Abstract
Background and Aims
Some otherwise promising selections of Actinidia chinensis (kiwifruit) have fruit that are too small for successful commercialization. We have therefore made the first detailed study in diploid kiwifruit of the effects of chromosome doubling induced by colchicine on fruit size, shape and crop loading.
Methods
Flow cytometric analysis of young leaves and chromosome analysis of flower buds and root tips was used to confirm the stability of induced autotetraploids. Fruit weight, size and crop load were measured in the third year after planting in the field and for three consecutive years. DNA fingerprinting was used to confirm the origin of the material.
Key Results
There was a very significant increase in fruit size in induced autotetraploids of different genotypes of A. chinensis. With the commercially important diploid cultivar ‘Hort16A’, most regenerants, Type A plants, had fruit which were much the same shape as fruit of the diploid but, at the same fruit load, were much larger and heavier. Some regenerants, Type B plants, produced fruit similar to ‘fasciated’ fruit. Fruit of the autotetraploids induced from three female red-fleshed A. chinensis selections were also 50–60 % larger than fruit of their diploid progenitors. The main increase in fruit dimensions was in their diameters. These improved fruit characteristics were stable over several seasons.
Conclusions
Chromosome doubling has been shown to increase significantly fruit size in autotetraploid A. chinensis, highlighting the considerable potential of this technique to produce new cultivars with fruit of adequate size. Other variants with differently shaped fruit were also produced but the genetic basis of this variation remains to be elucidated. Autoploids of other Actinidia species with commercial potential may also show improved fruit characteristics, opening up many new possibilities for commercial development.
Keywords: Actinidia chinensis, autotetraploid, chromosome doubling, chromosome number, colchicine, DNA fingerprinting, flow cytometry, fruit shape, fruit size, kiwifruit, red-fleshed kiwifruit, somaclonal variation
INTRODUCTION
Kiwifruit (Actinidia chinensis and A. deliciosa), have only relatively recently been introduced into cultivation and grown as commercial fruit crops (Ferguson and Huang, 2007), and they still make up <0·25 % of world total fresh fruit production (Belrose Inc., 2009). Their unique flavour and flesh colour and their high vitamin C, mineral and dietary fibre content (Ferguson and Huang, 2007), along with other health and wellness factors linked to their high antioxidant activity (Atkinson and MacRae, 2007) and disease-preventing elements, have helped to promote them to consumers.
There is considerable genetic diversity amongst the almost 60 species within the genus Actinidia, particularly in fruit skin type, skin colour, flesh colour and flavour (Cui, 1993; Ferguson and Huang, 2007). This diversity provides many opportunities for developing new types of kiwifruit.
Dioecy, long generation cycles, high heterozygosity and variation in ploidy make hybridization between Actinidia species difficult. Manipulation of ploidy could facilitate kiwifruit improvement (Wu et al., 2011). One method for manipulating plant ploidy levels is in vitro chromosome doubling by using antimitotic agents that disrupt mitosis. Recently, successful chromosome doubling of diploid Actinidia chinensis was achieved by in vitro colchicine treatment of somatic tissues from mature vines combined with use of flow cytometry to identify the autotetraploid plants produced (Wu et al., 2011). Such tetraploids can be used in a kiwifruit breeding programme as parents to facilitate interspecific Actinidia crosses between different ploidy levels (Wu et al., 2011). They also allow us to explore directly the effect of chromosome doubling on the fruit of A. chinensis.
Induction of autotetraploids is a procedure that has been used for at least 70 years (Blakeslee and Avery, 1937) and with several different fruit crops (Hancock, 1997), but there seems to have been little systematic evaluation of fruit quality and morphology or of the mature cropping potential of the induced tetraploids. In general, induced polyploidy has not directly resulted in producing larger fruits for commercial production (Hancock, 1997). Based on our preliminary report (Wu et al., 2009), autotetraploids of some selections may have commercial potential in their own right. In this paper we present systematic data collected over four years (2006–2009) for fruit of colchicine-induced autotetraploids from the yellow-fleshed cultivar ‘Hort16A’, which is diploid, and over two years (2008–2009) for fruit of autotetraploids induced from three red-fleshed female selections of A. chinensis, which are also diploid. Induced autotetraploids from a male of A. chinensis have been used as pollinizers in some experiments but, in this paper, these autotetraploid plants have not been compared in detail with the diploid plant from which they were derived.
MATERIALS AND METHODS
Plant management in the orchard
Regenerated tetraploid plants resulting from in vitro colchicine-treated petioles of Actinidia chinensis Planch. ‘Hort16A’ (Wu et al., 2011) were planted at the Plant & Food Research Orchard, Kerikeri, New Zealand together with grafted plants of the original diploid ‘Hort16A’. Autotetraploid and diploid plants (6:1) were planted randomly at a spacing of 5·5 × 2·5 m. A mixture of two tetraploid A. chinensis males from the Plant & Food Research germplasm collection and a diploid A. chinensis male cultivar (‘Meteor’ or ‘CK3’) (1:2) as pollinizers were planted at a ratio of 1 male : 8 female plants.
Colchicine-induced red-fleshed A. chinensis autotetraploids produced from three female selections (‘Hort22D’, Selection 1 and Selection 2) and one male (Selection 3) (Wu et al., 2011) were planted at the Plant & Food Research Orchard, Te Puke, New Zealand at a spacing of 4 × 0·8 m. The diploid male (‘Meteor’) was used as pollinizer and planted at a ratio of 1 male : 6 female plants along the same row.
Vines were grown on a modified winged T-bar as a support structure. Vines were managed according to standard orchard procedures.
Ploidy determination
Flow cytometry
Ploidy level was checked by flow cytometry as described by Wu and Mooney (2002) and Wu et al. (2011). Leaves of young shoot tips, collected from each plant during late spring and early summer, were used for ploidy level determination. Five shoot tips from each plant were analysed separately. Tetraploid A. chinensis and hexaploid A. deliciosa were used as reference standards; the ploidy of the standards had previously been confirmed by chromosome counting.
Counting of chromosomes in diploid and induced autotetraploids of ‘Hort16A’
Root tips from rooted cuttings or immature flower buds of in vitro cuttings were used for counting chromosomes.
