Abstract
Background and Aims
Subfamily Hyacinthoideae (Hyacinthaceae) comprises more than 400 species. Members are distributed in sub-Saharan Africa, Madagascar, India, eastern Asia, the Mediterranean region and Eurasia. Hyacinthoideae, like many other plant lineages, show disjunct distribution patterns. The aim of this study was to reconstruct the biogeographical history of Hyacinthoideae based on phylogenetic analyses, to find the possible ancestral range of Hyacinthoideae and to identify factors responsible for the current disjunct distribution pattern.
Methods
Parsimony and Bayesian approaches were applied to obtain phylogenetic trees, based on sequences of the trnL-F region. Biogeographical inferences were obtained by applying statistical dispersal-vicariance analysis (S-DIVA) and Bayesian binary MCMC (BBM) analysis implemented in RASP (Reconstruct Ancestral State in Phylogenies).
Key Results
S-DIVA and BBM analyses suggest that the Hyacinthoideae clade seem to have originated in sub-Saharan Africa. Dispersal and vicariance played vital roles in creating the disjunct distribution pattern. Results also suggest an early dispersal to the Mediterranean region, and thus the northward route (from sub-Saharan Africa to Mediterranean) of dispersal is plausible for members of subfamily Hyacinthoideae.
Conclusions
Biogeographical analyses reveal that subfamily Hyacinthoideae has originated in sub-Saharan Africa. S-DIVA indicates an early dispersal event to the Mediterranean region followed by a vicariance event, which resulted in Hyacintheae and Massonieae tribes. By contrast, BBM analysis favours dispersal to the Mediterranean region, eastern Asia and Europe. Biogeographical analysis suggests that sub-Saharan Africa and the Mediterranean region have played vital roles as centres of diversification and radiation within subfamily Hyacinthoideae. In this bimodal distribution pattern, sub-Saharan Africa is the primary centre of diversity and the Mediterranean region is the secondary centre of diversity. Sub-Saharan Africa was the source area for radiation toward Madagascar, the Mediterranean region and India. Radiations occurred from the Mediterranean region to eastern Asia, Europe, western Asia and India.
Keywords: Asparagaceae, biogeography, S-DIVA, Hyacinthoideae, Bayesian binary MCMC, RASP, Scilloideae
INTRODUCTION
Phylogenetically based historical biogeographical reconstructions are now an important way to illuminate the evolutionary history of organisms in space and time. The enormous growth of biogeographical studies has resulted from the rapid accumulation of phylogenetic data during the last two decades. Recently, model- and event-based approaches have been used for biogeographical inferences. The Lagrange (likelihood analysis of geographical range evolution) implementing dispersal-extinction cladogenesis (DEC) model (Ree et al., 2005; Ree and Smith, 2008) and the BIB (Bayesian island biogeography) method (Sanmartín et al., 2008, 2010) were recently applied to biogeographical analysis, but the event-based method dispersal vicariance analysis (DIVA; Ronquist, 1997, 2001) has remained the most popular and widely used method for reasons of simplicity. In the DIVA method, ancestral distributions are inferred based on a three-dimensional cost matrix derived from a simple biogeographical model (Ronquist, 1997). Two problems, uncertainty in phylogeny and uncertainty in ancestral area optimization, are attached to it. Nylander et al. (2008) proposed a new method, Bayes-DIVA, to overcome the uncertainties in DIVA analysis. Similarly, Harris and Xiang (2009) proposed their approach, an alternative to Bayes-DIVA. Their method differs in its ability to handle uncertainty at some nodes.
The Statistical DIVA (S-DIVA; Yan et al., 2010) method rectified the problems in DIVA analysis and the results are comparable with those obtained by Bayes-DIVA. RASP (Reconstruct Ancestral State in Phylogenies) (Yan et al., 2011) is a useful tool to reconstruct evolutionary histories in phylogeny. Three different methods, S-DIVA, Bayesian binary MCMC (BBM) and maximum-parsimony (MP) analysis, are implemented in RASP to obtain ancestral ranges at each node. S-DIVA and BBM methods suggest possible ancestral ranges at each node and also calculate probabilities of each ancestral range at nodes.
The family Hyacinthaceae [= Asparagaceae subfamily Scilloideae sensu APG III (2009)and Chase et al. (2009)] consists of approx. 900 species and 70 genera (Speta, 1998a, b) in temperate and tropical regions (e.g. Mwafongo et al., 2010). Members of this family can be found in different habitats, but most species are adapted to seasonal climates with pronounced dry and wet periods. Southern Africa has the highest diversity, followed by the Mediterranean region (Stedje, 1996). The Fynbos (Cape area) and succulent Karoo regions of southern Africa have the highest species diversity. The molecular analyses of Pfosser and Speta (1999) and Manning et al. (2004) resulted in the recognition of four monophyletic clades in the family Hyacinthaceae. These four clades are treated as subfamilies Oziroeoideae, Urgineoideae, Ornithogaloideae and Hyacinthoideae (Pfosser and Speta, 1999, 2001; Manning et al., 2004). Alternatively, Hyacinthaceae is nested within Asparagaceae sensu lato and can be treated as subfamily Scilloideae. Hyacinthaceae are monophyletic within Asparagaceae and the subfamilies mentioned above are then treated as tribes Hyacintheae, Ornithogaleae, Oziroëeae and Urgineeae (e.g. APG III, 2009; Chase et al., 2009). Here we use Hyacinthaceae at the family level. Subfamily Oziroeoideae is restricted to South America and consists of five species. The number of species in subfamily Urgineoideae is >100 (Manning et al., 2004), and Ornithogaloideae consists of 200–300 species (Manning et al., 2009; Martínez-Azorín et al., 2011); the remaining 400+ species form Hyacinthoideae. As most of the sub-Saharan African representatives of Hyacinthaceae occupy early branching positions in the phylogenetic analyses (Pfosser and Speta, 1999, 2001; Pfosser et al., 2003, 2006; Manning et al., 2004; Martínez-Azorín et al., 2011), it is commonly accepted that the whole family evolved from that region. Except for Oziroeoideae, all subfamilies exhibit a bimodal distribution pattern. A primary centre of diversity is located in sub-Saharan Africa, and a secondary centre of diversity occurs in the northern hemisphere around the Mediterranean, extending, at least for subfamily Hyacinthoideae, as far as East Asia. This split between northern and predominantly southern hemisphere taxa is most pronounced in subfamily Hyacinthoideae, which has been further subdivided into tribes Hyacintheae (northern hemisphere) and Massonieae (southern hemisphere, Madagascar, Arabia and India) (Pfosser and Speta, 1999; Pfosser et al., 2003; Wetschnig and Pfosser, 2003).
Pseudoprospero Speta occupies an early branching position in the phylogenetic tree and is sister to Massonieae and Hyacintheae. If Pseudoprospero is excluded from Massonieae, then molecular data suggest that Massonieae and Hyacintheae evolved independently (Pfosser et al., 2003). Tribe Massonieae is monophyletic upon the exclusion of genus Pseudoprospero (Pfosser et al., 2003), and it has been suggested that Pseudoprospero should be placed into a tribe of its own (Wetschnig et al., 2002; Pfosser et al., 2003). This led to the creation of a third monotypic tribe Pseudoprospereae (Manning et al., 2004).
Hyacinthaceae and its subfamilies (except Oziroeoideae), like many other plant lineages, show a disjunct distribution pattern. The Rand flora pattern is the best example of plant disjunction between the flora of the Mediterranean region/western Asia/north-west Africa and sub-Saharan Africa (Sanmartín et al., 2010). Vicariance and dispersal hypotheses have been proposed to explain the origin of this disjunct distribution pattern. According to the first hypothesis, due to aridification (Sahara region), the widespread African flora underwent partial extinction and created the current pockets of distribution of extant species. The dispersal hypothesis suggests long-distance dispersal among these regions, followed by local diversification. Two dispersal routes, southward and northward, have been proposed. The source area of the southward route is either the Mediterranean region or western Asia and the dispersal is directed toward sub-Saharan Africa (Levyns, 1964), whereas the northward route is directed from sub-Saharan Africa to the Mediterranean region (Galley et al., 2007).
