Abstract
BACKGROUND
Breast cancer development involves a series of mutations in a heterogeneous group of proto-oncogenes/tumor suppressor genes that alter mammary cells to create a microenvironment permissive to tumorigenesis. Exposure to hormones during infertility treatment may have a mutagenic effect on normal mammary epithelial cells, high-risk breast lesions and early-stage breast cancers. Our goal was to understand the association between infertility treatment and normal and cancerous breast cell proliferation.
METHODS
MCF-10A normal mammary cells and the breast cancer cell lines MCF-7 [estrogen receptor (ER)-positive, well differentiated] and HCC 1937 (ER-negative, aggressive, BRCA1 mutation) were treated with the weak ER activator clomiphene citrate and hormones that are increased during infertility treatment. Direct effects of treatment on cell proliferation and colony growth were determined.
RESULTS
While clomiphene citrate had no effect on MCF-10A cells or MCF-7 breast cancer cells, it decreased proliferation of HCC 1937 versus untreated cells (P= 0.003). Estrogen had no effect on either MCF-10A or HCC 1937 cells but, as expected, increased cell proliferation (20–100 nM; P≤0.002) and colony growth (10–30 nM; P< 0.0001) of MCF-7 cells versus control. Conversely, progesterone decreased both proliferation (P= 0.001) and colony growth (P= 0.01) of MCF-10A cells, inhibited colony size of MCF-7 cells (P= 0.01) and decreased proliferation of HCC 1937 cells (P= 0.008) versus control. hCG (100 mIU/ml) decreased both proliferation (P ≤ 0.01) and colony growth (P ≤ 0.002) of all three cell lines.
CONCLUSIONS
Although these data are preclinical, they support possible indirect estrogenic effects of infertility regimens on ER-positive breast cancer cells and validate the potential protective effect of pregnancy-related exposure to hCG.
Keywords: infertility, breast cancer, fertility treatment, estrogen, pregnancy
Introduction
In 2009, nearly 200 000 new invasive breast cancers were diagnosed in the USA (Breast Cancer Facts & Figures 2009–2010). Of these, approximately 18 000 cases occurred in women younger than 45 years (Breast Cancer Facts & Figures 2009–2010). Though age 35 years marks a time of declining fertility for women, increasingly, women are choosing to delay childbearing. Nearly 20% of US women will have their first child after age 35 years, and one-third of these women will have fertility issues (ASRM, 2003; Leridon, 2004). Thus, many women will opt for fertility treatment at a time when breast cancer incidence begins to increase. Some studies have found an increased risk of breast cancer in the setting of infertility treatment, particularly for patients at high risk for the disease, such as women with a family history (Gauthier et al., 2004). However, a causal relationship between breast cancer development and infertility treatment has not been well established.
Estrogen is elevated to supraphysiologic levels as a direct result of ovulation induction with clomiphene citrate and FSH/hCG during infertility treatment (Jee et al., 2006; Kyrou et al., 2009). Estrogen is a breast epithelial cell mitogen, and through interaction with other hormones and growth factors, contributes to activation of proto-oncogenes, such as c-myc, cyclin D1 and cyclin E (Mawson et al., 2005; Han et al., 2006; Butt et al., 2008). These genes mediate G1-to-S phase transition during normal cell-cycle progression. Thus, increased exposure to estrogens during infertility treatment may facilitate malignant potential of cell cycle regulatory proto-oncogenes in breast epithelium (Butt et al., 2008). This link between exposure to supraphysiologic levels of estrogen and risk of malignant mammary transformation forms a basis for concern about the impact that infertility treatments may have on mammary tumorigenesis. Potentially deleterious epigenetic events have been noted at early life time points, where young girls may be exposed to hormones, such as recombinant growth hormone and estradiol (E2), from additives found in some dairy and meat products, among a myriad of other environmental exposures that are thought to be cumulative over a patient's lifetime (Hiatt et al., 2009). Furthermore, lifetime exposure to estrogens, including early menarche and late menopause, is a known risk factor for breast cancer (Henderson and Feigelson, 2000; Hiatt et al., 2009). The time delay from deleterious exposure to malignant transformation is variable dependent on several factors, including genetic predilection, impact of harmful exposure and possible favorable modifying events. The additive effect of fertility treatments and the elevated hormone exposure associated with these treatments over time would thus be considered in the context of a patient's overall lifetime risk from hormone exposure. Some studies have shown a potential timeframe of over 10 years prior to malignant transformation for patients diagnosed with high-risk breast lesions (Collins et al., 2005). Interventions to prevent this transformation are thus recommended, such as the anti-estrogen tamoxifen (Eng-Wong et al., 2010).
