Abstract
Background and Aims
Determining the sources of variation in floral morphology is crucial to understanding the mechanisms underlying Angiosperm evolution. The selection of floral and reproductive traits is influenced by the plant's abiotic environment, florivores and pollinators. However, evidence that variations in floral traits result from mutualistic interactions with insects other than pollinators is lacking in the published literature and has rarely been investigated. We aimed to determine whether the association with either Camponotus femoratus or Pachycondyla goeldii (both involved in seed dispersal and plant protection) mediates the reproductive traits and allocation of Aechmea mertensii, an obligatory ant-garden tank-bromeliad, differently.
Methods
Floral and reproductive traits were compared between the two A. mertensii ant-gardens. The nitrogen flux from the ants to the bromeliads was investigated through experimental enrichments with stable isotopes (15N).
Key Results
Camponotus femoratus-associated bromeliads produced inflorescences up to four times longer than did P. goeldii-associated bromeliads. Also, the numbers of flowers and fruits were close to four times higher, and the number of seeds and their mass per fruit were close to 1·5 times higher in C. femoratus than in P. goeldii-associated bromeliads. Furthermore, the 15N-enrichment experiment showed that C. femoratus-associated bromeliads received more nitrogen from ants than did P. goeldii-associated bromeliads, with subsequent positive repercussions on floral development. Greater benefits were conferred to A. mertensii by the association with C. femoratus compared with P. goeldii ants.
Conclusions
We show for the first time that mutualistic associations with ants can result in an enhanced reproductive allocation for the bromeliad A. mertensii. Nevertheless, the strength and direction of the selection of floral and fruit traits change based on the ant species and were not related to light exposure. The different activities and ecological preferences of the ants may play a contrasting role in shaping plant evolution and speciation.
Keywords: Aechmea mertensii, Camponotus femoratus, bromeliad, Bromeliaceae, δ15N, floral traits, fruit-set, mutualistic ants, Pachycondyla goeldii, reproductive allocation, stable isotopes
INTRODUCTION
Floral traits play an important role in the dynamics of plant populations, primarily because their variations affect the attractiveness of flowers to pollinators and can subsequently influence plant fitness (Strauss et al., 1996; Mothershead and Marquis, 2000). Determining the sources of variation in floral morphology is therefore of crucial importance to broadening our understanding of the mechanisms underlying Angiosperm evolution. For a given plant species, the variations in floral traits result from pluralistic processes and causes (Galen, 1999). The proximate causes of phenotypic plasticity in plants concern the physical environment (inter alia: incident light, temperature, nutrient intake and elevation) (Frazee and Marquis, 1994; Strauss and Whittall, 2006). Ultimately, the process of diversification in floral traits must be carried out by pollinators (Fenster et al., 2004; Parachnowitsch and Kessler, 2010) and florivores (Cascante-Marin et al., 2009; Hanley et al., 2009). Variations in floral traits that increase plant fitness and involve mutualistic insects other than pollinators have not been reported to the best of our knowledge.
Ants are amongst the most abundant and ecologically important arthropods in tropical rain forests, accounting for 20–40 % of the arthropod biomass and up to one-third of all of the mutualisms between arthropods and woody plant species (Beattie, 1985). Ant–plant relationships range from simple opportunism and mutual benefit to complex, multiple interactions (Vazquez et al., 2009). Numerous studies have shown that ants play a major role in (1) seed dispersal (Howe and Smallwood, 1982; Brew et al., 1989), (2) the protection of leaves from herbivory (Fonseca, 1994; Heil and McKey, 2003), (3) macronutrient supply (Treseder et al., 1995; Fischer et al., 2003), (4) defending the plant's reproductive organs (Horvitz and Schemske, 1984; Vesprini et al., 2003), and, in some rare cases, (5) pollination (de Vega et al., 2009). Thus, an association with ants should a priori result in a higher plant reproductive output (Gaume et al., 2005b; but see Letourneau, 1998). Nevertheless, if ants can increase plant fitness by deterring phytophagous insects and by disseminating seeds, they may also impose reproductive costs on their host plants. Such costs are the result of either their predatory behaviour towards effective pollinators or damage caused directly to the reproductive parts of the plants (Yu and Pierce, 1998; Izzo and Vasconcelos, 2002; Gaume et al., 2005a; Ness, 2006; Frederickson, 2009; Orivel et al., 2011). Also, the sap-sucking Hemiptera exploited and disseminated by ants may dramatically affect flower structures and the plant's reproductive biology (Ivey and Carr, 2005). In obligate ant–plant interactions, the identity of the mutualistic ant species is therefore an important factor influencing (positively or negatively) plant fitness.
Among tropical plants, epiphytes represent a keystone resource in rain forests because of their important role in nutrient cycling and in providing habitats for many micro-organisms, invertebrates and small vertebrates (Nadkarni, 1994). Some epiphytic species have developed symbioses with ants, either by providing chambers (domatia) where ants nest (Davidson and Epstein, 1989) or by rooting in arboreal ant gardens (AGs) (Benzing, 2000; Orivel and Leroy, 2011). AGs are initiated by a few ant species whose founding queens and/or workers build arboreal carton nests. The main benefits for the plant combine the principal positive outcomes from both seed dispersal and protective mutualisms (Orivel and Leroy, 2011). The ants collect and incorporate the seeds of selected epiphyte species which then germinate and grow on the nest, so that the plant roots stabilize and anchor the entire structure to the supporting tree (Orivel et al., 1998).
