Abstract
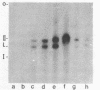
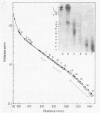
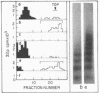
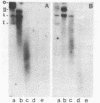
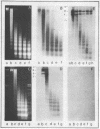
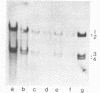
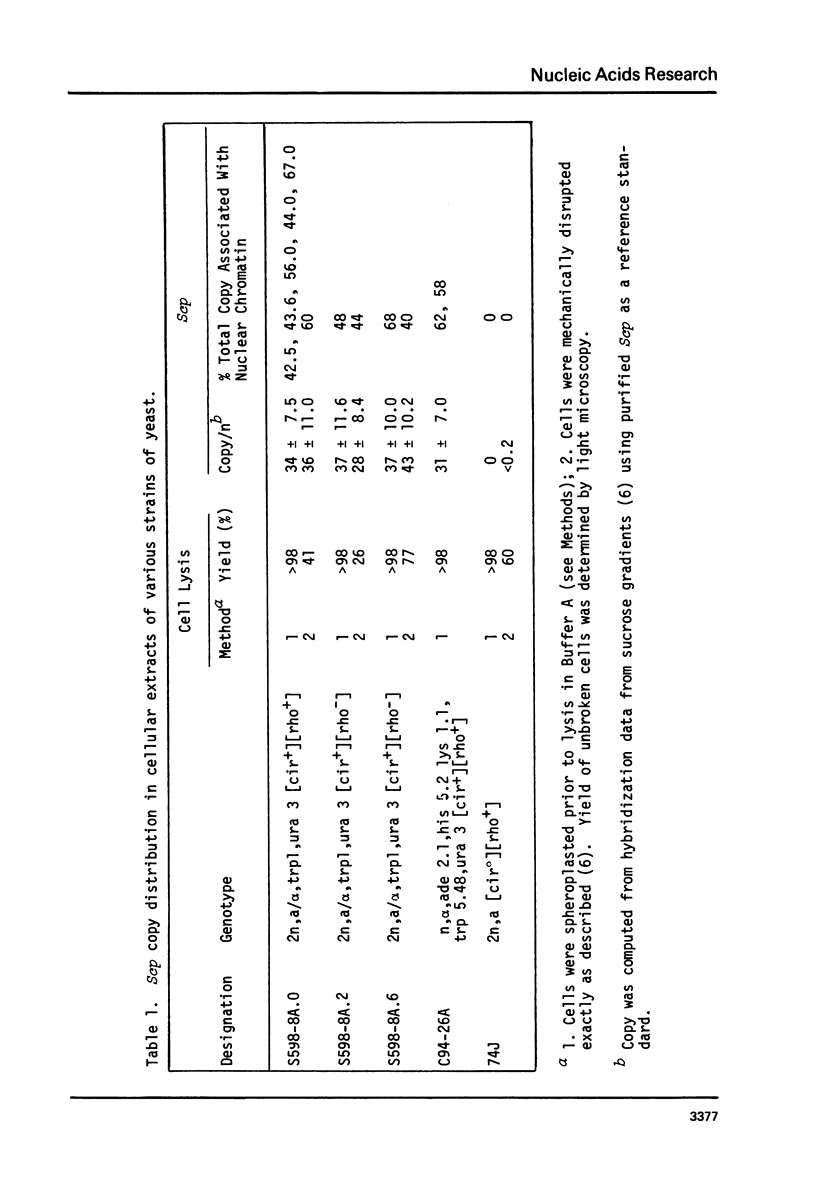
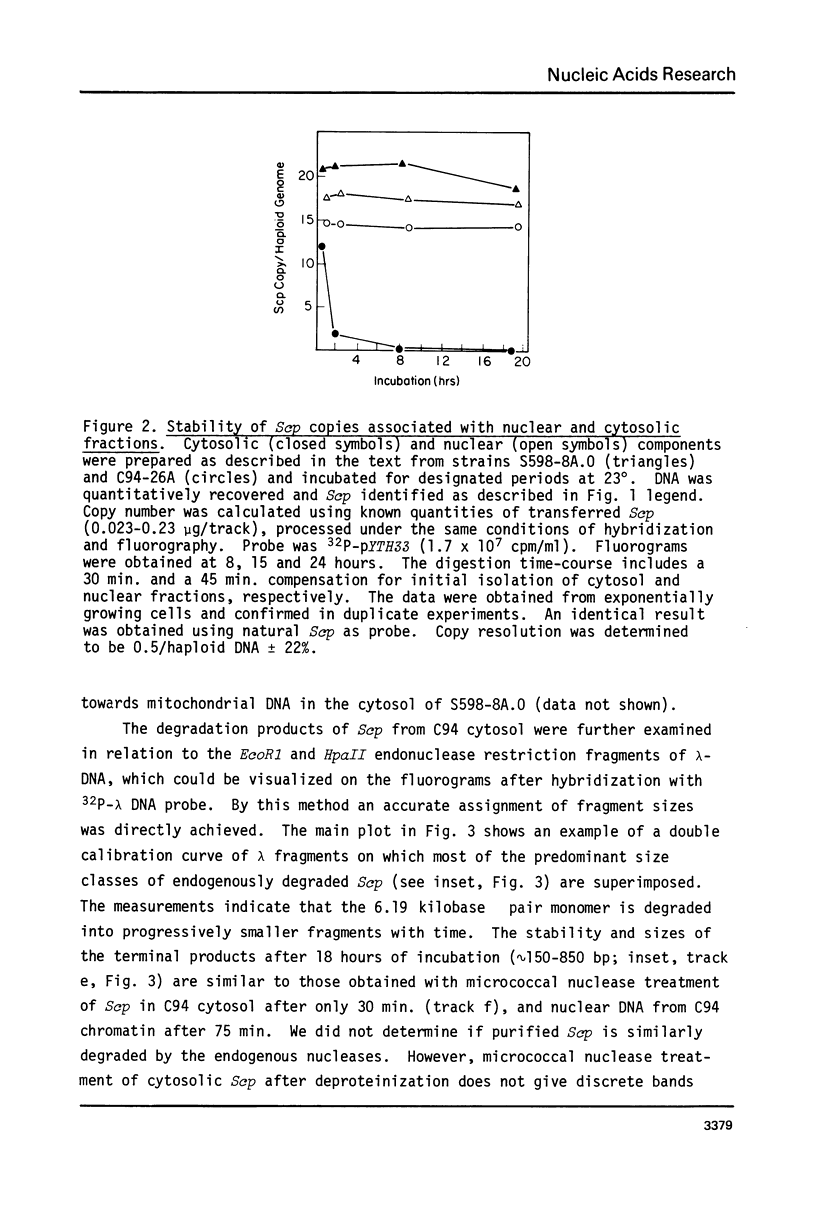
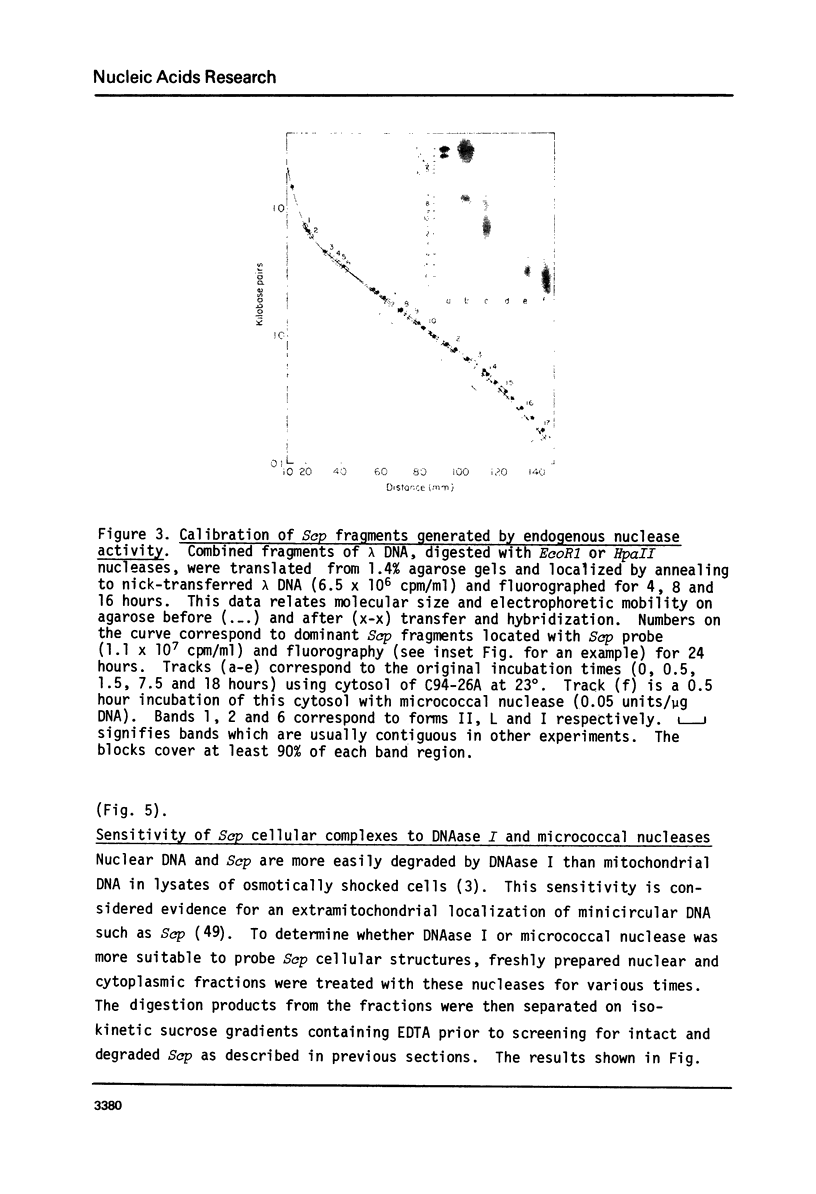
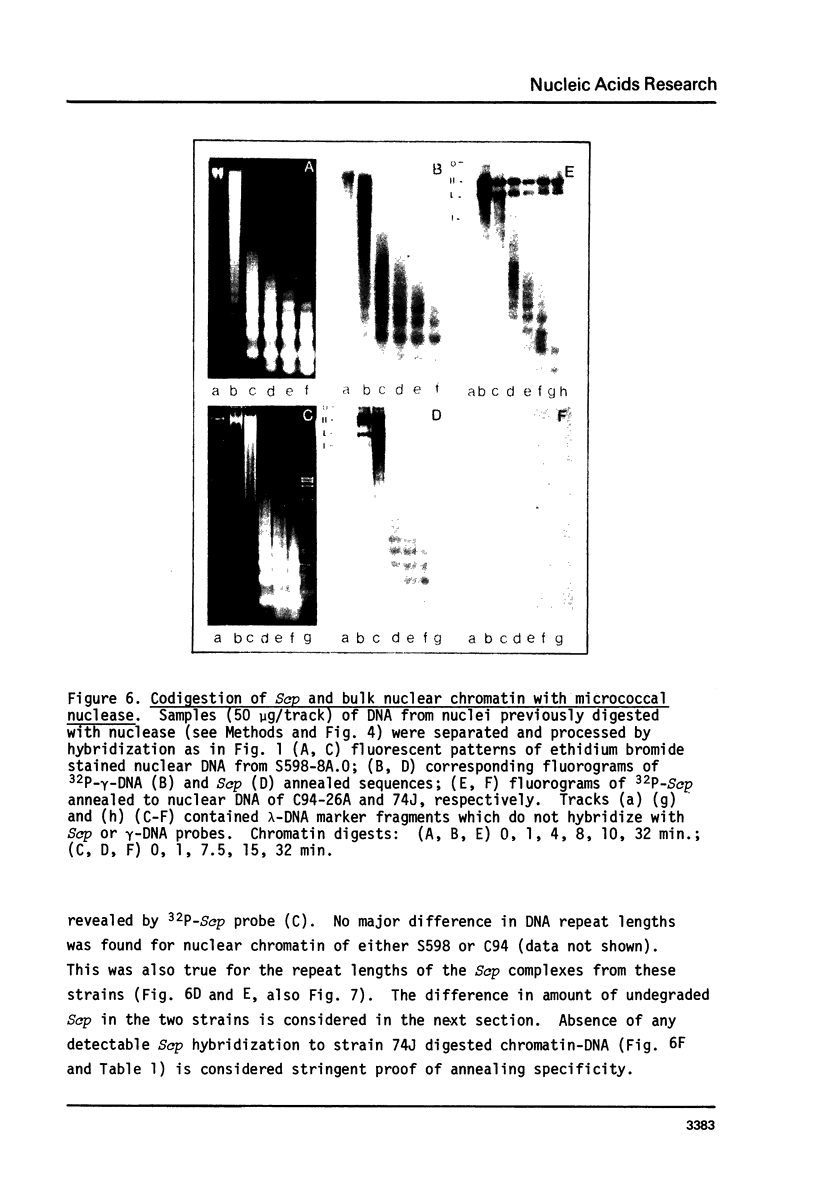
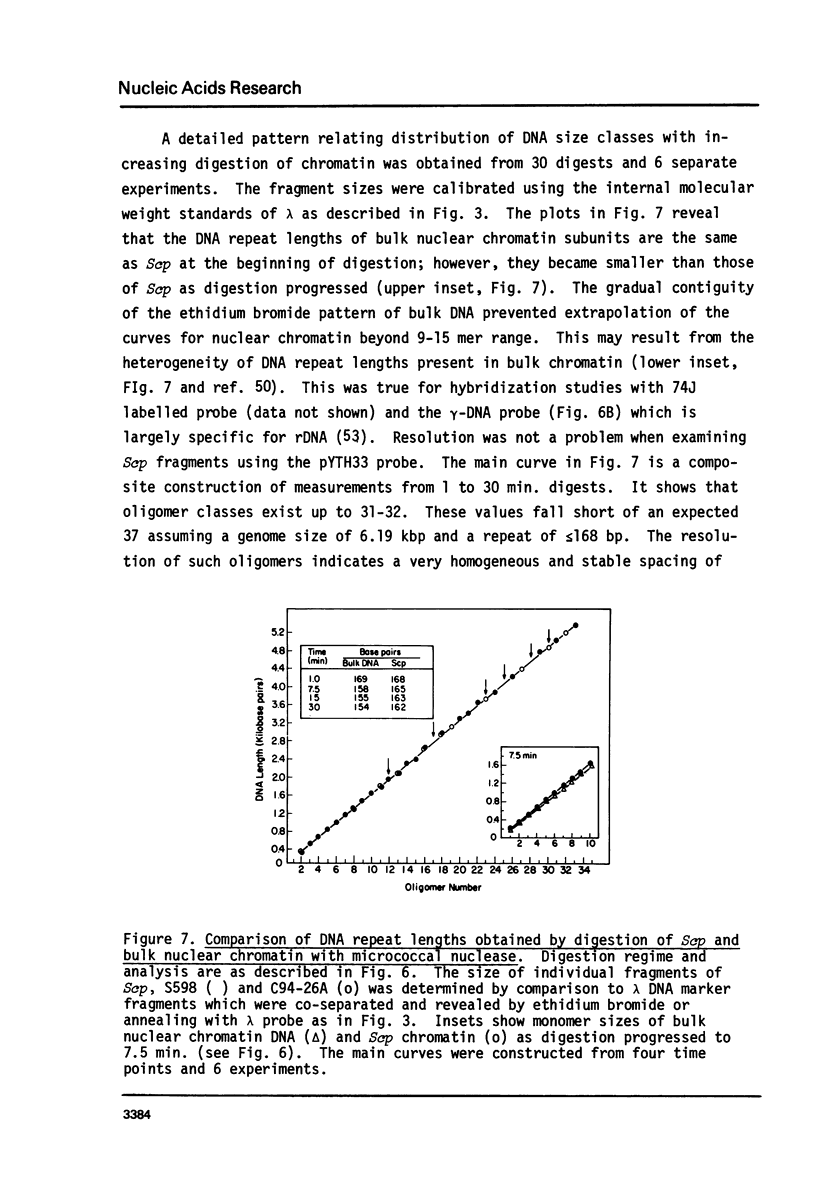
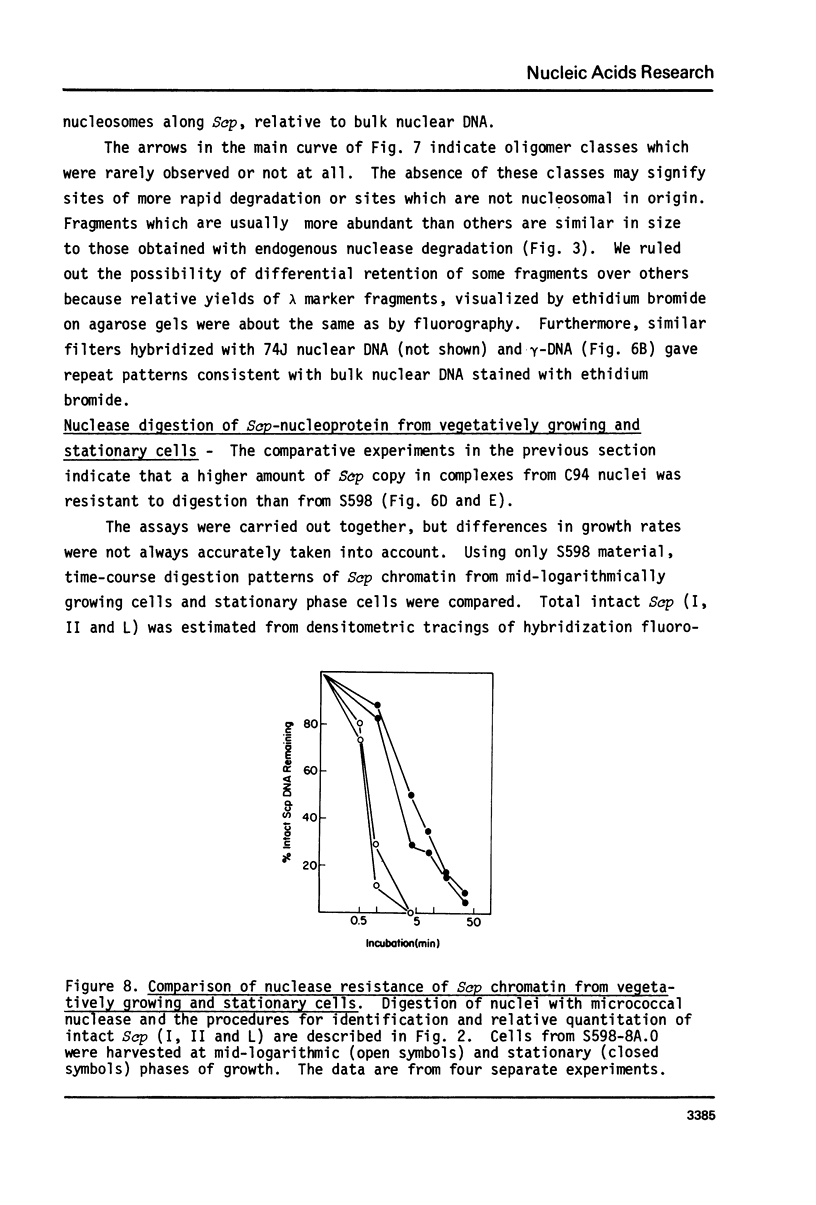
Cell fractions from yeast strains known to harbor the plasmic 2 mum or Scp were treated with nucleases used to probe eukaryotic chromosome structure. Scp and subfragments were identified by hybridization to natural or cloned Scp probes according to Southern (34). Specificity was confirmed with non-Scp probes. Copy/haploid nuclear genome(n) was estimated from reconstructions at a resolution of 0.5/n. About 43-67% of the total cellular copy exists as nucleoprotein complexes which separate from other debris on isokinetic sucrose gradients with s-values of 67-110. These complexes are totally degraded by DNAase I. Digestion with micrococcal nuclease produced integral-sized fragments; they are not generated by direct mixing of pure Scp with nuclear chromatin from a[cir] strain. Initial digests gave a repeat of 168 +/- 3 base pairs (bp) for both Scp and nuclear nucleoprotein; advanced digests reduced the nuclear repeat relative to Scp by 8 bp. Of a potential 37 repeat units/plasmid, 