Abstract
Background and Aims
The extreme complexity of asclepiad flowers (Asclepiadoideae–Apocynaceae) has generated particular interest in the pollination biology of this group of plants especially in the mechanisms involved in the pollination processes. This study compares two South American species, Morrenia odorata and Morrenia brachystephana, with respect to morphology and anatomy of flower structures, dynamic aspects of the pollination mechanism, diversity of visitors and effectiveness of pollinators.
Methods
Floral structure was studied with fresh and fixed flowers following classical techniques. The pollination mechanism was studied by visiting fresh flowers in the laboratory with artificial pollinator body parts created with an eyelash. Morphometric and nectar measurements were also taken. Pollen transfer efficiency in the flowers was calculated by recording the frequency of removed and inserted pollinia. Visitor activity was recorded in the field, and floral visitors were captured for subsequent analysis of pollen loads. Finally, pollinator effectiveness was calculated with an index.
Key Results
The detailed structure of the flowers revealed a complex system of guide rails and chambers precisely arranged in order to achieve effective pollinaria transport. Morrenia odorata is functionally specialized for wasp pollination, and M. brachystephana for wasp and bee pollination. Pollinators transport chains of pollinaria adhered to their mouthparts.
Conclusions
Morrenia odorata and M. brachystephana present differences in the morphology and size of their corona, gynostegium and pollinaria, which explain the differences in details of the functioning of the general pollination mechanism. Pollination is performed by different groups of highly effective pollinators. Morrenia species are specialized for pollination mainly by several species of wasps, a specialized pollination which has been poorly studied. In particular, pompilid wasps are reported as important pollinators in other regions outside South Africa. A putative new function of nectar in asclepiads is presented, as it would be contributing to the pollination mechanism.
Keywords: Morrenia odorata, Morrenia brachystephana, asclepiads, functional morphology, pollination mechanism, wasps, pollinator effectiveness
INTRODUCTION
The flowers of Asclepiadoideae, commonly known as ‘asclepiads’ (family Apocynaceae sensu Endress and Bruyns, 2000; Endress et al., 2007), caught the attention of early botanists, mainly because of their extremely elaborate floral arrangements (Brown, 1833; Corry, 1884; Endress, 1994). Several aspects of these arrangements involve a marked synorganization which is fairly uniform within and exclusive to this subfamily. The more outstanding features include: (1) a corona, a sterile whorl additional to the corolla; (2) a gynostegium, an organ formed by the post-genital fusion of androecium and gynoecium that is differentiated into five sectors arranged in a revolver-like system (‘Revolverblüte’), in which each sector constitutes a pollination unit which comprises a guide rail, a stigmatic chamber and the receptive area; and (3) pollinaria, constituted by two pollinia from adjacent anthers and linked by a solid bridge of stigmatic secretions, the translator apparatus (Bookman, 1981; Fallen, 1986; Endress, 1994; Kunze, 1981, 1996; Verhoeven and Venter, 2001). While the general bauplan is extremely conservative, floral diversity is driven by differences in these features and their relationship to each other (Endress, 1994).
In asclepiads, pollination is performed when an insect or, more exceptionally a bird, pursuing nectar gets first caught by the anther margins and then is forced by the guide rail to move its trapped body part upwards to reach the translator apparatus which clamps the pollinarium onto the pollen vector. When the pollen vector visits another flower, a pollinium is placed into the stigmatic chamber located between the margins of two adjacent anthers (Kunze, 1991, 1995; Liede, 1994; Ollerton and Liede, 1997; Pauw, 1998). This pollination mechanism is thought to minimize the mixing of pollen from different donors (Wyatt and Broyles, 1994; Wyatt and Lipow, 2007). While aspects of the general pollination mechanism are well understood, little is known about its dynamic aspects, i.e. details of the interaction between the floral parts and the pollinators to achieve the removal and the insertion of the pollinia. This lack of knowledge has hampered our understanding of some fundamental aspects in relation to floral morphology. To what extent are the morphology and anatomy of flowers involved in the way pollinaria are removed and deposited? Is the efficiency of pollinaria transference by pollinators associated with differences in structural details of the floral mechanism? Does floral morphology determine pollinaria deposition in different pollinator body parts? This knowledge would help to understand a question of more general interest, namely whether the diversity in corona, gynostegia and pollinaria morphology of closely related species is associated with different pollinators and pollination mechanisms.
Wasp-pollinated flowers are common among deceptive orchids and figs, but rare in nectar-rewarding plants (Nilsson, 1981; Ciotek et al., 2005; Johnson, 2005). Although species of several genera of Asclepiadoideae have been inferred to be pollinated by wasps, these insects have been confirmed as the main or exclusive pollinators for only a few species from southern Africa and tropical South America (Vieira and Shepherd, 1999; Ollerton et al., 2003; Shuttleworth and Johnson, 2006, 2008, 2009a, b, c; Wolff et al., 2008). Anecdotal records (Schulz, 1937; Ollerton et al., 2011) suggest that pollination by wasps may be more frequent in South American Asclepiadoideae than hitherto known, but detailed observations are lacking. It is thus interesting to test whether this prediction is confirmed and, if so, which floral characteristics have paralleled those of South African species that are known to be wasp pollinated. A number of floral features, such as dull colour, unusual odour and quite accessible and concentrated nectar, have been traditionally associated with wasp pollination (Faegri and van der Pijl, 1979; Heithaus, 1979; Proctor et al., 1996; Ollerton and Watts, 2000; Shuttleworth and Johnson, 2009a). Pompilid wasps (Hymenoptera: Pompilidae) have been reported as the main pollinators of several taxa of Asclepiadoideae such as species of Asclepias from North America (Kephart, 1979), species of Miraglossum, Xysmalobium and Pachycarpus from South Africa (Ollerton et al., 2003; Shuttleworth and Johnson, 2006, 2008, 2009a, b), Gomphocarpus physocarpus naturalized in Australia (Forster, 1994) and species of Oxypetalum from Brazil (Vieira and Shepherd, 1999). Vespid wasps (Hymenoptera: Vespidae) have been reported as pollinators of Oxypetalum species from Ecuador and Brazil (Vieira and Shepherd, 1999; Wolff et al., 2008), of Asclepias species from North America (Theiss et al., 2007), of Blepharodon nitidum in Guyana (J. Ollerton, University of Northampton, UK, pers. comm.) and of Cynanchum species in Japan (Yamashiro et al., 2008). Species of Asclepias have also been reported as pollinated by tiphiid wasps and sphecids (Hymenoptera: Tiphiidae and Sphecidae; Ollerton et al., 2003; Theiss et al., 2007).
Although Asclepiadoideae is a very speciose group (approx. 3000 species, Meve, 2002) and distributed worldwide, data on pollination biology are only available for a limited number of species (287 species, representing about 9·6 % of the total species number in the subfamily; see Table 1). Most data on pollination biology refer to the genus Asclepias and particularly to species from North America (Macior, 1965; Lynch, 1977; Bookman, 1981; Fishbein and Venable, 1996; Theiss et al., 2007, and references therein). In some parts of the world, such as Argentina, the lack of knowledge is particularly acute since only 1·3 % of the species have been studied in this respect (Kunze, 1995; Ezcurra, 1999). In addition, there is a scarcity of studies dealing with functional morphology, i.e. how floral structures are interpreted in terms of pollination mechanisms (but see Kunze, 1991, 1995; Kunze and Liede, 1991; Ollerton and Liede, 2003; Ollerton et al., 2003). Taking into account the scarce information in the literature on pollination biology of species of Asclepiadoideae, pollination studies on additional species should help to shed light on the diversity of pollination systems of the group.
Table 1.
Number of genera and species studied, and published and unpublished works in relation to pollination biology of Asclepiadoideae according to Ollerton et al. (2009, 2011)
| Continent | No. of genera | No. of species | Authors (published and unpublished works) |
|---|---|---|---|
| Africa | 38 | 121 | 39 |
| North America | 13 | 46 | 41 |
| Asia | 33 | 61 | 22 |
| Europe | 20 | 24 | 17 |
| Central and South America | 22 | 26 | 17 |
| Oceania | 7 | 9 | 10 |
| Total | 133 | 287 | 146 |
The aims of this work are to analyse the pollination biology of two species of Morrenia, M. brachystephana and M. odorata, which often grow sympatrically in southern South America. Aspects of particular interest include: (1) morphological and anatomical flower features related to the pollination mechanism; (2) comparison between both species of features such as nectar and morphometrics measurements; (3) dynamics of the pollination mechanism; (4) pollination success; and (5) flower visitors and pollinators, placement of pollen loads and pollinator effectiveness.
MATERIALS AND METHODS
Study species
Morrenia odorata (Hook. et Arn.) Lindl. and Morrenia brachystephana Griseb. are semi-succulent leafy and milky twiners common in the dry Chaco region of South America (Goyder, 2003). Both species are widely distributed in northern and central Argentina as well as in Uruguay, and M. odorata also reaches south-eastern Bolivia, Paraguay and southern Brazil (Ezcurra, 1999). These species do not form dense populations in nature and occur in several vegetation types including dense woodland, scrub and disturbed environments (Arenas, 1999). The flowering season of M. brachystephana is from October to February (Sérsic et al., 2006), and of M. odorata from November to March (A. N. Sérsic and A. A. Cocucci, pers. obs.).
Study sites
Field observations were carried out in three wild populations in Argentina, at the following sites: M. brachystephana in Cuesta Blanca village (31°28′55·9′′S, 64°34′54·2″W, 746 m; AAC 4280, 4281) during the flowering season from 2006 to 2010, and M. odorata in Chancaní Nature Reserve (31°22′33·74521′'S, 65°28′47·60553′′W, 347·9 m; AAC 4254), and near Quilino village (30°12′ S, 64°28′48′′W, 831 m) during 2007 and 2010. Plant specimens are deposited in CORD.
Floral structure analysis
To study the dynamics of the pollinarium removal and pollinium deposition processes, fresh flowers were analysed in the laboratory with the aid of a stereomicroscope. For anatomical studies adult flowers were fixed for 24 h in a (1:4) glutaraldehyde:phosphate buffer pH 7 solution, and then stored in 70 % ethanol for anatomical studies. A vacuum bomb was used to remove possible air bubbles from the material. Flowers were either embedded in synthetic resins according to the manufacturers' protocol (Kulzer Histo-Technique 7100), sectioned (5 µm thick) and stained with Toluidine Blue for 30 min (Sakai 1973), or embedded in Paraplast after dehydrating using a tertiary butyl alcohol series, sectioned 10–12 µm thick and stained with 1 % Safranin O for 10 min and 1 % Astra Blue for 20 min according to standard protocols (Gerlach, 1969). Light microscopy observations were carried out with Zeiss Axiophot and Olympus BX51 microscopes. Both techniques were used in M. odorata and only resins in M. brachystephana. Finally, samples of dissected flowers were viewed with a scanning electron microscope (SEM-Zeiss microscope) after critical point drying and sputter-coating using conventional protocols (Bozzola and Russel, 1991).