Over its entire (South American) range, the tank-bromeliad Aechmea mertensii occurs only in association with AGs (Benzing, 2000). In French Guiana, A. mertensii is found in secondary forest formations (pioneer growths) on AGs initiated either by Camponotus femoratus or by Pachycondyla goeldii ants (Corbara and Dejean, 1996; Vantaux et al., 2007). As dispersal and protective agents for this bromeliad, C. femoratus and P. goeldii indirectly influence its vegetative traits (i.e. plant shape and size, leaf anatomy) by determining the location of the seedling, from exposed to partially shaded areas, respectively (Leroy et al., 2009a). This recent study showed that variation in some vegetative traits (i.e. the size and shape of the bromeliad) were related to a light acclimation process whereas others (i.e. leaf thickness and leaf mass per unit area) were related to nutrient-stressed environments linked to the identity of the associated ant. Despite this variation in plant forms and vegetative traits, it was ascertained that both C. femoratus- and P. goeldii-associated bromeliads belong to the same species (Céréghino et al., 2011).
In the present study, we investigated the influence of its two mutualistic ant species, C. femoratus and P. goeldii, on the reproductive allocation of A. mertensii. Assuming that associations with ants having different ecological requirements affect the outcome of the mutualism for the plant (Leroy et al., 2009a; Céréghino et al., 2010), we hypothesized that the plant's reproductive traits (i.e. its flowers and seeds) depend more on the species of associated ant rather than on exposure to light. Furthermore, as δ15N values can be used as indicators of the nitrogen source (Leroy et al., 2009b), an experiment using 15N-enriched food provided to the ants was also carried out to investigate if ant-foraged nitrogen can enhance the plant's reproductive traits and allocation.
MATERIALS AND METHODS
Study site and species characteristics
This study was conducted from October 2008 to January 2009 and from October to November 2009 in pioneer growths along forest edges around the field station at Petit-Saut, Sinnamary, French Guiana (05°03′30·0″N, 52°58′34·6″W; elevation 100 m a.s.l.). The climate is tropical moist, with 3500 mm of yearly precipitation distributed over 280 d. A major drop in rainfall occurs between July and November (dry season), and another shorter, more irregular dry period occurs in March. The maximum monthly temperature averages around 33·5 °C, and the monthly minimum around 20·3 °C. The percentages of total incident light received by each of the plants studied were estimated using hemispherical photography (for a more detailed methodology, see Leroy et al., 2009a).
All of the plants studied were located adjacent to a dirt road on well-developed and easily accessible AGs inhabited by the ants Camponotus femoratus Fabr. and Crematogaster levior Longino or by Pachycondyla goeldii Forel. Camponotus femoratus is a polygynous (multiple queens), arboreal formicine species living in a parabiotic association with the myrmicine species Cr. levior; that is to say, they share the same nests and trails, but shelter in different cavities of the nests (Orivel et al., 1997; Vantaux et al., 2007). Their large polydomous (multiple nests) colonies and aggressiveness identify them as territorially dominant species in Neotropical rain forest canopies. Pachycondyla goeldii, by contrast, is a monogynous (single queen) arboreal ponerine species with smaller populations, although the colonies may be polydomous (Corbara and Dejean, 1996; Dejean et al., 2000).
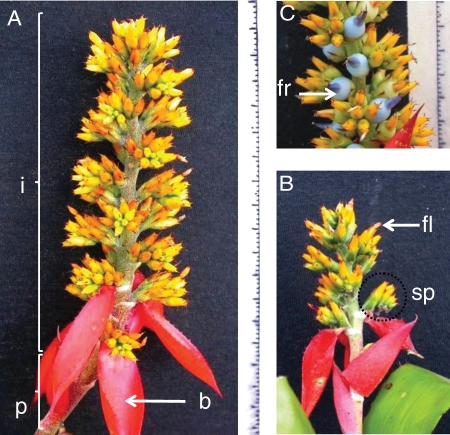
Aechmea mertensii Schult.f. (Bromeliaceae) (subfamily Bromelioideae) has tightly interlocking leaves forming compartments that collect water and organic detritus. These tanks, or phytotelmata (‘plant-held water’), provide a habitat for aquatic micro- and macro-organisms as well as for vertebrates (Richardson, 1999; Carrias et al., 2001; Brouard et al., 2011). Aechmea mertensii is characterized by sympodial branching that leads to a series of attached, compact, terminally flowered ramets (Benzing, 2000). Inflorescences are supported by a long reddish brown peduncle projecting the inflorescence above the rosette (Mori et al., 1997; Fig. 1). The peduncle has spirally arranged red to pink bracts (Fig. 1A, B). The inflorescences, one-branched, are composed of spikes with 4–12 flowers. The flowers are hermaphroditic and actinomorphic with fleshy yellow calyces composed of three sepals and red corollas composed of three petals. Stamens arise in two whorls of three members each, and are attached to the corolla. The gynoecium consists of three carpels with inferior ovaries. A septal nectary is present in the interlocular position. The fruits are blue, spine-armed berries containing naked seeds with appendages (Fig. 1C).
Fig. 1.
Morphology of Aechmea mertensii inflorescences on (A) Camponotus femoratus-associated bromeliads, and (B) Pachycondyla goeldii-associated bromeliads. Appearance of the first blue-coloured berries on a Camponotus femoratus-associated bromeliad with (C) extremity of an inflorescence with ripe fruits. Abbreviations: b = bract of the peduncle, fl = flower, fr = fruit, i = inflorescence, p = peduncle, sp = spike. Scale divisions in millimetres.
Floral trait measurements
Floral and fruit characteristics were measured during the reproductive period (October 2008 to January 2009) for C. femoratus- and P. goeldii-associated plants (n = 22 and 26 A. mertensii, respectively). We measured the diameter (two random measurements taken at 90°) of the reservoir and the height of the inflorescence, and recorded the number of spikes and flowers per inflorescence (Fig. 1A, B). We collected five flowers from the centre of the inflorescence for each of the plants studied (110 and 130 flowers from C. femoratus- and P. goeldii-associated plants, respectively). Flower length and width were measured using a stereomicroscope equipped with a micrometer.
