annual Review of Plant Physiology. 1971;22:23–44. [Google Scholar]
- Lüttge U. Ecophysiology of carnivorous plants. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Berlin: Springer; 1983. pp. 489–517. Encyclopedia of Plant Physiology: III physiological plant ecology responses to the chemical and biological environment. [Google Scholar]
- Marburger JE. Glandular leaf structure of Triphyophyllum peltatum (Dioncophyllaceae): a ‘fly-paper’ insect trapper. American Journal of Botany. 1979;66:404–411. [Google Scholar]
- Marmottant P, Vincent O, Quilliet C. Study of the ultrafast trap of an aquatic carnivorous plant. In: Thibant B., editor. Proceedings of the Plant Biomechanics Conference 16–20 Nov. 2009. Cayenne, French Guiana. 2009. pp. 170–175. Book of Abstracts. French Guyana: UMR EcoFoG. [Google Scholar]
- Matušiková I, Salaj J, Moravčiková J, Mlynárová L, Nap J-P, Libantová J. Tentacles of in vitro-grown round-leaf sundew (Drosera rotundifolia L.) show induction of chitinase activity upon mimicking the presence of prey. Planta. 2005;222:1020–1027. doi: 10.1007/s00425-005-0047-5. [DOI] [PubMed] [Google Scholar]
- McNally SF, Steward A, Wilson UE. The stimulation of acid phosphatase activity in the stalk gland of Drosera rotundifolia. Annals of Botany. 1988;61:289–292. [Google Scholar]
- McPherson S. Pitcher plants of the Old World. Poole: Redfern Natural History Productions Ltd; 2009. [Google Scholar]
- Merbach MA, Merbach DJ, Maschwitz U, Booth WE, Fiala B, Zizka G. Carnivorous plants: mass march of termites into the deadly trap. Nature. 2002;415:36–37. doi: 10.1038/415036a. [DOI] [PubMed] [Google Scholar]
- Midgley JJ, Stock WD. Natural abundance of δ15N confirms insectivorous habit of Roridula gorgonias, despite it having no proteolytic enzymes. Annals of Botany. 1998;82:387–388. [Google Scholar]
- Mithöfer A. Carnivorous pitcher plants: insights in an old topic. Phytochemistry. 2011;72:1678–1682. doi: 10.1016/j.phytochem.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Moody C, Green ID. Assimilation of Cd and Cu by the carnivorous plant Sarracenia leucophylla Raf. fed contaminated prey. Environmental Science and Technology. 2010;44:1610–1616. doi: 10.1021/es9019386. [DOI] [PubMed] [Google Scholar]
- Moran JA. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology. 1996;84:515–525. [Google Scholar]
- Moran JA, Clarke CM. The carnivorous syndrome in Nepenthes pitcher plants: current state of knowledge and potential future directions. Plant Signaling & Behavior. 2010;5:644–648. doi: 10.4161/psb.5.6.11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implications for prey capture. Annals of Botany. 1999;83:521–528. [Google Scholar]
- Moran JA, Clarke CM, Hawkins BJ. From carnivore to detritivore? Isotopic evidence for leaf litter utilization by the tropical pitcher plant Nepenthes ampullaria. Internataional Journal of Plant Science. 2003;164:635–639. [Google Scholar]
- Mouquet N, Daufresne T, Gray SM, Miller TE. Modelling the relationship between a pitcher plant (Sarracenia purpurea) and its phytotelma community: mutualism or parasitism? Functional Ecology. 2008;22:728–737. [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, Talbot MJ. Transfer cells: cells specialized for a special purpose. Annual Review of Plant Biology. 2003;54:431–454. doi: 10.1146/annurev.arplant.54.031902.134812. [DOI] [PubMed] [Google Scholar]