The direct effect of infertility treatment agents on mammary epithelial and/or stromal tissue, independent of downstream estrogen elevation, is not known. During cycles in which ovulation is induced, serum estrogen levels are greater than those occurring in naturally cycling women. Additionally, ovulation of multiple follicles leads to high progesterone levels, which may also increase breast cancer risk. Clomiphene citrate can act by binding to the estrogen receptor (ER) and exerting a weak estrogenic effect while blocking endogenous estrogen (Kurosawa et al., 2010). Potential carcinogenic effects of clomiphene citrate may be mediated through activation of estrogenic transcriptional activity, and these effects are likely dependent on clomiphene citrate exposure dose, endogenous tissue metabolism and the hormonal environment (Musgrove et al., 1989). One study of more than 5000 women has demonstrated an elevated breast cancer risk associated with clomiphene citrate, independent of family history (Lerner-Geva et al., 2006). In contrast, hCG, which is present at very high levels in early pregnancy, has both anti-invasive and anti-proliferative effects mediated through the breast epithelial LH/hCG receptor and down-regulation of nuclear factor-kappa B and activator protein-1 transcription factors in human breast cancer (Rao et al., 2004). In certain tissue beds, however, hCG has been identified as an inducer of angiogenesis through activation of the endothelial LH/hCG receptor (Zygmunt et al., 2002).
To gain a better understanding of the relationship between infertility treatment and the risk of breast malignancy, we characterized the effects of a widely used infertility agent—clomiphene citrate—as well as E2, progesterone and the pregnancy hormone, hCG, on mammary epithelial and cancer cell growth and proliferation in vitro. To this end, we utilized the MCF-10A cell line which approximates normal mammary cells, the MCF-7 cell line representative of ER+ well-differentiated breast cancer, and the HCC 1937 ER-, BRCA1-mutated breast cancer cell line reflecting hereditary, ER more aggressive breast cancer.
Materials and Methods
Cell lines, culture media and hormones
MCF-10A, MCF-7 and HCC 1937 cell lines were obtained from ATCC (Manassas, VA, USA). The MCF-10A cell line was maintained in Dulbecco's modified Eagle's medium (DMEM)-F12 and 10% horse serum, MCF-7 in DMEM-F12 and 10% fetal bovine serum (FBS) and HCC 1937 in RPMI with 10% FBS serum; all media were supplemented with penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). All media contained no phenol red; serum used in culture media was stripped using charcoal to remove estrogenic compounds. hCG, estrogen, progesterone and clomiphene citrate were obtained from Sigma (St. Louis, MO, USA).
2D cell culture and MTS assay
Cell proliferation was evaluated by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay per the manufacturer's instructions (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA). Briefly, for each cell line, cells were seeded in 96-well plates at 5 × 103 cells/100 μl media per well for MCF-10A cells and at 3 × 103/100 μl media for MCF-7 and HCC 1937 cells, which grow and reach confluency more rapidly than MCF-10A cells. Five wells were plated for each treatment for each time point and allowed to adhere overnight. Cells were treated as follows: no treatment (media plus ethanol diluent), 30 nM E2, 30 nM clomiphene citrate, 10 nM progesterone, 0.5 mIU/ml hCG, 10 mIU/ml hCG (Day 1 only) or 100 mIU/ml hCG (Day 1 only). To examine the impact of increasing dose of estrogen, MCF-7 cells were treated as follows: no treatment, 10, 20, 30, 60 and 100 nM E2. All experiments were performed in triplicate. For baseline reading (Day 0), 20 μl of MTS solution was added, plates were incubated for 3h and absorbance was then read on a Bio Rad Microplate Reader Model 680 (Hercules, CA, USA) at 490 nm. The MTS assay was repeated on Days 2, 4, 6 and 10. All experiments (five wells per treatment per time point) were repeated in triplicate.
3D Matrigel cell culture
In 96-well plates, 30 µl of growth factor-reduced, phenol red-free Matrigel (BD Biosciences, San Jose, CA, USA) was spread evenly over the entire well surface. Plates were then incubated at 37°C for 30min to solidify the Matrigel. Cells were trypsinized, spun down at 500g for 2min and resuspended in media with 2% v/v Matrigel added. Cell density was determined and 1.25 × 103 cells were plated in each well. This was considered Day 0. Media was freshly prepared and changed every other day beginning on Day 1. On Days 4 and 10, an image of each well was taken at ×10 magnification using AxioVision (Zeiss, Thornwood, NY, USA). Study cells were treated as described above. To examine the impact of increasing dose of estrogen, MCF-7 cells were treated as follows: no treatment (media plus ethanol diluent), 10, 20 and 30 nM E2.