The results of biogeographical histories of Hyacinthoideae obtained by S-DIVA and BBM analyses are presented here. The aims of this study are to find the possible ancestral range of Hyacinthoideae and to identify factors responsible for the current distribution pattern.
MATERIALS AND METHODS
Taxon sampling and outgroup selection
This analysis is based on material of subfamily Hyacinthoideae published in an earlier paper (Wetschnig et al., 2007). The trnL-F region is composed of the trnL (UAA) intron and the intergenic spacer (IGS) between the trnL (UAA)-3′ exon and the trnF (GAA) gene. In many groups of plants, the intron region evolves more slowly than the spacer region (Gielly and Taberlet, 1994, 1996; Kita et al., 1995; Gielly et al., 1996). The different evolving rates of these regions are helpful in providing useful information; slowly evolving regions support the older divergences and quickly evolving regions provide resolution among closer relatives (McDade and Moody, 1999). In this study, we include 59 taxa (58 ingroups and one outgroup). Voucher information for all plant accessions, geographical origin and EMBL database accession numbers are provided in the Appendix. Oziroe acaulis is closely related to Hyacinthoideae and was selected as the outgroup in this analysis. Nomenclature follows that of the most recent nomenclatural synopsis available for a particular group of taxa. In particular, we adopted the nomenclature of Speta (1998a, b) for tribe Hyacintheae of subfamily Hyacinthoideae, and that of Manning et al. (2004) for all other taxa, with the exception of the genera Ledebouria, Drimiopsis and Resnova, which have later been treated as separate genera by Lebatha et al. (2006).
Phylogenetic analysis
Clustal X (Jeanmougin et al., 1998) was used for DNA sequence alignment with a pairwise multiple alignment parameter. On average, <1 % of data matrix cells were scored as missing data and the region with ambiguous alignment was excluded in this analysis. Phylogenetic analysis using MP was performed in PAUP version 4·0b10 (Swofford, 2002). MP analyses were performed either without or with successive character weighting (rescaled consistency index) until tree lengths remained the same in two successive rounds. Most-parsimonious trees were obtained by 1000 replicates of random sequence addition using tree bisection-reconnection (TBR) branch swapping under the Fitch criterion (Fitch, 1971). Confidence limits for the resulting tree topologies were assessed by 10 000 fast bootstrap replicates (Felsenstein, 1985) and the jackknife algorithm (50 % deletion). The Bayesian analysis was conducted in MrBayes 3·1 (Hülsenbeck and Ronquist, 2001; Ronquist and Hülsenbeck, 2003) under the GTR + I + G model. MrModeltest version 2·2 (Nylander, 2004) was used to select the best fit model under the Akaike information criterion. The Markov chain Monte Carlo chains were run simultaneously for 1000 000 generations. Trees were sampled every 100 generations. The first 1000 trees were eliminated (burn-in) and the remaining trees were used to construct a 50 % majority rule consensus tree with posterior probability (PP) distribution. One of the post-burn trees was drawn using Mesquite software (Maddison and Maddison, 2010), shown in Fig. 1A. The tree topologies obtained by Bayesian and parsimony analyses are similar (no significant differences). The Bayesian tree with Bayesian PP and parsimony bootstrap (BS) values is shown in Fig. 1A.
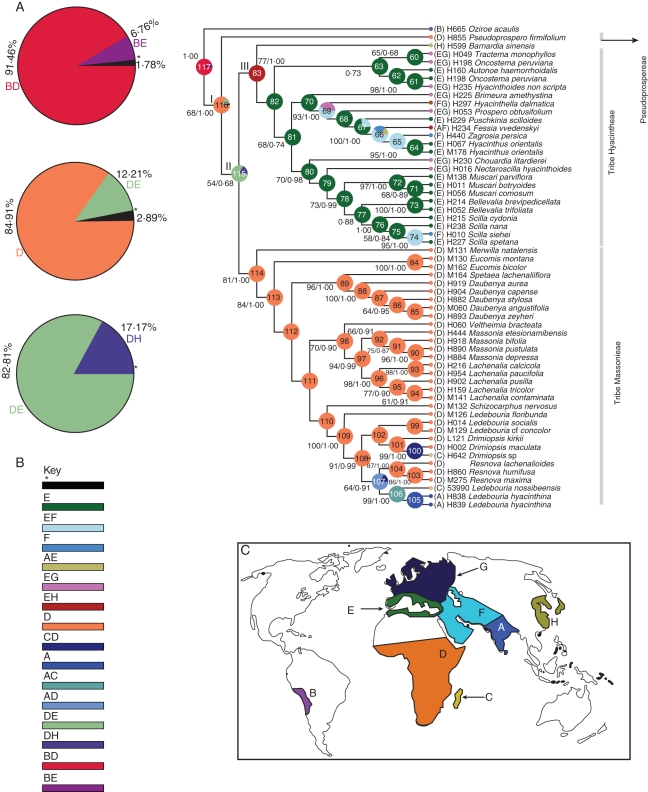
Fig. 1.
Graphical output from S-DIVA (RASP). (A) Graphical results of ancestral distributions at each node of the phylogeny of subfamily Hyacinthoideae obtained by S-DIVA. Alternative ancestral ranges of nodes 117, 116 and 115 (with frequency of occurrence) are shown in pie chart form. Bootstrap support values (50 % and higher) and Bayesian credibility values (PP) are indicated above the pie chart on one of the post-burn Bayesian trees. (B) Colour key to possible ancestral ranges at different nodes; black with an asterisk represents other ancestral ranges. (C) Biogeographical regions: A, India; B, South America; C, Madagascar; D, sub-Saharan Africa; E, Mediterranean; F, Western Asia; G, Europe; H, Eastern Asia.
Dating the tree
The lack of a fossil record is a major constraint in the estimation of the ages of Hyacinthaceae and its subfamilies. However, we used the Bayesian analyses to date the tree with BEAST v1·6·1 (Drummond et al., 2002; Drummond and Rambaut, 2007) under the hypothesis of the molecular clock based on the general substitution rates of the plastid sequence (u = 1·0 × 10−9 s s−1 year−1; Zurawski et al., 1984). Ten million generations of the MCMC chains were run, sampling every 1000 generations. Convergence of the stationary distribution was checked by visual inspection of plotted posterior estimates using the software Tracer v1·5 (Rambaut and Drummond, 2007). After discarding the first 1000 trees as burn-in, the samples were summarized in the maximum clade credibility tree using TreeAnnotator v1·6.1 (Drummond and Rambaut, 2007) with the PP limit set to 0 and summarizing mean node heights. The results were visualized using Figtree v1·3.1 (Rambaut, 2009).
Biogeographical analysis
The distribution range of Hyacinthoideae plus Oziroe was divided into eight areas, based on the presence of one or more endemic species, as shown in Fig. 1C. These areas are: A (India), B (South America), C (Madagascar), D (sub-Saharan Africa), E (Mediterranean region), F (Western Asia), G (Europe) and H (eastern Asia). In this analysis due to a lack of samples from eastern Africa, we treated sub-Saharan Africa as a single unit.
We used recently developed S-DIVA and BBM analyses implemented in RASP to reconstruct the possible ancestral ranges of subfamily Hyacinthoideae on the phylogenetic trees. In these methods, the frequencies of an ancestral range at a node in ancestral reconstructions are averaged over all trees (Yan et al., 2010). To account for uncertainties in phylogeny, we used 9000 trees from MCMC output and ran S-DIVA on all of them. The number of maximum areas was kept as 2. The possible ancestral ranges at each node on a selected tree were obtained. BBM analysis was also conducted in a similar way. The MCMC chains were run simultaneously for 5000 000 generations. The state was sampled every 100 generations. Fixed JC + G (Jukes-Cantor + Gamma) were used for BBM analysis with null root distribution. The maximum number of areas for this analysis was kept as 6. The ancestral ranges obtained by BBM analysis are shown in Fig. 2.