Induction and collection of root tips
Dormant canes of induced autotetraploids and their progenitor diploid ‘Hort16A’ were collected before winter pruning. The canes were immediately cut into four-node lengths. The basal end of each cutting was dipped in Clonex rooting hormone gel (3 g L−1 β-indolylbutrytic acid) (Yates New Zealand Ltd) and the cuttings were then placed in planter bags or small pots containing Daltons potting mix (Daltons, Matamata, New Zealand) and were held in the greenhouse under intermittent mist. After 6 weeks, when roots reached 2–3 cm, their tips were excised between 0900 h and 1100 h, then pre-treated in saturated p-dichlorobenzene for 3·5 h at room temperature, fixed in Carnoy's fixative solution (ethanol : glacial acetic acid, 3 : 1 v/v) overnight at 4 °C, and stored in 70 % ethanol, following the procedure described by Wu and Mooney (2002).
Collection of flower buds
Dormant canes of induced autotetraploids and their progenitor diploid ‘Hort16A’ were collected from the orchard and held at 4 °C for 5 weeks in sealed plastic bags to satisfy winter chilling requirements. The canes were then cut into five-node lengths and placed in water in 0·5-L jars in the greenhouse to allow buds to break dormancy. Water levels were checked every 2 d and the water was replaced every week. It took 4–5 weeks for flower buds to emerge. A week later, two flower buds were collected from each cutting at 1000 h and 1500 h. Anthers were removed and stained with aceto-carmine to check the stage of meiosis. Flower buds at metaphase I to anaphase I and/or telophase II stage were collected for chromosome analysis. Suitable flower buds were fixed and stored following the procedure used for roots.
Staining of chromosomes
A root tip or an anther stored in 70 % ethanol was placed onto a slide with a drop (45 µL) of distilled water, and then most of the water was carefully removed using strips of filter paper, leaving just a small amount of water surrounding the anther or root so that it did not completely dry out. A drop of water was again added and the slide was held at room temperature for 20 min, then the water was again removed with strips of filter paper in the manner described above, and replaced with 20 µL 1 m HCl. The slides were then suspended in a chamber, in which the humidity was maintained with paper towels dampened with distilled water. The humid chamber was held in an incubator at 37 °C for 45 min. The HCl was removed using strips of filter paper and a drop of water was added and removed with strips of filter paper, this procedure being repeated twice. Then 20 µL of an enzyme solution containing 5 % cellulase Onozuka R-10 (Yakult Honsha Co. Ltd) and 1 % pectolyase Y-23 (Seishin Pharm Ltd) in 0·01 M citrate buffer, pH 4·6 (Wu and Mooney, 2002) was added to the slide over the plant sample. Slides were re-suspended in the humid chamber, and held in the incubator at 37 °C for 4 h. A drop of distilled water was added to the side of the drop of enzyme solution and then carefully removed with strips of filter paper without disturbing the plant material. This procedure was repeated another two times to remove all remaining enzyme solution. A drop of fresh fixative solution (3 methanol : 1 acetic acid) was then added. After the plant sample was carefully macerated and spread, it was stained with 5 % Giemsa following the procedure described by Wu and Mooney (2002). A digital camera attached to an Olympus (Vanox-AHBT3) microscope was used to take photographs of chromosomes of disrupted cells at the desired stages.
Crop load and fruit number
Crop load (i.e. total fruit weight), and the total number of fruit and the average fruit weight were determined for each vine over four seasons (2006–2009) for diploid ‘Hort16A’ and its colchicine-induced autotetraploids and over two seasons (2008–2009) for the three genotypes of diploid red-fleshed A. chinensis and their colchicine-induced autotetraploids. All measurements started in the third year after planting.
After total fruit number from each vine was counted and total fruit weight from each vine was measured, a sub-sample of ten fruit was taken at random for determining individual fruit weights, dimensions and quality in the laboratory.
As kiwifruit are generally oval in equatorial cross section, the larger and smaller diameters were recorded. Fruit weight and dimensions were measured with a balance (Mettler Toledo, Switzerland) and digimatic callipers (Mitutoyo, Japan).
Propagation of selected colchicine-induced tetraploids of ‘Hort16A’ by grafting
Budwood of four selected colchicine-induced autotetraploid plants established at the Keriekri Research Orchard and of the progenitor diploid ‘Hort16A’ were grafted onto 2-year-old ‘Bruno’ seedling rootstocks at the Plant & Food Research Orchard, Te Puke in 2007. Four grafted plants of each were placed in a random selection trial at a spacing of 5 × 3 m with ‘Sparkler’, ‘Meteor’ and ‘Bruce’ (all diploid) in equal proportions as pollinizers, planted at a ratio of 1 male : 5 females. Harvest records were collected in 2011 when the fruiting canopy was becoming well established.
DNA fingerprinting
Genomic DNA was extracted from young leaves of 21 regenerants of colchicine-induced tetraploids and three vines of ‘Hort16A’ using DNeasy Plant Mini Kits (Qiagen, Germany) according to the manufacturer's instructions. Young leaves (100 mg) collected in spring from plants grown in the field were used for DNA extraction. Twenty-three fluorescent-labelled primer pairs, including three FPK primer pairs (FPK 722, FPK 723 and FPK 764) (Fraser et al., 2001) used for identification of ‘Hort16A’ and cultivar IP protection, and 20 pairs of Ke primers (Ke111, Ke188, Ke200, Ke211, Ke212, Ke216, Ke220, Ke247, Ke259, Ke264, Ke347, Ke383, Ke386, Ke396, Ke438, Ke455, Ke472, Ke527, Ke598, Ke738), used as microsatellite markers to construct linkage maps of the diploid species A. chinensis (Fraser et al., 2009), were selected for PCR amplification. FPK primers were amplified following Tsang et al. (2007) and separated by electrophoresis on 1 % agarose gels, stained with ethidium bromide and photographed under ultraviolet light (Wu et al., 2005). The 20 remaining Ke primers were amplified following the method of Fraser et al. (2009) and the allelic content of the regenerants and progenitors was determined by capillary electrophoresis in an ABI Prism® 3100 Genetic Analyser (Filter Set D, ROX™ GS500HD size standard), and analysed with GeneMapper™ Software Version 3·0 (Applied Biosystems).
