Abstract
Background and Aims
Obligate root parasitic plants of the Orobanchaceae do not germinate unless they chemically detect a host plant nearby. Members of this family, like Orobanche, Phelipanche and Striga, are noxious weeds that cause heavy damage to agriculture. In spite of their economic impact, only a few light microscopical studies of their minute seeds have been published, and there is no knowledge of their ultrastructure and of the role each tissue plays during the steps preceding germination. This paper describes the ultrastructure of Phelipanche seeds and contributes to our understanding of seed tissue function.
Methods
Seeds of P. aegyptiaca were examined under light, scanning electron, transmission electron and fluorescence microscopy following various fixations and staining protocols. The results were interpreted with physiological data regarding mode of water absorption and germination stimulation.
Key Results and Conclusions
The endothelium, which is the inner layer of the testa, rapidly absorbs water. Its interconnected cells are filled with mucilage and contain labyrinthine walls, facilitating water accumulation for germination that starts after receiving germination stimuli. Swelling of the endothelium leads to opening of the micropyle. The perisperm cells underneath this opening mediate between the rhizosphere and the embryo and are likely to be the location for the receptors of germination stimuli. The other perisperm cells are loaded with lipids and protein bodies, as are the endosperm and parts of the embryo. In the endosperm, the oil bodies fuse with each other while they are intact in the embryo and perisperm. Plasmodesmata connect the perisperm cells to each other, and the cells near the micropyle tightly surround the emerging seedling. These perisperm cells, and also the proximal embryo cells, have dense cytoplasmic contents, and they seem to represent the two seed components that are actively involved in transfer of reserve nutrients to the developing seedling during germination.
Keywords: Seed ultrastructure, seed coat, transfer cells, mucilage, germination, micropyle opening, Orobanchaceae, Phelipanche, root parasite, oil bodies
INTRODUCTION
One of the most specialized seed germination mechanisms belongs to some obligatory root parasitic angiosperms, including the genera Orobanche, Phelipanche and Striga, which do not germinate unless they detect the presence of a host plant nearby. These flowering plants are obligate root parasites and totally depend on a specific association with a host that provides them with nutrients and water, at least during the first stages of their life. Thus, the recognition system ensures that germination starts only when a suitable host root is available in the immediate vicinity. Structurally, these seeds are unique in many respects. They are very small, about 0·25 mm long (Fig. 1A), and contain only a reduced embryo that is composed of a spherical body without a plumule, radicle or cotyledons (Joel et al., 1995; Teryokhin, 1997). Lipids are the main storage material in the seed. The major fatty acids in the seed lipids of P. aegyptiaca are oleic and linoleic acids, although small amounts of stearic and palmitic acids also are found in dry seeds (Bar-Nun and Mayer, 2002). During germination, the emerging seedling grows to a limited extent, up to a few millimetres. When a host root is reached, the seedling tip develops into an attachment organ, and a haustorium is formed (Joel and Losner-Goshen, 1994). The haustorium penetrates the host root, forming an anatomical and physiological bridge between the parasite and the host (Kuijt, 1969; Losner-Goshen, et al. 1998). Subsequently, the parasite develops a flowering shoot that emerges above soil surface and produces numerous seeds.
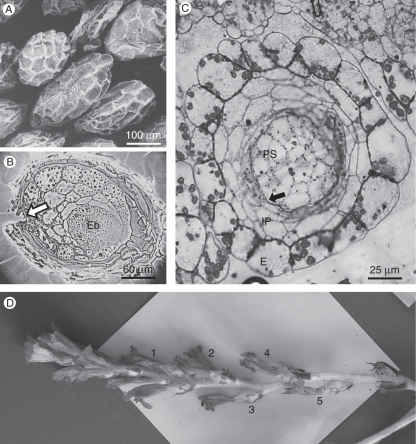
Fig. 1.
Mature and 4-d-old seeds of Phelipanche aegyptiaca. (A) SEM image of mature seeds. (B) Longitudinal section of seed, showing the small artificial gap (arrow) in the seed coat that was made to improve penetration of fixing solutions and embedding media. (C) Anatomical section of young seed, 4 d after pollination. The seed coat is composed of epidermis, inner parenchyma and endothelium (arrow). (D) Flowering stalk of P. aegyptiaca. The young seeds that were taken for microscopical analysis were collected from flowers on node 4. Abbreviations: E, epidermis; Eb, embryo; IP, inner parenchyma; Ps, perisperm.
A chemical stimulus is needed to trigger seed germination. This stimulus normally originates from nearby host roots (Vaucher, 1827; Xie et al., 2010). After imbibition, seeds respond to the germination stimuli only after several days with suitable temperatures. This preparatory period, during which the seed holds water that is needed for germination, is known as ‘seed conditioning’ (Joel et al., 1995, 2005, 2007). It has recently been established that seeds of Phelipanche aegyptiaca and Orobanche cumana are already receptive to germination stimulants before conditioning and that conditioning is not involved in stimulant receptivity (Plakhine et al., 2009).
The weedy obligate root parasites of the Orobanchaceae, and particularly species of the genera Phelipanche, Orobanche and Striga, cause heavy damage to many important crops worldwide (Parker and Riches, 1993; Holm et al., 1997; Joel, 2000; Parker, 2009) and their control is either problematic or impossible (Joel et al., 2007). Seed germination is a bottleneck in the life cycle of these parasitic plants, and therefore much research has been dedicated to physiological and biochemical aspects of the germination of these seeds.
Surprisingly, only few structural analyses of these seeds have been published so far. The most comprehensive structural analysis of seeds in this genus was published by Teryokhin (1997). According to Teryokhin, the main volume of the seed is occupied by the endosperm, with an external endosperm cell layer that is more differentiated. The embryo, which lies in the centre of the seed adjacent to the micropyle, was described as a non-differentiated, simple-spherical or elongated structure devoid of root cap and cotyledons. The seed coat was described as having an external epidermis with thickened radial and inner tangential walls and an inner ‘integumental tapetum’ or ‘endothelium’ with a cutinized inner tangential wall, and one or two additional parenchymatous layers in between (Kumar, 1977; Teryokhin, 1997).
To further understand seed structure and to elaborate the role of each seed component before and during germination, a more detailed analysis of the seed is required. This has been the objective of our light- and electron-microscopical study of P. aegyptiaca seeds.
MATERIALS AND METHODS
Plant material
Young seeds
Plants of Phelipanche aegyptiaca Pomel (syn. Orobanche aegyptiaca Pers.; Joel, 2009) were grown in a greenhouse at Newe-Ya'ar Research Center, from seeds that were collected in a tomato field in Israel. Living flowering stalks were taken to the laboratory (Fig. 1D). Our preliminary study confirmed the results of Musselman et al. (1982) and Teryokhin (1997) that when no cross-pollination of P. aegyptiaca occurs, stamens and stigma contact one another to self pollinate. Each flower remains open for 4 d, during which the position of the stamens and stigma changes to allow cross- and self-pollination. Each day, the flowers of the consecutive nodes close and turn brown. The young seeds for microscopical analysis were taken from node 4 that is shown in Fig. 1D, which is estimated to be about 4–6 d after fertilization. To collect young seeds and to avoid desiccation during dissection, the mature ovary was opened in water under the dissecting microscope. Then, a piece of the placenta with a group of young seeds attached, was removed with a fine needle and immediately immersed into fixing solution, as detailed below.