Statistical analysis
For the MTS assay in 2D cultures, proliferation was measured as absorbance at 490 nm and reported as raw values with or without treatment at each time point. For each image of the cells in 3D Matrigel cultures, number of colonies was recorded and colony size was measured and reported as a ratio of values for treated to untreated cells at each time point. Average values of absorbance at 490 nm (proliferation in 2D culture) and average ratios of colony area (growth in 3D culture) and SEM were calculated using GraphPad Prism 4 (La Jolla, CA, USA). Student's t-test was used to compare values between treated and untreated cells at each time point, generating two-sided P-values. P-values <0.05 were considered significant.
Results
Effects of infertility agents and pregnancy hormones on MCF-10A cells
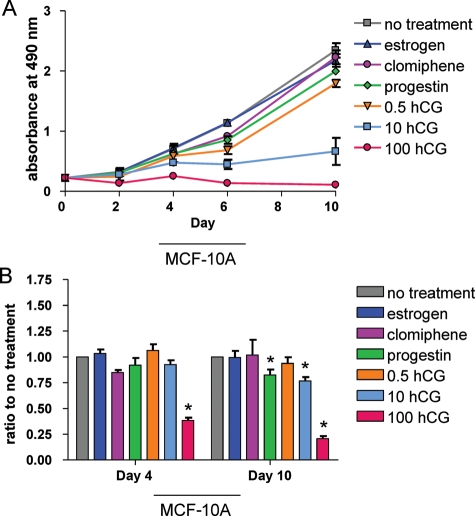
MCF-10A cells are ER- and approximate normal immortalized mammary epithelium. Compared with untreated cells, cell proliferation (Fig. 1A) was not affected by 10 days of treatment with E2 or clomiphene citrate, whereas progesterone significantly decreased cell proliferation as did any treatment with hCG. Overall, there was an inverse dose dependence on cell proliferation as concentration of hCG increased. For 3D cultures of MCF-10A cells, treatment with E2, clomiphene citrate or 0.5 IU/ml hCG did not have any effect on colony size (Fig. 1B); however, cells treated with progesterone, 10 mIU/ml hCG or 100 mIU/ml hCG had a relatively smaller colony size on study day 10 (Fig. 1B).
Figure 1.
Effects of clomiphene citrate and pregnancy hormones on MCF-10A cells. (A) MTS assay performed in 2D culture for MCF-10A cells; absorbance indicates number of viable cells at each time point as a measure of cell proliferation. Significant differences between treated and untreated values on Day 10 were determined by two-sided Student's t-test: estrogen, P= 0.34; clomiphene citrate, P= 0.16; progesterone, P= 0.001; 0.5 mIU/ml hCG, P= 0.001; 10 mIU/ml hCG, P= 0.001; 100 mIU/ml hCG, P< 0.0001. (B) Ratio of total area of colonies for treated versus untreated control cell 3D cultures (a measure of colony growth/size) on Day 4 and Day 10. Day 10 P-values are as follows: estrogen, P= 0.93; clomiphene citrate, P= 0.91; progesterone, P= 0.01; 0.5 mIU/ml hCG, P= 0.31; 10 mIU/ml hCG, P= 0.0001; 100 mIU/ml hCG, P< 0.0001. Asterisks indicate a significant difference between treated and untreated cells at each time point. All values are mean ± SEM for n = 3 experiments.
Effects of infertility agents and pregnancy hormones on MCF-7 cells
MCF-7 cells are well-differentiated, ER-positive breast cancer cells. In this cell line, clomiphene citrate, progesterone or 0.5 and 10 mIU/ml hCG did not affect cell proliferation compared with untreated cells, though a trend toward increased proliferation was observed with 30 nM E2 treatment and trend toward decreased proliferation was seen with low and moderate doses of hCG (Fig. 2A). For MCF-7 cells, escalating doses of E2 resulted in a significant incremental increase in cell proliferation for 20, 30, 60 and 100 nM when compared with untreated cells (Fig. 3A). The only significant decrease in cell proliferation compared with untreated cells occurred in MCF-7 cells treated with 100 mIU/ml hCG. In the 3D culture, a significant increase in colony size on study day 10 was seen in MCF-7 cells treated with E2 and a significant decrease was seen in cells treated with progesterone, 10 or 100 mIU/ml hCG (Fig. 2B). Additionally, for MCF-7 cells, escalating doses (10, 20 and 30 nM) of E2 also resulted in a statistically significant incremental increase in colony size on study day 10 (Fig. 3B).
