To the Editors:
Animal1,2 and human3 laboratory studies have suggested a possible etiologic association between in utero nucleoside analogue (NA) exposure and mitochondrial toxicity in HIV-uninfected children born to HIV-infected women. However, epidemiologic studies of children with clinical signs of mitochondrial dysfunction (MD) are limited, and confounding of an etiologic association cannot be disregarded.4,5 We conducted a study to estimate the association of in utero NA exposure and potential confounders and MD in the International Maternal Pediatric Adolescent AIDS Clinical Trials Group protocol P1025, a multisite US cohort of HIV-infected women and their infants.
The study population included HIV-uninfected or indeterminate infants born on or before November 1, 2006. This date restriction was used to allow 6 months of follow-up to evaluate the persistence or resolution of possible signs of MD at the time data were frozen for review. P1025 visits were conducted during routine prenatal and pediatric visits. Infant visits were scheduled at birth, 2, and 6 weeks of age, and at 4, 6, 9, and 12 months of age. Data were primarily abstracted from medical records of routine clinical care, and were supplemented by certain focused assessments including infant physical and neurological examinations, and Bayley neuropsychological testing. To identify possible or established cases of early MD according to the Enquête Perinatale Francçaise (EPF) screening definition, a retrospective review of clinical data recorded on protocol case report forms were performed by clinicians blinded to in utero exposures.4
The Fisher and Wilcoxon exact tests were used to assess differences in characteristics of cases and noncases. The Jonckheere–Terpstra test was used to estimate changes in maternal HIV viral loads by year of birth. A priori potential confounders of the association between in utero NA exposure and MD included timing of maternal prenatal care initiation, year of birth, preterm birth, neonatal zidovudine (ZDV) prophylaxis, maternal HIV viral load, and in utero alcohol, tobacco, cocaine, heroin, and prescription methadone exposure. Maternal HIV viral loads were defined as the highest recorded measurement in each trimester and overall during pregnancy. In utero NA exposure was categorized as exposure to any NA, to individual NAs, and to lamivudine/ZDV (3TC/ZDV); we only considered NAs that an infant with possible MD was exposed to.
As of November 1, 2006, 989 live born infants were delivered by 939 women in protocol P1025; 34 women enrolled for more than one pregnancy and there were 16 sets of twins. Five infants were HIV infected and excluded; 936 HIV uninfected and 48 indeterminate infants comprised the study population. Infants were followed to a median of 43 weeks of age (interquartile range [IQR]: 27, 51) in protocol P1025; 231 infants who subsequently enrolled in another study, Pediatric AIDS Clinical Trials Group protocol 219C, were followed to a median of 105 weeks (IQR: 57, 155).
Of the 984 infants in the study population, 125 were identified through computerized database screening as having a sign of MD. Upon clinical review of the medical histories of the 125 infants, 111 were classified as having clinical signs inconsistent with MD. Four infants were classified as having sudden infant death syndrome, and 7 were classified as having mental developmental delay only. Although these latter 2 groups of infants met the EPF screening criteria, the reviewing clinicians did not judge the infants as having possible MD without additional clinical manifestations given the higher background rate of sudden infant death syndrome and of cognitive delay in high-risk, low socioeconomic populations.6–9 Two infants were possible cases, one with motor and mental developmental delay and a later diagnosis of autism, and one with truncal hypotonia and repeated hospitalization for epilepsy, and one case was established through mitochondrial studies.10 The prevalence of possible MD was 0.30% (95% confidence interval: 0.06% to 0.89%).