Mature seeds
These were collected from capsules that opened about 20 d after pollination. The fixation and embedding of mature seeds was difficult. Both fixatives and embedding media could not easily penetrate the mature non-germinated seeds. To overcome this problem, we punctured each seed with a fine needle under the dissecting microscope during early fixation (arrow, Fig. 1B). This allowed proper infiltration, fixation and embedding prior to sectioning.
Light microscopy
For light microscopy, the plant material was fixed in FAA (5 % acetic acid, 4 % formalin, in 50 % ethanol), dehydrated in an ethanol series, and embedded in Paraplast®. The embedded material was sectioned with a Reicher-Jung 1150/Autocut rotary microtome and stained with alcian green–safranin (AGS) (Joel, 1983). Other material was fixed in 2 % paraformaldehyde and 2·5 % glutaraldehyde in 0·1 m sodium cacodylate buffer at pH 7·2 for 2 h, embedded in LR White, sectioned with a glass Ralph knife in a rotary microtome, and stained either in methylene blue–basic fuchsin (Aparicio and Marsden, 1969) or in toluidine blue (O'Brien and McCully, 1981). Additionally, imbibed seeds were cryo-sectioned with the rotary microtome, equipped with a freezing stage, and stained with AGS.
The sections were observed under an Olympus BX61 light microscope, and pictures were taken using an Olympus DP70 digital camera and AnalySIS B-mites image analysis software.
Electron microscopy (SEM and TEM)
Dry seeds were gold-coated and examined under a SEM (Jeol JSM T330).
For TEM, the plant material was fixed for 2 h in 2 % paraformaldehyde and 2·5 % glutaraldehyde in 0·1 m sodium cacodylate buffer at pH 7·2, and post-fixed either in 1 % OsO4 for an additional 1 h, or in KMnO4 for 5 min.
Post-fixation in OsO4 did not allow later infiltration of the embedding media (Epon or LR White) into the embryo. To overcome this problem, we used Spurr's embedding medium (Spurr, 1969), which produced sections of good quality for analysis with an electron microscope.
The embedded material was sectioned in an LKB ultra microtome and stained with uranyl acetate and lead citrate (Hall and Hawes, 1991) before examination under the TEM (Jeol 100CX at 80 kV).
Imbibition
Imbibition was followed by immersing tea bags, each containing 100 mg of P. aegyptiaca seeds (approx. 13 000 seeds), in water for different periods of time, up to 26 h. Each bag was then blotted on filter paper until the seed surface dried, and then its mass was recorded, and the percentage of net increase in seed mass was calculated. The results, which represent five replications, were subject to analysis of variance (ANOVA). Standard error of difference (SED) was used with Student's t-test to assess significant differences.
Route of water absorption by seeds
Movement of water into the mature seed apoplast was visualized by immersing seeds in a 0·1 % calcofluor white (Sigma) solution. Seeds were then washed in water and cryo-sectioned in a Reicher-Jung 1150/autocut rotary microtome equipped with a freezing stage. The sections were imaged using a Zeiss Standard 16 epifluorescence microscope under UV light excitation for calcofluor white localization.
RESULTS AND DISCUSSION
Structure of young seed
Seed development takes about 3 weeks from pollination to maturity. In the young seed sample, which was taken a few days after fertilization, all cells of the developing seed were living. The seed coat, which develops from the single integument (Fig. 1C), is composed of an external epidermis, a two- or three-layered inner parenchyma, and an internal endothelium (arrow) that is composed of flat small cells that face the perisperm. The epidermal cells are the largest, typically bounded by thick inner and lateral cell walls and containing a large vacuole surrounded by a thin cytoplasmic layer that contains large starch grains (Fig. 1C). The inner parenchyma is composed of one to three layers of thin-walled cells containing a large vacuole (Fig. 2A).
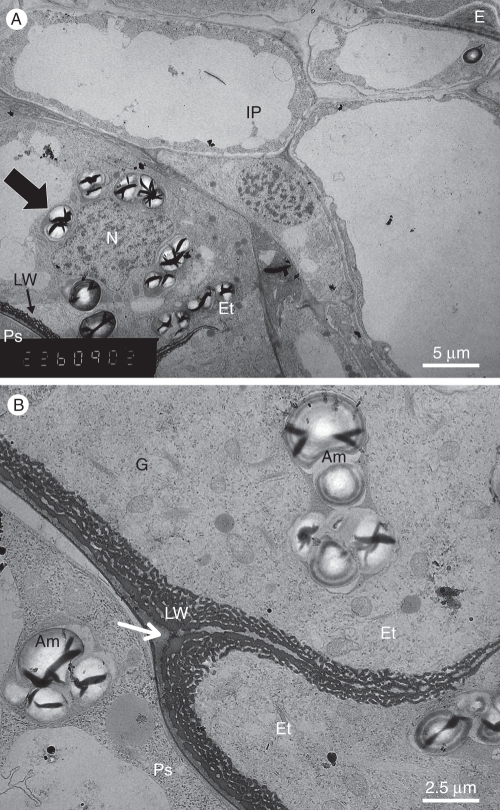
Fig. 2.
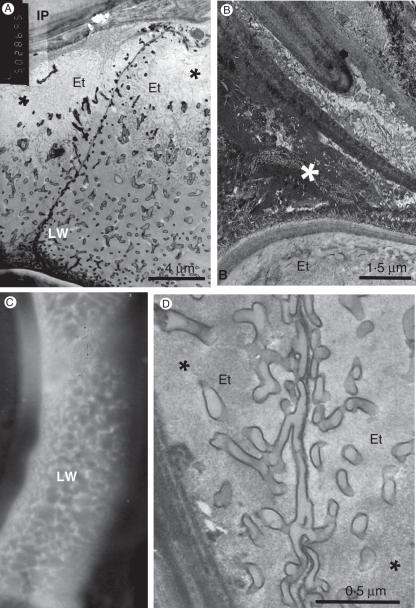
TEM image of the young seed that is shown in Fig. 1C. (A) The seed coat (arrow indicates amyloplast). (B) Details of the young endothelium shown in (A) showing two endothelial cells on the left, adjacent to a perisperm cell on the right. The arrow indicates the endothelial cuticle. Abbreviations: Am, amyloplast in both cell layers; E, inner tangential wall of an epidermal cell; Et, endothelium; G, Golgi bodies; IP, inner parenchyma; LW, labyrinthine wall; N, nucleus; Ps, perisperm.
In the endothelial cells the cytoplasm is dense, with a large nucleus that is often irregular and contains much heterochromatin (Fig. 2A). The cells contain a few vacuoles and many mitochondria, and are rich in ribosomes, some of which appear on the surface of the endoplasmic reticulum (Fig. 2B). Large amyloplasts are scattered in the cells, often located adjacent to the nucleus (Fig. 2A, B). The Golgi apparatus looks very active, with many dictyosomes (Fig. 2B). The endothelial cells are characterized with special inner tangential cell walls, facing the perisperm (Figs 2 and 3). During the first few days after fertilization, inner wall protuberances gradually develop on the primary cell walls, resembling the wall structure of transfer cells (Gunning and Pate, 1974; Offler et al., 2003; McCurdy et al., 2008). The wall protuberances appear dark after post-fixation with OsO4. The protuberances are inter-connected and develop a sponge-like three-dimensional network. The number of wall protuberances is relatively large. The protuberances are thicker adjacent to the primary tangential wall and thinner when facing the cell centre (Fig. 2B). Many protuberances stand in parallel to the primary wall.