- Osunkoya OO, Daud SD, Di-Giusto B, Wimmer FL, Holige TM. Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in Northern Borneo. Annals of Botany. 2007;99:895–906. doi: 10.1093/aob/mcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outenreath R, Dauwalder M. Ultrastructural and radioautographic studies of the digestive gland cells of Drosera capensis: I. Development and mucilage secretion. J Ultra Mol Str Res. 1982a;80:71–88. doi: 10.1016/s0022-5320(82)80033-6. [DOI] [PubMed] [Google Scholar]
- Outenreath R, Dauwalder M. Ultrastructural and radioautographic studies of the digestive gland cells of Drosera capensis: II. Changes induced by stimulation. Journal of Ultrastructure and Molecular Structure Research. 1982b;80:164–174. doi: 10.1016/s0022-5320(82)80033-6. [DOI] [PubMed] [Google Scholar]
- Owen TP, Jr, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Owen TP, Jr, Lennon KA, Santo MJ, Anderson AN. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Annals of Botany. 1999;84:459–466. [Google Scholar]
- Pavlovič A, Masarovičová E, Hudák J. Carnivorous syndrome in Asian pitcher plants of genus Nepenthes. Annals of Botany. 2007;100:527–536. doi: 10.1093/aob/mcm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Demko V, Hudak J. Trap closure and prey retention in Venus flytrap (Dionaea muscipula) temporarily reduces photosynthesis and stimulates respiration. Annals of Botany. 2010a;105:37–44. doi: 10.1093/aob/mcp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Singerová L, Demko V, Šantrůček J, Hudák J. Root nutrient uptake enhances photosynthetic assimilation in prey-deprived carnivorous pitcher plant Nepenthes talangensis. Photosynthetica. 2010b;48:227–233. [Google Scholar]
- Pavlovič A, Slovaková L, Pandolfi C, Mancuso S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis) Journal of Experimental Botany. 2011;62:1991–2000. doi: 10.1093/jxb/erq404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka M, Adlassnig W, Volgger M, Lendl T, Url W, Lichtscheidl I. Utricularia : a vegetarian carnivorous plant? Plant Ecology. 2008;199:153–162. [Google Scholar]
- Peterson CN, Day S, Wolfe BE, Ellison AM, Kolter R, Pringle A. A keystone predator controls bacterial diversity in the pitcher-plant (Sarracenia purpurea) microecosystem. Environmental Microbiology. 2008;10:2257–2266. doi: 10.1111/j.1462-2920.2008.01648.x. [DOI] [PubMed] [Google Scholar]
- Płachno BJ, Jankun A. Transfer cell wall architecture in secretory hairs of Utricularia intermedia. Acta Biologica Crecoviensia Series Botanica. 2004;46:193–200. [Google Scholar]
- Płachno B, Jankun A. Vienna, Austria: International Botanical Congress; 2005. Phosphatase activity in glandular structures of carnivorous plant traps; p. P1716. Abstracts p. 510, http://fyziologia.sav.sk/geophyte-colchicum/fran/papers/IBC2005.pdf . [Google Scholar]
- Płachno BJ, Wołowski K. Algae commensals community in Genlisea traps. Acta Societatis Botanicorum Poloniae. 2008;1:77–86. [Google Scholar]
- Płachno BJ, Adamus K, Faber J, Kozłowski J. Feeding behaviour of carnivorous Genlisea plants in the laboratory. Acta Botanica Gallica. 2005a;152:159–164. [Google Scholar]
- Płachno BJ, Faber J, Jankun A. Cuticular discontinuities in glandular hairs of Genlisea St.-Hil. in relation to their functions. Acta Botanica Gallica. 2005b;152:125–130. [Google Scholar]
- Płachno BJ, Adamec L, Lichtscheidl IK, Peroutka M, Adlassnig W, Vrba J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biology. 2006;8:813–820. doi: 10.1055/s-2006-924177. [DOI] [PubMed] [Google Scholar]