Data analysis
Regression analysis was used to test the relationship between the average fruit weight and natural logarithm of fruit number per vine from all surviving regenerants of autotetraploids and their diploid progenitor for four years using the GLM (general linear model) procedure in SAS software (SAS Institute Inc., 2000–2004). If necessary, autotetraploids were grouped according to their fruit characters in the field.
The pairwise t-test (Fisher's least significant difference test) was used to examine the differences observed between autotetraploids and their diploid progenitors among the same year, or the differences between two years for the autotetraploids or their diploid progenitors.
RESULTS
Stability of ploidy level
‘Hort16A’
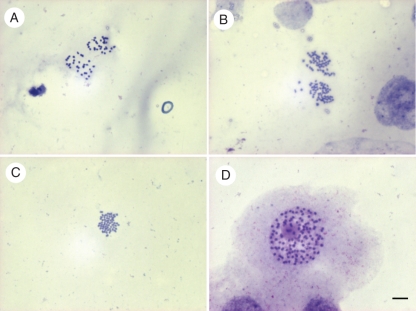
Of the 86 induced autotetraploid regenerants from ‘Hort16A’ that were planted in the orchard, 77 survived to maturity. Over four seasons, these individuals were tested four times by flow cytometry using young leaves from different parts of the vine canopy to confirm that they were indeed consistently tetraploid (data not shown). There was no evidence of chimeras or mixoploidy. The ploidy of selected plants was also confirmed by chromosome counts using root tips and pollen mother cells (Fig. 1).
Fig. 1.
Chromosome numbers from pollen mother cells and root tips of diploid Actinidia chinensis ‘Hort16A’ and its colchicine-induced autotetraploids: (A) meiotic anaphase I in a diploid showing the segregation of 29 chromosomes to each pole; (B) meiotic anaphase I in an autotetraploid showing the segregation of 58 chromosomes to each pole; (C) mitotic metaphase chromosomes of a diploid with 2n = 2x = 58; (D) mitotic metaphase chromosomes of an autotetraploid with 2n = 4x = 116. Scale bar = 10 µm.
Red-fleshed genotypes
A total of 98 colchicine-induced tetraploid regenerants from three females and one male selected from the Plant & Food Research red-fleshed A. chinensis breeding programme survived in the orchard: 20 autotetraploids induced from ‘Hort22D’, 32 autotetraploids from Selection 1, 36 autotetraploids from Selection 2, and 10 autotetraploids from the male, Selection 3. Testing by flow cytometry over four years using young leaves from different parts of the canopy indicated that they were all stable tetraploids and there was no evidence of chimeras.
Fruit shape and size
‘Hort16A’: all fruit from all vines
Three quarters (73 %) of the surviving colchicine-induced autotetraploid regenerants from ‘Hort16A’ had fruit that were the same general shape as that of the original diploid ‘Hort16A’ (Fig. 2A), but with much larger and more pronounced ‘beaks’ (stylar ends) (Fig. 2B). The autotetraploid regenerants with such fruit were grouped together and named as ‘Type A’. A quarter of the regenerants (27 %) had much squatter fruit, similar to fasciated fruit, with sunken beaks (Fig. 2C). Autotetraploid regenerants with such fruit were grouped and called ‘Type B’.
Fig. 2.
Fruit shapes and longitudinal and cross sections of diploid Actinidia chinensis ‘Hort16A’ (A), and its colchicine-induced autotetraploids Type A (B) and Type B (C). Scale bar = 1 cm.
Type A fruit were significantly heavier than those of diploid ‘Hort16A’ and the Type B fruit (P < 0·01). The difference in weight between the diploid ‘Hort16A’ and Type B fruit was not significant. These results were consistent over the four consecutive years of the study. The average weight of the Type A fruit over the four years was 148·8 g. Average fruit weight from all the fruit for most individual vines was 140–180 g (fruit data of individual regenerants not shown), although a few vines had particularly large fruit, averaging up to 250 g. By comparison, the average weight of fruit of diploid progenitor ‘Hort16A’ was 94·6 g, within the range 90–120 g for individual vines. The Type B fruit had an average weight of 102 g over the four years (Table 1).
Table 1.
Average fruit weight of diploid Actinidia chinensis ‘Hort16A’ and its colchicine-induced autotetraploids Type A and Type B, with all fruit from all plants from 2006 to 2009
| Group | Ploidy | Fruit weight (g) |
||||
|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | Average | ||
| ‘Hort16A’ | 2x | 96·3 ± 6·0b | 97·6 ± 2·6b | 95·3 ± 3·2b | 89·2 ± 2·8b | 94·6 ± 1·9 |
| Type A | 4x | 145·0 ± 3·4a | 145·9 ± 3·5a | 164·7 ± 3·2a | 140·0 ± 3·1a | 148·8 ± 5·6 |
| Type B | 4x | 102·1 ± 1·1b | 95·7 ± 4·5b | 107·4 ± 3·9b | 103·1 ± 3·1b | 102·1 ± 2·4 |
All data shown are averages ± s.e.
Values followed by different letters within the same column in the same year are significantly different (P < 0·01).
Subsample of fruit from all ‘Hort16A’ vines: average fruit weight from ten-fruit subsamples
Ten fruit (in 2006, 2007 and 2008) were taken as a subsample for quality evaluation. Similar conclusions on average fruit weights could be drawn from the subsamples as those from the total fruit from each vine (data not shown) and ten-fruit subsamples were also used for subsequent studies on fruit quality. In 2009, the subsample was reduced to five fruit but this was probably too small for consistent results (data not shown).
Subsample of fruit from all ‘Hort16A’ vines: fruit size and dimensions from ten-fruit subsamples
Type A fruit were significantly larger than those of their progenitor diploid ‘Hort16A’ and Type B in all three dimensions in 2006, 2007 and 2008 (P < 0·01), with a relatively greater increase in diameter than in length (Table 2). Type B fruit were squatter, with a greater increase in diameter (Table 2): all Type B fruit were significantly shorter than diploid ‘Hort16A’ and Type A fruit (P < 0·01). The ‘beak’ was also much wider (Fig. 2C). Diploid ‘Hort16A’ fruit had the greatest ratio of length to width, significantly greater than those of the Type A and Type B induced autotetraploids in all three years studied (P < 0·01). Type B fruit had the lowest ratio for the all three years (Table 2); the ratio was also significantly lower than that for Type A fruit (P < 0·01). The transverse section of Type A fruit was more likely to be round than that of ‘Hort16A’ fruit as the ratio of width 2 to width 1 was closer to 1.