31-32 were directly measured. A strain difference in Scp autodegradation was found. A partial nuclease resistant form was also demonstrated whose abundance was cell strain and growth stage dependent. Both Scp isomers exist in these complexes which are structurally similar to simian viral 40 minichromosomes.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Christiansen C., Christiansen G. Circular, repetitive DNA in yeast. Biochim Biophys Acta. 1972 May 29;269(3):527–530. doi: 10.1016/0005-2787(72)90144-x. [DOI] [PubMed] [Google Scholar]
- Beggs J. D., Guerineau M., Atkins J. F. A map of the restriction targets in yeast 2 micron plasmid DNA cloned on bacteriophage lambda. Mol Gen Genet. 1976 Nov 17;148(3):287–294. doi: 10.1007/BF00332903. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Bell L., Byers B. Occurrence of crossed strand-exchange forms in yeast DNA during meiosis. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3445–3449. doi: 10.1073/pnas.76.7.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billheimer F. E., Avers C. J. Nuclear and mitochondrial DNA from wild-type and petite yeast: circularity, length, and buoyant density. Proc Natl Acad Sci U S A. 1969 Oct;64(2):739–746. doi: 10.1073/pnas.64.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brasch K., Seligy V. L., Setterfield G. Effects of low salt concentration on structural organization and template activity of chromatin in chicken erythrocyte nuclei. Exp Cell Res. 1971 Mar;65(1):61–72. doi: 10.1016/s0014-4827(71)80050-2. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Atkins J. F., McGill C., Chow L. Identification and mapping of the transcriptional and translational products of the yeast plasmid, 2mu circle. Cell. 1979 Apr;16(4):827–839. doi: 10.1016/0092-8674(79)90098-9. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. Isolation of circular DNA from a mitochondrial fraction from yeast. Proc Natl Acad Sci U S A. 1972 Feb;69(2):388–392. doi: 10.1073/pnas.69.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Miklos G. L. Localization and quantification of circular DNA in yeast. Eur J Biochem. 1974 Jan 16;41(2):359–365. doi: 10.1111/j.1432-1033.1974.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D. Size distribution of circular DNA from petite-mutant yeast lacking rho DNA. Eur J Biochem. 1973 Jan 15;32(2):263–267. doi: 10.1111/j.1432-1033.1973.tb02606.x. [DOI] [PubMed] [Google Scholar]
- Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. II. Biochemical and electron microscopic analysis of simian virus 40 virion assembly. J Virol. 1979 Jul;31(1):199–208. doi: 10.1128/jvi.31.1.199-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G. C., Allison D. P., Niyogi S. K. Sites including those of origin and termination of replication are not freely available to single-cut restriction endonucleases in the supercompact form of simian virus 40 minichromosome. Biochem Biophys Res Commun. 1979 Jul 12;89(1):17–25. doi: 10.1016/0006-291x(79)90937-9. [DOI] [PubMed] [Google Scholar]