Morphometric and nectar measurements
Fourteen floral traits were measured from digital scaled photographs of fresh flowers using SigmaScan software (Copyright© 1987–1999 SPSS Inc.): corolla diameter, petal length, corolla tube length, corona diameter, corona length, gynostegium diameter, gynostegium length, guide rail length, guide rail entrance length, corpusculum length, corpusculum width, caudicle length, pollinium length and pollinium width. Measurements were taken on a total of 22 flowers (two flowers per plant of 11 plants) in M. odorata, and 20 flowers (two flowers per plant of ten plants) in M. brachystephana.
Nectar standing crop volume was measured with microcapillary tubes (Drummond Scientific Co.) and sugar concentration, as sucrose equivalents (% p/p), was measured using a hand-held refractometer (0–32 % Atago Co.) (Kearns and Inouye, 1993; Galetto and Bernardello, 2005). Nectar measurements were conducted for 12 plants (standing crop volume) and three plants (sugar concentration) in M. odorata, and for 17 plants (standing crop volume) and 11 plants (sugar concentration) in M. brachystephana.
To determine whether morphometric and nectar measurements significantly varied between species, an analysis of variance (ANOVA) was performed with InfoStat software (Di Rienzo et al., 2004). Corpusculum length and standing crop volume measurements were Log10 transformed. A non-parametric ANOVA (Kruskal–Wallis) was performed on corona length.
Flower–insect mechanical interaction
The mechanical interplay between flowers and pollinators was studied by simulating pollinator body parts with an artificial device created with a human eyelash adhered to the tip of a toothpick. Holm (1950) was the first to mention the use of a fine hair as a successful device in the study of the pollination process. However, eyelashes have the advantage of having a pointed end in comparison with hairs, and thus have, at a given height, the necessary thickness to operate the mechanism. The process was recorded with a Sony CCD-IRIS colour video camera mounted on a Leica M420 stereomicroscope for M. odorata.
Pollination success
In a random sample of flowers (M. brachystephana, n = 110; M. odorata, n = 104), the frequency of removed pollinaria (r) and pollinia inserted into the stigmatic chambers (i) per flower was scored under a stereomicroscope. The pollinia transfer efficiency was calculated as: PTE = i/r. The percentage of pollinated flowers (containing at least one inserted pollinium) was also calculated for each species (Johnson et al., 2004).
Visitors, pollinators and pollinator effectiveness
Insect activity was recorded between 0900 and 1800 h under optimal weather conditions, totalling 96 person-hours (47 for M. brachystephana and 49 for M. odorata). Insect behaviour visiting flowers was documented, and afterwards insects were netted to record the place of attachment of pollinaria on the pollinators' body under the stereomicroscope. Details on the mode of pollinaria attachment to the insects' body were studied with SEM as described above, omitting, however, the critical point drying procedure. Captured flower visitors were identified by specialists or with the use of the literature to genus or species level (Brewer and Argüello, 1980; Vardy, 2000, 2002, 2005; Fernandez and Sharky, 2006; Michener, 2007) [Supplementary Data Table S1, available online].
As a measure of pollinator effectiveness in removing pollinaria, the number of attached corpuscula, half-pollinaria and pollinaria (with zero, one and two pollinia, respectively) were counted and the number of missing pollinia was calculated from these data (Theiss et al., 2007). We developed an index expressing the efficiency of pollinia transference. Efficiency of pollen transference between flowers has to take into account the amount of pollen picked up from a flower by an insect and the amount of this pollen that is delivered onto the stigmas of other flowers by the same insect. Such an estimate regularly requires quantification, first, of the amount of pollen on flowers, because the amount picked by a pollinator is a fraction of the pollen available on flowers, and, secondly, quantification of the amount of pollen carried by each pollinator that is deposited onto the stigma (see Sahli and Conner, 2007). In plants with pollen grains that shed singly, this information may be cumbersome to obtain. In milkweeds, however, where pollen is shed in five discrete units, we can readily assess the amount of pollen that can be picked from flowers. In addition, because pollinaria of milkweeds consist, on the one hand, of parts that are transferable between flowers (pollinia) and, on the other hand, of parts that are not transferred and remain attached to the pollinator body (corpuscula), we can determine both the amount of pollen carried by a pollinator and the amount of this pollen that has been deposited on stigmas. Taking advantage of these particularities of the milkweeds floral mechanism, we derive a series of parameters to calculate the index of insect-mediated pollen transference efficiency. First, we considered the population's mean number of pollinaria in flowers from which the probability, P, of picking one pollinarium in one visit to a guide rail sector can be derived as P = p/5, where p is the mean number of pollinaria remaining unremoved per flower in the population and five is the total number of pollinaria per flower. Secondly, the number of corpuscula, C, informs of the number of times a pollinator has succeeded in removing pollinaria from the guide rails. Thus, the number of visits, R, to guide rails can be estimated as R = C/P. Finally, the number of pollinia that are missing from these corpuscula, I, represents an estimate of the efficiency of delivery, i.e. insertion in the flower's stigmatic sectors. The effectiveness of pollinia transference by an individual insect is the relationship between I and R, i.e. E = I/R. Since one pollinarium is removed in the same strike as a pollinium is delivered, E values close to 1 indicate that one pollinium is delivered for each pollinarium removed. Values of E <1 indicate that pollinia are delivered in a proportion lower than expected by the number of pollinaria picked up, and values of E >1 indicate that pollinaria are picked in a proportion lower that expected by the pollinia delivered. Thus values of E <1 and E >1 represent loss of efficiency through female and male functions, respectively. We performed a least-square linear regression between delivered pollinia (I) and the estimated number of visits (R) of both Morrenia species, and tested the significance of the departure from a slope of 1 with a t-test.
RESULTS
General description of the flowers
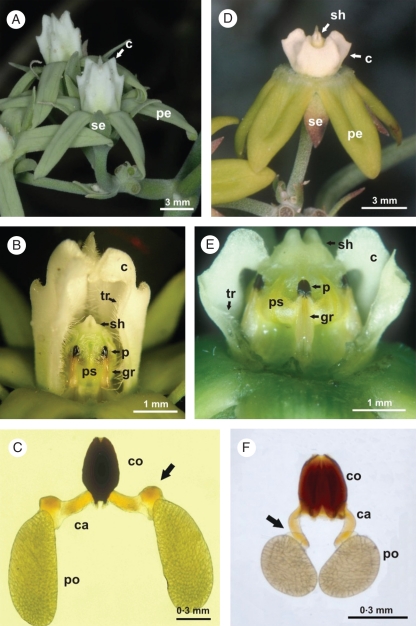
The corolla of both species is light green in colour, and rotates with horizontally spreading or sometimes slightly reflexed petals and a white tubular corona surrounding the fertile parts (Fig. 1A, D; Meyer, 1944). The androecium is adnate to a large and apically bifurcated style head to which it is post-genitally fused, forming a gynostegium (Figs 1B, E; 3B, C, G; 4A, B). The staminal filaments are fused into a filament tube on which anthers are borne. Each anther is constituted by a fertile central part formed by two theca, with one pollinium each, and three sterile expansions: two lateral anther wings, and an apical connective appendage, which covers part of the style head (Fig. 2). Between the anther wings and the connective appendage, two lateral indentations are noticeable (Figs 2, 4B). The edges of anther wings of adjacent anthers delimit the guide rail (Fig. 1B, E). The translator apparatus, located between adjacent anthers at the apical end of the guide rail, is constituted by a corpusculum and two caudicles, the former being a rounded solid structure with a cleft extending along its vertical axis and positioned in the same line as the guide rail (Figs 1E, 4B). The caudicles are two slender flexible arms attached laterally to the base of the corpusculum, and each one is connected to the pollinium of adjacent anthers (Fig. 1C, F).
Fig. 1.
External floral structure. (A–C) Morrenia odorata. (D–F) Morrenia brachystephana. (A, D) External lateral view of the flowers. (B, E) Gynostegium in lateral view; part of the corona was removed. (C, F) Pollinaria; arrows indicate the basal thickening of the caudicle. Abbreviations: caudicle (ca), corona (c), corpusculum (co), guide rail (gr), petal (pe), pollen sacs (ps), pollinarium (p), pollinium (po), sepal (se), style head (sh), trichomes (tr).
Fig. 3.
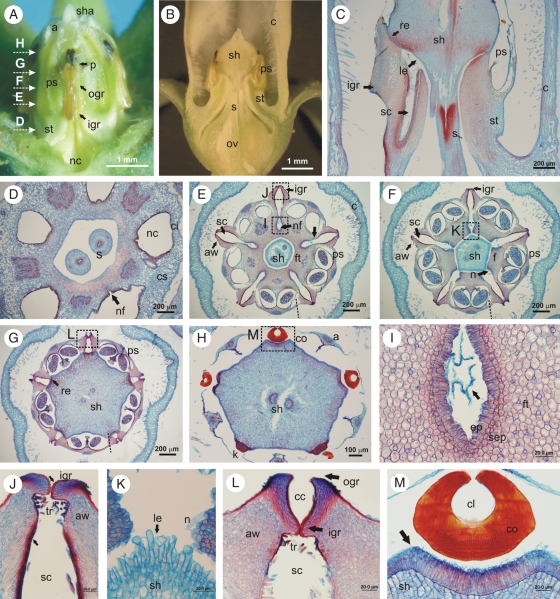
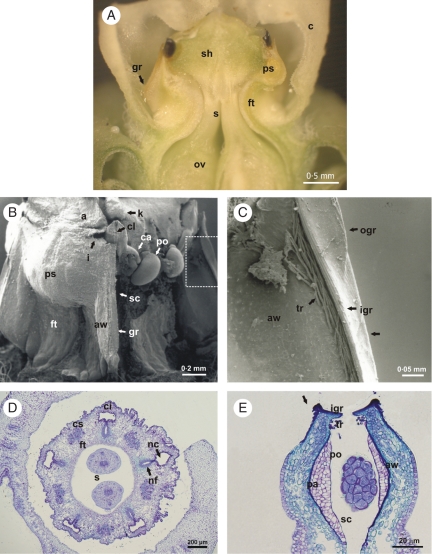
Internal floral structure of M. odorata. (A) Gynostegium in lateral view; the corona was removed. (B, C) Longitudinal section of the flower and gynostegium indicated in (E–G). (D–H) Cross sections of the gynostegium at the levels indicated in A. (I–M) Details marked in (E–H), respectively. The arrows indicate: in (E) the constriction between the anther and the filament tube, in (I) the amorphous phase secreted by the nectary, in (J) the denticles and the collapsed epidermal cells, and in (M) the epidermis which secretes the translator apparatus. Abbreviations: anther wings (aw), corona (c), corpusculum (co), corpuscular chamber (cc), corpusculum cleft (cl), epidermal cells of the nectary (ep), filament (f), filament tube (ft), connective appendage (a), inner guide rail (igr), interstaminal corona (ci), knob (k), lignified trichomes (tr), loose epidermal cells (le), nectary (n), nectar chamber (nc), nectar furrow (nf), outer guide rail (ogr), ovary (ov), pollen sacs (ps), pollinarium (p), retinaculum (re), staminal corona (cs), stigmatic chamber (sc), stipe (st), style head (sh), style head appendage (sha), styles (s), sub-epidermal cells of the nectary (sep).