Table 2.
Average fruit dimensions and ratios of pairs of two fruit dimensions of diploid Actinidia chinensis ‘Hort16A’and its colchicine-induced autotetraploids Type A and Type B, using ten-fruit subsamples from each vine, 2006 –2008
| Year | Group | Ploidy | Fruit dimensions |
Ratios for two fruit dimensions |
||||
|---|---|---|---|---|---|---|---|---|
| Length (mm) | Width 1 (mm) | Width 2 (mm) | Length: width 1 | Length: width 2 | Width 2: width 1 | |||
| 2006 | ‘Hort16A’ | 2x | 71·1 ± 0·6 | 49·7 ± 0·3 | 47·2 ± 0·2 | 1·43 ± 0·01 | 1·51 ± 0·01 | 0·95 ± 0·004 |
| 2007 | ‘Hort16A’ | 2x | 74·4 ± 0·7 | 52·5 ± 0·9 | 48·7 ± 0·8 | 1·43 ± 0·03 | 1·55 ± 0·04 | 0·93 ± 0·009 |
| 2008 | ‘Hort16A’ | 2x | 75·4 ± 1·2 | 51·7 ± 0·8 | 48·0 ± 0·9 | 1·46 ± 0·02 | 1·57 ± 0·02 | 0·93 ± 0·005 |
| Average | 2x | 73·6 ± 1·3 | 51·3 ± 0·8 | 48·0 ± 0·4 | 1·44 ± 0·01 | 1·54 ± 0·02 | 0·93 ± 0·006 | |
| 2006 | Type A | 4x | 76·0 ± 0·8 | 58·6 ± 0·5 | 56·0 ± 0·5 | 1·30 ± 0·01 | 1·36 ± 0·01 | 0·96 ± 0·002 |
| 2007 | Type A | 4x | 81·0 ± 0·6 | 61·6 ± 0·4 | 58·8 ± 0·4 | 1·32 ± 0·01 | 1·38 ± 0·01 | 0·96 ± 0·002 |
| 2008 | Type A | 4x | 86·8 ± 1·2 | 64·0 ± 0·4 | 59·9 ± 0·3 | 1·36 ± 0·02 | 1·45 ± 0·02 | 0·94 ± 0·002 |
| Average | 4x | 81·3 ± 3·1 | 61·4 ± 1·6 | 58·2 ± 1·2 | 1·33 ± 0·02 | 1·40 ± 0·03 | 0·95 ± 0·002 | |
| 2006 | Type B | 4x | 63·0 ± 1·5 | 54·6 ± 0·9 | 51·4 ± 0·8 | 1·15 ± 0·02 | 1·21 ± 0·02 | 0·95 ± 0·006 |
| 2007 | Type B | 4x | 64·0 ± 1·3 | 57·1 ± 0·8 | 53·0 ± 0·6 | 1·13 ± 0·02 | 1·21 ± 0·02 | 0·93 ± 0·005 |
| 2008 | Type B | 4x | 66·1 ± 1·1 | 59·0 ± 1·0 | 54·8 ± 0·7 | 1·13 ± 0·02 | 1·21 ± 0·02 | 0·93 ± 0·006 |
| Average | 4x | 64·4 ± 0·9 | 56·9 ± 1·3 | 53·1 ± 1·0 | 1·13 ± 0·01 | 1·21 ± 0·002 | 0·93 ± 0·005 | |
Width 1 is the greater equatorial diameter; width 2 is the lesser equatorial diameter.
All the data shown are averages ± s.e.
There were small differences of fruit size and dimensions between years within each group of fruit, but the trends in fruit size and dimensions were consistent across years.
Fruit weight in four Type A induced autotetraploids of ‘Hort16A’ propagated by grafting
The increased fruit weight of the selected Type A colchicine-induced autotetraploid regenerants of ‘Hort16A’ was retained on propagation by grafting (Table 3). Otherwise, no obvious differences were noted in morphology or vine growth between ‘Hort16A’ and the selected autotetraploid regenerants.
Table 3.
Average fruit weight of ‘Hort16A’ and four colchicine-induced autotetraplod Type A regenerants grafted into a selection trial at the Plant & Food Research Orchard, Te Puke (data are for the 2011 harvest 4 years after grafting)
| Plant | Ploidy | Fruit weight (g) |
|---|---|---|
| ‘Hort16A’ | 2x | 98·8 ± 1c |
| Regenerant 1 | 4x | 191·4 ± 2·7a |
| Regenerant 2 | 4x | 194·3 ± 3·1a |
| Regenerant 3 | 4x | 197·0 ± 3·1a |
| Regenerant 4 | 4x | 170·0 ± 2·6b |
The fruit weight data shown are averages ± s.e.
Values followed by different letters within the fruit weight column are significantly different (P < 0·01).
Red-fleshed genotypes
Induced autotetraploid vines of three female red-fleshed genotypes had much larger fruit (individual vine averages 100–120 g), 50 − 60 % greater (P < 0·01) than fruit of their respective diploid progenitors, which averaged 60–90 g (Table 4). There was not the same variation in fruit shape as in autotetraploids from ‘Hort16A’. The increase in fruit weight was due mainly to an increase in fruit diameter, not in fruit length (Table 5 and Fig. 3). As a result, fruit of all autotetraploids had significantly lower ratios of length to diameters than did fruit of the respective diploid progenitors (P < 0·01) (Table 5). Furthermore, the two transverse diameters became more equal and the fruit of the autotetraploids therefore became more round in cross section than those of their diploid progenitors.
Table 4.
