Abstract
T cell maturation was once thought to occur entirely within the thymus. Now, evidence is mounting that the youngest peripheral T cells in both mice and humans comprise a distinct population from their more mature, yet still naive, counterparts. These cells, termed recent thymic emigrants (RTEs), undergo a process of post-thymic maturation that can be monitored at the levels of cell phenotype and immune function. Understanding this final maturation step in the process of generating useful and safe T cells is of clinical relevance, given that RTEs are over-represented in neonates and in adults recovering from lymphopenia. Post-thymic maturation may function to ensure T cell fitness and self tolerance.
Recent thymic emigrants (RTEs) are T cells that have recently completed thymic development and egress, and they comprise a crucial but understudied population connecting thymic output to the recirculating T cell pool. RTEs are a clinically relevant population because they constitute a considerable proportion of the T cell pool in neonates, infants and young adults1–3. Moreover, they maintain T cell receptor (TCR) repertoire diversity in young and middle-aged adults4 and foster recovery from unintentional or therapeutic lymphoablation5. Thus, understanding RTE biology is key to predicting the responses to immune assault of neonates, ageing individuals and those recovering from lymphoablation. In addition, RTEs may have a role in several disease states, including ulcerative colitis6, chronic myeloid leukaemia7 and autoimmune thyroid disease8. A roadblock to studying the RTE compartment has been the lack of an unambiguous cell surface marker to distinguish RTEs from the remainder of the peripheral T cell pool (TABLE 1). However, newly developed tools are providing ways to specifically and reliably label RTEs3,9, thereby allowing immunologists to analyse for the first time the phenotype and function of RTEs from unmanipulated donors.
Table 1.
Methods for studying RTE biology
| Model system | Method | Advantages | Disadvantages | Refs |
|---|---|---|---|---|
| Intrathymic FITC injection | Labels developing thymocytes; FITC+ cells in the periphery are RTEs | Allows the phenotypic and functional analysis of the youngest RTEs by flow cytometry |
|
55,56 |
| BrdU incorporation | BrdU is taken up by dividing thymocytes; RTEs are BrdUlow | A non-surgical procedure that allows the analysis of RTE phenotype |
|
57 |
| TRECs | Quantification by PCR of excised DNA fragments generated during TCR rearrangement; RTEs are enriched for TRECs | Allows the determination of thymic output and the relative proportion of RTEs in the human T cell population |
|
58,59 |
| Thymic lobe grafts | Congenic thymic lobes are grafted under the kidney capsule | Allows the phenotypic and functional analysis of RTEs by flow cytometry for up to 3 weeks |
|
18,60 |
| Fetal thymus organ culture | Fetal thymic lobes are placed in culture; RTEs are harvested 1–12 days later | Allows the phenotypic and functional analysis of RTEs by flow cytometry |
|
24 |
| RAG2–GFP transgenic mice or RAG1–GFP knock-in mice | GFP signal remains detectable after RAG expression has ceased; GFP+ peripheral T cells are RTEs | Allows the phenotypic and functional analysis of RTEs from unmanipulated mice, and GFP signal intensity correlates with time since thymic egress |
|
9,47,61 |
| Cell surface phenotype | Mouse RTEs are QA2lowCD24hi | Allows for phenotypic and functional analysis of RTEs by flow cytometry |
|
9 |
| Human RTEs are CD31+PTK7+ |
|
3,62 |
BrdU, 5-bromodeoxyuridine; FITC, fluorescein isothiocyanate; GFP, green fluorescent protein; PTK7, protein tyrosine kinase 7; RAG, recombination-activating gene; RTE, recent thymic emigrant; TCR, T cell receptor; TREC, TCR, rearrangement excision circle.
In this Review, we highlight differences between adult mouse RTEs and their more mature, although still naive, T cell counterparts. Owing to space constraints, we do not cover in depth the biology of neonatal mouse RTEs or human RTEs, but these are the subjects of several recent and thorough reviews10–12.
RTE population dynamics
Maintenance of the peripheral T cell pool is achieved through both homeostatic turnover of peripheral T cells and thymic output, which provides new recruits to the pool of recirculating T cells. These new recruits are responsible for maintaining a diverse TCR repertoire, allowing useful T cell responses against newly encountered pathogens and antigens4. Indeed, female mice that have been thymectomized gradually lose reactivity to the male-specific HY antigen13. Moreover, during chronic viral infection, thymic output is necessary for efficient viral clearance14 and helps to preserve antiviral CD8+ T cell populations15. These data provide strong evidence that newly generated T cells play a crucial role in sustaining effective immunity.