Fig. 4.
Floral structure of M. brachystephana. (A) Longitudinal section of the flower. (B, C) SEM photographs. (B) Gynostegium; an anther was removed. (C) Detail marked in (B). Note the striated surface at the edges of the inner and outer guide rail. (D, E) Cross-sections. (D) Gynostegium at the level of the styles. (E) Inner guide rail with an inserted pollinium. Arrows indicate in (C) and (E) the ridge of concentrated sclerenchymatic cells. Abbreviations: anther wing (aw), caudicle (ca), connective appendage (a), corpusculum cleft (cl), filament tube (ft), guide rail (gr), indentation (i), inner guide rail (igr), interstaminal corona (ci), knob (k), lignified trichomes (tr), nectar furrow (nf), nectar chamber (nc), outer guide rail (ogr), ovary (ov), pad of multilayered epidermal cells (pa), pollen sacs (ps), pollinium (po), staminal corona (cs), stigmatic chamber (sc), style head (sh), styles (s).
Fig. 2.
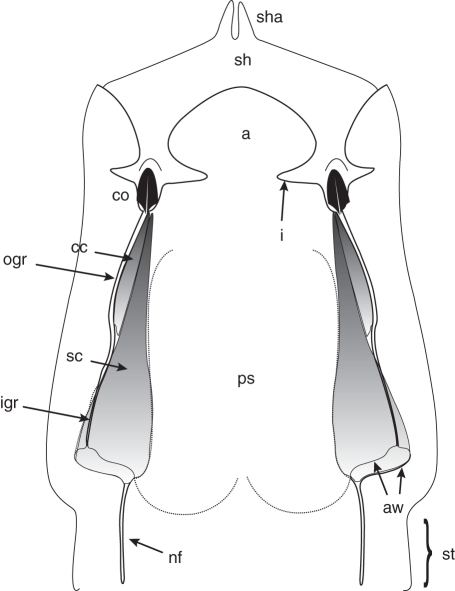
Diagram of the gynostegium of M. odorata. Abbreviations: anther wing (aw), connective appendage (a), corpuscular chamber (cc), corpusculum (co), indentation (i), inner guide rail (igr), outer guide rail (ogr), pollen sacs (ps), nectar furrow (nf), stigmatic chamber (sc), stipe (st), style head (sh), style head appendage (sha).
Floral structure analysis, and morphometric and nectar measurements
The corona consists of a tube that arises from the base of the gynostegium; at the top of the tube, five corona lobes close the entrance by folding inwards, towards the gynostegium (Fig. 1B, D, E), leaving a star-shaped access. In M. odorata the tubular corona surpasses the gynostegium in length and its entire inner surface is covered by smooth downward-pointing trichomes (Figs 1B, 5A–C). On the other hand, the bowl-shaped corona of M. brachystephana equals the gynostegium with the lobes almost in contact with the style head but not covering the style head appendage; the inner downward-pointing trichomes are arranged in five vertical strips, each one restricted to an interstaminal sector, opposite a guide rail (Fig. 1E). Below each guide rail, a nectar chamber is formed between the base of the filament tube and the corona; in M. brachystephana the nectar chambers are small and hidden below the guide rails, but in M. odorata, the nectar chambers are conspicuous and opened widely (Fig. 3A). Both Morrenia species differ in the extension of the filament tube and in the shape of the gynostegium. The filament tube of M. odorata elongates below the level of the anthers so that the cylindrical gynostegium is elevated from the base of the corona by a noticeable stipe (Figs 2, 3A, B), whereas in M. brachystephana the filament tube is not elongated, so its cone-shaped gynostegium is sessile (Fig. 4A). Concerning the nectar chambers, both species have a furrow that extends along the whole extension of the filament tube. This furrow has a noticeable glandular epithelium row which constitutes the nectary; the epithelium extends along the innermost part of the stigmatic chamber through the filament tube, reaching the free parts of the filaments (Fig. 3E, F, I). In M. brachystephana the nectar furrow discharges directly into the nectar chamber (Fig. 4D), while in M. odorata the secretory part of the furrow is connected to the nectar chamber by a non-secretory part extended along the stipe (Figs 2, 3A, D). The epidermal cells of the nectary of both species stained stronger in purple than their neighbouring cells, indicating secretory activity (Fig. 3I). Anatomically, these cells are elongated, well connected at the base and slightly loose at the top. Beneath the epidermis an additional layer of isodiametric smaller glandular cells is part of the nectary structure. Both layers show a cytoplasm rich in stroma and a large nucleus. The nectar furrow usually contains an amorphous phase secreted by these glands that stains blue in M. odorata when stained with Safranin and Astra Blue (Fig. 3I).
Fig. 5.
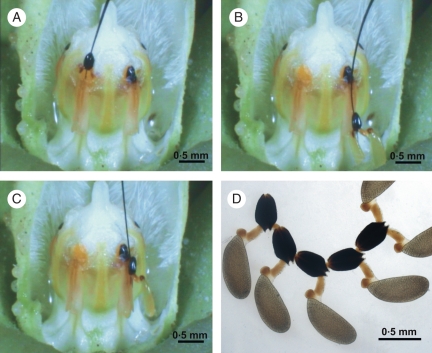
Pollination mechanism of M. odorata. (A) Pollinarium removal. (B) Insertion of one pollinium into the entrance of the inner guide rail. (C) The caudicle breaks and the pollinium becomes lodged in the stigmatic chamber. (D) Chain of pollinaria.
The margins of the anther wings form a double guide rail system constituted by a basal and inner part, named the inner guide rail, which is partially overlapped by an apical and external portion, named the outer guide rail. Each guide rail delimits an independent entrance by basally diverging edges which draw close together and run straight upwards (Fig. 2). A remarkable difference between the species is that in M. odorata the inner guide rail extension reaches the pollen sacs and its entrance is straight (Fig. 3A), whereas in M. brachystephana the inner guide rail is longer than the pollen sacs, which are gibbous, and its entrance is sloped (Fig. 4B). The inner guide rail leads to the receptive sector (below the style head), while the outer guide rail leads to the corpusculum of the pollinarium. Anatomically, the anther wings constitute many layers of sclerenchymatic cells strongly compressed towards the epidermis of the anther. The edges of the inner guide rail are covered by one layer of collapsed and sclerified epidermal cells with their surface covered by inward-oriented striae (Figs 3J, 4C). In M. brachystephana, each anther wing shows an evident rounded ridge not present in M. odorata (Fig. 4C, E). In upper levels, the edges of the inner guide rail become closer (Fig. 3G, L) until they make contact with each other at the beginning of the outer guide rail (Fig. 3L). As described for the inner guide rail, the outer guide rail is also composed of sclerenchymatic cells progressively compressed to the edges, and its sclerified epidermal cells also have inwardly oriented striae, but fewer than in the inner guide rail (Figs 3G, L, 4C).
Both guide rails delimit two internal chambers: the stigmatic chamber, which largely corresponds to the inner guide rail and partially overlaps with a previously unnamed chamber delimited by the outer guide rail, here called the corpuscular chamber (Figs 2, 3L). The lateral surface of the stigmatic chamber is sclerified, covered by collapsed layers of epidermal and sub-epidermal cells (Fig. 3J). Morrenia brachystephana is particular in showing a pad of non-sclerified multilayered tissue above the sclerified layers (Fig. 4E). Beneath the inner guide rail, a group of introrse and lignified upward-pointing trichomes is arranged (Figs 3J, L, 4C, E). The stigmatic chamber connects at its innermost part with the nectar furrow deep in the filament tube. The boundary between them is distinguishable by a marked constriction next to the pollen sacs (Fig. 3E). At the level where the filament tube becomes separated into five individual filaments, the inner part of the stigmatic chamber opens towards the lower surface of the style head where it has five patches of loosely arranged and elongated epidermal cells corresponding to the receptive surface (Fig. 3F, K). The innermost parts of the anther wings are in close contact with the style head epidermal cells forming the retinaculum (Fig. 3C, G).
At the top of the gynostegium, the style head shows five glandular areas adjacent to the five translator apparatus (Fig. 3H). This glandular area is constituted by strongly stained, elongated and tightly packed epidermal cells. This tissue is involved in the secretion of the translator apparatus. The corpusculum has an evident striped internal pattern presumably as a result of the secretory activity of individual epidermal cells (Fig. 3H, M). The edges that delimit the cleft of the corpusculum are sharp, inwardly oriented, becoming narrower at higher levels and slightly overlapping at a certain point. At the contact point between the caudicle and pollinium, a thickening of the caudicle is discernible (Fig. 1C, F). Each corpusculum is fixed in position by a style head knob located above it (Figs 3H, 4B). The two species also differ in their pollinaria morphology. Morrenia brachystephana has more compact pollinaria with a globose corpusculum and pendent, curved caudicles in which the thickened part is in contact at the middle of pollinia, whereas M. odorata has a rhomboid corpusculum, with horizontal caudicles that separate the oblong pollinia from each other, and with the thickened part at the apical end of the pollinia. Structural differences of Morrenia species are summarized in Table 2 and morphometric and nectar measurements in Table 3.
Table 2.
Main floral features differing between M. brachystephana and M. odorata flowers
| Floral features | M. brachystephana | M. odorata |
|---|---|---|
| Corona | ||
| Architecture | Bowl-shaped | Tubular |
| Relationship with gynostegium | Do not enclose the gynostegium completely | Enclose the gynostegium completely |
| Trichomes | Partitioned: in front of each guide rails | Not partitioned: all over the surface |
| Gynostegium (sensuLiede, 1996) | Sessile and cone-shaped | Stipitated and cylindrical |
| Nectar chambers | Small, hidden below the guide rails | Conspicuous, opened widely |
| Pollen sacs | ||
| Shape | Gibbous | Flat |
| Extent | Reaching the beginning of the outer guide rail | Reaching the beginning of the inner guide rail |
| Inner guide rail | ||
| Entrance | Sloped | Straight |
| Multilayered pad | Present | Absent |
| Ridge | Present | Absent |
| Extent of nectar furrow | Coupled to the nectar chambers | Uncoupled from the nectar chambers |
| Corpusculum | Globose | Rhomboid |
| Caudicles | Pendent and curved | Horizontal and slightly curved |
| Pollinia | Globose | Oblong |
Table 3.