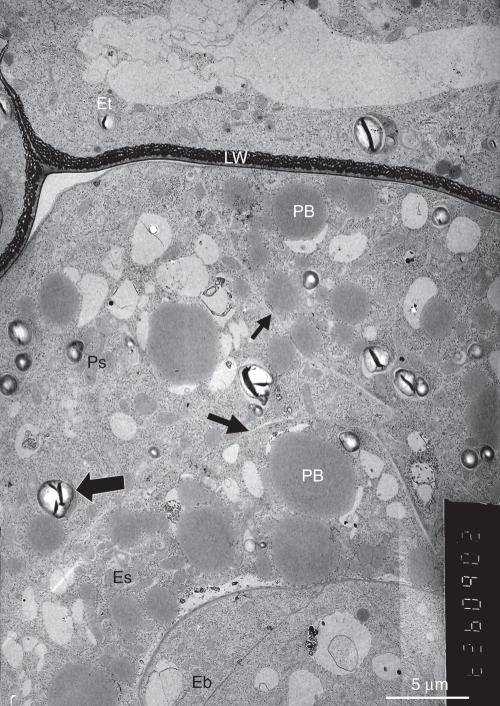
Fig. 3.
The internal parts of the young seed. Large protein bodies are seen in both the perisperm and the endosperm. Amyloplasts (large arrow) are mainly found in the perisperm. The endosperm and perisperm cell walls are very thin (small arrows). Abbreviations: Eb, embryo; Es, endosperm; Et, endothelium with labyrinthine wall (LW); PB, protein bodies; Ps, perisperm.
The radial walls of the endothelial cells are thicker at their basal side, and thinner toward the outer wall facing the inner parenchyma of the seed coat. Only a few wall protuberances develop on both sides of these thin walls. Dictyosomes are usually found near the developing wall protuberances (Fig. 2B). Typically, the outer wall of the endothelium is covered by a thin cuticle (arrow, Fig. 2B), as previously described by Kravtsova and Teryokhin (1987).
The young embryo is a small spherical body with thin primary walls separating the embryo cells (Fig. 3). The cells of the endosperm, which surrounds the embryo, contain a dense cytoplasm with large protein bodies that are associated with fenestrated endoplasmic reticulum and groups of small vacuoles. The unilayered perisperm that surrounds the endosperm and is enclosed by the seed coat is composed of large cells that are larger than those of the endosperm. They have a dense cytoplasm and, like the endothelium, typically contain amyloplasts with large starch grains (Fig. 3), resembling those occurring in Orobanche seedlings (Joel and Losner-Goshen, 1994).
Anatomy and ultrastructure of the mature seed
The mature seeds (Fig. 4), which are shed from the open capsules about 3 weeks after pollination, are composed of four distinct regions, each originating from a different part of the fertilized ovule. The embryo and the endosperm develop within the embryo-sac after the double fertilization, while the seed coat and the perisperm, respectively, arise from the integument and the nucellus, and are the maternal parts of the seed.
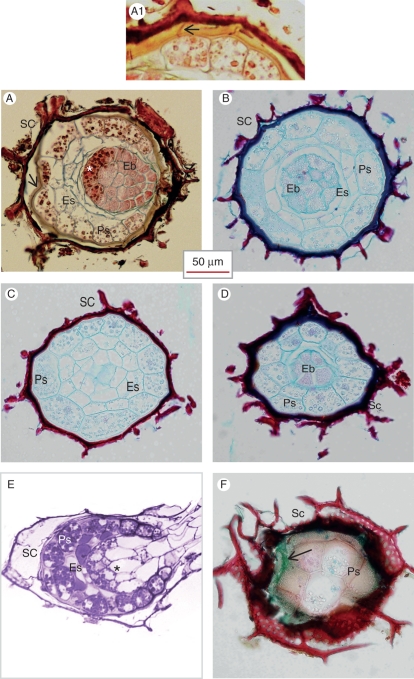
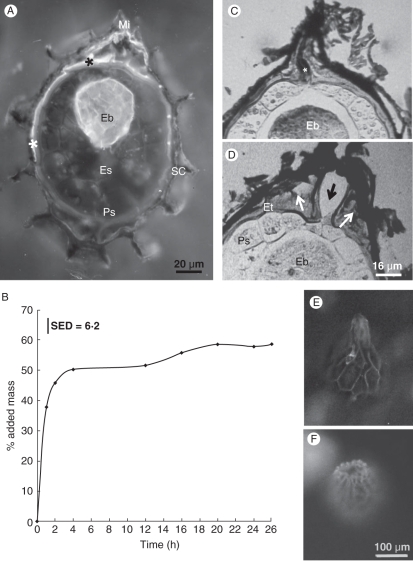
Fig. 4.
Light microscope image of mature seeds. (A–D) Paraffin-embedded sections. (A) Longitudinal section, showing the different parts of the seed. The arrow points to the endothelium layer of the seed coat, which stained yellow with AGS after FAA fixation. The asterisk indicates the proximal zone of the embryo. (A1) Details of the endothelium of a mature seed, stained with AGS after FAA fixation. The middle lamella of the wall between neighbouring endothelial cells stains red with safranin (arrow), while the labyrinthine wall stains yellow with alcian dyes. (B–D) Serial cross-sections of a single seed: (B) seed centre showing the proximal zone of the embryo; (C) behind the embryo; (D) near the micropyle showing the distal zone of the embryo. (E) Germinating seed in which the proximal zone of the embryo is indicated by an asterisk. LR White-embedded material, stained with methylene blue–basic fuchsin. (F) A thick tangential section of non-fixed seed, showing the outer cell layers. Cryo-sectioned material. AGS staining. The outer seed coat is lignified and stained red with safranin. The endothelium, which is the inner layer of the seed coat and partly overlapped by the outer layer, is filled with mucilage and stained with alcian green (arrow), while the outer wall of the perisperm, which includes a cuticular layer, stained red with safranin especially in the cell corners. Abbreviations: Eb, embryo; Es, endosperm; Ps, perisperm; SC, seed coat.
Embryo
The embryo in the mature seed of P. aegyptiaca is a globular body, which occupies the central part of the seed, close to the micropyle. It is composed of thin-walled small cells that are densely packed without intercellular spaces (Figs 1B, 4A, B and 5A–D). Unlike previous reports describing the embryo as an undifferentiated body, we found two distinct regions in the embryo. The distal cells, facing the micropyle, are smaller than those in the proximal embryonic side, and stain differently. The proximal cells are surrounded by the endosperm (Fig. 4A, B), while the distal cells are surrounded by perisperm cells (Fig. 4A, D).
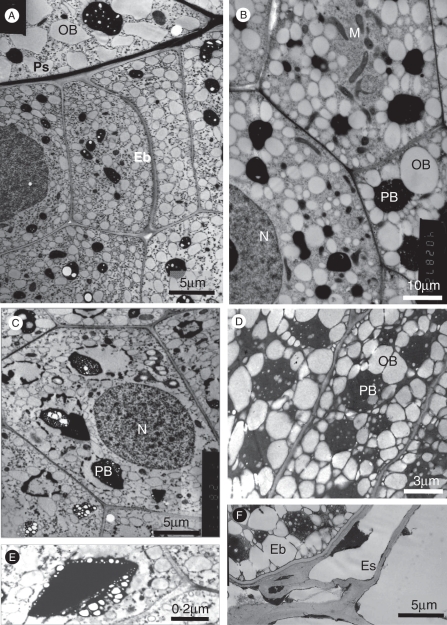
Fig. 5.