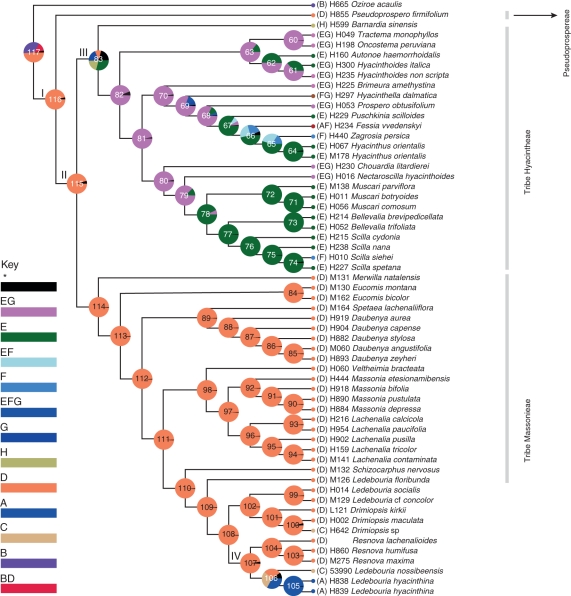
Fig. 2.
Graphical output from BBM analysis (exported from RASP). Graphical results of ancestral distributions at each node of the phylogeny of subfamily Hyacinthoideae obtained by BBM analysis. Pie charts at each node show probabilities of alternative ancestral ranges. Colour key to possible ancestral ranges at different nodes; black with an asterisk represents other ancestral ranges.
RESULTS
The aligned matrix consisted of 1613 characters, 282 of which were excluded from this analysis. Of the remaining 1331 characters, 1070 were constant, 119 were autapomorphic and 142 were potentially parsimony-informative. The mean G + C content was 33·22 %. The pairwise divergence estimate was 0–6·18 %.
More than 1000 equally parsimonious trees had 418 steps, consistency index (CI) = 0·72, retention index (RI) = 0·86 and rescaled consistency index (RC) = 0·62. Without uninformative characters the parsimony trees had 290 steps, CI = 0·60, RI = 0·86 and RC = 0·51.
PP and BS support for the majority of clades were >0·95 and >90 %, respectively, thus showing high support for the tree topology. The tree topology also reflects the classification of subfamily Hyacinthoideae into a strictly northern hemisphere tribe Hyacintheae, a southern hemisphere tribe Massonieae and a monotypic sub-Saharan African tribe Pseudopropereae. Within the Hyacinthoideae clade, three sub-Saharan African, two Mediterranean, one southern hemisphere and one Eurasian subclade can be distinguished.
S-DIVA suggests a complex biogeographical history in which dispersal and vicariance have been vital in the shaping of the current distribution pattern in Hyacinthoideae. S-DIVA postulates 18 dispersals, the majority of which are located on the backbone of the tree. S-DIVA suggests two possible ancestral ranges, D (sub-Saharan Africa) and DE (sub-Saharan Africa + Mediterranean region), for node 116 and the occurrence of these ranges are 84·91 and 12·21 %, respectively (Fig. 1A). S-DIVA postulates that the ancestors of Hyacinthoideae originated in sub-Saharan Africa (optimal area reconstruction at basal node 116). This node suggests an early dispersal to the Mediterranean region, as shown in Fig. 3. The possible ancestral ranges at node 115 are DE and DH, the frequency of occurrence of these ranges being 82·81 and 17·17 %, respectively, with 68 % Bayesian support value. The most favoured ancestral range at node 115 is DE (sub-Saharan Africa + Mediterranean). A vicariance event is evident at this node, resulting in the Eurasian and sub-Saharan African lineages.
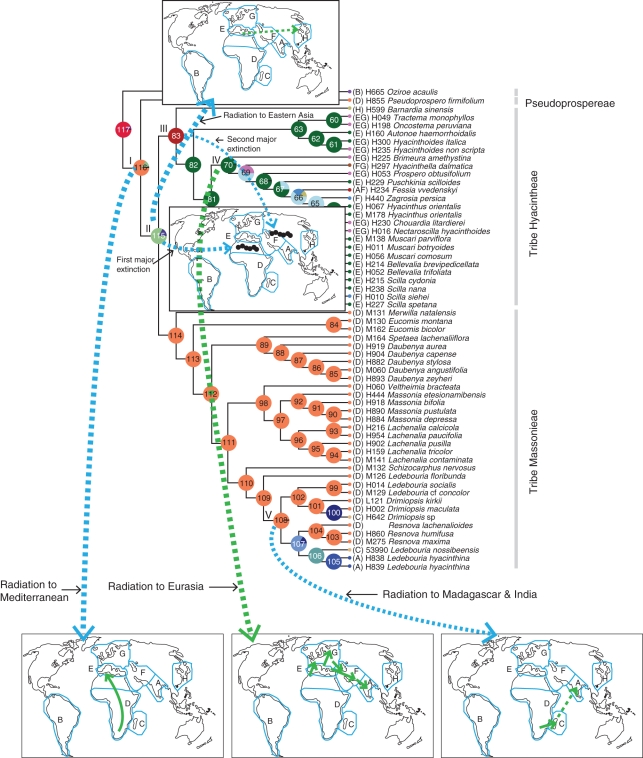
Fig. 3.
Major radiation and extinction events in subfamily Hyacinthoideae. Major radiations from southern Africa and the Mediterranean region are shown on the blank world maps.
Node 83 represents members of tribe Hyacintheae, and the possible ancestor range at this node is EH (Mediterranean + eastern Asia) with 100 % marginal probability. This node suggests a vicariance event. One descendant remained in eastern Asia and another linage underwent in situ diversification in the Mediterranean region. This in situ diversification resulted in two Mediterranean subclades. The ancestors of the node (82) of terminals 36–59 originated in the Mediterranean region (E) with 100 % marginal probability (Fig. 1A); however, the Bayesian support value for this node is low (35 %). This is followed by a series of dispersals to Europe and western Asia. One dispersal event occurred from western Asia to India. These dispersals resulted in a Eurasian subclade.
Node 114 of terminals 3–14 includes the taxa of tribe Massonieae. The ancestors of the node of terminals 3–14 originated in sub-Saharan Africa (D) with 100 % marginal probability. The members of this tribe underwent local radiation in sub-Saharan Africa. The ancestral area reconstruction at node 100 suggests sub-Saharan Africa (D) + Madagascar (C) as ancestral areas with 100 % support value; the probability of this result is 100 %. This probably indicates a trans-oceanic dispersal to Madagascar, with the source area of this dispersal being sub-Saharan Africa.
Node 107 of terminals 11–14 has two possible ancestral ranges, AD and CD. The most favoured ancestral range at this node is AD (India + sub-Saharan Africa) with 83·45 % marginal probability, indicating a second event of transoceanic dispersal (Fig. 3). The ancestral reconstruction at the next node is AC (India + Madagascar), suggesting another transoceanic dispersal. S-DIVA suggests that no dispersal occurred from sub-Saharan Africa to western Asia. Dispersals occurred to western Asia from the Mediterranean region. Similarly, no dispersal occurred from the Mediterranean region and western Asia to sub-Saharan Africa.
BBM analysis suggests slightly different ancestral ranges at basal nodes (Fig. 2). Node 116 represents all members of subfamily Hyacinthoideae. BBM analysis postulates that the ancestors of subfamily Hyacinthoideae originated in area D (sub-Saharan Africa). The marginal probability for D is 96·63 %. Similarly, at node 115, the ancestral reconstruction indicates that sub-Saharan Africa is the ancestral area of terminals 14–35, with 95·06 % marginal probability. BBM analysis suggests that the ancestors of Hyacinthoideae underwent in situ diversification in sub-Saharan Africa, as evident from ancestral ranges at basal nodes.