Figure 2.
Effects of clomiphene citrate and pregnancy hormones on MCF-7 cells. (A) MTS assay performed in 2D culture for MCF-7 cells; absorbance indicates number of viable cells at each time point as a measure of cell proliferation. Significant differences between treated and untreated values on Day 10 were determined by two-sided Student's t-test: estrogen, P= 0.23; clomiphene citrate, P= 0.57; progesterone, P= 0.99; 0.5 mIU/ml hCG, P= 0.34; 10 mIU/ml hCG, P= 0.06; 100 mIU/ml hCG, P= 0.01. (B) Ratio of total area of colonies for treated versus untreated control cell 3D cultures on Day 4 and Day 10. Day 10 P-values are as follows: estrogen, P= 0.04; clomiphene citrate, P= 0.82; progesterone, P= 0.01; 0.5 mIU/ml hCG, P= 0.35; 10 mIU/ml hCG, P< 0.0001; 100 mIU/ml hCG, P< 0.0001. Asterisks indicate a significant difference between treated and untreated cells at each time point. All values are mean ± SEM for n = 3 experiments.
Figure 3.
Effects of escalating doses of estrogen on MCF-7 cells. (A) MTS assay performed in 2D culture for MCF-7 cells; absorbance indicates number of viable cells at each time point as a measure of cell proliferation. Significant differences between treated and untreated values on Day 10 were determined by two-sided Student's t-test: 10 nM, P= 0.21; 20 nM, P= 0.002; 30 nM, P= 0.001; 60 nM, P= 0.0001; 100 nM, P= 0.0002. (B) Ratio of total area of colonies for treated versus untreated control cell 3D cultures on Day 4 and Day 10. Day 10 P-values are as follows: 10 nM, P< 0.0001; 20 nM, P< 0.0001; 30 nM, P< 0.0001. Asterisks indicate a significant difference between treated and untreated cells at each time point. All values are mean ± SEM for n = 3 experiments.
Effects of infertility agents and pregnancy hormones on HCC 1937 cells
HCC 1937 cells are ER-negative and have a BRCA1 mutation. For these cells, there was a significant decrease in cell proliferation observed after treatment with clomiphene citrate or progesterone (Fig. 4A). Increasing doses of hCG also resulted in significantly decreased HCC 1937 cell proliferation: 0.5, 10 and 100 mIU/ml hCG (Fig. 4A). In 3D culture, a significant decrease in colony size on study day 10 was seen in cells treated with clomiphene citrate or 100 mIU/ml hCG (Fig. 4B).
Figure 4.
Effects of clomiphene citrate and pregnancy hormones on HCC-1937 cells. (A) MTS assay performed in 2D culture for HCC-1937 cells; absorbance indicates number of viable cells at each time point as a measure of cell proliferation. Significant differences between treated and untreated values on Day 10 were determined by two-sided Student's t-test: estrogen, P= 0.80; clomiphene citrate, P= 0.003; progesterone, P= 0.008; 0.5 mIU/ml hCG, P= 0.009; 10 mIU/ml hCG, P= 0.002; 100 mIU/ml hCG, P< 0.0001. (B) Ratio of total area for colonies in treated versus untreated control cell 3D cultures on Day 4 and Day 10. Day 10 P-values are as follows: estrogen, P= 0.70; clomiphene citrate, P= 0.03; progesterone, P= 0.06; 0.5 mIU/ml hCG, P= 0.59; 10 mIU/ml hCG, P= 0.41; 100 mIU/ml hCG, P= 0.002. Asterisks indicate a significant difference between treated and untreated cells at each time point. All values are mean ± SEM for n = 3 experiments.