- Del Giudice L., Wolf K., Sassone-Corsi P., Mazza A. 2 micrometer covalently closed non-mitochondrial circular DNA in the petite-negative yeast Schizosaccharomyces pombe. Mol Gen Genet. 1979 May 4;172(2):165–169. doi: 10.1007/BF00268278. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Gerbaud C., Fournier P., Blanc H., Aigle M., Heslot H., Guerineau M. High frequency of yeast transformation by plasmids carrying part or entire 2-micron yeast plasmid. Gene. 1979 Mar;5(3):233–253. doi: 10.1016/0378-1119(79)90080-5. [DOI] [PubMed] [Google Scholar]
- Gubbins E. J., Newlon C. S., Kann M. D., Donelson J. E. Sequence organization and expression of a yeast plasmid DNA. Gene. 1977 May;1(3-4):185–207. doi: 10.1016/0378-1119(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Paoletti C., Slonimski P. Characterization of a new class of circular DNA molecules in yeast. Biochem Biophys Res Commun. 1971 Feb 5;42(3):550–557. doi: 10.1016/0006-291x(71)90406-2. [DOI] [PubMed] [Google Scholar]
- Guerineau M., Grandchamp C., Slonimski P. P. Circular DNA of a yeast episome with two inverted repeats: structural analysis by a restriction enzyme and electron microscopy. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3030–3034. doi: 10.1073/pnas.73.9.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley J. DNA sequences in a cloned restriction fragment containing the inverted repeat region from yeast 2 micrometer plasmid. FEBS Lett. 1977 Oct 1;82(1):121–124. doi: 10.1016/0014-5793(77)80900-9. [DOI] [PubMed] [Google Scholar]