Average fruit weight of three genotypes of diploid red-fleshed Actinidia chinensis and their colchicine-induced autotetraploids, 2007–2009
| Fruit weight (g) |
||||
|---|---|---|---|---|
| Genotype | Ploidy | 2007 | 2008 | 2009 |
| ‘Hort22D’ | 2x | 99·4 ± 6·1b | 63·0 ± 1·4b | 87·6 ± 3·0b |
| ‘Hort22D’ | 4x | 113·6 ± 8·3a | 101·2 ± 2·6a | 135·6 ± 4·0a |
| Selection 1 | 2x | no data | 66·0 ± 2·8b | 72·0 ± 0·9b |
| Selection 1 | 4x | no data | 102·3 ± 2·4a | 118·4 ± 9·7a |
| Selection 2 | 2x | 73·8 ± 5·1b | 77·2 ± 2·3b | 84·4 ± 0·0b |
| Selection 2 | 4x | 130·0 ± 3·3a | 99·9 ± 1·9a | 138·4 ± 5·3a |
The fruit weight data shown are averages ± s.e.
Values followed by different letters within the same column for the same genotype in the same year are significantly different (P < 0·01).
Table 5.
Average fruit dimensions and ratios of pairs of two fruit dimensions of three genotypes of diploid red-fleshed Actinidia chinensis and their colchicine-induced autotetraploids in 2008
| Genotype | Ploidy | Fruit dimensions |
Ratios for two fruit dimensions |
||||
|---|---|---|---|---|---|---|---|
| Length (mm) | Width 1 (mm) | Width 2 (mm) | Length: width 1 | Length: width 2 | Width 2: width 1 | ||
| ‘Hort22D’ | 2x | 48·2 ± 0·5a | 47·1 ± 0·5b | 42·1 ± 0·3b | 1·02 ± 0·01a | 1·14 ± 0·01a | 0·89 ± 0·01a |
| ‘Hort22D’ | 4x | 48·4 ± 0·5a | 58·5 ± 0·6a | 54·1 ± 0·4a | 0·83 ± 0·01b | 0·90 ± 0·01b | 0·93 ± 0·01a |
| Selection 1 | 2x | 56·5 ± 0·9a | 44·3 ± 0·6b | 42·6 ± 0·7b | 1·28 ± 0·03a | 1·34 ± 0·03a | 0·96 ± 0·01a |
| Selection 1 | 4x | 55·0 ± 0·6a | 54·9 ± 0·4a | 52·5 ± 0·4a | 1·00 ± 0·01b | 1·05 ± 0·01b | 0·95 ± 0·00a |
| Selection 2 | 2x | 58·8 ± 0·5a | 46·4 ± 0·7b | 43·6 ± 0·9b | 1·27 ± 0·02a | 1·35 ± 0·03a | 0·93 ± 0·01a |
| Selection 2 | 4x | 55·4 ± 0·4b | 54·2 ± 0·4a | 51·8 ± 0·3a | 1·02 ± 0·01b | 1·07 ± 0·01b | 0·95 ± 0·00a |
Width 1 is the greater equatorial diameter; width 2 is the lesser equatorial diameter.
All the data shown are averages ± s.e.
Values followed by different letters within the same column for the same genotype are significantly different (P < 0·01).
Fig. 3.
External view and longitudinal and equatorial cross sections of fruit: (A, B) original diploid Actinidia chinensis ‘Hort22D’ (A) and its colchicine-induced autotetraploid (B); (C, D) original diploid Actinidia chinensis Selection 1 (C) and its colchicine-induced autotetraploid (D); (E, F) original diploid Actinidia chinensis Selection 2 (E) and its colchicine-induced autotetraploid (F). Scale bar = 1 cm.
Fruit weight and fruit number in colchicine-induced autotetraploids and their diploid progenitors
‘Hort16A’
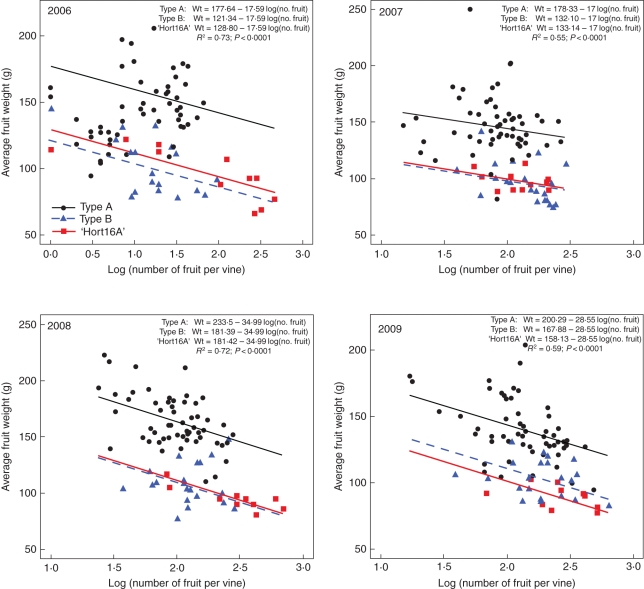
The relationship between average fruit weight and total number of fruit from a vine or regenerant (original diploid ‘Hort16A’ and the autotetraploid Types A and B) has been analysed from data for four consecutive years (2006–2009). As fruit number increased, average fruit weight tended to decrease. However, at the same crop load, average fruit weight of Type A was significantly greater than that of their progenitor diploid ‘Hort16A’ (P < 0·01) and Type B (P < 0·01) in all four years, whereas Type B fruit weight did not significantly differ from that of their progenitor diploid ‘Hort16A’ (Fig. 4).
Fig. 4.
Relationship of average fruit weight and natural logarithm of fruit number per vine or regenerant for all vines of diploid Actinidia chinensis ‘Hort16A’ and all regenerants of its colchicine-induced autotetraploids Type A and Type B in the 2006, 2007, 2008 and 2009 seasons separately. A single model incorporating the three types of fruit was fitted to each season's data. R2 and the P-value are given for each single model.
Red-fleshed genotypes
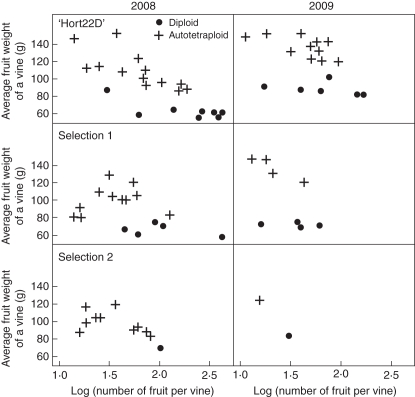
From two years' preliminary evaluation of three genotypes of red-fleshed induced autotetraploids, all autotetraploid regenerants had an average fruit weight significantly heavier than that of their respective diploid progenitor at the same crop load (P < 0·01) (Fig. 5). A difference existed between years. The weight of fruit in 2009 from all regenerants of three genotypes was heavier than that in 2008. There were insufficient numbers of the diploid progenitor plants as controls in the Selection 2 planting, which meant that it was not possible to analyse the results and as a consequence this genotype was excluded from fruit analysis.