Pollen/ovule ratio
The flowers examined for pollen/ovule ratios (P/O) were near anthesis; thus the pollen was mature, but the anthers had not dehisced. To estimate the number of pollen grains per stamen, we collected three stamens each with one anther per flower. Each anther was digested in 300 µL of 95 % sulphuric acid for 2 d at 24 °C. The solution was then homogenized, and 1 µL was collected and carefully placed on a microscope slide. The number of pollen grains (N) was counted for five independent replicates of 1 µL. The total number of pollen grains per stamen was obtained by multiplying the mean of the five replicate totals by 300 and multiplying the result by the average number of stamens (n). Thus, the P/O ratio used is 300 × N × n divided by the number of ovules in the ovary (Cruden, 1977).
Fruit- and seed-set
We periodically monitored fruit development until maturation. After counting the number of flowers, and then mature fruits, the evaluation of fruiting success was based on the ‘fruit-set’; i.e. the percentage of flowers developing into a mature fruit (Burne et al., 2003). For each of the plants studied, five ripe (blue-tinged) fruits were collected from the centre of the inflorescence (110 and 130 fruits from C. femoratus- and P. goeldii-associated plants, respectively). Using a stereomicroscope equipped with a micrometer, we measured the length and width of the fruits, and counted the number of seeds per fruit. We then distinguished mature (i.e. well-developed) seeds from aborted or unfertilized seeds (i.e. ovules that failed to form seeds). All of the mature seeds in each fruit were then dried and weighed using a quartz crystal microbalance.
15N enrichment
We investigated the role of the two mutualistic ant species in provisioning A. mertensii with nitrogen at the flowering stage by providing colonies with food artificially enriched with 15N. Between October and November 2009, we monitored 18 and 12 C. femoratus- and P. goeldii-associated plants, respectively, providing colonies ad libitum with food artificially enriched with 15N every 2 d for 3 weeks. The quantity of artificial food provided each time depended on the size of the AGs, itself related to the size of the colonies and the number of epiphytic plant species (C. femoratus-AGs are three to four times larger and host a more populous ant colony and number of plant species than P. goeldii-AGs). A preliminary study conducted using non-enriched foods permitted us to obtain (1) the preferred artificial food for both ant species, and (2) an approximate idea of the amount of food consumed by each ant colony. This varied from 6 to 12 g for C. femoratus colonies, and from 4 to 6 g for P. goeldii colonies. We then provided these colonies with the corresponding quantities of food artificially enriched with 15N. The food was placed in small plastic cups, covered to keep out food-robbing insects, attached to a branch on the host tree 20–40 cm away from the AGs. No physical contact occurred between these cups and the epiphytic plants or the carton nests. Before re-supplying each colony with a new, predefined quantity of fresh food artificially enriched with 15N, we cleaned the cups, removing any remaining food.
The food was artificially enriched with 15N as follows. We first boiled 375 mL of distilled water with 30 g Agar-Agar (Sigma, St Louis, MO, USA). Then, 750 g of mealworms was mixed into another 375 g of distilled water containing 3 g methyl 4-hydroxybenzoate (an antifungal agent used as a food preservative) plus 10 g ammonium nitrate (NH415NO3, 10 at.% 15N, Isotec, Sigma-Aldrich.com/isotec), 10 g ammonium nitrate (15NH4NO3, 10 at.% 15N, Isotec) and 10 g urea (H215NCO15NH2, 10 at.% 15N, Isotec). Finally, this preparation was mixed with the agar solution. This artificially enriched food was kept in a refrigerator at 4 °C during the entire experimental period.
Isotopic analysis
Pieces of leaves and flowers were collected before 15N enrichment and 1 week after the 3-week-long 15N enrichment experiment. All of the samples were vacuum-dried and ground into a homogeneous powder using a mixer mill. Around 1 g from each plant sampled was analysed for its total N and δ15N content. Stable isotope analyses were conducted at the Colorado Plateau Stable Isotope Laboratory (Northern Arizona University, USA) using a Thermo-Finnigan Deltaplus Advantage gas isotope-ratio mass spectrometer interfaced with a Costech Analytical ECS4010 elemental analyser. The natural abundances of 15N were calculated as follows:
where X is the element of interest, and Rsample and Rstandard are the molar ratios (i.e.15N/14N) of the sample and the standard, respectively (DeNiro and Epstein, 1978).
Statistical analyses
Preliminary tests showed that most of the variables were not normally distributed (Shapiro Wilk's test) even after transformation. Thus, Mann–Whitney U-tests (Statistica 8 software; Statsoft Inc, Tulsa, OK, USA) were used to test significant differences in incident light; inflorescence, and floral and fruit traits; and total leaf and flower N and δ15N based on ant species. Finally, a Wilcoxon matched-pairs test was used to compare the differential δ15N enrichment of the leaves and flowers.
RESULTS
Incident light
Camponotus femoratus-AGs received significantly less transmitted light than Pachycondyla goeldii-AGs (mean ± s.d. 38·57 ± 5·19 vs. 52·64 ± 5·34 %, respectively, U = –3·55, P < 0·0001). However, the distribution of the two AG-ant species showed a gradient from C. femoratus-AGs to P. goeldii-AGs with a clear overlapping of the incident light for the two ant species (Fig. 2). If we plot the length of A. mertensii inflorescences against incident light, it clearly appears that no relationship exists between these two variables either for C. femoratus-associated bromeliads (R2 = 0·0006, P = 0·99) or for P. goeldii-associated bromeliads (R2 = 0·027, P = 0·40).
Fig. 2.