The embryo. (A, B) Distal embryo cells (near the micropyle). (A) Lateral embryo cells (Eb) neighbouring a perisperm cell. Without post-fixation. (B) Apical embryo cells. OsO4 post-fixation. (C–F) Proximal (chalazal side) embryo cells showing large protein bodies and oil bodies filling the cells: (C) without post-fixation; (D) OsO4 post-fixation; (E) protein body containing a large crystal – without post-fixation; (F) proximal embryo cell neighbouring endosperm cells – KMnO4 post-fixation. Abbreviations: Eb, embryo; Es, endosperm cell; M, mitochondria; N, nucleus; OB, oil body; PB, protein body; Ps, perisperm cell.
Examination of the mature embryo with an electron microscope (Fig. 5) revealed that the embryo cells have a large nucleus, and the cytoplasm is translucent and rich in many small organelles and numerous elongated mitochondria (Fig. 5B) that are mainly located around the nucleus or near the cell walls. However, the most prominent structures within these cells are the oil bodies and the protein bodies. Both are much larger in the proximal than the distal embryo cells (Fig. 5C, D vs. A, B), which is consistent with the differences seen under the light microscope (see above; Fig. 4A). The protein bodies have an electron-dense matrix, containing unidentified material in the form of internal globoids. Some globoids are not well preserved during fixation and rupture under the electron beam. The protein bodies may also contain dark droplets or large electron-dense crystalloids, which were particularly prominent after KMnO4 post-fixation (Fig. 5C, E). The oil bodies are commonly organized in concentric circles around protein bodies (Fig. 5B, D). This arrangement is more obvious after osmium post-fixation and resembles the appearance of oil and protein bodies in seeds of various other plant families e.g. Solanaceae (Greenwood and Chrispeels, 1985) and Arecaceae (Moura et al., 2010). Each oil body is enclosed within a unilayered membrane, similar to oil bodies in other plant species. Seed oil bodies are usually surrounded by a layer of oleosins that prevents oil body coalescence and provides a large surface area to facilitate lipase binding and lipolysis during germination (Huang, 1996; Millar et al., 2000).
Perisperm
The perisperm of the mature seed is composed of a single layer of large cells with thick outer walls (Figs 4A–D and 6A), interconnected by numerous plasmodesmata (Fig. 6A–C). This cell layer surrounds the internal part of the seed, i.e. the embryo and the endosperm, and lies near to the seed coat. The perisperm cells are the largest in the mature seed (Fig. 4A–D) though at the micropyle the perisperm cells are smaller (Figs 4A, E and 7B). The perisperm cells have thin walls adjacent to distal embryo cells, while their cell walls near to the endothelium are thicker and covered by a cuticle. The cuticle is composed of a cuticular layer and a cutinized wall layer, which is rich in intracuticular wax in the form of discontinuous crystals that are mainly oriented perpendicular to the cell surface (Fig. 6D). The cuticle of the perisperm usually merges with the adjacent thin cuticle of the endothelium (Fig. 6A, D, black arrow), which has clearly been detected in the young seed (see above; Fig. 2B). The waxy cuticle seems to be major barrier to water (and fixative) movement into the dry seed interior.
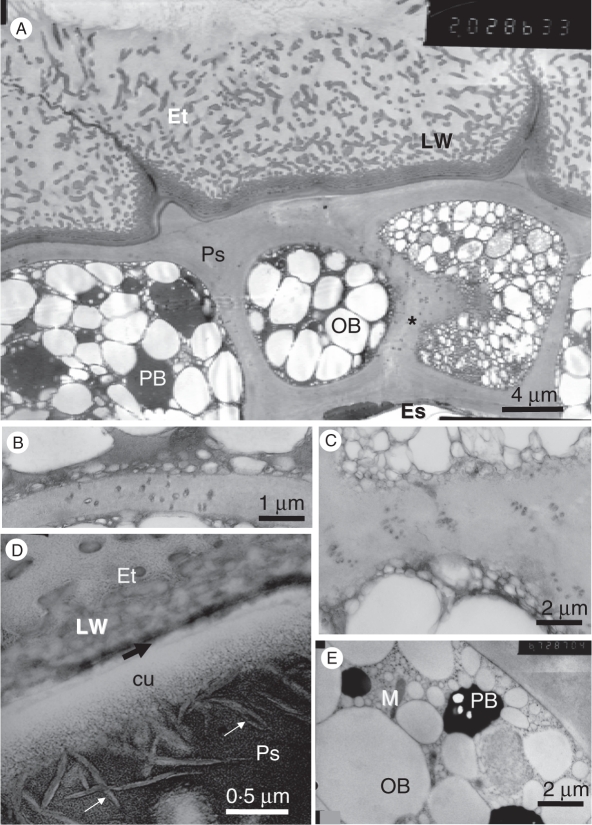
Fig. 6.
The perisperm. (A) Perisperm cells and neighbouring endothelium cells. The perisperm cell on the right was sectioned tangentially near a lateral wall, thus exposing cross-sectioned plasmodesmata (asterisk), and numerous small oil bodies of the cell periphery. KMnO4 post-fixation. (B) Plasmodesmata traversing the radial wall between two perisperm cells. KMnO4 post-fixation. (C) Groups of plasmodesmata in a radial wall between two perisperm cells. Magnified portion of the section shown in (A). (D) Portion of the cell wall at the boundary between a perisperm cell and an endothelial cell, showing the perisperm cuticle (cu) and the thin endothelial cuticle (black arrow). White arrows indicate wax crystals. The labyrinthine wall is in the endothelial cell. OsO4 post-fixation. (E) Peripheral portion of a perisperm cell. KMnO4 post-fixation. Abbreviations: cu, perisperm cuticle; Es, endosperm cell; Et, endothelium cells; Ps, perisperm cells; LW, labyrinthine wall; OB, oil body; PB, protein body.
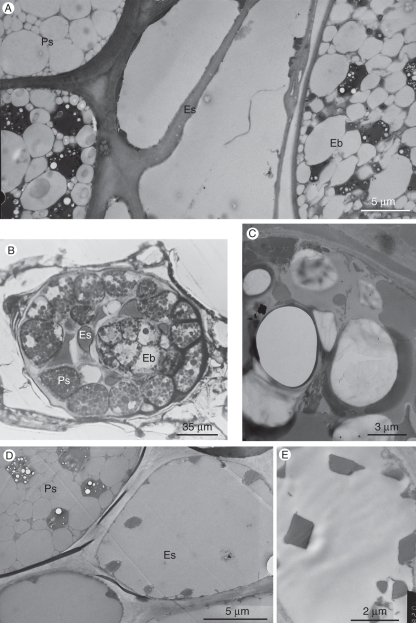
Fig. 7.
The endosperm. (A) Endosperm cells between the perisperm and the embryo. (B and C). OsO4 post-fixation. (B) Oblique longitudinal section of a seed, showing the diffuse appearance of the endosperm, while both the embryo and the perisperm contain well-defined protein and oil bodies. Light microscope image. (C) Portion of an endosperm cell with merging oil bodies. TEM image. (D and E) KMnO4 post-fixation. TEM image. (D) Endosperm cells with some crystals, presumably originating from protein bodies, and no visible membranes. A neighbouring nucellar cell clearly contains both oil and protein bodies. (E) Enlarged portion of an endosperm cell, with typical crystals. Abbreviations: Eb, embryo; Es, endosperm cell; Ps, perisperm cell.
The perisperm cells have a small nucleus and are densely filled with oil bodies that are arranged in concentric circles around large protein bodies (Figs 4A, C, 6A, E and 7A, D) in a manner similar to those appearing in the proximal pole of the embryo. The cell periphery is rich in smaller oil bodies (Fig. 6A, E).