- Hindley J., Phear G. A. Sequence of 1019 nucleotides encompassing one of the inverted repeats from the yeast 2 micrometer plasmid. Nucleic Acids Res. 1979 Sep 25;7(2):361–375. doi: 10.1093/nar/7.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Hollenberg C. P., Degelmann A., Kustermann-Kuhn B., Royer H. D. Characterization of 2-mum DNA of Saccharomyces cerevisiae by restriction fragment analysis and integration in an Escherichia coli plasmid. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2072–2076. doi: 10.1073/pnas.73.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg C. P., Kustermann-Kuhn B., Royer H. D. Synthesis of high molecular weight polypeptides in Escherichia coli minicells directed by cloned Saccharomyces cerevisiae 2-micron DNA. Gene. 1976;1(1):33–47. doi: 10.1016/0378-1119(76)90005-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P. Mapping of regions on cloned Saccharomyces cerevisiae 2-mum DNA coding for polypeptides synthesized in Escherichia coli minicells. Mol Gen Genet. 1978 Jun 1;162(1):23–34. doi: 10.1007/BF00333847. [DOI] [PubMed] [Google Scholar]
- Lauer G. D., Klotz L. C. Determination of the molecular weight of Saccharomyces cerevisiae nuclear DNA. J Mol Biol. 1975 Jun 25;95(2):309–326. doi: 10.1016/0022-2836(75)90397-6. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hahne S. Isolation of a condensed, intracellular form of the 2-micrometer DNA plasmid of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3727–3731. doi: 10.1073/pnas.76.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M. Inheritance of the 2 micrometer m DNA plasmid from Saccharomyces. Genetics. 1977 May;86(1):73–84. doi: 10.1093/genetics/86.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Klein H. L. Deoxyribonucleic acid sequence organization of a yeast plasmid. J Bacteriol. 1977 Jan;129(1):472–481. doi: 10.1128/jb.129.1.472-481.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Kupfer D. M. Control of Saccharomyces cerevisiae 2microN DNA replication by cell division cycle genes that control nuclear DNA replication. J Mol Biol. 1977 Oct 25;116(2):249–260. doi: 10.1016/0022-2836(77)90215-7. [DOI] [PubMed] [Google Scholar]
- Lohr D., Ide G. Comparison on the structure and transcriptional capability of growing phase and stationary yeast chromatin: a model for reversible gene activation. Nucleic Acids Res. 1979;6(5):1909–1927. doi: 10.1093/nar/6.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D., Van Holde K. E. Organization of spacer DNA in chromatin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6326–6330. doi: 10.1073/pnas.76.12.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardian J. K., Isenberg I. Yeast inner histones and the evolutionary conservation of histone-histone interactions. Biochemistry. 1978 Sep 5;17(18):3825–3833. doi: 10.1021/bi00611a023. [DOI] [PubMed] [Google Scholar]