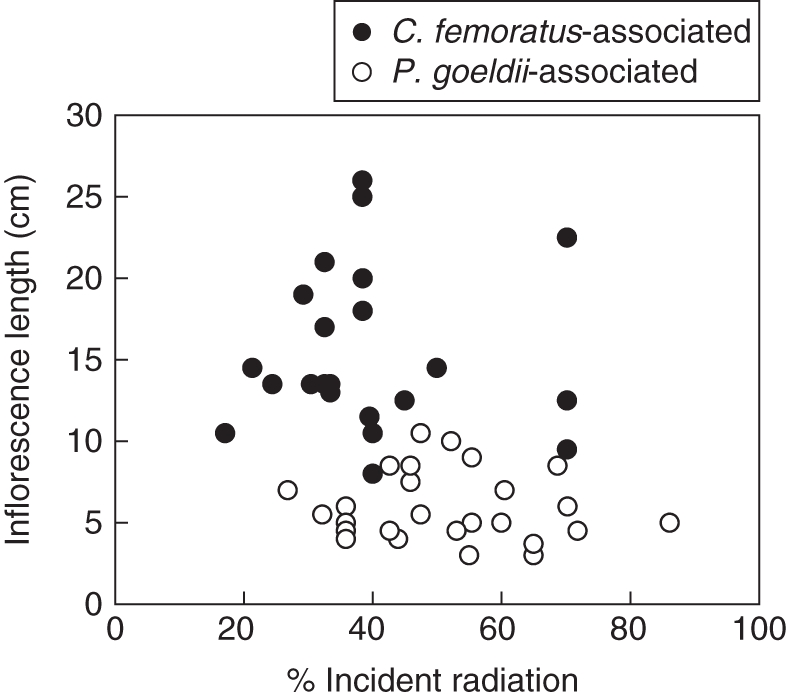
Relationship between the length of the bromeliad A. mertensii inflorescences and the light environment (% incident radiation) in relation to the distribution of its ant partner: Camponotus femoratus-associated bromeliads (n = 22), Pachycondyla goeldii-associated bromeliads (n = 26), as indicated.
Floral features
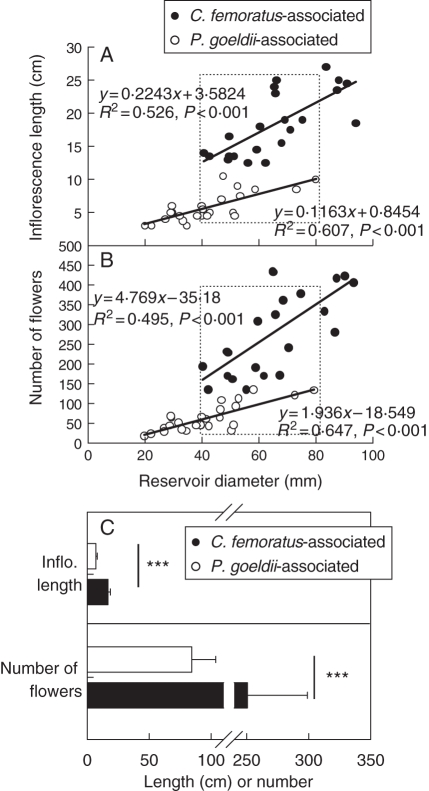
Compared with P. goeldii-associated plants, C. femoratus-associated plants had inflorescences three to four times longer, with up to four times more spikes and flowers per inflorescence, longer and wider flowers, and a higher number of ovules and pollen grains per flower (Table 1; see also Fig. 1). In both cases, the length of the inflorescences and the number of flowers per inflorescence showed a significant positive relationship with reservoir size (Fig. 3A, B). More interestingly, for a similar reservoir size, inflorescence length and the number of flowers per inflorescence were significantly higher for C. femoratus-associated bromeliads (Fig. 3C).
Table 1.
Inflorescence, flower and fruit traits of Aechmea mertensii associated with either Camponotus femoratus (C.f-bromeliads, n = 22) or Pachycondyla goeldii (P.g-bromeliads, n = 26)
| C.f-bromeliads | P.g-bromeliads | U-test | P | |
|---|---|---|---|---|
| Inflorescence traits | ||||
| Inflorescence length (cm) | 18·21 ± 2·03 | 5·71 ± 1·08 | 5·92 | <0·0001 |
| No. of spikes per inflorescence | 26·95 ± 4·10 | 10·11 ± 2·14 | 5·79 | <0·0001 |
| No. of flowers per inflorescence | 256·61 ± 61·55 | 64·24 ± 17·22 | 5·79 | <0·0001 |
| Flower traits | ||||
| Flower length (mm) | 1·30 ± 0·04 | 0·99 ± 0·04 | 5·92 | <0·0001 |
| Flower width (mm) | 0·36 ± 0·01 | 0·32 ± 0·01 | 5·52 | <0·0001 |
| No. of ovules per flower | 12·67 ± 0·53 | 9·29 ± 0·43 | 5·91 | <0·0001 |
| No. of pollen grains per flower | 5825 ± 1985·85 | 3238 ± 1103·41 | 3·42 | <0·0005 |
| P/O ratio | 479·25 ± 93·71 | 352·02 ± 60·96 | 1·92 | 0·054 |
| Fruit traits | ||||
| No. of fruits per inflorescence | 209·52 ± 45·21 | 56·18 ± 12·06 | 5·66 | <0·0001 |
| Fruit-set (%) | 84·43 ± 4·84 | 85·32 ± 2·88 | 0·55 | 0·577 |
| No. of seeds per fruit | 10·71 ± 0·89 | 7·50 ± 0·93 | 3·99 | <0·0001 |
| Seed mass per fruit (mg) | 2·67 ± 0·32 | 1·84 ± 0·24 | 3·41 | <0·0001 |
Comparison of both A. mertensii ant-garden variables (mean ± s.d.) were conducted using the non-parametric Mann–Whitney U-test.