Morphometric and nectar measurements of M. odorata and M. brachystephana (median ± s.e. for corona length and mean ± s.e. for the rest of the measurements)
| Measurement | M. odorata | M. brachystephana | Statistical value |
|---|---|---|---|
| Corolla diameter | 20·82 ± 0·38 | 13·65 ± 0·21 | F = 258·77*** |
| Corolla tube length | 2·73 ± 0·06 | 1·31 ± 0·07 | F = 239·79*** |
| Petal length | 8·37 ± 0·19 | 5·54 ± 0·10 | F = 168·16*** |
| Corona diameter | 4·66 ± 0·10 | 3·35 ± 0·05 | F = 133·40*** |
| Corona length (CNL) | 4·88 ± 0·20 | 2·01 ± 0·21 | F = 15·00*** |
| Gynostegium diameter | 2·11 ± 0·02 | 1·65 ± 0·02 | F = 256·24*** |
| Gynostegium length (GL) | 3·38 ± 0·06 | 2·27 ± 0·05 | F = 219·46*** |
| CNL/GL | 1·44 ± 0·03 | 0·86 ± 0·04 | F = 140·46*** |
| Style head appendage length | 0·53 ± 0·05 | 0·34 ± 0·02 | F = 12·95* |
| Guide rail length | 1·15 ± 0·01 | 0·83 ± 0·03 | F = 127·66*** |
| Entrance guide rail length | 0·30 ± 0·01 | 0·24 ± 0·01 | F = 24·38*** |
| Corpusculum length | 0·39 ± 0·01 | 0·30 ± 0·01 | F = 70·11*** |
| Corpusculum width | 0·26 ± 0·01 | 0·23 ± 0·01 | F = 19·34** |
| Caudicle length | 0·24 ±0 .00 | 0·17 ± 0·01 | F = 78·07*** |
| Pollinium length | 0·86 ± 0·01 | 0·33 ± 0·00 | F = 1910·65*** |
| Pollinium width | 0·36 ± 0·01 | 0·22 ± 0·01 | F = 247·76*** |
| Standing crop volume | 0·58 ± 0·23 | 0·45 ± 0·09 | F = 0·06* |
| Sugar concentration | 29·03 ± 0·34 | 23·52 ± 0·95 | F = 8·59* |
Morphometric measurements are in millimetres. Standing crop volume is in microlitres, and the sugar concentration is given as a percentage g/g.
***P < 0·0001; **P < 0·001; *P < 0·01.
Nectar presentation
Nectar presentation differs between the two species. In M. odorata, nectar flows from the nectary tissue through the nectar furrows and initially accumulates in the nectar chambers. If nectar secretion proceeds further, nectar chambers may be overflowing and nectar fills the space between the stipe and the corona, all around the base of the gynostegium (Figs 3A, 5A–C). Downward-pointing trichomes covering the inner surface of the corona tube seem to protect the stored nectar, since flowers can adopt a pendent position in the plant. The flowers of M. brachystephana lack such a continuous space around the base of the gynostegium due to the absence of a stipe and because nectar chambers are smaller and concealed below the guide rails. As a consequence, nectar is presented to pollinators partitioned among the five cavities opposite the anthers in that species. These cavities are flanked by the anther wings and topped by the pollen sacs (Fig. 4A, B). The vertical stripes of downward-pointing trichomes of the corona tube opposite the guide rails and the basal part of the corona in close contact with the entrance of the inner guide rail restrict the access to nectar of the five cavities below the pollen sacs (Fig. 4A).
Flower–insect mechanical interaction
The corona lobes are closed over the gynostegium so visitors must unfold them with their mouthparts to reach the nectar. In the case of M. brachystephana, the five corona lobes lie in contact with the gynostegium and restrict access to the nectar through five staminal sectors which lead to the five cavities filled with nectar (Figs 1E, 4B). In contrast, in M. odorata there is a single corona entrance delimited by the five staminal lobes, which are positioned at a higher level since the corona is almost double the length of the gynostegium (Fig. 1B; Table 3). In both species, trichomes covering the inner surface of the corona tube seem to play a role in guiding the mouthparts to the nectar and guide rails as they point downwards, but their distribution may be related to the way nectar is presented in both species. In M. odorata, nectar can occupy the whole base of the gynostegium so that the trichomes cover the entire surface. On the other hand, nectar in flowers of M. brachystephana is partitioned into the five staminal cavities so that the trichomes form vertical strips.
The video of M. odorata flowers (Supplementary Data Video) revealed that, in a first try, the artificial device is caught by the entrance of the outer guide rail and could only be moved upward into the corpuscular chamber. Downward movement is apparently prevented by the striae on the edges, and then the device is clasped by the corpusculum. The presence of this clasping mechanism is possibly favoured due to the decreasing narrowness and the slightly overlapping and inwardly curved edges of the corpusculum cleft (Figs 3M, 5A). It is evident that pollinating insects must be strong enough to pull out the pollinarium, since the style head knob locks the pollinarium, presumably contributing to the precise attachment of the corpusculum to the insects' mouthparts. A close match between the guide rail and the thickness of the device is probably needed. Thinner devices do not fit tightly enough into the guide rail and the corpusculum cleft, and can be passed through the guide rail without removing pollinaria. Conversely, thicker devices would be stuck into the guide rail when entering into it and could not be released, thus preventing efficient pollinaria removal. Caudicles of removed pollinaria do not reconfigure after removal and consequently do not change the orientation of the pollinia in relation to the corpusculum.
In subsequent trials, with a device carrying a pollinarium, one pollinium is caught by the inner guide rail and completely inserted into the stigmatic chamber while the other pollinium remains uninserted (Fig. 5B). The pollinium is apparently prevented from downward sliding by the striae and the introrse trichomes of the anther wings. The stipe and the relatively deep nectar chamber of this species provide the space required for the pollinium to fit below the guide rail entrance (Figs 3A, B, D, 5B). To insert the pollinium, the device has to perform an upward movement in order to guide the thickened part of the caudicle into the inner guide rail.
In M. brachystephana, pollinaria removal is performed as described above but there are marked differences from M. odorata in the way pollinia are inserted. As the gynostegium is sessile, the corona is in close contact with the entrance of the inner guide rail and the nectar chambers are smaller than in M. odorata. Consequently, the pollinium does not fit below the inner guide rails and must twist below and into the entrances of the guide rail to be inserted, moving from the staminal sector with nectar.
The thickened part of the caudicles seems to function as a guiding structure, as the pollinium can only be inserted after being first caught at the inner guide rail entrance (Figs 1C, F, 5B). The sclerenchymatic anther wings and the sclerified epidermal cells are probably necessary to withstand mechanical tensions exerted by the pollinators. The inwardly curved striated surface at the edges of the inner guide rail presumably improves the sliding of the inserting caudicle into the stigmatic chamber during the upward motion. The edges of the inner guide rail become close together towards the top and, with the contribution of the inwardly curved striate surface, the caudicle is gradually constrained and finally is cut. The introrse and lignified trichomes within the inner guide rail contribute to avoid a downward movement of the inserted caudicle (Figs 3J, L, 4C, E). At the base of the outer guide rail, the edges of the inner guide rail are in such close contact that the caudicle cuts off from the pollinium after it is left lodged in the stigmatic chamber (Figs 3L, 5C). Immediately, the remnant caudicle becomes lodged in the corpuscular chamber and slides towards the corpusculum cleft. Once the caudicle is caught by the corpusculum cleft, a second pollinarium is removed chained to the first one. In subsequent trials, longer pollinaria chains may be formed (Fig. 5D). The two lateral indentations at the base of the connective appendages provide enough flexibility to flip aside when the pollinarium slides outwards (Figs 2, 4B).
Pollination success
Multiple removals per flower were not frequent in the observed flowers. The maximum number of pollinaria removed was four in M. brachystephana, and three in M. odorata (Table 4). The mean number of removed pollinaria per flower was 0·55 and 0·31 in M. brachystephana and in M. odorata, respectively. In most of the flowers, only one pollinarium per flower was removed, and less often two pollinaria were removed per flower. In both species, the number of removed pollinaria was higher than the number of inserted pollinia. The mean number of inserted pollinia per flower was 0·19 and 0·10 in M. brachystephana and M. odorata, respectively. Insertions were successful mainly in only one stigmatic chamber per flower, i.e. one pollinium per stigmatic chamber (single insertion), with insertions of two pollinia in one stigmatic chamber being very rare. In M. odorata, 100 % of insertions occurred together with the removal of the corresponding pollinarium, whereas in M. brachystephana insertions occurred 47·6 % of the times, the remainder occurring without pollinarium removal. The percentage of pollinated flowers in M. brachystephana was 19·1 %, corresponding to flowers with one insertion. Pollination success on M. odorata was much lower, only 9·6 % of the flowers were pollinated, 8·7 % corresponding to flowers with one insertion and 0·96 % to flowers with two insertions. The PTE was 0·35 and 0·32 for M brachystephana and M. odorata, respectively, which means that at least 35 and 32 % of the removed pollinaria inserted, at least, one pollinium.
Table 4.
Pollinaria removal and pollinia insertion rates
| Percentage of flowers with 0, 1, 2, 3, 4 or 5 pollinaria removed/pollinia inserted |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | n | 0 | 1 | 2 | 3 | 4 | 5 | pr | r | pi* | i | i/r |
| M. brachystephana | 110 | 57·3/80·9 | 32·7/19·1 | 8/0 | 0·91/0 | 0·91/0 | 0/0 | 61 | 0·55 | 21 (47·62) | 0·19 | 0·35 |
| M. odorata | 104 | 75/90·4 | 20·2/8·65 | 3·85/0·96 | 0·96/0 | 0/0 | 0/0 | 32 | 0·31 | 11 (100) | 0·10 | 0·32 |
*The number in parentheses is the percentage of pollinia inserted where the corresponding pollinarium was already missing from that flower sector.
n, number of flowers studied; pr, total number of removed pollinaria; r, mean number of removals per flower; pi, total number of inserted pollinia; i, mean number of insertions per flower; i/r, insertion rate (pollinia transfer efficiency).