- Płachno BJ, Adamec L, Huet H. Mineral nutrient uptake from prey and glandular phosphatase activity as a dual test of carnivory in semi-desert plants with glandular leaves suspected of carnivory. Annals of Botany. 2009;104:649–54. doi: 10.1093/aob/mcp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płachno BJ, Kozieradzka-Kiszkurno M, Świątek P. Functional utrastructure of Genlisea (Lentibulariaceae) digestive hairs. Annals of Botany. 2007;100:195–203. doi: 10.1093/aob/mcm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer GL, Jackson TH. Bacterial activities within the sarcophagus of the insectivorous plant Sarracenia flava. American Midland Naturalist. 1963;69:462–469. [Google Scholar]
- Poppinga S, Koch K, Bohn HF, Barthlott W. Comparative and functional morphology of hierarchically structured anti-adhesive surfaces in carnivorous plants and kettle trap flowers. Functional Plant Biology. 2010;37:952–961. [Google Scholar]
- Putalun W, Udomsin O, Yusakul G, Juengwatanatrakul T, Sakamoto S, Tanaka H. Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnology Letters. 2010;32:721–724. doi: 10.1007/s10529-010-0202-3. [DOI] [PubMed] [Google Scholar]
- Rahman MO. Use of Random PCR (RAPD) technology to analyze systematic relationships in terrestrial bladderworts (Utricularia L.) Bangladesh Journal of Botany. 2010;39:97–102. [Google Scholar]
- Ratsirarson J, Silander JA. Structure and dynamics in Nepenthes madagascariensis pitcher plant micro-communities. Biotropica. 1996;28:218–227. [Google Scholar]
- Rea PA, Whatley FR. The influence of secretion elicitors and external pH on the kinetics of D-alanine uptake by the trap lobes of Dionaea muscipula Ellis (Venus's Flytrap) Planta. 1983;158:312–319. doi: 10.1007/BF00397333. [DOI] [PubMed] [Google Scholar]
- Rice BA. The Carnivorous Plant FAQ. 2007 v. 11·5. http://www.sarracenia.com/faq/faq5250.html . [Google Scholar]
- Rice BA. Part 7: Carnivorous plants: reversing the roles of predator and prey. A review of carnivory in the botanical world. In: Seckbach J, Dubinski Z, editors. All flesh is grass. Plant-animal interrelationships. Cellular origin, life in extreme habitats and astrobiology. Dordrecht: Springer Science + Business Media B. V.; 2011. pp. 493–518. [Google Scholar]
- Richards J. Bladder function in Utricularia purpurea (Lentibulariaceae): is carnivory important? American Journal of Botany. 2001;88:170–176. [PubMed] [Google Scholar]
- Rischer H, Hamm A, Bringmann G. Nepenthes insignis uses a C2-portion of the carbon skeleton of L-alanine acquired via its carnivorous organs, to build up the allelochemical plumbagin. Phytochemistry. 2002;59:603–609. doi: 10.1016/s0031-9422(02)00003-1. [DOI] [PubMed] [Google Scholar]
- Robins RJ. The nature of the stimuli causing digestive juice secretion in Dionaea muscipula Ellis (Venus's flytrap) Planta. 1976;128:263–265. doi: 10.1007/BF00393238. [DOI] [PubMed] [Google Scholar]
- Robins RJ, Juniper BE. The secretory cycle of Dionaea muscipula Ellis. I. The fine structure and the effects of stimulation on the fine structure of the digestive gland cells. New Phytologist. 1980a;86:279–296. [Google Scholar]
- Robins RJ, Juniper BE. The secretory cycle of Dionaea muscipula Ellis. II. Storage and the synthesis of the secretory proteins. New Phytologist. 1980b;86:297–311. [Google Scholar]
- Robins RJ, Juniper BE. The secretory cycle of Dionaea muscipula Ellis. III. The mechanism of the release of digestive secretion. New Phytologist. 1980c;86:313–327. [Google Scholar]
- Robins RJ, Juniper BE. The secretory cycle of Dionaea muscipula Ellis. IV. The enzymology of the secretion. New Phytologist. 1980d;86:401–412. [Google Scholar]