Discussion
Increasingly, women are delaying childbearing to an age that coincides with an increased risk of developing breast cancer. A significant percentage of these women will undergo treatment for infertility, and the impact of hormonally based fertility treatments on the development of breast cancer is a concern. Additionally, female cancer survivors who experience a fertility threat through exposure to chemotherapy and/or radiation may choose to use assisted reproduction technologies to help conceive a child (Jeruss and Woodruff, 2009). For young breast cancer survivors, it is important to consider both the baseline risk of recurrence as well as the potential added risk for recurrence that may be associated with hormonally based infertility treatments. Given these clinical scenarios, we examined the effects of infertility treatments on both normal breast cell and breast cancer cell proliferation in vitro. Overall, our data largely support the minimal direct effect of infertility agents on normal and cancerous breast cell proliferation. Specifically, for MCF-10A cells there was no hyperproliferative effect demonstrated under any treatment condition. For the ER+ MCF-7 cells, there was a dose-dependent increase in cell proliferation and colony growth found when the cells were exposed to E2, though no other direct proliferative effect was shown as a result of the other study treatments. Interestingly, for the ER-, BRCA1-mutated HCC 1937 cells, treatment with clomiphene citrate or progesterone caused a decrease in cell proliferation. Importantly, exposure to hCG had a highly significant direct effect on decreasing cell proliferation, regardless of cell type or hormone receptor status. Thus, given the mitogenic impact of E2 exposure on ER+ cells, these findings highlight the possible protective importance of hCG exposure attained through successful pregnancy after treatment for infertility. Additionally, this work further validates previous studies suggesting a potential protective effect of pregnancy on reducing breast cancer risk (Blakely et al., 2004; Russo et al., 2006).
Clomiphene citrate is a non-steroidal, selective ER modulator with both agonistic and antagonistic activity; it is widely used for ovulation induction in women with anovulatory causes of infertility (Klip et al., 2000). Several studies have pointed toward an association between clomiphene citrate exposure and breast cancer risk, and while a recent meta-analysis did not confirm this finding, this result was hampered by the lack of long-term follow-up for the majority of available studies used to power the analysis (Zreik et al., 2010). Clomiphene citrate binds ER at multiple locations in the body. As an estrogen antagonist at the level of the hypothalamus, clomiphene citrate interrupts ER recycling and thus decreases the number of active ERs, reduces negative feedback by estrogen and increases gonadotrophin release (Klip et al., 2000). As a result, the mean serum estrogen level is increased up to 3-fold above its normal level (Klip et al., 2000). Because estrogen is mitogenic for mammary epithelial cell proliferation and because of the weak estrogenic transcriptional activity induced by clomiphene citrate, it was important to question whether the use of clomiphene citrate may secondarily cause breast cell proliferation that could increase the risk for malignant transformation. In vitro studies have demonstrated that several clomiphene citrate analogs have a direct anti-proliferative activity in MCF-7 and LY2 breast tumor cell lines; in vivo studies showed clomiphene citrate-related inhibition of MCF-7 breast tumor xenografts in nude mice (Baumann et al., 1998). However, other studies have found that low doses of clomiphene citrate exert a weakly proliferative effect on MCF-7 cells (Han et al., 2002). Our data did not indicate a lasting direct proliferative effect of clomiphene citrate treatment in any of the cell types examined, regardless of ER expression and actually resulted in a decrease in cell proliferation and colony size for the HCC 1937 cells. The direct anti-proliferative effects of clomiphene citrate on ER-negative, BRCA 1-mutated HCC 1937 cells are compelling. Clearly the impact of clomiphene citrate on breast cancer risk is complex and requires further investigation in the context of forthcoming in vivo studies, where the indirect effects of clomiphene citrate through estrogen exposure will also be addressed.
hCG is typically injected toward the end of the follicular phase in order to induce ovulation (Filicori et al., 2002). Subsequently, during a successful pregnancy, endogenous hCG levels rise rapidly in the first trimester to support the corpus luteum (Rao et al., 2004). To better understand the impact of hCG exposure, we studied the direct effects of hCG on normal and cancerous breast eptihelial cells at doses that reflected normal serum levels during early pregnancy. We observed that treatment with the highest concentration of hCG (100 mIU/ml) decreased both proliferation and colony growth of normal and ER+ and ER-breast cancer cells. These findings are consistent with previous work demonstrating that MCF-7 breast cancer cells contain high levels of hCG/LH receptors, which are responsive to hCG (Lojun et al., 1997). Indeed, hCG has been shown to prevent tumor formation induced by E2 in non-cancerous MCF-10F cells, while increasing terminal branching and differentiation of the cells (Kocdor et al., 2009). These observations, in addition to the rapid rise in hCG levels—from 50 mIU/ml to over 200 000 mIU/ml—during pregnancy, led to the hypothesis that if a healthy pregnancy could be achieved and breast epithelium could be exposed to hCG in a normal pattern, the deleterious estrogen-related effects associated with ovulation-inducing agents might be mitigated.