Visitors, pollinators and pollinator effectiveness
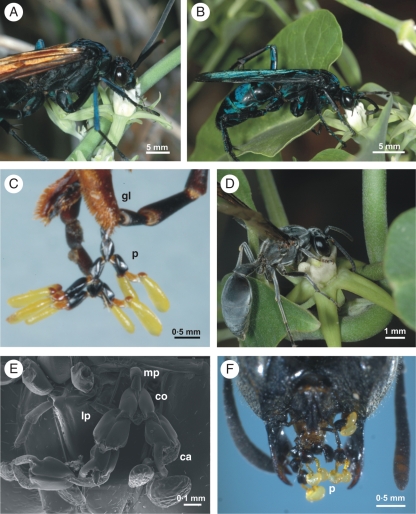
A total of 92 and 138 insects were collected on M. odorata and M. brachystephana, respectively. Hymenopterans were the most frequent visitors, but it was mainly wasps that carried pollinaria. In particular, Pompilidae represents the most diverse family of pollinators of both Morrenia species (Table 5). Some Apis mellifera were also pollinators of M. brachystephana (Table 5) [Table in Supplementary Information online]. Other visitors observed were nectar thieves or insects that only alighted on the flowers without removing pollinaria. For both Morrenia species, hymenopterans sipped nectar by introducing their mouthparts into the corona. When visiting M. odorata flowers, the hymenopterans introduced their heads once through the single corona entrance and gathered nectar by moving their heads circularly inside the tube of the corona (Fig. 6A, B). When visiting M. brachystephana, the hymenopterans separately probed in a ‘Revolverblüte’ fashion the individual staminal sectors with nectar (Fig. 6D). Observations of pollinators under a stereomicroscope showed that carried pollinaria often formed chains on different areas of their mouthparts (Fig. 6C, E, F; Table 5). All of the captured hymenopterans that pollinate M. brachystephana flowers transported pollinaria on the glossa and paraglossa, and approximately half of the individuals also had pollinaria on maxillary and labial palps. On the other hand, M. odorata pollinators transported pollinaria only on the glossa.
Table 5.
Insect visitors, their mean pollen loads and pollinator effectiveness
| Insect visitor* | Individuals captured† | Pollinaria placement | Co | Hp | Po | TPl (C) | I | R | E |
|---|---|---|---|---|---|---|---|---|---|
| M. brachystephana | |||||||||
| Hymenoptera | |||||||||
| Apidae (2) | 12 (9) | gl, gal | 1·11 | 1·00 | 2·89 | 4·89 | 3·22 | 5·86 | 0·38 |
| Pompilidae (9) | 16 (13) | l | 1·29 | 0·40 | 1·00 | 2·71 | 3·00 | 3·14 | 0·76 |
| Scoliidae (3) | 8 (6) | gl | 1·00 | 0·67 | 2·00 | 3·67 | 2·67 | 4·40 | 0·39 |
| Sphecidae (3) | 12 (3) | l | 1·67 | 0·33 | 0·00 | 2·00 | 3·67 | 2·40 | 1·53 |
| Tiphiidae (2) | 8 (4) | l | 2·00 | 0·50 | 0·75 | 3·25 | 4·50 | 3·78 | 1·46 |
| Vespidae (3) | 79 (74) | gl, pgl, lp, mp | 4·27 | 0·84 | 2·15 | 7·23 | 9·39 | 8·47 | 1·12 |
| Colletidae (3) | 22 (0) | – | – | – | – | – | – | – | – |
| Crabronidae (1) | 12 (0) | – | – | – | – | – | – | – | – |
| Halictidae (2) | 13 (0) | – | – | – | – | – | – | – | – |
| Unknown wasps | 2 (0) | – | – | – | – | – | – | – | – |
| Diptera | |||||||||
| Bombyllidae (1) | 1 (0) | – | – | – | – | – | – | – | – |
| Sarcophagidae (1) | 3 (0) | – | – | – | – | – | – | – | – |
| Calliphoridae(1) | 1 (0) | – | – | – | – | – | – | – | – |
| Lepidoptera | |||||||||
| Hesperiidae (1) | 1 (0) | – | – | – | – | – | – | – | – |
| Noctuidae (3) | 5 (0) | – | – | – | – | – | – | – | – |
| Coleoptera | |||||||||
| Melyridae (1) | 2 (0) | – | – | – | – | – | – | – | – |
| M. odorata | |||||||||
| Hymenoptera | |||||||||
| Pompilidae (7) | 64 (33) | gl | 2·12 | 0·84 | 0·94 | 3·87 | 5·19 | 4·28 | 1·14 |
| Scoliidae (1) | 2 (1) | gl | 0·00 | 0·00 | 2·00 | 2·00 | 0·00 | 2·50 | 0·00 |
| Sphecidae (2) | 26 (11) | gl | 1·64 | 0·36 | 0·54 | 2·50 | 3·60 | 2·96 | 1·19 |
| Colletidae (2) | 2 (0) | – | – | – | – | – | – | – | – |
| Crabronidae (1) | 2 (0) | – | – | – | – | – | – | – | – |
| Vespidae (1) | 1 (0) | – | – | – | – | – | – | – | – |
| Tiphiidae (1) | 1 (0) | – | – | – | – | – | – | – | – |
| Apidae (2) | – | – | – | – | – | – | – | – | – |
| Diptera | |||||||||
| Sarcophagidae | 2(0) | – | – | – | – | – | – | – | – |
| Syrphidae | 1(0) | – | – | – | – | – | – | – | – |
| Lepidoptera | |||||||||
| Noctuidae | 2 (0) | – | – | – | – | – | – | – | – |
| Sphingidae | 3 (0) | – | – | – | – | – | – | – | – |
| Coleoptera | |||||||||
| Melyridae | 1 (0) | – | – | – | – | – | – | – | – |
The number in parentheses is the number of species within each family (*) and number of individuals carrying pollinaria (†).
Co, mean number of corpuscula (0 pollinia); Hp, half pollinaria (one pollinium); Pl, complete pollinaria (two pollinia); TPl (C), total pollinaria on the pollinator's body; I, missing (or delivered) pollinia; R, corrected number of corpuscula (pollinaria) carried; E, effectiveness of pollinia transference; gl, glossa; pgl, paraglossa; lp, labial palp; mp, maxillary palp; l, labium; gal, galea.
Fig. 6.
Pollinators. (A–C) Pollinators of M. odorata. (A) Pepsis albocincta. (B) Pepsis sp. 1. (C) Detail of mouthparts with chain of pollinaria. (D–F) Pollinator of M. brachystephana, Polybia ignobilis. (E) SEM of the mouthparts. (F) Head of the wasp showing the chain of pollinaria on the mouthparts. Abbreviations: corpusculum (co), caudicle (ca), pollinia (po), pollinaria (p), maxilary palp (mp), labial palp (lp), glossa (gl).
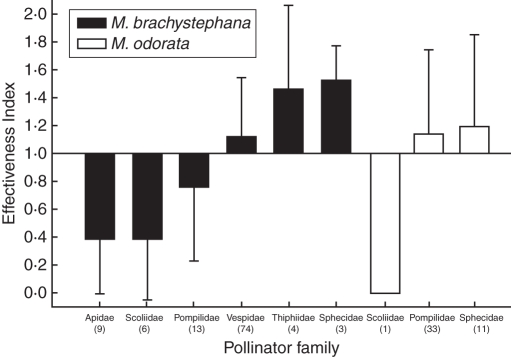
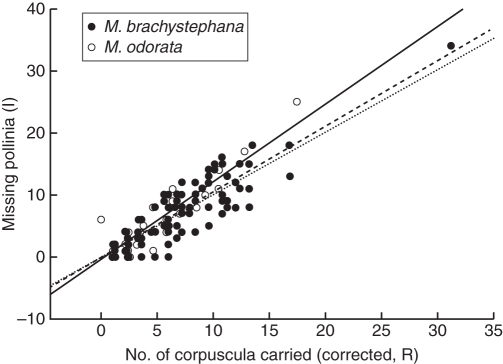
Regarding pollinator effectiveness, wasps were the most effective pollinators of M. brachystephana, in which vespids were the most effective taxa (E = 1·12; Table 5, Fig. 7). In second place, pompilid wasps were intermediately effective (E = 0·76), whereas thiphiid wasps (E = 1·46) were mostly ineffective since they may have either wasted pollinia or may have not achieved proper insertions. In the case of pollinators of M. odorata, pompilid wasps (E = 1·14) and sphecids (E = 1·19) were equally effective (Table 5, Fig. 7). The least-square linear regression (Fig. 8) shows that (1) hymenopterans that pollinate both Morrenia flowers remove and deposit approximately the same amount of pollen load; (2) due to the dispersion of each individual along the regression line, it is evident that the more pollinaria the pollinators remove, the more pollinia they insert on the flowers, showing that individual effectiveness in pollinia transfer is constant along the axes (approx. E = 1); and (3) the regression line of pollinators of M. brachystephana did not show statistical differences from the hypothetical regression line (T = 0·83; P = 0·406), whereas pollinators of M. odorata showed differences from a statistical standpoint (T = 4·14; P < 0·001).
Fig. 7.
Effectiveness of pollinia transference by different pollinator families of M. brachystephana and M. odorata. Numbers in parentheses are insects that carry pollinaria. See text for details of Effectiveness Index.
Fig. 8.
Least-square linear regression between missing pollinia (I) and corrected number of corpuscula carried by individual pollinators (R). The dashed line is the least-square linear regression the effectiveness of pollinia transference by individual pollinators of M. brachystephana. (F = 320·6, R2 = 0·75, P < 0·0001). The solid line is the least-square linear regression the effectiveness of pollinia transference by individual pollinators of M. odorata (F = 325·71, R2 = 0·89, P < 0·0001). The dotted line is a hypothetical regression where all transported pollinaria by each individual insect inserted all their pollinia.
DISCUSSION
Flower analysis and flower–insect mechanical interaction
In Morrenia species, corona morphology is such that pollinators can only introduce their mouthparts into the flowers; thus they function in mechanically filtering pollinator body parts. The inner corona trichomes of M. odorata apparently also function as a ‘nectarostegium’, preventing nectar from dripping out and, in both species, may further contribute to the guidance of pollinators towards the guide rails in a similar way to that which has been already suggested for the trichomes in the corolla tube of Sisyranthus, Leptadenia, Microloma calycinum (Kunze, 1991) and Sisyranthus trichostomus (Ollerton et al., 2003). In most cases, the corona is involved in several essential functions of the pollination mechanism, such as nectar protection and storage, in a similar way to that previously observed in Cynanchum flowers (Ollerton and Liede, 2003). An interesting aspect to note is that Morrenia belongs to a clade (Oxypetalinae) in which wasp pollination seems to be very common and possibly the dominant pollination system (Ollerton and Liede, 1997; Ollerton et al., 2011). The evolution of the corona into an organ to restrict pollinators similar to Cynanchum could be considered an interesting case of convergent evolution as Oxypetalinae and Cynanchinae sub-tribes are not that closely related (Liede, 1997; Rapini et al., 2003, 2007).