Interestingly, the perisperm was previously interpreted as the external layer of the endosperm (Privat, 1960; Teryokhin et al., 1993; Teryokhin, 1997). However, we found clear structural evidence that this cell layer differs significantly from the endosperm, in both cell arrangement and cytoplasm differentiation. Moreover, while many plasmodesmata bridge between perisperm cells and a few plasmodesmata are present between endosperm cells, no plasmodesmata were found between perisperm and endosperm cells. Based on a recent genetic study (D. Plakhine et al., unpubl. res.) some germination characteristics related to stimulant reception of Orobanche hybrids are segregated only in the F3 generation, indicating that a living maternal tissue exists in the mature seed. After senescence of the seed coat during seed maturation, the only maternal tissue that may potentially survive in a seed would be the perisperm, providing further evidence for the nucellar origin of this cell layer.
The high lipid content of the seeds, as seen under the electron microscope, in both the embryo and the perisperm, is consistent with the results of the chemical analysis of Phelipanche seeds that have previously been published, indicating that almost 30 % of the seed dry mass is composed of lipids, mainly oleic acid (Valesco et al., 2000; Bar-Nun and Mayer, 2002).
Endosperm
The endosperm, which fills the space between the embryo and the perisperm (Figs 4A–C, E and 7A, B), is composed of cells that are smaller than those of the perisperm (Figs 1B and 4C). The endosperm cells contain large amounts of oil but, unlike the oil bodies in the neighbouring embryo and perisperm cells, the oil bodies in the endosperm cells were clearly seen to have diffuse boundaries and to fuse with each other when observed following post-fixation with osmium (Fig. 7B, C), i.e. when the lipids were fixed and not extracted during tissue dehydration and embedding. Our findings are consistent with those of Heneen et al. (2008), who elegantly showed that the fusion of oil bodies that occurs in the endosperm of oat seeds does not occur in the embryo, scutellum and aleurone cells. They have shown that this phenomenon is correlated with a reduced amount of proteins associated with the oil bodies. Indeed, following permanganate post-fixation of P. aegyptiaca seeds, which allowed staining of the boundary membranes of the oil bodies in both perisperm and embryo cells (but did not prevent extraction of the oil during dehydration and embedding), no such boundary membranes could be detected in endosperm cells of the same seeds (Fig. 7A, D, E). Under these tissue-preparation conditions, no protein bodies or other organelles could be detected in the endosperm cells, apart from large electron-dense crystals (Fig. 7D, E). This is consistent with the role of the endosperm as a storage tissue. Since there are no organelles in the endosperm and compartmentization seems to be broken, we assume that the endosperm cells are non-living at maturity.
Mature seed coat
The mature seed coat is composed of two main cell layers, the epidermis and the endothelium (Fig. 8A, C). The inner parenchyma cells (hypodermis), which are present in the ovule integument (Fig. 1C) and in the young seed (Fig. 2A), collapse during seed development and do not occupy a significant volume of the seed coat of the mature seed, but their cell walls remain and can be seen in some places between the epidermis and the endothelium (Fig. 8C, E). Inner parenchyma cells are not present at the micropyle (Fig. 8D). Similar collapse of hypodermal cells was previously found in members of the closely related family Scrophulariaceae, where the complete degeneration of the hypodermis in mature seeds is characteristic of section Leptoplectron (Juan et al., 1999).
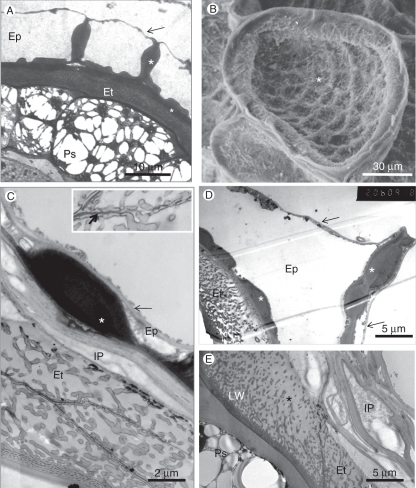
Fig. 8.
The seed coat. (A) TEM image. Cross-section of seed showing a general view of the seed coat and perisperm. In this case, the outer epidermal cell wall is still intact (arrow). Both the radial walls and the inner tangential walls are unevenly thickened (asterisks). The large perisperm cells are filled with oil bodies that surround protein bodies. (B) SEM image of the concave outer surface of a single epidermal cell in which the outer tangential wall collapsed and clings to the inner tangential wall, which has reticulate thickenings (asterisk). (C) The seed coat: an epidermal cell with internal cell wall carrying a large wall thickening (asterisk) to which the external thin cell wall tightly clings (arrow). The endothelium, with diagonal lateral walls, contains many wall protuberances. The appearance of multiple layers is due to the angle of the section. The collapsed inner parenchyma cells sit between these two tissues. Inset: enlarged portion of the cell wall between endothelial cells, showing the dark middle lamella (arrow), traversed by protuberances. (D) Cross-section of the seed coat showing the uneven thickening of the radial wall and the inner tangential walls of an epidermal cell (asterisk). The outer tangential wall of the epidermis collapsed only on one side of the radial wall. The endothelium contains a well-developed labyrinthine wall. (E) The inner layers of the seed coat. In this electron micrograph the collapsed inner parenchyma (IP) is composed of the cell walls of several cells. The endothelium contains labyrinthine wall complex with a density gradient of wall protuberances toward the inner parenchyma. Abbreviations: Ep, epidermis; Et, endothelium; IP, inner parenchyma cell; LW, labyrinthine wall complex; Ps, perisperm.
Epidermal cells
The epidermal cells of the mature seed are dead and devoid of any cytoplasm (Fig. 8A, D). The internal tangential walls and the radial cell walls are particularly thickened, with reticulate wall thickenings, and therefore the surface view of each epidermal cell looks fenestrated under the light and scanning electron microscopes (Figs 1A and 8B). The external tangential wall is very thin (Fig. 8A, C, D, arrow) and usually collapses and clings to the inner walls soon after the seeds are released. The outer tangential wall first becomes concave, and then it splits and disintegrates or tightly clinging to the inner walls (Fig. 8B, C) (Joel, 1987a; Kravtsova and Teryokhin, 1987). In this way, each cell eventually shows a typical external cup-like shape with a reticulate surface (Fig. 8B). The seed surface is typical of each species and can be used as a taxonomic character (Joel, 1987b; Teryokhin et al., 1993).
Endothelium
The endothelium, which lies under the crushed inner parenchyma cells and surrounds the perisperm (Figs 4A, arrow, 6A and 8E), is an interesting characteristic of the seeds. As already described above for the young seed, the endothelium is usually made up of a single-cell layer, the cells of which are sometimes larger near the micropyle. The cells are narrow and much elongated, up to 60 µm long, so that in some seeds we found only four or five endothelium cells along the seed length. The lateral walls of the endothelial cells are usually diagonal (Figs 4A, 8C, E and 10A).
Fig. 10.
The endothelium. (A) Longitudinal section of two neighbouring endothelial cells carrying labyrinthine wall. The radial wall between them is thinner towards the inner parenchyma. (B) Longitudinal section through the micropyle, showing the filling substance (white asterisk) near an endothelial cell (Et). (C) Fluorescence light micrograph of the endothelium after calcofluor white staining, showing the labyrinthine wall complex under UV light excitation. (D) Electron micrograph of portion of a cross-section in the endothelium, showing the continuity of wall protuberances on both sides of the thin radial wall between adjacent endothelial cells. Abbreviations: Et, endothelium; IP, inner parenchyma cell; LW, labyrinthine wall.