The ER is normally expressed at low levels in breast epithelial cells; levels of the receptor increase during the transformation to some premalignant lesions and can increase even further with progression to carcinoma (Shoker et al., 1999). It has been suggested that in the normal breast, a small population of ER-positive cells may act as stem cells that self-renew and differentiate into other cell types and that pathological proliferation of these cells leads to overexpression of ER in the majority of breast cancers (Clarke et al., 2005). The link between estrogen exposure and malignant transformation of mammary cells is partially explained by ultimate activation of c-myc, cyclin D1 and cyclin E proto-oncogenes in response to estrogen and other cytokines and growth factors (Butt et al., 2008). These genes regulate the G1-to-S phase transition during cell-cycle progression, thus estrogens may accelerate cell-cycle progression and mitosis (Butt et al., 2008). Progesterone, which is administered as part of IVF treatment, and is selectively used for the prevention of early miscarriage, has effects on the cell cycle and carcinogenic transformation that are less well defined (Dodd et al., 2008). Progesterone has been shown to increase the invasive potential of breast cancer cells, partly mediated by the up-regulation of tissue factor (Kato et al., 2005). Progesterone has also been implicated in cell-cycle progression and pro-survival signaling of epithelial cells through direct transcription and activation of mitogen-activated protein kinase signaling pathways (Dressing et al., 2009). Furthermore, it has been suggested that progesterone may reactivate occult mammary cancer stem cells, which will lead to cancerous lesions in the combined presence of estrogen (Horwitz and Sartorius, 2008). We hypothesized that the direct effects of fertility treatments would have a minimal effect on breast epithelial cell proliferation but that indirect effects, mediated by increased estrogen and/or progesterone, would stimulate growth in hormone receptor-positive cells. As a result, patients with multiple or prolonged exposures to these agents would be at elevated risk for breast tumorigenesis. In fact, we showed that E2 had no effect on either normal breast epithelial cells (MCF-10A) or ER-breast cancer cells (HCC 1937) but escalating doses of E2 did correlate to increased cell proliferation and colony growth of ER+ breast cancer cells (MCF-7). Conversely, progesterone decreased both proliferation and colony growth of normal cells (MCF-10A), had an inhibitory effect on colony size of ER+ breast cancer (MCF-7) cells and decreased proliferation of ER-breast cancer cells (HCC 1937). Discrepancies in the magnitude between proliferation and colony growth responses may be related to the behavior of each cell line in the 2D and 3D Matrigel culture conditions. Overall, while data on the role of progesterone in breast cancer remains controversial, our data confirm that E2 stimulates growth of ER+ breast cancer cells in a dose-dependent fashion, and lend support to concerns regarding an indirect effect of elevated estrogen, secondary to infertility treatment in women at risk for breast cancer.
An interaction between infertility treatment regimen and cause for infertility has also been shown for patients with non-ovulatory infertility who were exposed to higher doses of clomiphene citrate (Orgeas et al., 2009). For young breast cancer survivors, one study of women aged 35 years and younger suggested a decrease in disease recurrence after a successful pregnancy, when compared with women who did not have a post-treatment pregnancy (Blakely et al., 2004). This decreased recurrence rate could be attributed to the growth inhibitory effects associated with the hCG exposure of pregnancy. Ultimately, however, the association between recurrence risk and pregnancy is difficult to ascertain owing to the lack of prospective trials to examine this question. These data point toward the complex interplay of several factors that contribute to the difficulty of assessing the impact of infertility treatment on risk for breast cancer development.
Although this work is preclinical and limited in scope, the data provide evidence that there are minimal direct effects of infertility treatment on breast cell proliferation. Simultaneously, it may be advisable for patients at higher risk for breast cancer, such as those with a history of breast cancer or atypical ER+ lesions, to limit infertility treatment course and minimize exposure to supraphysiologic estrogen levels. This conclusion has been substantiated by the finding that women treated with ovulation-inducing agents for >12 months before conceiving had a 2-fold increased risk of breast cancer when compared with women treated for <12 months (Calderon-Margalit et al., 2009). Furthermore, the safety of alternative fertility treatment regimens that result in lower estrogen levels are actively being studied in breast cancer patients (Azim et al., 2007, 2008). Lastly, our data further confirm findings that hCG has an anti-proliferative effect on normal and malignant breast cells and supports the potential protective effect of successful pregnancy on offsetting breast cancer risk. Future work will focus on in vivo effects of fertility treatments on breast cell transformation and breast cancer progression.
Authors’ roles
A.C. and J.S.J. contributed toward study design, execution, analysis, manuscript drafting and critical discussion. L.M. and A.H. were involved in study execution, analysis, manuscript drafting and critical discussion. S.Z. participated study design, execution, analysis and critical discussion.
Funding
J.S.J. is a Lynn Sage Scholar supported by the Lynn Sage and Dixon Foundations and NIH UL1 RR024926 and K22 CA138776 research grants.