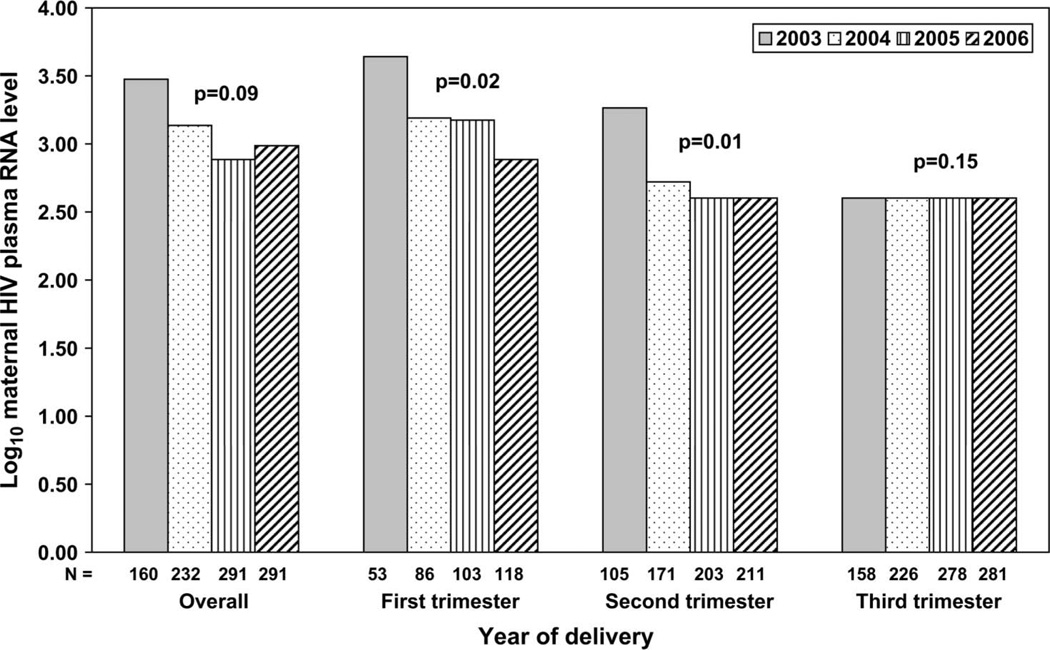
Possible and established cases (N = 3) were significantly more likely to be born in earlier years (all born in 2003) than noncases (N = 979, born in 2002–2006), P = 0.02. Among infants with maternal HIV viral loads recorded, the log median maternal HIV viral load during the first trimester was significantly higher among cases [N = 2, 5.2 (IQR: 4.6, 5.7)] than noncases [N = 355, 3.2 (IQR ≤ 2.6, 4.1), P = 0.01]. The maternal HIV viral load of the established case was 187 copies/mL at the time of the maternal HIV diagnosis early in the second trimester]. No significant difference in the distribution of other potential confounders was detected. Overall, peak maternal HIV viral loads in the first and second trimesters significantly decreased with increasing year of delivery (Fig. 1). No significant difference was observed in peak maternal HIV viral load in the third trimester by year of delivery: half of all women were below the limit of detection of 400 copies/mL in 2003 through 2006. We did not detect any significant differences in the in utero NA exposure of possible cases and noncases (Table 1).
FIGURE 1.
Median maternal log10 plasma HIV viral load by year of delivery of 974 infants in protocol P1025*. *Includes the highest maternal HIV RNA measure available per pregnancy period.
TABLE 1.
In Utero NA Exposure of Cases and Noncases*
| In Utero Exposure | Cases (N = 3) |
Noncases (N = 979) |
P† | Cases Median Days of Exposure (IQR) |
Noncases Median Days of Exposure (IQR) |
P‡ | ||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | |||||
| Any antiretroviral | ||||||||
| Exposed | 3 | 100 | 970 | 99.1 | 1.00 | 161 (29, 197) | 165 (120, 254) | 0.49 |
| Unexposed | 0 | 0 | 9§ | 0.9 | — | — | — | — |
| Any NA | ||||||||
| Exposed | 3 | 100 | 968 | 98.9 | 1.00 | 161 (29, 197) | 165 (119, 253) | 0.51 |
| Unexposed | 0 | 0 | 11 | 1.1 | — | — | — | — |
| 3TC | ||||||||
| Exposed | 2 | 66.7 | 882 | 90.1 | 0.27 | 96.5 (30, 163) | 158 (115, 235) | 0.31 |
| Unexposed | 1 | 33.3 | 97 | 9.9 | — | — | — | — |
| Abacavir | ||||||||
| Exposed | 1 | 33.3 | 260 | 26.6 | 1.00 | 163 | 155 (99, 222) | 0.88 |
| Unexposed | 2 | 66.7 | 719 | 73.4 | — | — | — | — |
| Stavudine | ||||||||
| Exposed | 1 | 33.3 | 69 | 7.0 | 0.20 | 31 | 161 (81, 266) | 0.14 |
| Unexposed | 2 | 66.7 | 910 | 93.0 | — | — | — | — |
| Didanosine | ||||||||
| Exposed | 1 | 33.3 | 75 | 7.7 | 0.21 | 169 | 153 (79, 250) | 0.82 |
| Unexposed | 2 | 66.7 | 904 | 92.3 | — | — | — | — |
| Tenofovir | ||||||||
| Exposed | 2 | 66.7 | 189 | 19.3 | 0.10 | 30 (29, 31) | 142 (61, 245) | 0.06 |
| Unexposed | 1 | 33.3 | 790 | 80.7 | — | — | — | — |
| ZDV | ||||||||
| Exposed | 3 | 100 | 825 | 84.3 | 1.00 | 163 (30, 169) | 152 (109, 199) | 0.61 |
| Unexposed | 0 | 0 | 154 | 15.7 | — | — | — | — |
| ZDV/3TC | ||||||||
| Exposed | 2 | 66.7 | 804 | 82.1 | 0.45 | 96.5 (30, 163) | 152 (109, 200) | 0.37 |
| Unexposed | 1 | 33.3 | 175 | 17.9 | — | — | — | — |
Two noncases with unknown exposure excluded.