Fig. 5.
Scatter plots of average fruit weight and natural logarithm of fruit number per vine for all vines from three genotypes of diploid red-fleshed Actinidia chinensis and their colchicine-induced autotetraploids in the 2008 (left) and 2009 (right) seasons separately.
Fingerprinting analysis of ‘Hort16A’ and the two types of induced tetraploid
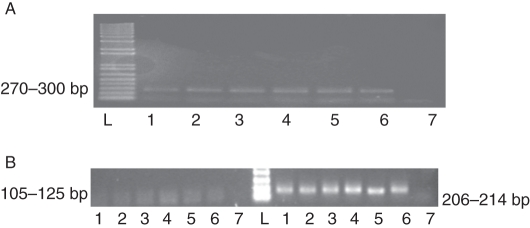
The separation of colchicine-induced autotetraploids from ‘Hort16A’ into two distinct types (Type A and Type B) based on fruit shape and size raised questions as to the origin of the plant material used for chromosome doubling – could there have been confusion in the labelling of cultures? Fourteen Type A regenerants, seven Type B regenerants and three vines of the progenitor diploid ‘Hort16A’ were sampled for DNA fingerprinting. The banding profiles produced by the three FPK primers of the sampled induced autotetraploids regenerated from ‘Hort16A’ matched that of diploid ‘Hort16A’ (Fig. 6). No differences were found using another 20 pairs of Ke microsatellite primers, as they amplified the same-sized alleles in all samples of the two types of induced autotetraploid regenerants and their progenitor diploid ‘Hort16A’ (data not shown). There was no evidence that the two types of induced autotetraploid differed at all in their origin: the results are consistent with all tetraploid regenerants having been derived from diploid ‘Hort16A’.
Fig. 6.
DNA fingerprinting analysis using three primers, (A) FPK 764 (270–300 bp), and (B) FPK 722 (105–125 bp) and FPK 723 (206–214 bp), for diploid Actinidia chinensis ‘Hort16A’ (lane 6) and its colchicine-induced autotetraploids Type A (lanes 1–4) and Type B (lane 5), control without any DNA template (lane 7), and 1 kb plus ladder (lane L).
DISCUSSION
Fruit size is important commercially, as customers pay a premium for larger kiwifruit (Belrose Inc., 2009). Maximizing fruit growth and, therefore, the proportion of premium-sized fruit, is a key objective for growers (Minchin et al., 2003; Chamberlain et al., 2010). Cultural practices that increase fruit size have been integrated into orchard management, including the use of plant growth regulators and chemical sprays (Woolley and Cruz-Castillo, 2006; Famiani et al., 2007), trunk and cane girdling (Woolley and Cruz-Castillo, 2006; Assar et al., 2009; Chamberlain et al., 2010) and fruit thinning (Lahav et al., 1989; Richardson et al., 2007). Even when such practices are used, fruit size has still been a concern in some years (Patterson et al., 2003). Furthermore, it is unlikely that all these management techniques can be maintained long term: excessive application of plant growth regulators to increase kiwifruit size can result in a marked deterioration in fruit quality (Jiang et al., 2009); the use of plant growth regulators and chemical sprays is under scrutiny by consumers owing to food safety issues and environmental concerns; organic orchards are not able to use plant growth regulators; girdling and fruit thinning also carry labour costs and there are some doubts as to the long-term effects of girdling on kiwifruit vines.
Fruit of Actinidia species vary greatly in size, colour and nutrient content. Some species or genotypes have good quality fruit and/or novel flesh colours and/or novel flavours but their fruit size is not sufficient for commercial marketing, e.g. many red-fleshed selections of A. chinensis (Cheng et al., 2007; Ferguson, 2009). None of the Actinidia hybrids so far produced by crossing species at different ploidy levels (diploid and tetraploid) has been considered for use as a commercial cultivar. The hybrids often have traits that have commercial potential but are currently lacking in kiwifruit cultivars and the successful commercialization of such hybrids is dependent on the ability to develop plants with larger fruit, heavier crop loads and better fertility (Beatson et al., 2007). Our results confirm that ploidy manipulation could be a way of solving some of these problems.
With ‘Hort16A’, fruit of the induced autotetraploids (Type A) are on average 50–60 % larger than those of diploid progenitors, but on some autotetraploid vines, they are as much as 100 % bigger. It would be difficult to achieve such large fruit with diploid ‘Hort16A’ through management techniques or by normal hybridization at the diploid level. The larger fruit of autotetraploids are not simply a result of reduced crop load, although the autotetraploid vines do follow the usual trend that fruit size increases as the number of fruit decreases. The changes observed are genetically and morphologically stable on vegetative propagation. We do not know whether chromosome doubling has resulted in cell proliferation or in larger cells nor do we know why there are such marked differences in fruit size among the individual autotetraploid regenerants.
Fruit size is also often a critical commercial trait in other fruit crops, e.g. apple, blueberry, grape, strawberry and tomato. Final fruit size is determined by cell proliferation and cell expansion. Endoreduplication (replication of the nuclear genome without cell division) results in an increase in ploidy and is often correlated with increases in cell size (Malladi and Hirst, 2010). Fruit weight in turn can be correlated with the degree of endoreduplication and cell size (Cheniclet et al., 2005). It is likely that there is a common mechanism by which endoreduplication and induced polyploidization both increase fruit weight.
Fruit shape also changed on chromosome doubling. Type A autotetraploid regenerants from ‘Hort16A’ had fruit which were larger in all dimensions, but with a relatively larger increase in equatorial diameters than in length. This resulted in the fruit appearing less cylindrical and rounder in cross section. Fruit from autotetraploid regenerants of red-fleshed A. chinensis genotypes were more spherical, less cylindrical than fruit of their diploid progenitors. Chromosome doubling could be a way of modifying fruit shape in some diploid selections with long, cylindrical fruit. The diploid progenitors ‘Hort22D’ and Selection 2 had fruit that were noticeably flat. Their autotetraploid regenerants had fruit that were more spherical as well as being larger. This could make them more readily accepted commercially than the small flat fruit of their diploid progenitors.