- Müller W., Gautier F. Interactions of heteroaromatic compounds with nucleic acids. A - T-specific non-intercalating DNA ligands. Eur J Biochem. 1975 Jun;54(2):385–394. doi: 10.1111/j.1432-1033.1975.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Nelson R. G., Fangman W. L. Nucleosome organization of the yeast 2-micrometer DNA plasmid: a eukaryotic minichromosome. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6515–6519. doi: 10.1073/pnas.76.12.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Williamson D. H. Replicating circular DNA molecules in yeast. Cell. 1975 Mar;4(3):249–253. doi: 10.1016/0092-8674(75)90172-5. [DOI] [PubMed] [Google Scholar]
- Pinon R., Leney E. Studies on deoxyribonucleases from Saccharomyces cerevisiae. Characterization of two endonuclease activities with a preference for double-stranded DNA. Nucleic Acids Res. 1975 Jul;2(7):1023–1042. doi: 10.1093/nar/2.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer H. D., Hollenberg C. P. Saccharomyces cerevisiae 2-mum DNA. An analysis of the monomer and its multimers by electron microscopy. Mol Gen Genet. 1977 Feb 15;150(3):271–284. doi: 10.1007/BF00268126. [DOI] [PubMed] [Google Scholar]
- Saunders G. W., Rank G. H., Kustermann-Kuhn B., Hollenberg C. P. Inheritance of multiple drug resistance in Saccharomyces cerevisiae: linkage to leu1 and analyses of 2 micron DNA in partial revertants. Mol Gen Genet. 1979 Aug;175(1):45–52. doi: 10.1007/BF00267854. [DOI] [PubMed] [Google Scholar]