The ancestral reconstruction at node 83 is ambiguous and suggests four possible ancestral ranges, E, H, G and D. The occurrence of these ranges is: E, 29·00 %; H, 20·41 %; G, 19·67 %; and D, 10·07 %. BBM analysis suggests three dispersal events (internode leading to node 83) to eastern Asia, the Mediterranean region and Europe. Dispersal to the Mediterranean region occurred from sub-Saharan Africa. It is not clear whether dispersal to Europe and eastern Asia occurred from sub-Saharan Africa or the Mediterranean region. This node also suggests a vicariance event and one descendant underwent in situ diversification in EG (Mediterranean region + Europe), followed by dispersal to western Asia and India.
The ancestral reconstruction at node 106 is ambiguous, suggesting a number of possible ancestral ranges. Three ranges, A (India), C (Madagascar) and D (sub-Saharan Africa), had higher marginal probabilities of 32·43, 29·17 and 28·63 %, respectively. This suggests two transoceanic dispersals (Fig. 3).
DISCUSSION
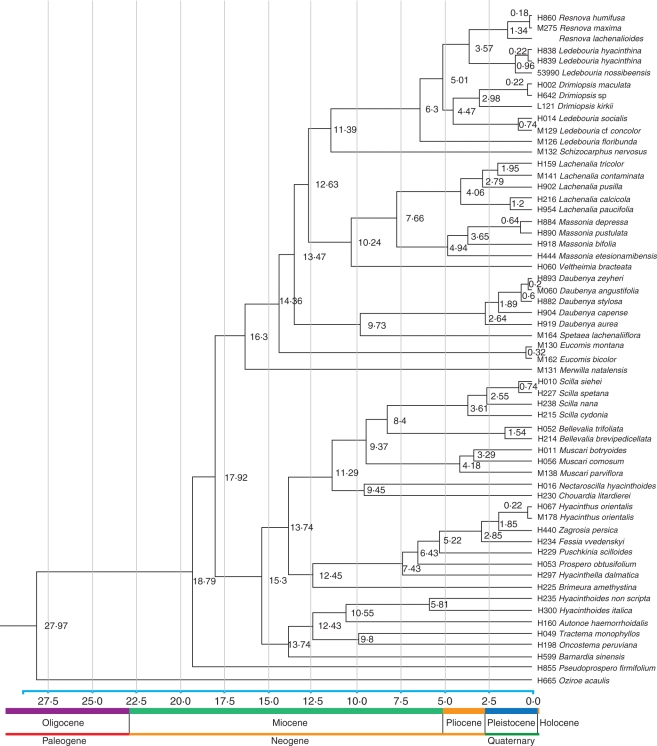
Several biogeographical inferences can be made from this analysis. S-DIVA suggests that the ancestors of Hyacinthoideae originated in range D (sub-Saharan Africa, 84·91 %). This was followed by an early dispersal to the Mediterranean region indicated by the ancestral ranges at node 115. The favoured ancestral range at node 115 is DE (82·81 %), and the support value and this node also suggests a vicariance event between sub-Saharan Africa and the Mediterranean. Aridification of the Sahara region from the Miocene onward could explain this vicariance event, which resulted in the partial extinction of the widespread African flora and the creation of the current pockets of distribution of extant species. A similar vicariance pattern has been suggested within Adenocarpus (Fabaceae) (see Sanmartín et al., 2010). The age of this event based on dated tree material in Fig. 4 is 17·92 Ma, consistent with the accelerated African aridification in the early Miocene due to the uplift of the continent and the formation of the East African Rift Valley (Axelrod, 1972; Baker et al., 1972).
Fig. 4.
Molecular clock based on the general substitution rates of the plastid sequence. Divergence age is shown below each node. Geological epoch is shown below the tree.
BBM analysis suggests that Hyacinthoideae originated in sub-Saharan Africa (D) with marginal probability >96 % (node 116). Dispersal to the Mediterranean region and eastern Asia took place early in the history of Hyacinthoideae (nodes 115 and 83). A similar pattern of sub-Saharan Africa origin and dispersal to the Mediterranean region has been suggested within Androcymbium (Colchicaceae) (del Hoyo et al., 2007; Sanmartín et al., 2010) and Senecio flavus (Coleman et al., 2003). The arrival of members of Hyacinthaceae in the Mediterranean region would have been possible in the Oligocene/Miocene through north-west Africa (Pfosser and Speta, 2004). The dated tree in Fig. 4 suggests that Hyacinthoideae originated in the early Miocene (19 Ma) and arrived in the Mediterranean region between 19 and 18 Ma. Further northward movement was not possible due to the large Tethys Ocean (Rögl, 1998, 1999) barrier. The formation of the Gomphotherium land bridge (19 Ma) allowed the free exchange of floras and faunas between Africa and Eurasia (Rögl, 1998). The tree data in Fig. 4 suggests that dispersal to Eurasia took place about 15·3 Ma.
This single colonization from sub-Saharan Africa to the Mediterranean region, followed by rapid diversification and movements, resulted in the monophyletic Eurasian tribe Hyacintheae (Pfosser et al., 2003; Wetschnig and Pfosser, 2003). Pseudoprospero occupies an early branching position in Hyacinthoideae in most of the trnL-F trees and is sister to the rest of the subfamily.
Barnardia sinensis occupies the earliest branching position in tribe Hyacintheae and suggests an early evolution of this genus. Its presence at an early diverging branch indicates that its ancestor was among the first members to colonize the Mediterranean region. Pfosser and Speta (1999) suggested that Barnardia was distributed from northern Africa to eastern Asia. Barnardia sinensis is found in Korea, Japan, China and Russia, whereas Barnardia numidica is distributed in northern Africa and the Balearic Islands. However, it is now believed that B. numidica is not related to Barnardia (data not shown) and should be transferred to a genus of its own.
S-DIVA suggests EH (Mediterranean + eastern Asia) as a possible ancestral range at node 83 with a 100 % marginal probability, and the most favoured ancestral range at node 115 is DE. Thus, S-DIVA suggests dispersal from the Mediterranean region to eastern Asia. BBM analysis suggests ambiguous ancestral ranges at node 83, but the Mediterranean is the most favoured ancestral range at this node. Node 83 also suggests a vicariance event between the Mediterranean and eastern Asia. Geological and climatic changes (aridification and the uplift of Tibet) were instrumental in the extinction of widespread flora and created the Mediterranean–eastern Asia disjunct distribution pattern. The aridification of central Asia and North China occurred between 22·0 and 6·2 Ma (Guo et al., 2002). The dated tree in Fig. 4 suggests that this extinction event occurred about 13·74 Ma. A similar Mediterranean–eastern Asia disjunct distribution pattern has been suggested within Helleborus (Ranunculaceae) (Sun et al., 2001).
According to S-DIVA, one descendant underwent local radiation in the Mediterranean region, followed by a series of dispersals to Europe (G), western Asia (F) and India (A). BBM analysis suggests an early dispersal to Europe. In situ diversification took place in these areas and then dispersals occurred to western Asia and India. Only one dispersal event was directed to India via western Asia. The Mediterranean region acted as a source area for these dispersals toward Europe and western Asia in tribe Hyacintheae.
The node (114) of terminals 3–14 represents all members of tribe Massonieae. Both S-DIVA and BBM analysis suggest D (sub-Saharan Africa) as the ancestral range at this node, with 100 and 99·05 % marginal probability, respectively. The sub-Saharan African ancestors underwent local radiation and in situ diversification within sub-Saharan Africa. Three transoceanic dispersals occurred in Massonieae to Madagascar and India. Ledebouria nossibeensis (Madagascar) shows strong affinity to L. hyacinthina from India and to members from the Arabian Peninsula, such as L. grandifolia from Socotra. The monophyly of L. nossibeensis from the north of Madagascar with L. hyacinthina from India is well supported (99/98 % bootstrap support and PP of 1·00).