Acknowledgements
The authors thank Samir Boukaidi, MD, Elizabeth Tarasewicz and Stacey C. Tobin, PhD, for editorial assistance.
References
- ASRM B. Alabama. Age Fertil. 2003:1–20. [Google Scholar]
- Azim AA, Costantini-Ferrando M, Lostritto K, Oktay K. Relative potencies of anastrozole and letrozole to suppress estradiol in breast cancer patients undergoing ovarian stimulation before in vitro fertilization. J Clin Endocrinol Metab. 2007;92:2197–2200. doi: 10.1210/jc.2007-0247. [DOI] [PubMed] [Google Scholar]
- Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- Baumann RJ, Bush TL, Cross-Doersen DE, Cashman EA, Wright PS, Zwolshen JH, Davis GF, Matthews DP, Bender DM, Bitonti AJ. Clomiphene analogs with activity in vitro and in vivo against human breast cancer cells. Biochem Pharmacol. 1998;55:841–851. doi: 10.1016/s0006-2952(97)00574-1. [DOI] [PubMed] [Google Scholar]
- Blakely LJ, Buzdar AU, Lozada JA, Shullaih SA, Hoy E, Smith TL, Hortobagyi GN. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer. 2004;100:465–469. doi: 10.1002/cncr.11929. [DOI] [PubMed] [Google Scholar]
- Breast Cancer Facts & Figures 2009-2010. Atlanta: American Cancer Society; [Google Scholar]
- Butt AJ, Caldon CE, McNeil CM, Swarbrick A, Musgrove EA, Sutherland RL. Cell cycle machinery: links with genesis and treatment of breast cancer. Adv Exp Med Biol. 2008;630:189–205. doi: 10.1007/978-0-387-78818-0_12. [DOI] [PubMed] [Google Scholar]
- Calderon-Margalit R, Friedlander Y, Yanetz R, Kleinhaus K, Perrin MC, Manor O, Harlap S, Paltiel O. Cancer risk after exposure to treatments for ovulation induction. Am J Epidemiol. 2009;169:365–375. doi: 10.1093/aje/kwn318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer. 2005;103:1778–1784. doi: 10.1002/cncr.20979. [DOI] [PubMed] [Google Scholar]
- Dodd JM, Flenady VJ, Cincotta R, Crowther CA. Progesterone for the prevention of preterm birth: a systematic review. Obstet Gynecol. 2008;112:127–134. doi: 10.1097/AOG.0b013e31817d0262. [DOI] [PubMed] [Google Scholar]
- Dressing GE, Hagan CR, Knutson TP, Daniel AR, Lange CA. Progesterone receptors act as sensors for mitogenic protein kinases in breast cancer models. Endocr Relat Cancer. 2009;16:351–361. doi: 10.1677/ERC-08-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng-Wong J, Costantino JP, Swain SM. The impact of systemic therapy following ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;41:200–203. doi: 10.1093/jncimonographs/lgq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filicori M, Cognigni GE, Samara A, Melappioni S, Perri T, Cantelli B, Parmegiani L, Pelusi G, DeAloysio D. The use of LH activity to drive folliculogenesis: exploring uncharted territories in ovulation induction. Hum Reprod Update. 2002;8:543–557. doi: 10.1093/humupd/8.6.543. [DOI] [PubMed] [Google Scholar]
- Gauthier E, Paoletti X, Clavel-Chapelon F. Breast cancer risk associated with being treated for infertility: results from the French E3N cohort study. Hum Reprod. 2004;19:2216–2221. doi: 10.1093/humrep/deh422. [DOI] [PubMed] [Google Scholar]
- Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci Biotechnol Biochem. 2002;66:1479–1487. doi: 10.1271/bbb.66.1479. [DOI] [PubMed] [Google Scholar]
- Han HJ, Heo JS, Lee YJ. Estradiol-17beta stimulates proliferation of mouse embryonic stem cells: involvement of MAPKs and CDKs as well as protooncogenes. Am J Physiol Cell Physiol. 2006;290:C1067–C1075. doi: 10.1152/ajpcell.00222.2005. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Haslam SZ, Osuch J. The breast cancer and the environment research centers: transdisciplinary research on the role of the environment in breast cancer etiology. Environ Health Perspect. 2009;117:1814–1822. doi: 10.1289/ehp.0800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93:3295–3298. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee BC, Ku SY, Suh CS, Kim KC, Lee WD, Kim SH. Use of letrozole versus clomiphene citrate combined with gonadotropins in intrauterine insemination cycles: a pilot study. Fertil Steril. 2006;85:1774–1777. doi: 10.1016/j.fertnstert.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–911. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Pinto M, Carvajal A, Espinoza N, Monso C, Sadarangani A, Villalon M, Brosens JJ, White JO, Richer JK, et al. Progesterone increases tissue factor gene expression, procoagulant activity, and invasion in the breast cancer cell line ZR-75-1. J Clin Endocrinol Metab. 2005;90:1181–1188. doi: 10.1210/jc.2004-0857. [DOI] [PubMed] [Google Scholar]