The autotetraploid Type B vines from ‘Hort16A’, had fruit similar to fasciated fruit, which are commercially unacceptable. Such ‘fan’ or ‘flat’ fruit have been observed in both A. deliciosa and A. chinensis and they appear to result from fusion of the terminal floral bud with one or more of the lateral buds (Brundell, 1975; Cooper and Marshall, 1986; Watson and Gould, 1993, 1994). The fruit of the Type B vines were quite different. All their fruit of these vines were misshapen and, in general, there was no indication that they resulted from flower fusion. We are, however, confident that both Type A and Type B autotetraploids were derived from the one genotype, ‘Hort16A’. What then is the cause of the difference? Is it environmental, is it a delayed effect of the colchicine or is it a response to regeneration by tissue culture? It seems unlikely that the cause is environmental because this type of fruit appears consistently on the same vines year by year. Has the mutation from these types of fruit been isolated with stable mutation after in vitro tissue culture? This would imply that ‘Hort16A’ could be a chimera or genetically unstable. Although unlikely, this is not impossible, as at least one kiwifruit genotype has been shown to be such a chimera: the male rootstock selection from Italy, ‘D uno’ (‘D1’) is a plastid periclinal chimera whose existence was revealed only by molecular studies (Chat et al., 2002). Colchicine treatment has been shown to induce mutation in ryegrass (Hague and Jones, 1987). Epigenetic and genetic variation has also often been detected as a result of in vitro tissue culture (Bairu et al., 2011; Smulders and de Klerk, 2011), including the appearance of fasciated organs (Iliev and Kitin, 2011). A better understanding of the origin of the two types of autotetraploids from ‘Hort16A’ will probably require studies at the molecular level (Yang et al., 2011). No such ‘fasciated’ fruit have been observed in any of the induced autotetraploid regenerants from red-fleshed A. chinensis, although their fruit shape was changed on chromosome doubling.
Generally tetraploid plants show some characteristic vegetative morphological features, e.g. colchicine-induced autotetraploid Citrus plants have broader, thicker leaf blades which are deep green (Wu and Mooney, 2002). However, we have not observed any differences in vegetative morphology between induced tetraploid A. chinensis regenerants and their respective diploid progenitors. In Actinidia, difference in ploidy cannot be consistently predicted by differences in morphology (Wu et al., 2011).
Doubling the chromosome number of diploid kiwifruit selections resulted in an increase in fruit size and also affected fruit shape. We do not yet know whether other increases in ploidy, e.g. from tetraploid to octoploid, would have a similar effect on fruit size, or whether chromosome doubling would have a similar effect in other Actinidia species. This remains to be tested. Fruit quality after chromosome doubling will be discussed in future papers.
In conclusion, colchicine-induced autotetraploids of A. chinensis appeared to be genetically stable over four consecutive years of ploidy determination by flow cytometry and chromosome counting. Doubling the number of chromosomes of four female diploid A. chinensis genotypes resulted in significantly larger fruit. The fruit of the autotetraploid regenerants were, on average, 50–60 % larger than fruit of their respective diploid progenitors, a much bigger increase than would be produced by vine management techniques. The fruit of autotetraploids are more likely to be round in transverse section than those of their diploid progenitors. Both fruit size and fruit shape might therefore be improved by chromosome doubling and the change is stable on vegetative propagation. Variation in fruit characteristics exists among each group of autotetraploid fruit. A wider range of autotetraploids should yield more desirable fruit types for selection.
ACKNOWLEDGEMENTS
We thank Tim Lawrence, Erik Rikkerink and Gianna Tsang for assistance with DNA fingerprinting, Arier Lee and Nihal de Silva with initial statistics analysis, Hong Ding with flow cytometer operation, Tony Corbett, Donna Gibson and Tim Holmes for photography and design, Canhong Cheng, Alison Duffy, Roger Hamilton, Tim Machin, Philip Martin, Meng Meng, Peter Sutton, Eric Rui Wu and Hao Wu for harvesting fruit and collecting some data, Anne Gunson for editing, and Nihal de Silva and Dan Cohen for critical comments on the manuscript.