- Robins RJ, Juniper BE. The secretory cycle of Dionaea muscipula Ellis. V. The absorption of nutrients. New Phytologist. 1980e;86:413–422. [Google Scholar]
- Robinson AS, Fleischmann AS, McPherson SR, Heinrich VB, Gironella EP, Neña CQ. A spectacular new species of Nepenthes L. (Nepenthaceae) pitcher plant from central Palawan, Philippines. Botanical Journal of the Linnean Society. 2009;159:195–202. [Google Scholar]
- Rogers W, Cruse-Sanders J, Determann R, Malmberg R. Development and characterization of microsatellite markers in Sarracenia L. (pitcher plant) species. Conservation Genetics Resource. 2010;2:75–79. doi: 10.1007/s12686-009-9165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottloff S, Müller U, Kilper R, Mithöfer A. Micropreparation of single secretory glands from the carnivorous plant Nepenthes. Annals of Biochemistry. 2009;394:135–137. doi: 10.1016/j.ab.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Sanders D. Behind the curtain: Treat and Austin's contributions to Darwin's work on insectivorous plants and subsequent botanical studies. Jahrbuch Europaische Wissenschaftskultur. 2009/2010;5:285–298. [Google Scholar]
- Sasago A, Sibaoka T. Water extrusion in the trap bladders of Utricularia vulgaris. I. A possible pathway of water across bladder wall. Journal of Plant Research. 1985a;98:55–66. [Google Scholar]
- Sasago A, Sibaoka T. Water extrustion in the trap bladders of Utricularia vulgaris. II. A possible mechanism of water outfow. Journal of Plant Research. 1985b;98:113–124. [Google Scholar]
- Schaefer HM, Ruxton GD. Fatal attraction: carnivorous plants roll out the red carpet to lure insects. Biology Letters. 2008;4:153–155. doi: 10.1098/rsbl.2007.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze W, Frommer WB, Ward JM. Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes. Plant Journal. 1999;17:637–646. doi: 10.1046/j.1365-313x.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Schulze I, Oren R. Quantification of insect nitrogen utilization by the venus fly trap Dionaea muscipula catching prey with highly variable isotope signatures. Journal of Experimental Botany. 2001;52:1041–1049. doi: 10.1093/jexbot/52.358.1041. [DOI] [PubMed] [Google Scholar]
- Schwab DW, Simmons E, Scala J. Fine structure changes during function of the digestive glands of Venus's flytrap. American Journal of Botany. 1969;56:88–100. [Google Scholar]
- Shaw PJ, Shackleton K. Carnivory in the teasel Dipsacus fullonum – the effect of experimental feeding on growth and seed set. PLoS One. 2011;6:e17935. doi: 10.1371/journal.pone.0017935. http://dx.doi.org/10.1371/journal.pone.0017935 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibaoka T. Action potentials in plant organs. Symposia of the Society of Experimental Biology. 1966;20:49–74. [PubMed] [Google Scholar]
- Sibaoka T. Rapid plant movements triggered by action potentials. Journal of Plant Research. 1991;104:73–95. [Google Scholar]
- Sirová D, Adamec L, Vrba J. Enzymatic activities in traps of four aquatic species of the carnivorous genus Utricularia. New Phytologist. 2003;159:669–675. doi: 10.1046/j.1469-8137.2003.00834.x. [DOI] [PubMed] [Google Scholar]
- Sirová D, Borovec J, Černá B, Rejmánková E, Adamec L, Vrba J. Microbial community development in the traps of aquatic Utricularia species. Aquatic Botany. 2009;90:129–136. [Google Scholar]
- Sirová D, Borovec J, Šantručková H, Šantruček J, Vrba J, Adamec L. Utricularia carnivory revisited: plants supply photosynthetic carbon to traps. Journal of Experimental Botany. 2010;61:99–103. doi: 10.1093/jxb/erp286. [DOI] [PubMed] [Google Scholar]