Since nectar is contained in areas distinct from that of nectar production, i.e. the nectar furrow, the nectar chambers of M. odorata and the staminal cavities of M. brachystephana are here interpreted as secondary nectar presenters. Nectar-holding structures have also been described for several other asclepiads, although they are also formed by the corolla tube (Kunze, 1991, 1995). In Asclepias and Calotropis, the corona constitutes a complex system, where nectar is guided to the secondary nectar-holding cups by an elaborate capillary mechanism (Galil and Zeroni, 1965; Kunze, 1997). The nectar system is much simpler in the studied species of Morrenia: nectar just flows downwards through the nectar furrow and accumulates in the secondary presenters. The anatomy of the nectar tissue of M. brachystephana and M. odorata is similar to that described for Cynanchum vincetoxicum [=Vincetoxicum hirundinaria] (Christ and Schnepf, 1985), Asclepias curassavica (Galil and Zeroni, 1965), Oxypetalum banksii subsp. banksii (Vieira and Shepherd, 2002a), Blepharodon bicuspidatum (Demarco, 2005), Leptadenia, Tylophora and Sisyranthus (Kunze, 1991). The present results show that the anatomy appears to be more complex than that previously described for M. odorata by Galetto (2006). It is well known that in asclepiads, nectar not only functions as a reward for pollinators, but also constitutes the medium for pollen tube germination (Eisikowitch, 1986; Kevan et al., 1989; Kunze, 1995, 1997; Vieira and Shepherd, 2002a). A mixed mucilage secretion favours pollen tube germination in Oxypetalum banksii subsp. banksii (Vieira and Shepherd, 2002a) and in C. vincetoxicum (Christ and Schnepf, 1985). Sections made in the present study showed the secretion of an amorphous Astra Blue-positive material by the nectary, which suggests that mucilage is also secreted in Morrenia. This result must be taken cautiously, however, since histochemical tests for nectary tissue secretions have not been performed and the chemical nature of the nectar of these species remains unknown. An additional and previously unnoticed function of nectar that can be deduced from this study is the lubrication of the pollinia entering into the guide rail, probably favouring the sliding during insertion into the stigmatic chambers. Nevertheless, more studies are needed to test this function of nectar. The function of the multilayered pad of epidermal cells located inside the two anther wings of M. brachystephana remains unclear.
In Asclepiadoideae, the guide rails perform two different functions: (1) they catch a pollinator body part and guide it towards the corpusculum; and (2) they receive the pollinium and guide it into the stigmatic chamber (Kunze, 1981, 1991). These two functions are linked with the structural differentiation of the guide rails into two parts: the inner and the outer guide rails, respectively, as recognized for Morrenia species (Liede, 1996) and for other asclepiads (Kunze, 1981, 1991, 1995, 1996). The increasing narrowness and the inwardly curved striated surface on the edges of the inner guide rail force the inserted pollinium to be cut off from the caudicle; likewise, the striae on the outer guide rail would function as a guiding structure to secure insect parts into the guide rail. The guiding function of these striae has also been observed in the not structurally differentiated guide rails of the Mexican Sarcostemma (Kunze and Liede, 1991), and striae have previously been regarded as a highly adaptive character of species in the genus Astephanus and Microloma (Kunze, 1991; Kunze and Liede, 1991). The development of such an elaborate system of guide rails and chambers, i.e. an overlapped arrangement of guide rails with striated edges which conduct to a stigmatic and a corpuscular chamber, are key features that control the chain formation of pollinaria. This structural evidence, associated with detailed insight into dynamic aspects of the pollination mechanism, has shed light on understanding the formation of chains of pollinaria, which has never been reported in previous works. There have been a few previous attempts to link internal floral structure, anatomical aspects and interplay of the flowers with pollinators as presented here. In Morrenia, the function of the style head knob as a lock fixing the corpusculum for the correct attachment to a pollinator's body parts for removal was also recognized in Vincetoxicum, Astephanus and Microloma (Kunze, 1991). On the other hand, in Sarcostemma australe, a species that does not form pollinaria chains, the knob acts as a lock for the inserted pollinium as a way of stopping it, preventing removal of the original pollinarium. As a consequence, the inserted pollinium may prevent further successful removal of the pollinarium (Kunze and Liede, 1991). In Morrenia, the locking of the inserted pollinia is performed by the stigmatic chamber itself, which becomes narrow at higher levels, producing a tight fit of the pollinia inside it. A noteworthy difference in the two Morrenia species here studied from other asclepiads is that the caudicles do not turn after removal, so pollinaria do not reconfigure, as also documented in other species (Kunze, 1991). The turning of pollinia seems not to be essential for the insertion of pollinia in M. brachystephana and M. odorata, probably because the flexibility shown by the caudicles allows free movement of the pollinia. In addition, the basal thickened part of the caudicles probably contributes in this function as a guiding structure of pollinia at the entrance of the inner guide rail.
The distinct morphology and size of the corona, gynostegium and pollinaria of M. brachystephana and M. odorata could explain the observed differences in their pollination mechanism. The corona of M. odorata allows the pollinator's mouthparts to enter once to gather the nectar located at the base of the gynostegium, whose stipe and wide nectar chambers provide the space for the inserting pollinia. The pollinaria are larger and wider because horizontal caudicles position the pollinia far from the corpusculum, and the apical thickened connection of the caudicles to the pollinia favours the upward movement during insertion into the inner guide rails. On the other hand, the five-partitioned corona entrance of M. brachystephana forces the wasps to insert their mouthparts separately, probing the individual sectors below the anthers. As the gynostegium is sessile and the base of the corona is in close contact with the entrance of the guide rail, the pollinia of M. brachystephana are not able to fit inside the nectar chambers as they are more concealed below the guide rails, so that the pollinia have to reach the entrance of the inner guide rail laterally from the flank of the entrance guide rails. Besides, the reduced space for the insertion observed below the guide rails in M. brachystephana would explain the smaller size of the whole pollinarium. Also, the pendent and curved caudicles that position the pollinia below the corpusculum making a narrower pollinaria and the thickened connection of the caudicles to the middle of pollinia would favour the lateral movement and confer more flexibility during insertion.
Although Morrenia species grow sympatrically, they seem to be mechanically isolated for hybridization; the differences in nectar presentation between the two species and consequent difference in the feeding behaviour of pollinators may be relevant in the prevention of interspecific pollen transfer.
Visitors, pollinators, pollinator effectiveness and pollination success
Wasps are often associated with generalist flower types, so floral specialization for wasp pollination is unusual among nectar-rewarding plants (Nilsson, 1981; Kephart, 1983). However, several works are beginning to recognize specialized pollination systems operated by wasps (Heithaus, 1979; Nilsson, 1981; Vieira and Shepherd, 1999; Ollerton et al., 2003; Johnson, 2005; Shuttleworth and Johnson, 2006, 2008, 2009a, b, c, d; Johnson et al., 2007). In these works, flowers are characterized as dull and cryptic in coloration, bearing short corollas, relatively accessible nectar and specific fragrance composition. In addition, it has been demonstrated for Xysmalobium orbiculare and Pachycarpus grandiflorus, both species from South Africa, that floral scent plays a more important role in attracting pompilid wasps as pollinators than floral colour, and that nectar is unpalatable for the non-pollinating visitors (Shuttleworth and Johnson, 2009a, b). In this work, it is shown that M. brachystephana is effectively pollinated by different types of wasps, mainly vespids and pompilids, and also bees to some extent, and in M. odorata the most effective pollinators were pompilid wasps followed by sphecids, which were less frequent visitors. This last taxon has been classified as the most basal clade within the Apoidea, being the bee's nearest phylogenetic group in relation to wasp groups (Engler, 2005). Nevertheless, sphecids as a group represent functionally similar pollinators to wasps for M. odorata when several bionomic aspects are taken into account. Considering the overall floral features and the fact that wasps were the most frequent pollinators captured, M. odorata seem to be phenotypically and ecologically specialized for wasp pollination and M. brachsytephana mainly for wasps and to some extent for bees (most bees were A. mellifera, a non-native pollinator). Pompilid and vespid wasps have been recorded among the pollinators of some generalist North American Asclepias (Kephart, 1979), South American Oxypetalum (Vieira and Shepherd, 1999; Wolff et al., 2008) and Gomphocarpus physocarpus naturalized in Australia (Forster, 1994). Also, several South African species of the genera Miraglossum, Pachycarpus and Xysmalobium seem to have specialized wasp pollination systems (Ollerton et al., 2003; Shuttleworth and Johnson, 2006, 2008, 2009a, b). The ASCLEPOL database (Ollerton et al., 2011) contains records of Pepsis wasps as pollinators of M. odorata in Argentina, and also species of this genus are reported as pollinators of North American Asclepias (Punzo, 2006). Schulz (1937) also observed the ‘cabahatá’ wasp as a floral visitor of M. odorata. Galetto (1993) observed wasps, bees and butterflies as floral visitors of M. brachystephana, although it was unclear whether they acted as legitimate pollinators. Tiphiid wasps and sphecids were also found as pollinators of Asclepias (Kephart and Theiss, 2004; Theiss et al., 2007) and the generalist Sarcostemma viminale (Liede and Whitehead, 1991).
With regard to nectar, the studied Morrenia species with mean nectar concentration of 23·5 and 29 % are near to the relatively high concentrated nectar of X. orbiculare and P. grandiflorus with 29 and 30–45 %, respectively (Shuttleworth and Johnson, 2009a, b). Nevertheless, nectar concentration values found here are relatively low compared with those found by Ollerton and co-workers (2003) for wasp-pollinated species. Floral colour of other wasp-pollinated species, such as species of asclepiads, Disa (Orchidaceae) and Eucomis (Hyacinthaceae) from South Africa, are similar to the dull cryptic colour of the flowers studied in this work (Ollerton et al., 2003; Johnson, 2005; Shuttleworth and Johnson, 2009a, b, d). Morrenia flowers produce a strong, spicy-sweet, jasmine-like odour. Although there is evidence that floral fragrances are a key feature for wasp specialization in South African species (Shuttleworth and Johnson, 2009a, b), in the Morrenia species studied here, the role of fragrances remains to be clarified.
In the studied Morrenia species the arrangement of the tubular corona and nectar location is associated with the place of pollinaria deposition onto the mouthparts in a similar manner to that which occurs in Cynanchum adalinae (Ollerton and Liede, 2003). The corona morphology in Morrenia species studied here and the proportions between corona and gynostegium length seem to determine different insect feeding behaviours: in M. brachystephana there are five independent entrances to reach the nectar, and insects visit flowers in a ‘Revolverblüte’ fashion, while in M. odorata insects enter their mouthparts only once inside the corona tube, however also gathering nectar with lateral movements of their heads.