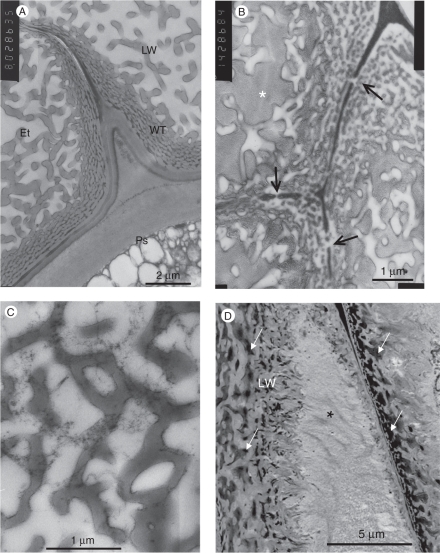
The endothelial cells resemble ‘transfer cells’, in which numerous inner wall protuberances form an extensive labyrinthine surface (Figs 8C and 9A, B). In the mature seed, the cell volume in many endothelial cells is fully occupied by these structures (Fig. 8C–E). While the ultrastructural features of these cells can only be seen under the electron microscope, the labyrinthine wall can also be identified under the light microscope because it stains with calcofluor white and can clearly be seen under UV excitation (see Fig. 10C).
Fig. 9.
The endothelium. (A) Basal part of two endothelial cells. The cell wall carries densely packed wall protuberances. The labyrinthine wall complex extends into the cell lumen. The radial wall between the endothelial cells becomes thinner towards the inner parenchyma (upper left). Whereas the primary cell wall of the endothelium is thin, the adjacent perisperm cell wall is thicker. (B) The middle lamella between endothelial cells is interrupted where wall protuberances are continuous in adjacent cells (arrows). The space between the protuberances is occupied by a granular substance. (C) Tangential section through the labyrinthine wall complex, showing horizontally interconnected protuberances. (D) Densely packed labyrinthine wall enclosing an electron-dense substance at the base of endothelial cells (arrows). The other part of the cells is occupied by a granular and fibrillar substance (asterisk). Abbreviations: Et, endothelial cell; LW, labyrinthine wall complex; Ps, perisperm cell wall; WT, wall protuberances.
A density gradient of the protuberances can be seen in these cells, where the tangential wall facing the perisperm is covered with densely packed protuberances that are inter-connected (Fig. 9B, C). Towards the cell centre, and even more so toward the tangential wall that faces the inner parenchyma cells, the protuberances are sparse and much shorter than those on the tangential walls facing the perisperm (Figs 8E, 9A, B and 10A).
The middle lamella between endothelial cells stains red with safranin (Fig. 4A1) and appears dark under the electron microscope (Fig. 8C, inset), which may indicate that it is lignified. However, the protuberances that cover these walls are often continuous in contiguous cells, and in these sites the middle lamella is not seen (Figs 8C, inset, and 10D), indicating the existence of a continuous apoplastic compartment along this tissue.
The mature endothelium cells are devoid of any cytoplasm. They are filled with a substance that seems to be mucilage. With alcian green it stains green in non-fixed seed sections, and yellow in FAA-fixed seeds (Fig. 4A, F). Under the electron microscope, this mucilage looks granular (Figs 9B and 10A) or partly to densely fibrillar in the cell centres of the thicker endothelium cells at the micropyle regions (Figs 9D and 10A).
Starch grains were not found in the mature seed. Our ultrastructural evidence, which indicates that starch is abundant only in immature seeds (Fig. 2A), contradicts former reports by Kumar (1977) that starch is typically accumulated in the outer endosperm cells of mature P. aegyptiaca seeds, which is identified in our study as the perisperm. It seems that starch, which is accumulated in early stages of seed development, is consumed during the development of the labyrinthine wall and the accumulation of mucilage. The Golgi bodies, which are typically abundant in the young endothelial cells, also seem to be involved in these processes.
The wall protuberances of Orobanchaceae seeds were previously described by Kravtsova and Teryokhin (1987) as a ‘coral-like structure’, but they assumed that these structures are composed of cutin. Other researchers interpreted these wall protuberances as pigment bodies [Aber and Sallé (1983) in O. crenata seeds], fungal infection [Sauerborn et al. (1996) in Striga seeds] or degenerated perisperm [Thomas et al. (1999) in O. cumana], rather than a cell layer that differentiates into transfer-like cells during seed maturation. By re-examining the micrographs in the publications cited above, it is clear that the seed coats of all these Orobanche and Striga species exhibit typical wall labyrinths, similar to those found in Phelipanche. The occurrence of similar ‘spongy’ endothelial cells in Verbascum (a non-parasitic species of the related family Scrophulariaceae) (Juan et al., 1997) may indicate that this character is widespread at least within the Lamiales. Similar spongy structures were also found in members of the Hydrophyllaceae (of the closely related Boraginales) (Diane et al., 2002).
The endothelial cells of the Orobanchaceae clearly resemble transfer cells that have previously been associated with short-distance transfer of solutes (Gunning and Pate, 1974). In previous studies, transfer cells in seeds were mainly found at the maternal/filial interface, where active symplast/apoplast transport of nutrients occurs either during seed development or during germination (Gunning and Pate, 1974; Diane et al., 2002; Offler et al., 2003; Zheng and Wang, 2010). Does this apply to the seeds of the Orobanchaceae? Our findings that the cells possessing labyrinthine walls comprise part of the seed coat and are filled with a mucilage, point to a possible alternative function. While transfer cells in mature legume and cereal seeds are living cells and do not have wall barriers with neighbouring tissues, the endothelium cells in mature seeds of the Orobanchaceae are dead cells with no cytoplasm. Further, the cell walls between endothelium cells and the neighbouring perisperm cells are cutinized and not permeable to water. Although the living endothelial cells in the young seeds do have wall protuberances, the labyrinthine walls of these cells do not occupy much of the cell volume. Due to the presence of the cuticle between this cell layer and the seed interior, the labyrinthine wall of the Orobanchaceae cannot serve as a ‘classical’ transfer cell in transmitting water and nutrients to the developing seed. Instead, these young cells have numerous mitochondria, an active Golgi apparatus and large amyloplasts that seem to be involved in the construction of further wall protuberances and in the internal secretion of the mucilage that eventually fills the whole cell volume. In this respect, the endothelial cells can be regarded as glandular.
Functions of the endothelium
This labyrinthine wall system develops during seed maturation rather than in early stages of seed development, and the wall protuberances are continuous in neighbouring endothelial cells (Fig. 10D). In this respect, the endothelium resembles a conductive tissue. This brings us to question the possible role of the endothelium in the seed physiology. Would the endothelium serve in water transfer in the ripe seed?
To determine the routes of water movement during imbibition, we immersed P. aegyptiaca seeds in a solution of the apoplastic marker calcofluor white. After 10 min the endothelial cells near the micropyle fluoresced under UV excitation, and only 10 min later the dye also had reached the embryo (Fig. 11A) but did not reach any other seed tissue. Full imbibition of P. aegyptiaca seeds takes about 20 h and includes two main phases (Fig. 11B). The first phase takes 3–4 h, but during its first hour water absorption is very fast, adding almost 50 % to seed mass. The second phase starts a few hours later and leads to a gradual and moderate additional increase in seed hydration. It therefore seems that, when water is available to the seed, the endothelium that is loaded with mucilage acts as a sponge and is the first to rapidly absorb water. Only then the embryo gets water, while the endosperm and the perisperm, which are loaded with lipids, gradually absorb smaller amounts of water sometime later.