BBM analysis suggests different ancestral ranges at nodes 107 and 106. The ancestral reconstruction at node 107 suggests sub-Saharan Africa as the sole ancestor area. The most recent common ancestors of nodes 106 and 104 are distributed in sub-Saharan Africa. Discussing multiple overseas dispersals in amphibians, Vences et al. (2003) mentioned that the important part of the Malagasy fauna was the result of dispersal from Africa. This dispersal was probably facilitated by currently submerged islands via a stepping-stone mechanism (McCall, 1997). Important examples of long-distance dispersal between Africa, Madagascar and the Seychelles are provided by multiple lineages of frogs (Vences et al., 2003, 2004), chameleons (Raxworthy et al., 2002), snakes (Nagy et al., 2003) and lemurs (Yoder and Yang, 2004). Slow climbing lorises distributed in Africa and Asia provide another striking example of dispersal between Africa and Asia (Masters et al., 2005). A number of recent studies have attempted to explain the plant disjunction in Africa, Madagascar and Asia. The stepping-stone mechanism, birds capable of long-distance flight and monsoon trade winds coupled with oceanic currents are important tools of dispersal. Dispersal by a stepping-stone mechanism occurred through the Seychelles, Comoros and Chagos Archipelago between Africa and Asia. Diaspore dispersal and successful speciation occurred in both directions. Dispersal from Africa to Asia has been suggested in Osbeckia (Melastomataceae) (Renner and Meyer, 2001; Renner, 2004), Gaertnera (Rubaceae) (Malcomber, 2002), Exacum (Gentianaceae; from Madagascar to Asia) (Yuan et al., 2005), Cucumis (Renner et al., 2007) and tribe Sonerileae (Melastomataceae; from Africa to Madagascar and Asia) (Renner, 2004). Dispersal has been suggested from Asia to Africa in Uvaria (Annonaceae) (Richardson et al., 2004), Bridelia (Phyllanthaceae) (Li et al., 2009), and Macaranga and Mallotus (Euphorbiaceae) (Kulju et al., 2007).
The second route of dispersal was opened after the collision of the African and Eurasian plates. In the Miocene, the collision of the African and Eurasian plates was followed by a series of palaeogeographical reorganizations in the circum-Mediterranean region (Rögl, 1998; Scotese et al., 1988). Finally, a new dispersal route was established in the early Pliocene (5 Ma) between Africa and south-western Asia via the Arabian Peninsula and the Levant region (Thompson, 2000; Fernandes et al., 2006) due to the closing of the Red Sea to the Mediterranean. Extensive faunal exchange occurred through this route between Africa and Eurasia (Vrba, 1993; Cox and Moore, 2005; Nylander et al., 2008). The dispersal route between Africa and Eurasia via the Arabian Peninsula and the Levant region was used by the ancestors of current Campanulaceae (Roquet et al., 2009) and other plants (Mummenhoff et al., 2001; Oberprieler, 2005; Inda et al., 2008; Mansion et al., 2008).
Birds are considered to be important tools of dispersal. Birds that can cross the 400-km Mozambique Channel include the Madagascan squacco heron, the Madagascan cuckoo, the Madagascan pratincole, the broad-billed roller and the Mascarene martin (Moreau, 1966; Renner, 2004). The first three species spend winters in eastern Africa and the last one is a casual visitor (Moreau, 1966; Renner, 2004).
S-DIVA and BBM analyses suggest two independent transoceanic dispersals to Madagascar from the source area of sub-Saharan Africa. According to S-DIVA reconstruction, African ancestors colonized the Mediterranean region (115), Madagascar (nodes 106 and 100) and India (node 107). BBM analysis suggests that African ancestors could have colonized Eurasia (node 83), Madagascar and India (node 106).
S-DIVA suggests early dispersal to the Mediterranean region in Hyacinthoideae, followed by a vicariance event (due to aridification of the Sahara region) between sub-Saharan Africa and the Mediterranean region, which resulted in sub-Saharan African and the Mediterranean lineages. A southward dispersal route is not plausible for members of Hyacinthoideae because the results of S-DIVA and BBM analyses suggest that sub-Saharan Africa is the ancestral area of the subfamily. Results also suggest an early dispersal to the Mediterranean region, and thus the northward route of dispersal is plausible for members of the subfamily. A similar dispersal route was shown for Androcymbium (Colchicaceae) (Sanmartín et al., 2010). In the present study, no samples from eastern Africa were available; therefore, we treated the region south of Sahara as a single block (sub-Saharan Africa). The absence of taxa from eastern Africa will surely have had impact on the results because without representation from this area, a northward route of dispersal would not be clarified. Sanmartín et al. (2010) suggest that southern Africa has highest dispersal rates with eastern Africa, while eastern Africa has highest dispersal rates with north-west Africa (Macaronesia). Therefore, sub-Saharan Africa was treated as a single block in this study, to understand the northward dispersal scenario from southern Africa to the Mediterranean region in the absence of taxa from the important eastern African region. In their latest study on Ornithogaloideae, Martínez-Azorín et al. (2011) included taxa from eastern Africa, which nested within predominantly southern African taxa, probably suggesting dispersal from southern Africa to eastern Africa. The formation of the eastern African mountains was instrumental in the migration of some southern African lineages to eastern Africa via the Grand Rift and the Drakensberg mountains, which resulted in the eastern African endemic flora (Linder, 2005; Galley et al., 2007; Sanmartín et al., 2010). No nuclear gene data are available for this study; however, Martínez-Azorín et al. (2011) included the ITS region of nuclear ribosomal DNA in their study of Ornithogaloideae, a closely related group to Hyacinthoideae. They found the position of two clades were different in the phylogenetic tree based on the ITS region compared with the combined tree. Whether we include or exclude a nuclear region, sub-Saharan Africa is the ancestral area, but inclusion of this region will be helpful to explain some other dispersal and vicariance scenarios.
ACKNOWLEDGEMENTS
We are grateful to the handling editor, Dr Michael F. Fay, and two anonymous reviewers for their valuable suggestions and constructive criticisms. We thank John Munson for suggested corrections to the English text.