- Sinclair J. H., Stevens B. J., Sanghavi P., Rabinowitz M. Mitochondrial-satellite and circular DNA filaments in yeast. Science. 1967 Jun 2;156(3779):1234–1237. doi: 10.1126/science.156.3779.1234. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens B. J., Moustacchi E. ADN satellite gamma et molécules circulaires torsadées de petite taille chez la levure Saccharomyces cerevisiae. Exp Cell Res. 1971 Feb;64(2):259–266. doi: 10.1016/0014-4827(71)90075-9. [DOI] [PubMed] [Google Scholar]
- Tabak H. F. Absence of 2 micrometer DNA sequences in Saccharomyces cerevisiae Y 379-5D. FEBS Lett. 1977 Dec 1;84(1):67–70. doi: 10.1016/0014-5793(77)81058-2. [DOI] [PubMed] [Google Scholar]
- Tekamp P. A., Valenzuela P., Maynard T., Bell G. I., Rutter W. J. Specific gene transcription in yeast nuclei and chromatin by added homologous RNA polymerases I and II. J Biol Chem. 1979 Feb 10;254(3):955–963. [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Nedospasov S. A., Georgiev G. P. On the structure of eukaryotic, prokaryotic, and viral chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):457–473. doi: 10.1101/sqb.1978.042.01.049. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Smith P., Letnansky K. Yeast chromatin. Preparation from isolated nuclei, histone composition and transcription capacity. Eur J Biochem. 1973 Feb 15;33(1):123–130. doi: 10.1111/j.1432-1033.1973.tb02663.x. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]