Fig. 11.
Water absorption by P. aegyptiaca seeds. (A) Cryo-tangential section of a dry P. aegyptiaca seed after immersion in a solution of calcofluor white for 20 min. Fluorescence light micrograph under UV excitation. Both the endothelium (asterisks) and the embryo (Eb) fluoresce, indicating water absorption. (B) Graph describing water absorption during imbibition by P. aegyptiaca seeds (P < 0·05). (C, D) Light microscopy image of the seed micropyle before (C) and after (D) imbibition, showing swelling of the endothelium (white arrows), and the gap which opened at the seed apex (black arrow). The asterisk indicates a sealing substance in the closed micropyle. (E, F) Fluorescence light micrographs of the seed surface under blue excitation: (E) dry seed; (F) seed imbibed for 30 min showing the micropyle water gap. Abbreviations: Es, endosperm; Ps, perisperm; Eb, embryo; Et, endothelium.
The micropyle of dry seeds is a cylindrical gap between endothelial cells that is filled with a complex sealing substance (Figs 10B and 11C), which stains red with safranin but has yet to be identified. A comparison of longitudinal sections of dry seeds and of imbibed seeds under the light microscope (Fig. 11C vs. D, respectively) shows that during imbibition the endothelium near the micropyle considerably swells, which is consistent with the presence of mucilage in these cells. The presence of a thicker endothelium at the micropyle may enhance water uptake and swelling. These sections also show a gap that opens between the micropylar endothelium cells so that the closed micropyle opens up, allowing water to enter the seed. Water gaps of various sorts have previously been studied in several families, and they were shown to allow the initial uptake of water in many physically dormant seeds (Baskin et al., 2000; Gama-Arachchige et al., 2011). However, Phelipanche seeds do not have physical dormancy – they have a water-absorbing seed coat, not a water-impermeable palisade cell layer. While in physically dormant seeds the water gap is the first thing to open, before any water is absorbed, in Phelipanche the micropyle opens after enough water has accumulated in the seed coat. In this case the endothelial cells, which surround the seed interior, take up water that forces the micropyle to open, thereby allowing water to enter the seed interior. It therefore seems that an initial role of the endothelium in the Orobanchaceae, is to help create a large opening for water to enter the seed interior.
The water capacity of the mucilaginous endothelium allows for the rapid imbibition of water when it is available. This facilitates ‘seed conditioning’ by keeping the seed tissues hydrated so the receptor cells in the seed interior can perceive the germination stimulants, if available from nearby host roots, for germination to occur. Often the period of time between imbibition and germination is 6–21 d, and the seed can respond to germination stimuli during that period if a signal is not received during imbibition (Plakhine et al., 2009). Therefore the accumulation of large amounts of water in the mucilaginous endothelium should be an advantage, especially in dry habitats. Mucilage in seed coats of various other plants has already been shown to assist in maintaining water supply during germination. Penfield et al. (2001) have shown that germination rates decreased under water-limiting conditions in the arabidopsis mutant myb61 that lacks mucilage in its seed coat.
Whereas a waxy cuticle between the perisperm and the endothelium of Phelipanche seeds should blocks water transfer to the seed prior to germination, the young seedling that emerges out of the perisperm during germination is in direct contact with the endothelium, allowing gradual and possible controlled absorption of the endothelial water, which should ensure that water is supplied to the seedling even in dry soil.
Putative receptor cells
The living cells located immediately below the micropyle are small perisperm cells that mediate between the micropylar opening and the embryo (Fig. 11D). We therefore hypothesize that these perisperm cells are the sensory cells that receive the germination stimuli (e.g. strigolactones) from host roots, and transmit the germination signal to the embryo. Flash-stimulation experiments established that dry seeds have the ability to respond to a short chemical stimulation of 2 h after the onset of imbibition (Plakhine et al., 2009), which matches the timing of micropyle opening and the first phase of water absorption. Unlike the thick cell walls of the large perisperm cells that are located adjacent to the endosperm (Fig. 6A), the perisperm cell walls facing the embryo are thin (Fig. 5A). This is also consistent with the putative ability of this group of small perisperm cells to transmit the germination signal to the embryo. These cells are also the site through which the elongating distal part of the embryo passes during germination.
Role of seed tissues during germination
During germination the central and the distal embryo cells elongate and form the emerging seedling (Fig. 4E). Subsequently, the small perisperm sheath cells at the micropyle, which tightly surround the emerging seedling, seem to supply it with their nutrients. The perisperm cell walls facing the embryo are thin (Fig. 5A), which is consistent with reduced resistance in nutrient transport through the apoplast. Similarly, the interface between the embryo and the endosperm is also composed of thin cell walls (Figs 5F and 7A), unlike the other outer cell walls of the endosperm. Thus, we assume that seed food reserves are transferred to the developing seedling through two main routes: (1) from the living perisperm, along its symplastic route, to the seedling ‘neck’ through the perisperm sheath at the micropyle; and (2) from the presumably non-living endosperm to the proximal parts of the seedling. Interestingly, during early germination the contents of the proximal embryo cells resemble those of the perisperm sheath cells (Fig. 4F). Both are rich in protein bodies and not in oil bodies, which hints to a common role in actively transporting nutrients to the developing parts of the seedling.
ACKNOWLEDGEMENTS
We thank Dr Dalia Losner-Goshen for preparation of the section shown in Fig. 4E. We are also grateful to the two anonymous referees for their helpful comments. This research was supported by BSF, United States–Israel Binational Science Foundation, Project No. 1999372, and by BARD, the US-Israel Agricultural Research and Development Fund, Project no. IS-1421-88.