APPENDIX
List of taxa investigated in this study, with vouchers, citation information and EMBL accession numbers
| Species | Voucher | Locality | EMBL acc. no(s). |
|---|---|---|---|
| Autonoe haemorrhoidalis (Webb & Berthel) Speta | Klenner H160 | Spain | AJ232518/AJ2326411 |
| Barnardia scilloides Lindl. | Pfosser H599 | Korea | AJ507998 |
| Bellevalia brevipedicellata Turrill | Jahn H214 | Greece | AJ232547/AJ2326701 |
| Bellevalia trifoliata Kunth | Speta H052 | Greece | AJ232548/AJ2326711 |
| Brimeura amethystina (L.) Chouard | Pfosser H225 | cult. ex B.G. Tallinn | AJ232510/AJ2326331 |
| Chouardia litardierei (Breistr.) Speta | Pfosser H230 | Croatia | AJ232541/AJ2326641 |
| Daubenya angustifolia (L.f.) A.M.van der Merwe & J.C.Manning | Wetschnig 1101 | South Africa | AJ507960 |
| Daubenya aurea Lindl. | Wetschnig 1162 | South Africa | AJ507956 |
| Daubenya capensis (Schltr.) A.M.van der Merwe & J.C. Manning | Wetschnig 1129 | South Africa | AJ507955 |
| Daubenya stylosa (W.F.Barker) A.M.van der Merwe & J.C.Manning | Wetschnig 1160 | South Africa | AJ507957 |
| Daubenya zeyheri (Kunth) J.C.Manning & A.M.van der Merwe | Wetschnig 1153 | South Africa | AJ507961 |
| Drimiopsis maculata Lindl. & Paxton | Speta H002 | cult. Linz | AJ232502/AJ2326251 |
| Drimiopsis sp. | Pfosser H642 | Madagascar | AJ507953 |
| Drimiopsis kirkii Baker | Pfosser L121 | cult. B.G. Vienna | AJ507952 |
| Eucomis bicolor Baker | Schnabel M162 | South Africa | AJ507933 |
| Eucomis montana Compton | Schnabel M130 | South Africa | AJ507932 |
| Fessia vvedenskyi (Pazij) Speta | Speta H234 | Uzbekistan | AJ232535/AJ2326581 |
| Hyacinthella dalmatica (Baker) Chouard | Gutermann H297 | Croatia | AJ232526/AJ2326491 |
| Hyacinthoides italica (L.) Rothm. | Pfosser H300 | France | AJ232519/AJ2326421 |
| Hyacinthoides non-scripta (L.) Chouard ex W.Rothmaler | Pfosser H235 | France | AJ232524/AJ2326471 |
| Hyacinthus orientalis L. | Speta H067 | Romania | AJ232539/AJ2326621 |
| Hyacinthus orientalis L. var. alba | J. Plass M178 | Syria | AJ508002 |
| Lachenalia calcicola (U.Müll.-Doblies & D.Müll.-Doblies) J.C.Manning & Goldblatt | Müller-Doblies H216 | South Africa | AJ232506/AJ2326291 |
| Lachenalia contaminata (Soland.) | Pfosser M141 | South Africa | AJ507985 |
| Lachenalia paucifolia (W.F.Barker) J.C.Manning & Goldblatt | Wetschnig 1154 | South Africa | AJ507990 |
| Lachenalia pusilla Jacq. | Wetschnig 1115 | South Africa | AJ507986 |
| Lachenalia tricolor Jack. ex Murray | Pfosser H159 | cult. B.G. Vienna | AJ232508/AJ2326311 |
| Ledebouria cf. concolor (Baker) Jessop | Wetschnig 1412 | South Africa | AJ507946 |
| Ledebouria floribunda (Baker) Jessop | Wetschnig 1433 | South Africa | AJ507937 |
| Ledebouria hyacinthina Roth | Jha H838 | India | AJ507944 |
| Ledebouria hyacinthina Roth | Jha H839 | India | AJ507945 |
| Ledebouria nossibeensis (H.Perrier) J.C.Manning & Goldblatt | Andriantiana 53990 | Madagascar | AN109129 |
| Ledebouria socialis (Baker) Jessop | Pfosser H014 | cult. B.G. Vienna | AJ232501/AJ2326241 |
| Massonia bifolia (Jacq.) J.C.Manning & Goldblatt | Wetschnig 1130 | South Africa | AJ507966 |
| Massonia depressa Houtt. | Wetschnig 1142 | South Africa | AJ507980 |
| Massonia etesionamibensis (U.Müll.-Doblies & D.Müll.-Doblies) J.C.Manning & Goldblatt | Lavranos & Pehle-mann H444 | Namibia | AJ232504/AJ2326271 |
| Massonia pustulata Jacq. | Wetschnig 1148 | South Africa | AJ507970 |
| Merwilla natalensis (Planch.) Speta | Wetschnig 1534 | South Africa | AJ507931 |
| Muscari botryoides (L.) Mill. | Kleesadl H011 | Austria | AJ232545/AJ2326681 |
| Muscari comosum (L.) Mill. | Neuner H056 | Italy | AJ232546/AJ2326691 |
| Muscari parviflorum Desf. | J. Plass M131 | Syria | AJ508003 |
| Nectaroscilla hyacinthoides Parl. | Scheiblreiter H016 | Portugal | AJ232542/AJ2326651 |
| Oncostema peruviana (L.) Speta | Pfosser H198 | Portugal | AJ232516/AJ2326391 |
| Oziroe acaulis (Baker) Speta | Weigend s.n. | Peru | AJ507921 |
| Prospero obtusifolium (Poir.) Speta | HC H053 | Morocco | AJ232529/AJ2326521 |
| Pseudoprospero firmifolium (Baker) Speta | Wetschnig 1322 | South Africa | AJ507928 |
| Puschkinia scilloides Adams var. libanotica | Pfosser H229 | Lebanon | AJ508688 |
| Resnova humifusa (Baker) U.Müll.-Doblies & D.Müll.-Doblies | Wetschnig 1524 | South Africa | AJ507942 |
| Resnova lachenalioides (Baker) Van der Merwe | Lebatha 019 (PUC) | South Africa | DQ313330 |
| Resnova maxima Van der Merwe | Hankey M275 | South Africa | AJ507943 |
| Schizocarphus nervosus (Burch.) Van der Merwe | Saunders M132 | South Africa | AJ507936 |
| Scilla cydonia Speta | Jahn et al. H215 | Greece | AJ232549/AJ2326721 |
| Scilla nana (Schultes f.) Speta | Speta H238 | Greece | AJ232552/AJ2326751 |
| Scilla siehei (Stapf) Speta ‘Pink Giant’ | Speta H010 | cult. Linz | AJ232551/AJ2326741 |
| Scilla spetana Kereszty | Speta H227 | Austria | AJ232556/AJ2326791 |
| Spetaea lachenaliiflora Wetschnig & Pfosser | Saunders M164 | South Africa | AJ507954 |
| Tractema monophyllos (Link) Speta | Raus H049 | Spain | AJ232513/AJ2326361 |
| Veltheimia bracteata Harv. ex Baker | Speta H060 | cult. Linz | AJ232503/AJ2326261 |
| Zagrosia persica (Hausskn.) Speta | Leep H440 | Turkey | AJ232537/AJ2326601 |
LITERATURE CITED
- APG III. An update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Axelrod DI. Ocean-floor spreading in relation to ecosystematic problems. Occasional Paper of the University of Arkansas. 1972;4:15–68. [Google Scholar]
- Baker BH, Mohr PA, Williams LAJ. Geology of the eastern rift system of Africa. Special Paper of the Geological Society of America. 1972;136:1–67. [Google Scholar]
- Chase MW, Reveal JL, Fay MF. A subfamilial classification for the expanded asparagalean families, Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Botanical Journal of the Linnean Society. 2009;161:132–136. [Google Scholar]
- Coleman M, Liston A, Kadereit JW, Abbott RJ. Repeat intercontinental dispersal and Pleistocene speciation in disjunct Mediterranean and desert Senecio (Asteraceae) American Journal of Botany. 2003;90:1446–1454. doi: 10.3732/ajb.90.10.1446. [DOI] [PubMed] [Google Scholar]
- Cox BC, Moore PD. Biogeography: an ecological and evolutionary approach. 7th edition. London: Blackwell Publishing; 2005. [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology. 2007;7:214. doi: 10.1186/1471-2148-7-214. http://dx.doi.org/10.1186/1471-2148-7-214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Nicholls GK, Rodrigo AG, Solomon W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics. 2002;161:1307–1320. doi: 10.1093/genetics/161.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fernandes CA, Rohling EJ, Siddall M. Absence of post-Miocene Red Sea land bridges: biogeographic implications. Journal of Biogeography. 2006;33:961–966. [Google Scholar]
- Fitch WM. Toward defining the course of evolution: minimum change for a specific tree topology. Systematic Zoology. 1971;20:406–416. [Google Scholar]
- Galley C, Bytebier B, Bellstedt DU, Linder HP. The Cape element in the Afrotemperate flora: from Cape to Cairo? Proceedings of the Royal Society of London Series B. Biological Sciences. 2007;274:535–543. doi: 10.1098/rspb.2006.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielly L, Taberlet P. The use of chloroplast DNA to resolve plant phylogenies: noncoding versus rbcL sequences. Molecular Biology and Evolution. 1994;11:769–777. doi: 10.1093/oxfordjournals.molbev.a040157. [DOI] [PubMed] [Google Scholar]