- Sirová D, Borovec J, Picek T, Adamec L, Nedbalová L, Vrba J. Ecological implications of organic carbon dynamics in the traps of aquatic carnivorous Utricularia plants. Functional Plant Biology. 2011;38:583–593. doi: 10.1071/FP11023. [DOI] [PubMed] [Google Scholar]
- Skotheim JM, Mahadevan L. Physical limits and design principles for plant and fungal movements. Science. 2005;308:1308–1310. doi: 10.1126/science.1107976. [DOI] [PubMed] [Google Scholar]
- Souza PCB, Bove CP. A new species of Utricularia (Lentibulariaceae) from Chapada dos Veadeiros (Central Brazil) Systematic Botany. 2011;36:465–469. [Google Scholar]
- Steinhauser G, Adlassnig W, Peroutka M, et al. Application of radiotracers in an exotic field of botany: how to feed carnivorous plants. Journal of Radioanal and Nuclear Chemistry. 2007;274:403–409. [Google Scholar]
- Stewart CN, Jr, Nilsen ET. Responses of Drosera capensis and D. binata var. multifida (Droseraceae) to manipulations of insect availability and soil nutrient levels. New Zealand Journal of Botany. 1993;31:385–390. [Google Scholar]
- Studnička M. Several ecophysiological observations in Genlisea. Carnivorous Plant Newsletter. 1996;25:14–16. [Google Scholar]
- Suksathan P, Parnell JAN. Three new species and two new records of Utricularia L. (Lentibulariaceae) from Northern Thailand. Thailand Forestry Bulletin. 2010;38:23–32. [Google Scholar]
- Sydenham PH, Findlay GP. The rapid movement of the bladder of Utricularia spp. Australian Journal of Biological Science. 1973;26:1115–1126. [Google Scholar]
- Sydenham PH, Findlay GP. Transport of solutes and water by resetting bladders of Utricularia. Australian Journal of Plant Physiology. 1975;2:335–351. [Google Scholar]
- Ślesak E. The effects of jasmonic acid on nastic movements in Dionaea muscipula and Drosera capensis. Cell Molecular Biology Letters. 2002;7:334. [Google Scholar]
- Thorén LM, Tuomi J, Kämäräinen T, Laine K. Resource availability affects investment in carnivory in Drosera rotundifolia. New Phytologist. 2003;159:507–511. doi: 10.1046/j.1469-8137.2003.00816.x. [DOI] [PubMed] [Google Scholar]
- Thurston EL, Seabury F. A scanning electron microscopic study of the utricle trichomes in Utricularia biflora Lam. Botanical Gazette. 1975;136:87–93. [Google Scholar]
- Treat M. Plants that eat animals. American Naturalist. 1875;9:658–662. [Google Scholar]
- Treat M. Is the valve of Utricularia sensitive? Harpers New Monthly Magazine. 1876;52:382–387. [Google Scholar]
- Trewavas A. Green plants as intelligent organisms. Trends in Plant Science. 2005a;10:413–419. doi: 10.1016/j.tplants.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Trewavas A. Plant intelligence. Naturwissenschaften. 2005b;92:401–413. doi: 10.1007/s00114-005-0014-9. [DOI] [PubMed] [Google Scholar]
- Trębacz K, Sievers A. Action potentials evoked by light in traps of Dionaea muscipula Ellis. Plant Cell Physiology. 1998;39:369–372. [Google Scholar]
- Trębacz K, Busch MB, Hejnowicz Z, Sievers A. Cyclopiazonic acid disturbs the regulation of cytosolic calcium when repetitive action potentials are evoked in Dionaea traps. Planta. 1996;198:623–626. doi: 10.1007/BF00262650. [DOI] [PubMed] [Google Scholar]
- Trębacz K, Dziubińska H, Król E. Eletrical signals in long-distance communication in plants. In: Baluska F, Mancuso S, Volkmann D, editors. Communication in plants. Berlin: Springer-Verlag; 2006. pp. 277–290. [Google Scholar]
- Ueda M, Nakamura Y. Metabolites involved in plant movement and ‘memory’: nyctinasty of legumes and trap movement in the Venus flytrap. Nat Prod Rep. 2006;23:548–557. doi: 10.1039/b515708k. [DOI] [PubMed] [Google Scholar]