LITERATURE CITED
- Aber M, Sallé G. Graine et procaulome d'Orobanche crenata Forsk: Etude histocytologique et cytochimique. Canadian Journal of Botany. 1983;61:3302–3313. [Google Scholar]
- Aparicio SR, Marsden P. Methylene blue-basic fuchsin. Journal of Microscroscopy. 1969;89:139–141. doi: 10.1111/j.1365-2818.1969.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Bar-Nun N, Mayer AM. Composition of and changes in storage compounds in Orobanche aegyptiaca seeds during preconditioning. Israel Journal of Plant Science. 2002;50:277–279. [Google Scholar]
- Baskin JM, Baskin CC, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. [Google Scholar]
- Diane N, Hilger HH, Gottschling M. Transfer cells in the seeds of Boraginales. Botanical Journal of the Linnean Society. 2002;140:155–164. [Google Scholar]
- Gama-Arachchige NS, Baskin JM, Geneve RL, Baskin CC. Acquisition of physical dormancy and ontogeny of the micropyle-water-gap complex in developing seeds of Geranium carolinianum (Geraniaceae) Annals of Botany. 2011;108:51–64. doi: 10.1093/aob/mcr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JS, Chrispeels MJ. Correct targeting of the bean storage protein phaseolin in the seeds of transformed tobacco. Plant Physiology. 1985;79:65–71. doi: 10.1104/pp.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BE, Pate JS. Transfer cells. In: Robards A, editor. Dynamic aspects of plant ultrastructure. Maidenhead: McGraw Hill; 1974. pp. 441–480. [Google Scholar]
- Hall JL, Hawes C. Electron microscopy of plant cells. London: Academic Press; 1991. [Google Scholar]
- Heneen WK, Karlsson G, Brismar K, et al. Fusion of oil bodies in endosperm of oat grains. Planta. 2008;228:589–599. doi: 10.1007/s00425-008-0761-x. [DOI] [PubMed] [Google Scholar]
- Holm L, Doll J, Holm E, Pancho J, Herberger J. World weeds: natural histories and distribution. New York, NY: John Wiley & Sons; 1997. [Google Scholar]
- Huang AHC. Oleosins and oil bodies in seeds and other organs. Plant Physiology. 1996;110:1055–1061. doi: 10.1104/pp.110.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel DM. AGS (alcian green safranin) – a simple differential staining of plant material for the light microscope. Proceedings of the Royal Microscopical Society. 1983;183:149–151. [Google Scholar]
- Joel DM. Proceedings of the 4th Symposium of the International Society for Parasitic Flowering Plants (ISPFP) Marburg: FRG; 1987a. Identification of Orobanche seeds. Parasitic flowering Plants; pp. 437–444. In: Weber H. Ch, Forstreuter W. eds. [Google Scholar]
- Joel DM. Detection and identification of Orobanche seeds using fluorescence microscopy. Seed Science & Technology. 1987b;15:119–124. [Google Scholar]
- Joel DM. The long-term approach to parasitic weeds control: manipulation of specific developmental mechanisms of the parasite. Crop Protection. 2000;19:753–758. [Google Scholar]
- Joel DM. The new nomenclature of Orobanche and Phelipanche. Weed Research. 2009;49(Suppl 1):6–7. [Google Scholar]
- Joel DM, Losner-Goshen D. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Canadian Journal of Botany. 1994;72:564–574. [Google Scholar]
- Joel DM, Steffens JC, Matthews DE. Germination of weedy root parasites. In: Kigel J, Galili G, editors. Seed development and germination. New York, NY: Marcel Dekker; 1995. pp. 567–598. [Google Scholar]
- Joel DM, Bar H, Mayer AM, Verdoucq V, Welbaum G, Westwood J. Characterization of a dioxygenase gene with a potential role in steps leading to germination of the root parasite Orobanche aegyptiaca. In: Adkins SW, Ashmore S, Navie SC, editors. Seeds: biology, development and ecology. Wallingford, UK: CAB International; 2005. pp. 296–306. [Google Scholar]
- Joel DM, Hershenhorn Y, Eizenberg H, et al. Biology and management of weedy root parasites. In: Janick J, editor. Horticultural Reviews. Vol. 33. Hoboken, NJ: John Wiley & Sons; 2007. pp. 267–349. [Google Scholar]
- Juan R, Fernandez I, Pastor J. Systematic consideration of microcharacters of fruits and seeds in the genus Verbascum (Scrophulariaceae) Annals of Botany. 1997;80:591–598. [Google Scholar]
- Juan R, Pastor J, Fernandez I. Morphological and anatomical studies of Linaria species from south-west Spain: seeds. Annals of Botany. 1999;84:11–19. [Google Scholar]
- Kravtsova TI, Teryokhin ES. Histochemical analysis of the seed coat in some representatives of the Orobanchaceae of the flora USSR. Botanicheskii zhurnal SSSR. 1987;72:644–651. [in Russian]. [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berekeley, CA: University of California Press; 1969. [Google Scholar]
- Kumar U. Morphogenetic regulation of seed germination in Orobanche aegyptiaca Pers. Canadian Journal of Botany. 1977;55:2613–2621. [Google Scholar]
- Losner-Goshen D, Portnoy V, Mayer AM, Joel DM. Pectolytic activity by the haustorium of the parasitic plant Orobanche L. (Orobanchaceae) in host roots. Annals of Botany. 1998;81:319–326. [Google Scholar]
- McCurdy DW, Patrick JW, Offler CE. Wall ingrowth formation in transfer cells: novel examples oflocalized wall deposition in plant cells. Current Opinion in Plant Biology. 2008;11:653–661. doi: 10.1016/j.pbi.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Millar AA, Smith MA, Kunst L. All fatty acids are not equal: discrimination in plant membrane lipids. Trends in Plant Science. 2000;5:95–101. doi: 10.1016/s1360-1385(00)01566-1. [DOI] [PubMed] [Google Scholar]
- Moura EF, Ventrella MC, Motoike SY. Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae) Scientia Agricola. 2010;67:399–407. [Google Scholar]
- Musselman LJ, Parker C, Dixon N. Notes on autogamy and flower structure in agronomically important species of Striga (Scrophulariaceae) and Orobanche (Orobanchaceae) Beitrage zur Biologie der Pflanzen. 1982;5:329–343. [Google Scholar]
- O'Brien TP, McCuelly ME. The study of plant structure principles and selected methods. Melbourne: Termarcarphi; 1981. [Google Scholar]
- Offler CE, McCurdy DW, Patrick JW, Talbot MJ. Transfer cells: cells specialized for a special purpose. Annual Review of Plant Biology. 2003;54:431–454. doi: 10.1146/annurev.arplant.54.031902.134812. [DOI] [PubMed] [Google Scholar]
- Parker C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Management Science. 2009;65:453–459. doi: 10.1002/ps.1713. [DOI] [PubMed] [Google Scholar]
- Parker C, Riches CR. Parasitic weeds of the world: biology and control. Wallingford, UK: CAB International; 1993. [Google Scholar]
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. The Plant Cell. 2001;13:2777–2791. doi: 10.1105/tpc.010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakhine D, Ziadna H, Joel DM. Is seed conditioning essential for Orobanche germination? Pest Management Science. 2009;65:492–496. doi: 10.1002/ps.1716. [DOI] [PubMed] [Google Scholar]
- Privat G. Recherches sur les phanérogames parasites (étude d'Orobanche hederae Duby) Annales des Sciences Naturelles, Botanique et Biologie Végétale. 1960;12:722–865. [Google Scholar]
- Sauerborn J, Dörr I, Abbasher A, Thomas H, Kroschel J. Electron microscopic analysis of the penetration process of Fusarium nygamai, a hyperparasite of Striga hermonthica. Biological Control. 1996;7:53–59. [Google Scholar]
- Spurr AR. A low viscosity resin embedding medium for electron microscopy. Journal of Ultrastructure Researach. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Teryokhin ES. Weed broomrapes: systematics ontogenesis biology evolution. München: Aufstieg-Verlag; 1997. [Google Scholar]
- Teryokhin ES, Shibakina GV, Serafimovitch NB, Kravtsova TI. Determination of broomrapes in the USSR flora. Sankt-Peterburg: Nauka [in Russian]; 1993. [Google Scholar]
- Thomas H, Heller A, Sauerborn J, Müller-Stöver D. Fusarium oxysporum f. sp. orthoceras, a potenial mycoherbicide, parasitizes seeds of Orbanches cumana (sunflower broomrape): a cytological study. Annals of Botany. 1999;83:453–458. [Google Scholar]
- Vaucher JP. Monographie des Orobanches. Genève: Chez les héritiers de J.J. Paschoud, imprimeurs – librares; 1827. [Google Scholar]
- Velasco L, Goffman FD, Pujadas Salva AJ. Fatty acids and tocochromanols in seeds of Orobanche. Phytochemistry. 2000;54:295–300. doi: 10.1016/s0031-9422(00)00085-6. [DOI] [PubMed] [Google Scholar]
- Xie X, Yoneyama K, Yoneyama K. The strigolactone story. Annual Review of Phytopathology. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wang Z. Current opinions on endosperm transfer cells in maize. Plant Cell Reports. 2010;29:935–942. doi: 10.1007/s00299-010-0891-z. [DOI] [PubMed] [Google Scholar]