With reference to pollinator effectiveness, it seems evident that pollinator individuals of both Morrenia species are effective in performing pollination, although visitors of M. brachystephana are more effective. This means that the pollination mechanism in both Morrenia species is highly effective, regardless of structural differences of their flowers and their different pollinators. An interesting aspect to consider is that the more pollinaria the pollinators carry, the longer the pollinaria chains could be, and consequently these chains may interfere with the pollination mechanism, complicating the insertion, as suggested for Asclepias syriaca (Morse, 1982). This interference, however, seems not to occur in M. brachystephana and M. odorata because a clump of pollinia seems not to reduce the transference effectiveness (A. A. Cocucci et al., unpubl. res.). It is highly probable that a critical threshold in the number of pollinaria that participate in the formation of chains may restrict the effective amount of pollinaria transferred for both Morrenia species.
Pollinarium removal and pollinium insertion give an estimate of insect activity and pollination success in asclepiads (Willson and Rathke, 1974; Kunze and Liede, 1991; Ollerton et al., 2003). In Morrenia, pollinia insertions into the guide rails are less frequent than pollinaria removals as recorded from other asclepiads (Wyatt, 1978; Vieira and Shepherd, 2002b; Ollerton et al., 2003; Wolff et al., 2008). In M. odorata, all pollinia were inserted where the corresponding pollinarium was already missing from that flower sector, showing that it is highly probable that every insertion is linked effectively with a removal. Nevertheless, it should be taken into account that pollinia insertions into the guide rails may have occurred after the corresponding pollinarium has been removed, not allowing for the chance of pollinaria chain formation. The mechanism of pollinaria forming chains seems to have a limited efficiency in M. brachystephana, since only approx. 50 % of the insertions were performed where the corresponding pollinarium was missing. So, this situation of a reduction in the frequency in pollinaria forming chains could be the result of a differential selection of a specific pollinarium character (A. A. Cocucci et al., unpubl. res.). The fact that in the studied species the pollinia are transferred in chains on the pollinator's body may increase the chances of carrying pollinaria. In species that do not form chains of pollinaria (e.g. Amblyopetalum coccineum; Wiemer and Sérsic, 2006), the number of transported pollinaria is comparatively lower. Bearing this in mind, the presence of a divided guide rail – inner and outer – allows two events in one visit, an initial insertion of pollinia from a different flower and the subsequent removal of a pollinarium. The presence of a complex system of guide rails and connected chambers (nectar, stigmatic and corpuscular chambers) provides strong evidence that the pollination mechanisms of M. odorata and M. brachystephana have achieved a high level of specialization of pollen transfer.
In conclusion, M. odorata and M. brachystephana, two closely related species growing sympatrically, present differences in the morphology and size of their corona, gynostegium and pollinaria, which explain differences in details of the functioning of the general pollination mechanism. Morrenia species are specialized for pollination by different species of hymenopterans, which are highly effective. Morrenia odorata is specialized for several species of wasps and M. brachystephana also for wasps and to some extent bees. The dull colouring of their flowers may suggest a selective attraction of wasps in comparison with other wasp-pollinated asclepiads. This works reveals that wasp pollination may be more common than previously expected in asclepiads.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This contribution is part of A.P.W.'s PhD thesis at Universidad Nacional de Córdoba (UNC). The authors wish to thank Sigrid Liede-Schumann for helpful comments and revision of the manuscript; Noel Wiemer and Nick Olle for linguistic advice; Mariana Musicante and Arturo Roig-Alsina for identifying the insects; Lucrecia Díaz, Sandra Obando, Federico Sazatornil and Matias Baranzelli for field assistance; and Laboratorio de Anatomia Vegetal (IB-UNICAMP) for technical support. Funding was provided by Consejo Nacional de Ciencia y Tecnología (PIP 11220080101264 CONICET, Argentina), Agencia Nacional de Promoción Científica y Tecnológica (PICT 01-33755 MINCyT, Argentina) and Secretaría de Ciencia y Tecnología of the Universidad Nacional de Córdoba (UNC, Argentina). A.P.W. is a professor at UNC. A.N.S. and A.A.C. are researchers at the Instituto Multidiscipliario de Biología Vegetal, jointly funded by CONICET and UNC. A.O.S. is a professor at USP.
LITERATURE CITED
- Arenas P. Morrenia odorata (Asclepiadaceae), an edible plant of the Gran Chaco. Economic Botany. 1999;53:89–97. [Google Scholar]
- Bookman SS. The floral morphology of Asclepias speciosa (Asclepiadaceae) in relation to pollination and a clarification in terminology for the genus. American Journal of Botany. 1981;68:675–679. [Google Scholar]
- Bozzola JJ, Russel LD. Electron microscopy – principles and techniques for biologists. Boston: Jones & Batlett Publishers; 1991. [Google Scholar]
- Brewer MM, Arguello NV. Guía ilustrada de insectos comunes de la Argentina. Tucumán, Argentina: Fundación Miguel Lillo; 1980. [Google Scholar]
- Brown R. On the organs and mode of fecundation in Orchideae and Asclpepiadeae. Transactions of the Linnean Society of London. 1833;16:685–745. [Google Scholar]
- Christ P, Schnepf E. The nectaries of Cynanchum vincetoxicum (Asclepiadaceae) Israel Journal of Botany. 1985;34:79–90. [Google Scholar]
- Ciotek L, Giorgis P, Benitez-Vieyra S, Cocucci AA. First confirmed case of pseudocopulation in terrestrial orchids of South America: pollination of Geoblasta penicillata (Orchidaceae) by Campsomeris bistrimacula (Hymenoptera, Scoliidae) Flora. 2005;201:365–369. [Google Scholar]
- Corry TH. On the structure and development of the gynostegium and the mode of fertilisation in Asclepias cornuti Decaisne (A. syriaca L.) Transactions of the Linnean Society of London. 1884;2:173–207. [Google Scholar]
- Demarco D. 2005 Estruturas secretoras florais e coléteres foliares em espécies de cerrado de Aspidosperma Mart. e Blepharodon Decne. (Apocynaceae s.l.). Masters Thesis, University of Campinas, Brasil. [Google Scholar]
- Di Rienzo JA, Robledo CW, Balzarini MG, Casanoves F, Gonzales L, Tablada M. InfoStat, Software estadístico Versión 2004·1. 2004 Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba, Argentina. [Google Scholar]
- Eisikowitch D. Morpho-ecological aspects on the pollination of Calotropis procera (Asclepiadaceae) in Israel. Plant Systematics and Evolution. 1986;152:185–194. [Google Scholar]
- Endress ME, Bruyns P. A revised classification of the Apocynaceae sens. lat. Botanical Review (Lancaster) 2000;66:1–56. [Google Scholar]
- Endress ME, Liede-Schumann S, Meve U. Advances in Apocynaceae: the enlightment, an introduction. Annals of the Missouri Botanical Garden. 2007;94:259–267. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Engler MS. Family-group names for bees (Hymenoptera: Apoidea) American Museum Novitates. 2005;3476:1–33. [Google Scholar]
- Ezcurra C. Asclepiadaceae. In: Zuloaga FO, Morrone O, editors. Catálogo de las plantas vasculares de la República Argentina 2. St Louis, MO: Missouri Botanical Garden Press; 1999. pp. 78–98. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Fallen ME. Floral structure in the Apocynaceae: morphological, functional, and evolutionary aspects. Botanische Jahrbücher für Systematik. 1986;106:245–286. [Google Scholar]
- Fernandez F, Sharkey MJ. Introducción a los hymenoptera de la región neotropical. Bogotá: Universidad Nacional de Colombia; 2006. [Google Scholar]
- Fishbein M, Venable DL. Diversity and temporal change in the effective pollinators of Asclepias tuberose. Ecology. 1996;77:1061–1073. [Google Scholar]
- Forster PI. Diurnal insects associated with the flowers of Gomphocarpus physocarpus E. May. (Asclepiadaceae), an introduced weed in Australia. Biotropica. 1994;26:214–217. [Google Scholar]
- Galetto L. Estudio sobre el néctar en Asteridae argentinas: análisis químico e histología comparada de las estructuras secretoras. 1993 Tesis Doctoral, Universidad Nacional de Córdoba, Argentina. [Google Scholar]
- Galetto L. Morfología y anatomía floral en especies de Apocynaceae-Asclepiadoidea. Kurtziana. 2006;32:5–11. [Google Scholar]
- Galetto L, Bernardello G. Néctar. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge, Ontario, Canada: Enviroquest, Ltd; 2005. pp. 261–313. [Google Scholar]
- Galil J, Zeroni M. Nectar system of Asclepias curassavica. Botanical Gazette. 1965;126:144–148. [Google Scholar]
- Gerlach D. Botanische Mikrotechnik: eine Einführung. Stuttgart: Thieme; 1969. [Google Scholar]
- Goyder DJ. A synopsis of Morrenia Lindl. (Apocynaceae subfam. Asclepiadoideae) Kew Bulletin. 2003;58:713–721. [Google Scholar]
- Heithaus ER. Flower visitation records and resource overlap of bees and wasps in northwest Costa Rica. Brenesia. 1979;16:9–52. [Google Scholar]
- Holm RW. The American species of Sarcostemma R. Br. (Asclepiadaceae) Annals of the Missouri Botanical Garden. 1950;37:477–560. [Google Scholar]
- Johnson SD. Specialized pollination by spider-hunting wasps in the African orchid Disa snkeyi. Plant Systematics and Evolution. 2005;251:153–160. [Google Scholar]
- Johnson SD, Peter CI, Ågren J. The effects of nectar addition on pollen removal and geitonogamy in the non-rewarding orchid Anacamptis morio. Proceedings of the Royal Society B: Biological Sciences. 2004;271:803–809. doi: 10.1098/rspb.2003.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Ellis A, Dötterl S. Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae) American Journal of Botany. 2007;94:47–55. doi: 10.3732/ajb.94.1.47. [DOI] [PubMed] [Google Scholar]