Fig. 3.
(A) Inflorescence length and (B) number of flowers per inflorescence as a function of the diameter of the A. mertensii reservoir. Camponotus femoratus-associated bromeliads (n = 22); Pachycondyla goeldii-associated bromeliads (n = 26), as indicated. Dotted squares indicate C. femoratus- and P. goeldii-associated bromeliads with a similar range of tank diameters selected for (C). (C) Mean inflorescence length and number of flowers per inflorescence for A. mertensii with similar tank diameters on both C. femoratus- and P. goeldii-associated AGs (individuals inside the dotted squares in parts A, B): C. femoratus-associated bromeliads (n = 17), P. goeldii-associated bromeliads (n = 14), as indicated. Statistical analyses were conducted using the Mann–Whitney U-test. ***P < 0·001.
The P/O ratio, which was not significantly different between the two ant–bromeliad associations, ranged from 168 to 852. Based on Cruden's (1977) P/O categories, the breeding system used by A. mertensii ranges from facultative autogamous (i.e. P/O = 168·5 ± 22·1) to facultative allogamous (i.e. P/O = 796·6 ± 87·7), whereas obligate allogamous species are characterized by a higher P/O ratio (i.e. 5859·2 ± 936·5).
Fruit- and seed-set
The number of fruits per inflorescence was over three times higher for C. femoratus-associated plants than for P. goeldii-associated plants (Table 1). Fruit-set is very high under natural conditions and was not significantly different in either ant–bromeliad association with more than 80 % of the flowers developing into a mature fruit. This high fruit-set associated with the observed P/O ratio indicates that A. mertensii spontaneously self-pollinates regardless of the ant partner. The number of seeds and the seed mass per fruit were significantly higher in C. femoratus-associated plants than in P. goeldii-associated plants (Table 1).
Tracing ant-foraged nitrogen through the addition of a 15N tracer
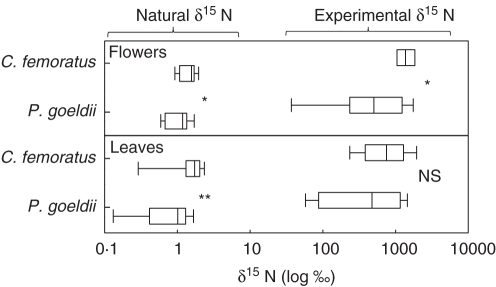
Bromeliads associated with C. femoratus showed significantly higher total N and natural δ15N values for leaf tissues than those associated with P. goeldii (N = 0·61 ± 0·06 vs. 0·50 ± 0·06 %, U = 2·271, P = 0·022; δ15N = 1·61 ± 0·35 vs. 0·88 ± 0·24 ‰, U = 3·005, P = 0·002). Provisioning the ants with 15N-enriched food resulted in a more than 600 % increase in leaf and flower δ15N (Fig. 4). The δ15N in the leaves did not vary significantly between the plants based on the plant's ant mutualist (δ15N = 824·09 ± 279·60 vs. 613·10 ± 307·29 ‰ for C. femoratus- and P. goeldii-associated bromeliads, respectively; U = 1·481, P = 0·146). However, flowers on C. femoratus-associated plants were significantly more enriched with 15N than those on P. goeldii-associated plants (δ15N = 1459·59 ± 296·58 vs. 732·97 ± 387·25 ‰; U = 2·428, P = 0·014). Moreover, δ15N was significantly higher in flowers than in leaves at the end of the experiment for C. femoratus-associated plants (Wilcoxon matched-pairs test; z = 2·665, P = 0·007; Fig. 3); this was not the case for those bromeliads associated with P. goeldii (z = 1·540, P = 0·123).
Fig. 4.
Effect of 15N provisioning of ants on Aechmea mertensii vegetative and floral parts. Natural and experimental δ15N values for leaves (n = 18) and flowers (n = 9) on Camponotus femoratus-bromeliads and for leaves (n = 12) and flowers (n = 9) on Pachycondyla goeldii-bromeliads. Error bars indicate the 90th and 10th percentiles, the ends of the boxes indicate the 25th and 75th percentiles. Statistical analyses were conducted using the Mann–Whitney U-test: NS = no significant differences, *P < 0·05, **P < 0·005.
DISCUSSION
In tandem with previous investigations, our results show that the two AG-ant species have a contrasting impact on the structural plasticity of A. mertensii for both vegetative (see Leroy et al., 2009a) and floral traits (present study). While numerous studies have shown that the identity of the associated ant species can differentially affect (1) plant protection (Gaume et al., 2005b), (2) plant size and growth (Frederickson, 2005) and (3) reproductive allocation (Horvitz and Schemske, 1984; Vesprini et al., 2003) or flower castration (Yu and Pierce, 1998; Gaume et al., 2005a; Malé et al., 2011; Orivel et al., 2011), to the best of our knowledge, no previous study has demonstrated variations in floral and reproductive traits linked to the identity of the mutualistic ant species. Thus, the present study brings new insights into how mutualistic ants impact the floral traits of their host plant. The production of flowers, fruits and seeds by the bromeliad A. mertensii, as well as the structural characteristics of these organs, clearly depend on the species of its associated ant. Interestingly, while the incident light received by plants can explain phenotypic plasticity in the vegetative traits, we showed that the floral traits of A. mertensii were not primarily influenced by this factor. As association with AGs is obligatory for the bromeliad, our results suggest that greater benefits are conferred to this plant by the association with C. femoratus compared with the association with P. goeldii.