- Ueda M, Tokunaga T, Okada M, et al. Trap-closing chemical factors of the Venus Flytrap (Dionaea muscipulla Ellis) ChemBioChem. 2010;11:2378–2383. doi: 10.1002/cbic.201000392. [DOI] [PubMed] [Google Scholar]
- Vassilyev AE. Dynamics of ultrastructural characters of Drosophyllum lusitanicum (Droseraceae) link digestive glands during maturation and after stimulation. Taiwania. 2005;50:167–182. [Google Scholar]
- Vassilyev AE, Muravnik LE. The ultrastructure of the digestive glands in Pinguicula vulgaris L. (Lentibulariaceae) relative to their function. I. The changes during maturation. Annals of Botany. 1988a;62:329–341. [Google Scholar]
- Vassilyev AE, Muravnik LE. The ultrastructure of the digestive glands in Pinguicula vulgaris L. (Lentibulariaceae) relative to their function. II. The changes on stimulation. Annals of Botany. 1988b;62:343–351. [Google Scholar]
- Vincent O, Roditchev I, Marmottant P. Spontaneous firings of carnivorous aquatic Utricularia traps: temporal patterns and mechanical oscillations. PLoS One. 2011;6:e20205. doi: 10.1371/journal.pone.0020205. http://dx.doi.org/10.1371/journal.pone.0020205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt D, Gorb S. Desiccation resistance of adhesive secretion in the protocarnivorous plant Roridula gorgonias as an adaptation to periodically dry environment. Planta. 2010;232:1511–1515. doi: 10.1007/s00425-010-1270-2. [DOI] [PubMed] [Google Scholar]
- Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiology. 2008;146:694–702. doi: 10.1104/pp.107.108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Carrell H, Baldwin A, Markin VS. Electrical memory in Venus flytrap. Bioelectrochemistry. 2009a;75:142–147. doi: 10.1016/j.bioelechem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Volkov AG, Carrell H, Markin VS. Molecular electronics of the Dionaea muscipula trap. Plant Signaling & Behavior. 2009b;4:353–354. doi: 10.4161/psb.4.4.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Pinnock MR, Lowe DC, Gay MS, Markin VS. Complete hunting cycle of Dionaea muscipula: consecutive steps and their electrical properties. Journal of Plant Physiology. 2011;168:109–120. doi: 10.1016/j.jplph.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Wells K, Lakim MB, Schulz S, Ayasse M. Pitchers of Nepenthes rajah collect faecal droppings from both diurnal and nocturnal small mammals and emit fruity odour. Journal of Tropical Ecology. 2011;27:347–353. [Google Scholar]
- Williams SE, Pickard BG. Properties of action potentials in Drosera tentacles. Planta. 1972a;103:222–240. doi: 10.1007/BF00386845. [DOI] [PubMed] [Google Scholar]
- Williams SE, Pickard BG. Receptor potentials and action potentials in Drosera tentacles. Planta. 1972b;103:193–221. doi: 10.1007/BF00386844. [DOI] [PubMed] [Google Scholar]
- Williams SE, Pickard BG. Connections and barriers between cells of Drosera tentacles in relation to their electrophysiology. Planta. 1974;116:1–16. doi: 10.1007/BF00390198. [DOI] [PubMed] [Google Scholar]
- Williams S, Spanswick R. Propagation of the neuroid action potential of the carnivorous plant Drosera. Journal of Comparative Physiology. 1976;108:211–223. [Google Scholar]
- Zamora R, Gomez JM, Hodar JA. Fitness responses of a carnivorous plant in contrasting ecological scenarios. Ecology. 1998;79:1630–1644. [Google Scholar]
- Zamudio S, Olvera M. A new species of Utricularia (Lentibulariaceae) from Guerrero, Mexico. Brittonia. 2009;61:119–125. [Google Scholar]