- Gielly L, Taberlet P. A phylogeny of the European gentians inferred from chloroplast trnL (UAA) intron sequences. Botanical Journal of the Linnean Society. 1996;120:57–75. [Google Scholar]
- Gielly L, Yuan Y, Kupfer P, Taberlet P. Phylogenetic use of noncoding regions in the genus Gentiana L.: chloroplast trnL (UAA) intron versus nuclear ribosomal internal transcribed spacer sequences. Molecular Phylogenetics and Evolution. 1996;5:460–466. doi: 10.1006/mpev.1996.0042. [DOI] [PubMed] [Google Scholar]
- Guo ZT, Ruddiman WF, Hao QZ, et al. Onset of Asian desertification by 22 Myr ago inferred from loess deposits in China. Nature. 2002;416:159–163. doi: 10.1038/416159a. [DOI] [PubMed] [Google Scholar]
- Harris AJ, Xiang Q-Y. Estimating ancestral distributions of lineages with uncertain sister groups: a statistical approach to dispersal–vicariance analysis and a case using Aesculus L (Sapindaceae) including fossils. Journal of Systematics and Evolution. 2009;47:349–368. [Google Scholar]
- del Hoyo A, Garcia-Marin JL, Pedrola-Monfort J. Temporal and spatial diversification of the African disjunct genus Androcymbium (Colchicaceae) Molecular Phylogenetic and Evolution. 2007;53:848–861. doi: 10.1016/j.ympev.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Hülsenbeck JP, Ronquist F. MrBayes, Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Inda LA, Segarra-Moragues JG, Müller J, Peterson PM, Catalán P. Dated historical biogeography of the temperate Loliinae (Poaceae, Pooideae) grasses in the northern and southern hemispheres. Molecular Phylogenetics and Evolution. 2008;46:932–957. doi: 10.1016/j.ympev.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends in Biochemical Sciences. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Kita Y, Ueda K, Kadota Y. Molecular phylogeny and evolution of the Asian Aconitum subgenus Aconitum (Ranunculaceae) Journal of Plant Research. 1995;108:429–442. [Google Scholar]
- Kulju KKM, Sierra SEC, Draisma SGA, Samuel R, van Welzen PC. Molecular phylogeny of Macaranga, Mallotus, and related genera (Euphorbiaceae S.S.): insights from plastid and nuclear DNA sequence data. American Journal of Botany. 2007;94:1726–1743. doi: 10.3732/ajb.94.10.1726. [DOI] [PubMed] [Google Scholar]
- Lebatha P, Buys MH, Stedje B. Ledebouria, Resnova and Drimiopsis: a tale of three genera. Taxon. 2006;55:643–652. [Google Scholar]
- Levyns MR. Migrations and origin of the Cape flora. Transactions of the Royal Society of South Africa. 1964;37:85–107. [Google Scholar]
- Li Y, Dressler S, Zhang D, Renner SS. More Miocene dispersal between Africa and Asia—the case of Bridelia (Phyllanthaceae) Systematic Botany. 2009;34:521–529. [Google Scholar]
- Linder HP. Evolution of diversity: the Cape Flora. Trends in Plant Science. 2005;10:536–541. doi: 10.1016/j.tplants.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2010 Version 2·74. Available at: http://mesquiteproject.org/mesquite/mesquite.html . [Google Scholar]
- Malcomber ST. Phylogeny of Gaertnera Lam. (Rubiaceae) based on multiple DNA markers: evidence of a rapid radiation in a widespread, morphologically diverse genus. Evolution. 2002;56:42–57. doi: 10.1111/j.0014-3820.2002.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Manning JC, Goldblatt P, Fay MF. A revised generic synopsis of Hyacinthaceae in sub-Saharan Africa based on molecular evidence, including new combinations and the new tribe Pseudoprospereae. Edinburgh Journal of Botany. 2004;60:533–568. [Google Scholar]
- Manning JC, Forest F, Devey D, Fay M, Goldblatt P. A molecular phylogeny and a revised classification of Ornithogaloideae (Hyacinthaceae) based on an analysis of four plastid DNA regions. Taxon. 2009;58:77–107. [Google Scholar]
- Mansion G, Rosenbaum G, Schoenenberger N, Bacchetta G, Rosselló JA, Conti E. Phylogenetic analysis informed by geological history supports multiple, sequential invasions of the Mediterranean basin by the angiosperm family Araceae. Systematic Biology. 2008;57:269–285. doi: 10.1080/10635150802044029. [DOI] [PubMed] [Google Scholar]
- Martínez-Azorín M, Crespo MB, Juan A, Fay MF. Molecular phylogenetics of subfamily Ornithogaloideae (Hyacinthaceae) based on nuclear and plastid DNA regions, including a new taxonomic arrangement. Annals of Botany. 2011;107:1–37. doi: 10.1093/aob/mcq207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters JC, Anthony N, De Wit M, Mitchell A. Reconstructing the evolutionary history of the Lorisidae using morphological, molecular, and geological data. American Journal of Physical Anthropology. 2005;127:465–480. doi: 10.1002/ajpa.20149. [DOI] [PubMed] [Google Scholar]
- McCall RA. Implications of recent geological investigations of the Mozambique channel for the mammalian colonization of Madagascar. Proceedings of the Royal Society of London Series B. Biological Sciences. 1997;264:663–665. doi: 10.1098/rspb.1997.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade LA, Moody ML. Phylogenetic relationships among Acanthaceae: evidence from non-coding trnL-trnF chloroplast DNA sequences. American Journal of Botany. 1999;86:70–80. [PubMed] [Google Scholar]
- Moreau RE. The bird faunas of Africa and its islands. New York: Academic Press; 1966. [Google Scholar]
- Mummenhoff K, Brüggemann H, Bowman JL. Chloroplast DNA phylogeny and biogeography of Lepidium (Brassicaceae) American Journal of Botany. 2001;88:2051–2063. [PubMed] [Google Scholar]
- Mwafongo E, Nordal I, Magombo Z, Stedje B. Ethnobotanical study of Hyacinthaceae and non-hyacinthaceous geophytes in selected districts of Malawi. Ethnobotany Research and Applications. 2010;8:75–93. [Google Scholar]
- Nagy ZT, Joger U, Wink M, Glaw F, Vences M. Multiple colonization of Madagascar and Socotra by colubrid snakes: evidence from nuclear and mitochondrial gene phylogenies. Proceedings of the Royal Society of London Series B. Biological Sciences. 2003;270:2613–2621. doi: 10.1098/rspb.2003.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JAA. MrModeltest. 2004 version 2·2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University (http://www.abc.se/~nylander/ ) [Google Scholar]
- Nylander JAA, Olsson O, Alström P, Sanmartín I. Accounting for phylogenetic uncertainity in biogeography: a Bayesian approach to dispersal–vicariance analysis of the thrushes (Aves: Turdus) Systematic Biology. 2008;57:257–268. doi: 10.1080/10635150802044003. [DOI] [PubMed] [Google Scholar]
- Oberprieler C. Temporal and spatial diversification of circum-Mediterranean Compositae–Anthemidae. Taxon. 2005;54:951–966. [Google Scholar]
- Pfosser M, Speta F. Phylogenetics of Hyacinthaceae based on plastid DNA sequences. Annals of the Missouri Botanical Garden. 1999;86:852–875. [Google Scholar]
- Pfosser M, Speta F. Bufadienolides and DNA sequences: on lumping and smashing of subfamily Urgineoideae (Hyacinthaceae) Stapfia. 2001;75:177–250. [Google Scholar]
- Pfosser M, Speta F. From Scilla to Charybdis – is our voyage safer now? Plant Systematics and Evolution. 2004;246:245–263. [Google Scholar]