In epiphytic tank-bromeliads, the main source of nutrients comes from the phytotelmata formed by the tightly interlocking leaves which collect water, leaf litter and other organic detritus, and provide habitat for invertebrates (Benzing, 2000). By having larger phytotelmata, C. femoratus-associated plants host higher numbers of aquatic invertebrate species and individuals (Céréghino et al., 2010), so that greater amounts of nitrogen from invertebrate faeces are made available to the bromeliad (Leroy et al., 2009a). Camponotus femoratus-associated plants have the potential for greater nutrient allocation to inflorescences, flowers and seeds than do P. goeldii-associated plants, presumably due in part to the difference in the size of the phytotelmata. However, as we show here, with a similar phytotelm size, C. femoratus-associated plants produce more flowers and fruits per inflorescence than P. goeldii-associated plants. This observation suggests that ants indirectly influence A. mertensii nutrition via the phytotelm, but, on the other hand, it underlines the differential effect that the two main mutualistic ants have on the reproductive biology of the host plant. In the present study, we provide new evidence that ants could play a direct role in the transfer of nutrients to the plants probably through the plants' roots. Indeed, we found a significant increase in the relative abundance of the 15N isotope in plant tissues after both ant species were supplied with 15N-enriched food. Interestingly, C. femoratus-associated plants had the higher δ15N values, indicating that members of this ant species might be better able to pass nitrogen to the host plant compared with P. goeldii. In addition, C. femoratus-associated plants allocated more resources to male (pollen grain number) and female (ovule number) functions compared with P. goeldii-associated plants. These results are in keeping with the resource–cost hypothesis postulated by Galen (1999) that predicts that reduced flower (inflorescence) size is advantageous under resource-poor conditions. Bromeliads may thus adjust to differences in resource availability through plastic changes in allocation to flowering.
According to Wyatt (1982), the function of the inflorescence in attracting insects is best fulfilled through large, showy floral displays, so that the inflorescences on A. mertensii associated with C. femoratus should be more attractive to diurnal pollinators than those on individuals associated with P. goeldii. Yet, during observations and measurements made on A. mertensii inflorescences throughout the day, no pollinating visitors were noted on the flowers on either kind of AG despite their bright red and yellow colours, so that we deduced that they are infrequent at least during daytime. Outcrossing (i.e. pollination by bats or nocturnal insects) cannot be ruled out as no observations were conducted at night, but it is likely that this species is mostly self-pollinated based on the P/O ratio and high fruit-set. In addition to a deficiency in pollinator services, several other selective factors may promote the evolution of self-pollination, such as the cost of outcrossing, low population density and selection for local adaptation (Lande and Schemske, 1985; Charlesworth and Charlesworth, 1987). For the majority of AG epiphytes, the high incidence of autogamy may be triggered by the aggressive behaviour of the ants toward pollinators (Madison, 1979). In C. femoratus-associated AGs, the ants are actively present on the inflorescence but never damage the flowers, while their presence and aggressive behaviour could deter both pollinators and flower and fruit feeders. Thus, the bright colour of A. mertensii inflorescences might be an ancestral trait with no or very little importance vis-à-vis pollinators (Saito and Harborne, 2001). These bright-coloured inflorescences (red bracts) and blue fruits probably rather play a role in seed dispersal by birds as they can be attracted by these colours (Stiles, 1976).
Based on our results, C. femoratus seems to be the best mutualistic ant partner for A. mertensii when compared with P. goeldii. As C. femoratus-associated bromeliads produce more seeds, natural selection should favour plants associated with C. femoratus more than plants associated with P. goeldii. Thus, we might wonder why the A. mertensii–P. goeldii association persists. An evolutionary response that might eliminate the P. goeldii-associated plants over evolutionary time can only occur if variations in the traits related to the mutualism are heritable; specifically, variation must exist in traits that might determine whether a plant is associated with one or the other ant species. Studies have demonstrated that at least some AG-plants are equipped with aromatic compounds, perhaps genetically determined, that might make their seeds more or less attractive to ants (Seidel et al., 1990), and potentially differentially attractive to different ant species. If there is genetic variation in plant traits that influence the identity of the mutualistic ant, several hypotheses might explain the continued persistence of the P. goeldii association. First, trade-offs may exist between resource acquisition and inbreeding, with C. femoratus-associated plants acquiring more resources but also suffering more inbreeding than P. goeldii-associated plants. Pachycondyla goeldii workers, which very rarely leave their nest during the daytime, might be less aggressive towards pollinators than C. femoratus workers that – day and night – may instantaneously react to any motion or disturbance of the AG. In this way, P. goeldii-associated plants might experience an outcrossing advantage through the ant mutualist's impact on pollinators. Second, the fruits and seeds of P. goeldii-associated bromeliads are dispersed by insects/animals whereas those associated with C. femoratus are, in part, harvested by the ants which then disperse the seeds in their own AG (J. Orivel, UMR Ecologie des Forêts de Guyane, French Guiana, pers. comm.). If the seeds of P. goeldii-associated bromeliads are dispersed further away than those from C. femoratus-associated bromeliads, P. goeldii-associated plants may benefit from advantages related to enhanced seed dispersal (Howe and Smallwood, 1982). Third, traits that might specialize plants to one mutualist (C. femoratus) may themselves be selected against compared with traits that permit more generalization in mutualist identity because specialization can reduce the assurance that the seeds become part of any AG (an argument analogous to the concept in pollination biology that generalist pollination systems are favoured due to reproductive assurance; Waser et al., 1996). Aechmea mertensii seed dispersal, even by an apparently less favourable ant species, is important because this bromeliad species occurs exclusively in association with arboreal ants, and has never been found growing outside AGs (Madison, 1979; Benzing, 2000).
Divergence in flower size and shape among a plant population is largely explained on the basis of pollinator- or florivore-mediated selection. In the present study, we provide evidence for the first time of the importance of the identity of the mutualistic ants on inflorescence, floral and fruit traits. The strength and direction of this selection on floral and fruit traits change depending on the ant species, which may play a contrasting role in shaping plant evolution and speciation. However, as the reproductive biology of A. mertensii is still very poorly known, further experiments and studies are needed to better understand its breeding system and the mechanisms of microevolution such as gene flow, as well as genetic drift and selection in the context of ant–plant interactions.