LITERATURE CITED
- Assar P, Eshghi S, Tafazoli E, Rahemi M, Khazaeipoul YG, Monfared AS. Improving fruit quality in ‘Hayward’ kiwifruit using proper leaf to fruit ratios and girdling. Horticulture, Environment and Biotechnology. 2009;50:481–486. [Google Scholar]
- Atkinson RG, MacRae EA. Kiwifruit. In: Pua EC, Davey MR, editors. Biotechnology in agriculture and forestry. Vol. 60. Transgenic crops V. Berlin: Springer-Verlag; 2007. pp. 329–346. [Google Scholar]
- Bairu MW, Aremu AO, van Staden J. Somaclonal variation in plants: causes and detection methods. Plant Growth Regulation. 2011;63:147–173. [Google Scholar]
- Beatson RA, Datson PM, Harris-Virgin PM, Graham LT. Progress in the breeding of novel interspecific Actinidia hybrids. Acta Horticulturae. 2007;753:147–151. [Google Scholar]
- Belrose Inc. World kiwifruit review. 2009 edn. Pullman, WA, USA: Belrose Inc.; 2009. [Google Scholar]
- Blakeslee AF, Avery AG. Methods of inducing doubling of chromosomes in plants: by treatment with colchicine. The Journal of Heredity. 1937;28:393–411. [Google Scholar]
- Brundell DJ. Flower development of the Chinese gooseberry (Actinidia chinensis Planch.). II. Development of the flower bud. New Zealand Journal of Botany. 1975;13:485–496. [Google Scholar]
- Chamberlain J, Drummond L, Adams J, Parkes B, Mowat A. Key outcomes of ZESPRI'S innovation programme. New Zealand Kiwifruit Journal. 2010;198:21–25. [Google Scholar]
- Chat J, Decroocq S, Decroocq V, Petit RJ. A case of chloroplast heteroplasmy in kiwifruit (Actinidia deliciosa) that is not transmitted during sexual reproduction. The Journal of Heredity. 2002;93:293–300. doi: 10.1093/jhered/93.4.293. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Seal AG, Murphy SJ, Lowe RG. Red-fleshed kiwifruit (Actinidia chinensis) breeding in New Zealand. Acta Horticulturae. 2007;753:139–146. [Google Scholar]
- Cheniclet C, Rong WY, Causse M, et al. Cell expansion and endoreduplication show a large genetic variability in pericarp and contribute strongly to tomato fruit growth. Plant Physiology. 2005;139:1984–1994. doi: 10.1104/pp.105.068767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K, Marshall R. Why so many flats and fans? New Zealand Kiwifruit Journal. 1986;39:23–26. [Google Scholar]
- Cui ZX. Zhongguo Mihoutao [Actinidia in China] Jinnan: Shandong Science and Technology Publisher; 1993. [Google Scholar]
- Famiani F, Proietti P, Pilli M, Battistelli A, Moscatello S. Effects of application of thidiazuron (TDZ), gibberellic acid (GA3), and 2,4-dichlorophenoxyacetic acid (2,4-D) on fruit size and quality of Actinidia deliciosa ‘Hayward. New Zealand Journal of Crop and Horticultural Science. 2007;35:341–347. [Google Scholar]
- Ferguson AR. Kiwifruit cultivars, 2009. Italus Hortus. 2009;16(5):252–258. [Google Scholar]
- Ferguson AR, Huang H. Genetic resources of kiwifruit: domestication and breeding. Horticultural Reviews. 2007;33:1–121. [Google Scholar]
- Fraser LG, Harvey CF, Gill GP. Application of microsatellite-based DNA profiling in Actinidia species. Acta Horticulturae. 2001;546:401–405. [Google Scholar]
- Fraser LG, Tsang GK, Datson PM, et al. A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genomics. 2009;10:102. doi: 10.1186/1471-2164-10-102. doi:10.1186/1471-2164-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague LM, Jones RN. Cytogenetics of Lolium perenne. 4. Colchicine induced variation in diploids. Theoretical and Applied Genetics. 1987;74:233–241. doi: 10.1007/BF00289974. [DOI] [PubMed] [Google Scholar]
- Hancock JF. The colchicine story. HortScience. 1997;32:1011–1012. [Google Scholar]
- Iliev I, Kitin P. Origin, morphology, and anatomy of fasciation in plants cultured in vivo and in vitro. Plant Growth Regulation. 2011;63:115–129. [Google Scholar]
- Jiang Z, Huang H, Zhong C, Zhang Z, Wang S. The development of the Chinese kiwifruit industry. Italus Hortus. 2009;16(5):245–251. [Google Scholar]
- Lahav E, Korkin A, Adar G. Thinning stage influences fruit size and yield of kiwifruit. HortScience. 1989;24:438–440. [Google Scholar]
- Malladi A, Hirst PM. Increase in fruit size of a spontaneous mutant of ‘Gala’ apple (Malus × domestica Borkh.) is facilitated by altered cell production and enhanced cell size. Journal of Experimental Botany. 2010;61:3003–3013. doi: 10.1093/jxb/erq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin PEH, Richardson AC, Patterson KJ, Martin PJ. Prediction of final weight for Actinidia chinensis ‘Hort16A’ fruit. New Zealand Journal of Crop and Horticultural Science. 2003;31:147–157. [Google Scholar]
- Patterson K, Burdon J, Lallu N. ‘Hort16A’ kiwifruit: progress and issues with commercialisation. Acta Horticulturae. 2003;610:267–273. [Google Scholar]
- Richardson AC, Dawson TE, Kelly H, Boldingh H, MacRae EA. Modifying carbon partitioning in ‘Hort16A’ fruit. Acta Horticulturae. 2007;753:397–398. [Google Scholar]
- SAS Institute Inc. Cary, NC: SAS Institute Inc; 2000–2004 SAS 9·1·3 Help and documentation. [Google Scholar]
- Smulders MJM, de Klerk GJ. Epigenetics in plant tissue culture. Plant Growth Regulation. 2011;63:137–146. [Google Scholar]
- Tsang GK, Fraser LG, McNeilage MA, De Silva HN, MacRae EA. Genetic markers: their transferability and usefulness across Actinidia. Acta Horticulturae. 2007;753:177–184. [Google Scholar]
- Watson M, Gould KS. The development of fruit shape in kiwifruit: growth characteristics and positional differences. Journal of Horticultural Science. 1993;68:185–194. [Google Scholar]
- Watson M, Gould KS. Development of flat and fan-shaped fruit in Actinidia chinensis var. chinensis and Actinidia deliciosa. Annals of Botany. 1994;74:59–68. doi: 10.1093/aob/74.1.59. [DOI] [PubMed] [Google Scholar]
- Woolley D, Cruz-Castillo JG. Stimulation of fruit growth of green and gold kiwifruit. Acta Horticulturae. 2006;727:291–293. [Google Scholar]
- Wu J-H, Mooney P. Autotetraploid tangor plant regeneration from in vitro Citrus somatic embryogenic callus treated with colchicine. Plant Cell, Tissue and Organ Culture. 2002;70:99–104. [Google Scholar]
- Wu J-H, Ferguson AR, Mooney PA. Allotetraploid hybrids produced by protoplast fusion for seedless triploid Citrus breeding. Euphytica. 2005;141:229–235. [Google Scholar]
- Wu J-H, Ferguson AR, Murray BG. In vitro induction of autotetraploid Actinidia plants and their field evaluation for crop improvement. Acta Horticulturae. 2009;829:245–250. [Google Scholar]
- Wu J-H, Ferguson AR, Murray BG. Manipulation of ploidy for kiwifruit breeding: in vitro chromosome doubling in diploid Actinidia chinensis Planch. Plant Cell, Tissue and Organ Culture. 2011;106:503–511. [Google Scholar]
- Yang X, Ye C-Y, Cheng Z-M, et al. Genomic aspects of research involving polyploid plants. Plant Cell, Tissue and Organ Culture. 2011;104:387–397. [Google Scholar]