- Pfosser M, Wetschnig W, Ungar S, Prenner G. Phylogenetic relationships among genera of Massonieae (Hyacinthaceae) inferred from plastid DNA and seed morphology. Journal of Plant Research. 2003;116:115–132. doi: 10.1007/s10265-003-0076-8. [DOI] [PubMed] [Google Scholar]
- Pfosser M, Wetschnig W, Speta F. Drimia cryptopoda, a new combination in Hyacinthaceae from Madagascar. Linzer Biologische Beiträge. 2006;38:1731–1739. [Google Scholar]
- Rambaut A. FigTree. 2009 v1·3·1. Available from: http://tree.bio.ed.ac.uk/software/figtree . [Google Scholar]
- Rambaut A, Drummond AJ. Tracer. 2007 version 1·5. Available at: http://beast.bio.ed.ac.uk/Tracer . [Google Scholar]
- Raxworthy CJ, Forstner MRJ, Nussbaum RA. Chameleon radiation by oceanic dispersal. Nature. 2002;415:784–787. doi: 10.1038/415784a. [DOI] [PubMed] [Google Scholar]
- Ree RH, Smith SA. A maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- Ree RH, Moore BR, Webb CO, Donoghue MJ. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution. 2005;59:2299–2311. [PubMed] [Google Scholar]
- Renner SS. Multiple Miocene Melastomataceae dispersal between Madagascar, Africa and India. Philosophical Transactions of the Royal Society of London. Series B. 2004;359:1485–1494. doi: 10.1098/rstb.2004.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner SS, Meyer K. Melastomataceae come full circle: biogeographic reconstruction and molecular clock dating. Evolution. 2001;55:1315–1324. doi: 10.1111/j.0014-3820.2001.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Renner SS, Schaefer H, Kocyan A. Phylogenetics of Cucumis (Cucurbitaceae): cucumber (C. sativus) belongs in an Asian/Australian clade far from melon (C. melo) BMC Evolutionary Biology. 2007;7:58. doi: 10.1186/1471-2148-7-58. http://dx.doi.org/10.1186/1471-2148-7-58 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JE, Chatrou LW, Mols JB, Erkens RHJ, Pirie MD. Historical biogeography of two cosmopolitan families of flowering plants: Annonaceae and Rhamnaceae. Philosophical Transactions of the Royal Society of London Series B. 2004;359:1495–1508. doi: 10.1098/rstb.2004.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rögl F. Paleogeographic considerations for Mediterranean and Paratethys seaways (Oligocene to Miocene) Annalen des Naturhistorischen Museums in Wien. 1998;99:279–310. [Google Scholar]
- Rögl F. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview) Geologica Carpathica. 1999;50:339–349. [Google Scholar]
- Ronquist F. Dispersal-vicariance analysis: a new approach to the quantification of historical biogeography. Systematic Biology. 1997;46:195–203. [Google Scholar]
- Ronquist F. DIVA. 2001 version 1·2. Computer program for MacOS and Win32. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Ronquist F, Hülsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Roquet C, Sanmartín I, Garcia-Jacas N, Susanna A, Wikström N, Aldasoro JJ. Reconstructing the history of Campanulaceae with a Bayesian approach to molecular dating and dispersal–vicariance analyses. Molecular Phylogenetics and Evolution. 2009;52:575–587. doi: 10.1016/j.ympev.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Sanmartín I, van der Mark P, Ronquist F. Inferring dispersal: a Bayesian, phylogeny-based approach to island biogeography, with special reference to the Canary Islands. Journal of Biogeography. 2008;35:428–449. [Google Scholar]
- Sanmartín I, Anderson C, Alarcon M, Ronquist F, Aldasoro J. Bayesian island biogeography in a continental setting: the Rand flora case. Biology Letters. 2010;6:703–707. doi: 10.1098/rsbl.2010.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotese CR, Gahagan LM, Larson RL. Plate tectonic reconstructions of the Cretaceous and Cenozoic ocean basins. Tectonophysics. 1988;155:27–48. [Google Scholar]
- Speta F. Systematische Analyse der Gattung Scilla l. (Hyacinthaceae) Phyton (Horn) 1998a;38:1–141. [Google Scholar]
- Speta F. Hyacinthaceae. In: Kubitzki K, editor. The families and genera of vascular plants 3. Berlin: Springer-Verlag; 1998b. pp. 261–285. [Google Scholar]
- Stedje B. Hyacinthaceae. In: Polhill RM, editor. Flora of tropical East Africa. Rotterdam: A.A. Balkema; 1996. pp. 1–32. [Google Scholar]
- Sun H, McLewin W, Fay MF. Molecular phylogeny of Helleborus (Ranunculaceae), with an emphasis on the East Asian-Mediterranean disjunction. Taxon. 2001;50:1001–1018. [Google Scholar]
- Swofford DL. PAUP*: Phylogenetic analysis using parsimony (and other methods) Sunderland, MA: Sinauer Associates; 2002. version 4. [Google Scholar]
- Thompson A. Origins of Arabia. Dubai: Oriental Press; 2000. [Google Scholar]
- Vences M, Vieites DR, Glaw F, et al. Multiple overseas dispersal in amphibians. Proceedings of the Royal Society of London. 2003;270:2435–2442. doi: 10.1098/rspb.2003.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vences M, Kosuch J, Rödel MO, et al. Phylogeography of Ptychadena mascareniensis suggests transoceanic dispersal in a widespread African-Malagasy frog lineage. Journal of Biogeography. 2004;31:593–601. [Google Scholar]
- Vrba ES. Mammal evolution in the African Neogene and a new look at the great American interchange. In: Goldblatt P, editor. Biological relationships between Africa and South America. New Haven: Yale University Press; 1993. pp. 393–434. [Google Scholar]
- Wetschnig W, Pfosser M. The Scilla plumbea puzzle – present status of the genus Scilla sensu lato in Southern Africa and description of Spetaea lachenaliiflora, a new genus and species of Massonieae (Hyacinthaceae) Taxon. 2003;52:75–91. [Google Scholar]
- Wetschnig W, Pfosser M, Prenner G. Zur Samenmorphologie der Massonieae Baker 1871 (Hyacinthaceae) im Lichte phylogenetisch interpretierter molekularer Befunde. Stapfia. 2002;80:349–379. [Google Scholar]
- Wetschnig W, Pfosser M, Ali S, Knirsch W. Systematic position of three little known and frequently misplaced species of Hyacinthaceae from Madagascar. Phyton (Austria) 2007;47:321–337. [Google Scholar]
- Yan Y, Harris AJ, Xingjin H. S-DIVA (Statistical Dispersal-Vicariance Analysis): a tool for inferring biogeographic histories. Molecular Phylogenetics and Evolution. 2010;56:848–850. doi: 10.1016/j.ympev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Yan Y, Harris AJ, Xingjin H. RASP (Reconstruct Ancestral State in Phylogenies) 2011 doi: 10.1016/j.ympev.2015.03.008. 1·1. Available at: http://mnh.scu.edu.cn/soft/blog/RASP . [DOI] [PubMed] [Google Scholar]
- Yoder AD, Yang Z. Divergence dates for Malagasy lemurs estimated from multiple gene loci: geological and evolutionary context. Molecular Ecology. 2004;13:757–773. doi: 10.1046/j.1365-294x.2004.02106.x. [DOI] [PubMed] [Google Scholar]
- Yuan YM, Wohlhauser S, Möller M, Klackenberg J, Callmander MW, Küpfer P. Phylogeny and biogeography of Exacum (Gentianaceae): a disjunctive distribution in the Indian Ocean basin resulted from long distance dispersal and extensive radiation. Systematic Biology. 2005;54:21–34. doi: 10.1080/10635150590905867. [DOI] [PubMed] [Google Scholar]
- Zurawski G, Clegg MT, Brown AHD. The nature of nucleotide sequence divergence between barley and maize chloroplast DNA. Genetics. 1984;106:735–749. doi: 10.1093/genetics/106.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]