ACKNOWLEDGEMENTS
We are grateful to the members of the ‘Laboratoire Hydréco de Petit-Saut’ for their logistical help, to Olivier Roux for his help in the field and to Andrea Yockey-Dejean for proofreading the manuscript. We would also like to thank the three anonymous reviewers for valuable comments on previous versions of the manuscript. Financial support for this study was provided through the Programme Amazonie II of the French Centre National de la Recherche Scientifique (Project 2ID), and the Programme Convergence 2007–2013 (Région Guyane) from the European Community (Project DEGA).
LITERATURE CITED
- Beattie AJ. The evolutionary ecology of ant-plant mutualisms. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- Benzing DH. Bromeliaceae: profile of an adaptative radiation. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Brew CR, O'Dowd DJ, Rae ID. Seed dispersal by ants: behaviour-releasing compounds in elaiosomes. Oecologia. 1989;80:490–497. doi: 10.1007/BF00380071. [DOI] [PubMed] [Google Scholar]
- Brouard O, Le Jeune A-H, Leroy C, et al. Are algae relevant to the detritus-based food web in tank-bromeliads? PLoS ONE. 2011;6:e20129. doi: 10.1371/journal.pone.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne HE, Yates CJ, Ladd PG. Comparative population structure and reproductive biology of the critically endangered shrub Grevillea althoferorum and two closely related more common congeners. Biological Conservation. 2003;114:53–65. [Google Scholar]
- Carrias J-F, Cussac ME, Corbara B. A preliminary study of freshwater Protozoa in tank-bromeliads. Journal of Tropical Ecology. 2001;17:611–617. [Google Scholar]
- Cascante-Marin A, Wolf JHD, Oostermeijer JGB. Wasp florivory decreases reproductive success in an epiphytic bromeliad. Plant Ecology. 2009;203:149–153. [Google Scholar]
- Céréghino R, Leroy C, Dejean A, Corbara B. Ants mediate the structure of phytotelm communities in an ant-garden bromeliad. Ecology. 2010;91:1549–1556. doi: 10.1890/09-1534.1. [DOI] [PubMed] [Google Scholar]
- Céréghino R, Leroy C, Carrias J-F, et al. Ant–plant mutualisms promote functional diversity in phytotelm communities. Functional Ecology. 2011;25:954–963. [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Corbara B, Dejean A. Arboreal nest building and ant-garden initiation by a ponerine ant. Naturwissenschaften. 1996;83:227–230. [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Davidson DW, Epstein WW. Epiphytic associations with ants. In: Lüttge U, editor. Vascular plant as epiphytes. New York: Springer; 1989. pp. 201–233. [Google Scholar]
- Dejean A, Corbara B, Orivel J, Snelling RR, Delabie JHC, Belin-Depoux M. The importance of ant gardens in the pioneer vegetal formations of French Guiana. Sociobiology. 2000;35:425–439. [Google Scholar]
- DeNiro MJ, Epstein S. Carbon isotopic evidence for different feeding patterns in two Hyrax species occupying the same habitat. Science. 1978;201:906–908. doi: 10.1126/science.201.4359.906. [DOI] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson PJ, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 2004;35:375–403. [Google Scholar]
- Fischer RC, Wanek W, Richter A, Mayer VE. Do ants feed plants? A 15N labelling study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Piper. Journal of Ecology. 2003;91:126–134. [Google Scholar]
- Fonseca CR. Herbivory and the long-lived leaves of an Amazonian ant-tree. Journal of Ecology. 1994;82:833–842. [Google Scholar]
- Frazee JE, Marquis RJ. Environmental contribution to floral trait variation in Chamaecrista fasciculata (Fabaceae: Caesalpinoideae) American Journal of Botany. 1994;81:206–215. [Google Scholar]
- Frederickson ME. Ant species confer different partner benefits on two neotropical myrmecophytes. Oecologia. 2005;143:387–395. doi: 10.1007/s00442-004-1817-7. [DOI] [PubMed] [Google Scholar]
- Frederickson ME. Conflict over reproduction in an ant–plant symbiosis: why Allomerus octoarticulatus ants sterilize Cordia nodosa trees. The American Naturalist. 2009;173:675–681. doi: 10.1086/597608. [DOI] [PubMed] [Google Scholar]
- Galen C. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience. 1999;49:631–640. [Google Scholar]
- Gaume L, Zacharias M, Borges RM. Ant–plant conflicts and a novel case of castration parasitism in a myrmecophyte. Evolutionary Ecology Research. 2005a;7:435–452. [Google Scholar]
- Gaume L, Zacharias M, Grosbois V, Borges RM. The fitness consequences of bearing domatia and having the right ant partner: experiments with protective and non-protective ants in a semi-myrmecophyte. Oecologia. 2005b;145:76–86. doi: 10.1007/s00442-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Armbruster WS. Pollination and plant defence traits co-vary in Western Australian Hakeas. New Phytologist. 2009;182:251–260. doi: 10.1111/j.1469-8137.2008.02709.x. [DOI] [PubMed] [Google Scholar]
- Heil M, McKey D. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology, Evolution and Systematics. 2003;34:425–453. [Google Scholar]
- Horvitz CC, Schemske DW. Effects of ants and ant-tented herbivore on seed production of a neotropical herb. Ecology. 1984;65:1369–1378. [Google Scholar]
- Howe HF, Smallwood J. Ecology of seed dispersal. Annual Review of Ecology and Systematics. 1982;13:201–228. [Google Scholar]