P value from Fisher’s exact test.
P value from Wilcoxon exact test.
Eight of 9 infants unexposed to ARV during gestation were exposed to maternal intravenous ZDV during labor and delivery.
Few infants in our study had confirmed MD, although the prevalence of 0.30% was higher than that of 0.01% in the general pediatric population.4 Unadjusted results from the EPF suggested that this increase might be due to combination NA exposure, and findings from the Pediatric AIDS Clinical Trials Group 219/219C study suggested that it might be due to first 3TC or 3TC/ZDV exposure in the third trimester.5 In this latter study, confounding from year of birth was evident, and possible confounding from maternal drug use and HIV viral loads was suggested. Our study also provides evidence of confounding of the association between NA exposure and MD: possible cases were significantly more likely to be born in earlier years than noncases, and median maternal HIV viral load—which is associated with antiretroviral use that has changed over time11—in the first trimester was significantly higher among cases than noncases. Further, first and second trimester maternal HIV viral loads decreased with increasing year of delivery overall. Laboratory studies have shown HIV-uninfected infants have depletions in mtDNA without in utero ARV exposure.12
Our study was limited by a small number of cases, which precluded a multivariate analysis of the association between in utero NA exposure and MD. Further, our findings could substantially change if additional cases with different profiles were identified. Screening for MD in our cohort was difficult due to the high background rate of related clinical conditions, and infants with transient abnormalities were not considered cases. Future studies should use a rigorous case definition and control of potential confounders identified in our study.
ACKNOWLEDGMENT
We thank the mothers and infants for their participation in International Maternal Pediatric Adolescent AIDS Clinical Trials protocol P1025 and the individuals and institutions involved in the conduct of P1025.
Supported by National Institute of Allergy and Infectious Diseases grants U01AI068632 and 1 U01 AI068616 and contract number HHSN2722008000014C; and the International and Domestic Pediatric and Maternal HIV Clinical Trials Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development under contracts number N01-HD-3-3365 and HHSN267200800001C (control # N01-DK-8-0001).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Divi R, Leonard S, Kuo M, et al. Cardiac mitochondrial compromise in 1-yr old Erythrocebus patas monkeys perinatally exposed to nucleoside reverse transcriptase inhibitors. Cardiovasc Toxicol. 2005;5:333–346. doi: 10.1385/ct:5:3:333. [DOI] [PubMed] [Google Scholar]
- 2.Olivero O, Anderson L, Diwan B, et al. Transplacental effects of 3′-azido-2′,3′-dideoxythimidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J Natl Cancer Inst. 1997;89:1602–1608. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 3.Côté HC, Raboud J, Bitnun A, et al. Perinatal exposure to antiretroviral therapy is associated with increased blood mitochondrial DNA levels and decreased mitochondrial gene expression in infants. J Infect Dis. 2008;198:851–859. doi: 10.1086/591253. [DOI] [PubMed] [Google Scholar]
- 4.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brogly S, Ylitalo N, Mofenson L, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 6.Bee H, Barnard K, Eyres S, et al. Prediction of IQ and language skill from perinatal status, child performance, family characteristics, and mother-infant interaction. Child Dev. 1982;53:1134–1156. [PubMed] [Google Scholar]
- 7.Arendt R, Angelopoulos J, Salvator A, et al. Motor development of cocaine-exposed children at age two years. Pediatrics. 1999;103:86–92. doi: 10.1542/peds.103.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhasin TK, Brocksen S, Avchen RN, et al. Prevalence of four developmental disabilities among children aged 8 years: Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1996 and 2000. MMWR Surveill Summ. 2006;55(SS01):1–9. [PubMed] [Google Scholar]
- 9.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–1587. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 10.Cooper ER, DiMauro S, Sullivan M, et al. Biopsy-confirmed mitochondrial dysfunction in an HIV-exposed infant whose mother received combination antiretrovirals during the last 4 weeks of pregnancy. Presented at: 15th International AIDS Conference; July 13, 2004; Bangkok, Thailand. Abstract TUPEB4394. [Google Scholar]
- 11.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 12.Poirier M, Divi R, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]