- Klip H, Burger CW, Kenemans P, van Leeuwen FE. Cancer risk associated with subfertility and ovulation induction: a review. Cancer Causes Control. 2000;11:319–344. doi: 10.1023/a:1008921211309. [DOI] [PubMed] [Google Scholar]
- Kocdor H, Kocdor MA, Russo J, Snider KE, Vanegas JE, Russo IH, Fernandez SV. Human chorionic gonadotropin (hCG) prevents the transformed phenotypes induced by 17 beta-estradiol in human breast epithelial cells. Cell Biol Int. 2009;33:1135–1143. doi: 10.1016/j.cellbi.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa T, Hiroi H, Momoeda M, Inoue S, Taketani Y. Clomiphene citrate elicits estrogen agonistic/antagonistic effects differentially via estrogen receptors alpha and beta. Endocr J. 2010;57:517–521. doi: 10.1507/endocrj.k09e-368. [DOI] [PubMed] [Google Scholar]
- Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, Van Landuyt L, Devroey P. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24:2902–2909. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–1553. doi: 10.1093/humrep/deh304. [DOI] [PubMed] [Google Scholar]
- Lerner-Geva L, Keinan-Boker L, Blumstein T, Boyko V, Olmar L, Mashiach S, Rabinovici J, Potashnik G, Lunenfeld E, Schenker JG, et al. Infertility, ovulation induction treatments and the incidence of breast cancer—a historical prospective cohort of Israeli women. Breast Cancer Res Treat. 2006;100:201–212. doi: 10.1007/s10549-006-9238-4. [DOI] [PubMed] [Google Scholar]
- Lojun S, Bao S, Lei ZM, Rao CV. Presence of functional luteinizing hormone/chorionic gonadotropin (hCG) receptors in human breast cell lines: implications supporting the premise that hCG protects women against breast cancer. Biol Reprod. 1997;57:1202–1210. doi: 10.1095/biolreprod57.5.1202. [DOI] [PubMed] [Google Scholar]
- Mawson A, Lai A, Carroll JS, Sergio CM, Mitchell CJ, Sarcevic B. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Mol Cell Endocrinol. 2005;229:161–173. doi: 10.1016/j.mce.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Musgrove EA, Wakeling AE, Sutherland RL. Points of action of estrogen antagonists and a calmodulin antagonist within the MCF-7 human breast cancer cell cycle. Cancer Res. 1989;49:2398–2404. [PubMed] [Google Scholar]
- Orgeas CC, Sanner K, Hall P, Conner P, Holte J, Nilsson SJ, Sundfeldt K, Persson I, Chia KS, Wedren S, et al. Breast cancer incidence after hormonal infertility treatment in Sweden: a cohort study. Am J Obstet Gynecol. 2009;200:72 e71–77.. doi: 10.1016/j.ajog.2008.08.066. [DOI] [PubMed] [Google Scholar]
- Rao Ch V, Li X, Manna SK, Lei ZM, Aggarwal BB. Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-kappaB and AP-1 activation. J Biol Chem. 2004;279:25503–25510. doi: 10.1074/jbc.M400683200. [DOI] [PubMed] [Google Scholar]
- Russo J, Balogh GA, Heulings R, Mailo DA, Moral R, Russo PA, Sheriff F, Vanegas J, Russo IH. Molecular basis of pregnancy-induced breast cancer protection. Eur J Cancer Prev. 2006;15:306–342. doi: 10.1097/00008469-200608000-00006. [DOI] [PubMed] [Google Scholar]
- Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP. Estrogen receptor-positive proliferating cells in the normal and precancerous breast. Am J Pathol. 1999;155:1811–1815. doi: 10.1016/S0002-9440(10)65498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zreik TG, Mazloom A, Chen Y, Vannucci M, Pinnix CC, Fulton S, Hadziahmetovic M, Asmar N, Munkarah AR, Ayoub CM, et al. Fertility drugs and the risk of breast cancer: a meta-analysis and review. Breast Cancer Res Treat. 2010;124:13–26. doi: 10.1007/s10549-010-1140-4. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]