- Kearns CA, Inouye WD. Techniques for pollination biologist. Colorado: University Press of Colorado; 1993. [Google Scholar]
- Kephart SR. The floral ecology and reproductive isolation of three sympatric species of Asclepias. 1979. PhD dissertation, Indiana University, Bloomington. [Google Scholar]
- Kephart SR. The partitioning of pollinators among 3 species of Asclepias. Ecology. 1983;64:120–133. [Google Scholar]
- Kephart S, Theiss K. Pollinator-mediated isolation in sympatric milkweeds (Asclepias): do floral morphology and insect behavior influence species boundaries? New Phytologist. 2004;161:265–277. [Google Scholar]
- Kevan PG, Eisikowitch D, Rathwell B. The role of nectar in the germination of pollen in Asclepias syriaca L. Botanical Gazette. 1989;150:266–270. [Google Scholar]
- Kunze H. Morphogenese und Synorganization des Bestäubungsapparates einiger Asclepiadaceen. Beiträge zur Biologie der Pflanzen. 1981;56:133–170. [Google Scholar]
- Kunze H. Structure and function in asclepiad pollination. Plants Systematics and Evolution. 1991;176:227–253. [Google Scholar]
- Kunze H. Floral morphology of some Gonolobeae (Asclepiadaceae) Botanische Jahrbücher für Systematik. 1995;117:211–238. [Google Scholar]
- Kunze H. Morphology of the stamen in the Asclepiadaceae and its systematic relevance. Botanische Jahrbucher fur Systematik. 1996;118:547–579. [Google Scholar]
- Kunze H. Corona and nectar system in Asclepiadinae (Asclepiadaceae) Flora. 1997;192:175–183. [Google Scholar]
- Kunze H, Liede S. Observation on pollination in Sarcostemma (Asclepiadaceae) Plant Systematics and Evolution. 1991;178:95–105. [Google Scholar]
- Liede S. Some observations on pollination in Mexican Asclepiadecae. Madroño. 1994;41:266–276. [Google Scholar]
- Liede S. Cynanchum–Rhodostegiella–Vincetoxicum–Tylophora (Asclepiadaceae): new considerations on an old problem. Taxon. 1996;45:193–211. [Google Scholar]
- Liede S. Subtribes and genera of the tribe Asclepiadeae (Apocynaceae, Asclepiadoideae) – a synopsis. Taxon. 1997;46:233–247. [Google Scholar]
- Liede S, Whitehead V. Studies in the pollination biology of Sarcostemma viminale R. Br. sensu lato. South African Journal of Botany. 1991;57:115–122. [Google Scholar]
- Lynch SP. The floral ecology of Asclepias solanoana. Madroño. 1977;24:159–177. [Google Scholar]
- Macior L. Insect adaptation and behavior in Asclepias pollination. Bulletin of the Torrey Botanical Club. 1965;92:114–126. [Google Scholar]
- Meve U. Species numbers and progress in asclepiad taxonomy. Kew Bulletin. 2002;57:459–464. [Google Scholar]
- Meyer T. Asclepiadaceae. In: Descole HR, editor. Genera et Species Plantarum Argentinarum 2. Buenos Aires: Kraft; 1944. pp. 1–273. [Google Scholar]
- Michener CD. The bees of the world. 2nd edn. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Morse DH. The turnover of milkweed pollinia on bumble bees and implications on outcrossing. Oecologia. 1982;53:187–196. doi: 10.1007/BF00545662. [DOI] [PubMed] [Google Scholar]
- Nilsson LA. The pollination ecology of Listera ovata (Orchidaceae) Nordic Journal of Botany. 1981;1:461–480. [Google Scholar]
- Ollerton J, Liede S. Pollination systems in the Asclepiadaceae: a survey and preliminary analysis. Biological Journal of the Linnean Society. 1997;62:593–610. [Google Scholar]
- Ollerton J, Liede S. Corona structure in Cynanchum: linking morphology to function. Ecotropica. 2003;9:107–112. [Google Scholar]
- Ollerton J, Watts S. Phenotype space and floral typology: towards an objective assessment of pollination syndromes. Det Norske Videnskaps-Akademi I. Matematisk Naturvitenskapelig Klasse, Avhandlinger, Ny Serie. 2000;39:149–159. [Google Scholar]
- Ollerton J, Johnson SD, Cranmer L, Kellie S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Annals of Botany. 2003;92:807–834. doi: 10.1093/aob/mcg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Masinde S, Meve U, Picker M, Whittington A. Fly pollination in Ceropegia (Apocynaceae: Asclepiadoideae): biogeographic and phylogenetic perspectives. Annals of Botany. 2009;103:1501–1514. doi: 10.1093/aob/mcp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Meve U, Liede S. ASCLEPOL database on line of plant–pollinator interactions in the Asclepiadaceae. 2011 At http://www.uni-bayreuth.de/departments/planta2/research/pollina/as_pol_t.html . Department of Plant Systematics, University of Bayreuth, Germany. [Google Scholar]
- Pauw A. Pollen transfer on bird's tongues. Nature. 1998;394:731–732. [Google Scholar]
- Proctor M, Yeo P, Lack A. The natural history of pollination. Portland: Timber Press; 1996. [Google Scholar]
- Punzo F. Plants whose flowers are utilized by adults of Pepsis grossa Fabricius (Hymenoptera: Pompilidae) as a source of nectar. Journal of Hymenoptera Research. 2006;15:171–176. [Google Scholar]
- Rapini A, Chase MW, Goyder DJ, Griffiths J. Asclepiadeae classification: evaluating the phylogenetic relationships of New World Asclepiadoideae (Apocynaceae) Taxon. 2003;52:33–50. [Google Scholar]
- Rapini A, van der Berg C, Liede-Schumann S. Diversification of Asclepiadoideae (Apocynaceae) in the New World. Annals of the Missouri Botanical Garden. 2007;94:407–434. [Google Scholar]
- Sahli HF, Conner JK. Visitation, effectiveness and efficiency of 15 genera of visitors to wild radish, Raphanus raphanistrum (Brassicaceae) American Journal of Botany. 2007;94:203–209. doi: 10.3732/ajb.94.2.203. [DOI] [PubMed] [Google Scholar]
- Sakai WS. Simple method for differential staining of paraffin embedded plant material using toluidine blue. Stain Technology. 1973;48:247–249. doi: 10.3109/10520297309116632. [DOI] [PubMed] [Google Scholar]
- Schulz AG. Las Asclepiadáceas del territorio del Chaco (Argentina) Lilloa. 1937;1:347–391. [Google Scholar]
- Sérsic A, Cocucci A, Benítez-Vieyra S, et al. Flores del Centro de Argentina. Una guía ilustrada para conocer 141 especies típicas. Córdoba: Academia Nacional de Ciencias; 2006. [Google Scholar]
- Shuttleworth A, Johnson SD. Specialized pollination by large spider-hunting wasps and self-incompatibility in the African milkweed Pachycarpus asperifolius. International Journal of Plant Sciences. 2006;167:1177–1186. [Google Scholar]
- Shuttleworth A, Johnson SD. Bimodal pollination by wasps and beetles in the African milkweed Xysmalobium undulatum. Biotropica. 2008;40:568–574. [Google Scholar]
- Shuttleworth A, Johnson SD. The importance of scent and nectar filters in a specialized wasp-pollination system. Functional Ecology. 2009a;23:931–940. [Google Scholar]
- Shuttleworth A, Johnson SD. Specialized pollination in the African milkweed Xysmalobium orbiculare: a key role for floral scent in the attraction of spider-hunting wasps. Plant Systematics and Evolution. 2009b;280:37–44. [Google Scholar]
- Shuttleworth A, Johnson SD. Palp-faction: an African milkweed dismembers its wasp pollinators. Environmental Entomolgy. 2009c;38:741–747. doi: 10.1603/022.038.0326. [DOI] [PubMed] [Google Scholar]
- Shuttleworth A, Johnson SD. A key role for floral scent in a wasp-pollination system in Eucomis (Hyacinthaceae) Annals of Botany. 2009d;103:715–725. doi: 10.1093/aob/mcn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss K, Kephart S, Ivey CT. Pollination effectiveness on co-ocurring milkweed (Asclepias; Apocynaceae, Asclepiadoideae) Annals of the Missouri Botanical Garden. 2007;94:507–518. [Google Scholar]
- Vardy CR. The New World tarantula-hawk wasp genus Pepsis Fabricius (Hymenoptera: Pompilidae). Part 1. Introduction and the P. rubra species-group. Zoologische Verhandelingen. 2000;332:1–86. [Google Scholar]
- Vardy CR. The New World tarantula-hawk wasp genus Pepsis Fabricius (Hymenoptera: Pompilidae). Part 2. The P. grossa- to P. deaurata-groups. Zoologische Verhandelingen. 2002;338:1–134. [Google Scholar]
- Vardy CR. The New World tarantula-hawk wasp genus Pepsis Fabricius (Hymenoptera: Pompilidae). Part 3. The P. inclyta- to P. auriguttata-groups. Zoologische Mededelingen. 2005;79:1–305. [Google Scholar]
- Verhoeven RL, Venter HJT. Pollen morphology of the Periplocoideae, Secamonoideae, and Asclepiadoideae (Apocynaceae) Annals of the Missouri Botanical Garden. 2001;88:569–582. [Google Scholar]
- Vieira MF, Shepherd GJ. Pollinators of Oxypetalum (Asclepiadaceae) in southeastern Brazil. Revista Brasileira de Biologia. 1999;59:693–704. doi: 10.1590/s0034-71081999000400018. [DOI] [PubMed] [Google Scholar]
- Vieira MF, Shepherd GJ. Oxypetalum banksii subsp. banksii: a taxon of Asclepiadaceae with an extragynoecial compitum. Plant Systematics and Evolution. 2002a;233:199–206. [Google Scholar]
- Vieira MF, Shepherd GJ. Removal and insertion of pollinia in flowers of Oxypetalum (Asclepiadaceae) in southeastern Brazil. Revista Brasileira de Biologia. 2002b;50:37–43. [PubMed] [Google Scholar]
- Wiemer AP, Sérsic AN. Polinización en tres especies de Asclepiadoideae (Apocynaceae) de la Provincia de Córdoba. Proceedings of XII Reunión Argentina de Ecología. 2006 Córdoba, Argentina. [Google Scholar]
- Willson MF, Rathke BJ. Adaptive design of the floral display in Asclepias syriaca L. American Midland Naturalist. 1974;92:47–57. [Google Scholar]
- Wolff D, Meve U, Liede-Schumann S. Pollination ecology of Ecuadorian Asclepiadoideae (Apocynaceae): how generalized are morphologically specialized flowers? Basic and Applied Ecology. 2008;9:24–34. [Google Scholar]
- Wyatt R. Experimental evidence concerning the role of the corpusculum in Asclepias pollination. Systematic Botany. 1978;3:313–321. [Google Scholar]
- Wyatt R, Broyles SB. Ecology and evolution of reproduction in milkweeds. Annual Review of Ecology and Systematics. 1994;25:423–441. [Google Scholar]
- Wyatt R, Lypow SR. A new explanation for the evolution of pollinia and loss of carpel fusion in Asclepias and the Apocynacear s.l. Annals of the Missouri Botanical Garden. 2007;94:474–484. [Google Scholar]
- Yamashiro T, Yamashiro A, Yokoyama Y, Maki M. Morphological aspects and phylogenetics analyses of pollination systems in the Tylophora–Vincetoxicum complex (Apocynaceae-Asclepiadoideae) in Japan. Biological Journal of the Linnean Society. 2008;93:325–341. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.