- Ivey CT, Carr DE. Effects of herbivory and inbreeding on the pollinators and mating system of Mimulus guttatus (Phrymaceae) American Journal of Botany. 2005;92:1641–1649. doi: 10.3732/ajb.92.10.1641. [DOI] [PubMed] [Google Scholar]
- Izzo TJ, Vasconcelos HL. Cheating the cheater: domatia loss minimizes the effects of ant castration in an Amazonian ant-plant. Oecologia. 2002;133:200–205. doi: 10.1007/s00442-002-1027-0. [DOI] [PubMed] [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Leroy C, Corbara B, Dejean A, Céréghino R. Ants mediate foliar structure and nitrogen acquisition in a tank-bromeliad. New Phytologist. 2009a;183:1124–1133. doi: 10.1111/j.1469-8137.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- Leroy C, Corbara B, Dejean A, Céréghino R. Potential sources of nitrogen in an ant-garden tank-bromeliad. Plant Signaling & Behavior. 2009b;4:868–870. doi: 10.4161/psb.4.9.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau DK. Ants, stem-borers, and fungal pathogens: experimental tests of a fitness advantage in Piper ant-plants. Ecology. 1998;79:593–603. [Google Scholar]
- Madison M. Additional observations on ant-gardens in Amazonas. Selbyana. 1979;5:107–115. [Google Scholar]
- Malé P-JG, Leroy C, Dejean A, Quilichini A, Orivel J. An ant symbiont directly and indirectly limits its host plant's reproductive success. Evolutionary Ecology. 2011 in press. doi:10.1007/s10682-011-9485-7. [Google Scholar]
- Mori SA, Cremers G, Gracie C, de Granville J-J, Hoff M, Mitchell JD. Guide to the vascular plants of central French Guiana, Part 1. Pteridophytes, gymnosperms, and monocotyledons. New York: The New York Botanical Garden; 1997. [Google Scholar]
- Mothershead K, Marquis RJ. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in Oenothera macrocarpa. Ecology. 2000;81:30–40. [Google Scholar]
- Nadkarni NM. Diversity of species and interaction in the upper tree canopy of forest ecosystems. American Zoologist. 1994;34:70–78. [Google Scholar]
- Ness JH. A mutualism's indirect costs: the most aggressive plant bodyguards also deter pollinators. Oikos. 2006;113:506–514. [Google Scholar]
- Orivel J, Leroy C. The diversity and ecology of ant gardens (Hymenoptera: Formicidae; Spermatophyta: Angiospermae) Myrmecological News. 2011;14:73–85. [Google Scholar]
- Orivel J, Errard C, Dejean A. Ant gardens: interspecific recognition in parabiotic ant species. Behavioral Ecology and Sociobiology. 1997;40:87–93. [Google Scholar]
- Orivel J, Dejean A, Errard C. Active role of two ponerine ants in the elaboration of ant gardens. Biotropica. 1998;30:487–491. [Google Scholar]
- Orivel J, Lambs L, Malé P-JG, et al. Dynamics of the association between a long-lived understory myrmecophyte and its specific associated ants. Oecologia. 2011;165:369–376. doi: 10.1007/s00442-010-1739-5. [DOI] [PubMed] [Google Scholar]
- Parachnowitsch AL, Kessler A. Pollinators exert natural selection on flower size and floral display in Penstemon digitalis. New Phytologist. 2010;188:393–402. doi: 10.1111/j.1469-8137.2010.03410.x. [DOI] [PubMed] [Google Scholar]
- Richardson BA. The bromeliad microcosm and assessment of faunal diversity in a neotropical forest. Biotropica. 1999;31:321–336. [Google Scholar]
- Saito N, Harborne JB. A cynidin glycoside giving scarlet coloration in plants of the Bromeliaceae. Photochemistry. 2001;22:1735–1740. [Google Scholar]
- Seidel JL, Epstein WW, Davidson DW. Neotropical ant garden: I. Chemical constituents. Journal of Chemical Ecology. 1990;16:1791–1816. doi: 10.1007/BF01020495. [DOI] [PubMed] [Google Scholar]
- Stiles FG. Taste preferences, color preferences, and flower choice in hummingbirds. The Condor. 1976;78:10–26. [Google Scholar]
- Strauss SY, Whittall JB. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 120–138. [Google Scholar]
- Strauss SY, Conner JK, Rush SL. Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. The American Naturalist. 1996;147:1098–1107. [Google Scholar]
- Treseder KK, Davidson DW, Ehleringer JR. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature. 1995;375:137–139. [Google Scholar]
- Vantaux A, Dejean A, Dor A, Orivel J. Parasitism versus mutualism in the ant-garden parabiosis between Camponotus femoratus and Crematogaster levior. Insectes Sociaux. 2007;54:95–99. [Google Scholar]
- Vazquez DP, Blüthgen N, Cagnolo L, Chacoff NP. Uniting pattern and process in plant–animal mutualistic networks: a review. Annals of Botany. 2009;103:1445–1457. doi: 10.1093/aob/mcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega C, Arista M, Ortiz PL, Herrera CM, Talaver S. The ant-pollination system of Cytinus hypocistis (Cytinaceae), a Mediterranean root holoparasite. Annals of Botany. 2009;103:1065–1075. doi: 10.1093/aob/mcp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesprini JL, Galetto L, Bernardello G. The beneficial effect of ants on the reproductive success of Dyckia floribunda (Bromeliaceae), an extrafloral nectary plant. Canadian Journal of Botany. 2003;81:24–27. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- Wyatt R. Inflorescence architecture: how flower number, arrangement, and phenology affect pollination and fruit-set. American Journal of Botany. 1982;69:585–594. [Google Scholar]
- Yu DW, Pierce NE. A castration parasite of an ant–plant mutualism. Proceedings of the Royal Society, B. 1998;265:375–382. [Google Scholar]