Although the absolute number of RTEs in mice peaks at approximately 6 weeks of age, the proportion of the peripheral T cell population comprised of RTEs drops from 100% at 0–3 weeks of age to ~20% in the young adult mouse1. The number of T cells leaving the thymus decreases as the thymus involutes, but RTEs are readily detectable even in aged mice1. Adoptive transfer experiments show that, once in the periphery, RTEs accumulate in the spleen less well than mature naive T cells do16. This imbalance is ameliorated by overexpression of either the interleukin-7 receptor α-chain (IL-7Rα) or the anti-apoptotic molecule B cell lymphoma 2 (BCL-2), suggesting that RTEs exhibit diminished survival compared with mature naive T cells. The transcriptional regulator early growth response protein 1 (EGR1) may also have a role in RTE survival, because EGR1-deficient mice show poor accumulation of RTEs17.
Do RTEs occupy a distinct cellular niche?
Studies of RTEs from hyperthymic (thymus-grafted) mice suggest that, on a population level, RTEs are preferentially incorporated into the peripheral T cell pool at the expense of their mature naive T cell counterparts18. More recently, however, adoptive transfer experiments have demonstrated that RTEs and mature naive T cells occupy largely overlapping niches16. Depleting the RTE compartment by thymectomy only marginally improves the accumulation of RTEs relative to co-transferred mature naive T cells. Thus, in the healthy adult, RTEs and mature naive T cells compete for a limiting niche, and RTEs, whether polyclonal or TCR transgenic, are the disadvantaged competitors16.
RTE homeostasis is context dependent
RTEs can enter a periphery that is either empty, as in neonates or in adults recovering from lymphoablation, or full, as in healthy lymphoreplete adults. Depending on the lymphocyte occupancy of the immediate environment, RTEs can be expected to receive distinct homeostatic signals. Adoptive transfer of RTEs and mature naive T cells into lymphoreplete or lymphopenic mice demonstrates that RTEs preferentially accumulate only in a lymphopenic environment16. Perhaps their higher expression level of CD24 (REF. 9) — a molecule required for optimal T cell homeostatic proliferation in a lymphopenic setting19 — allows RTEs to settle into an empty compartment more readily than their mature counterparts. Although the ability of RTEs to undergo homeostasis is influenced by their environment, whether CD4+ and CD8+ RTEs can sense the occupancy of their separate compartments remains to be tested.
RTEs are a distinct T cell subset
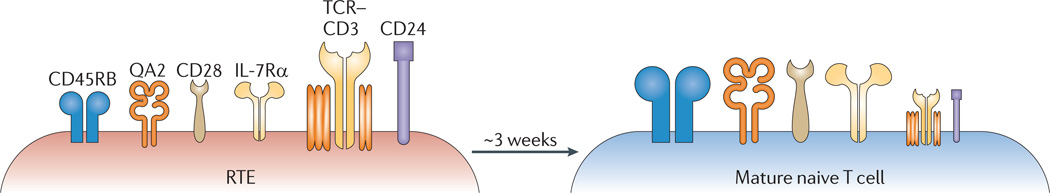
A long-standing question in T cell development is whether intrathymic maturation generates fully functional thymic emigrants20. Early studies focused on similarities between the RTE compartment and mature peripheral T cells, with the intention of elevating the thymus from a mere factory for hormone production to its unique status as the site for T cell maturation21,22. More recent studies, however, have shown that RTEs display a cell surface phenotype distinct from that of mature naive T cells (FIG. 1), with higher expression levels of CD24 and TCR–CD3 and lower expression levels of QA2, CD45RB, IL-7Rα and CD28 (REFS 9,23,24). That functional differences underlie this distinct phenotype has been proven experimentally.
Figure 1. RTEs exhibit a distinct cell surface phenotype.
Over a period of approximately 3 weeks, recent thymic emigrants (RTEs) undergo phenotypic maturation in the lymphoid periphery. This is characterized by the downregulation of surface CD24 and T cell receptor (TCR)–CD3 expression and the upregulation of surface expression of CD45RB, QA2, interleukin-7 receptor α-chain (IL-7Rα) and CD28. Symbol sizes indicate the relative cell surface expression level on each cell type.
CD8+ RTEs are functionally distinct
CD8+ RTEs are unique in their high-level expression of αE integrin and CC-chemokine receptor 9 (CCR9) compared with mature naive T cells, and this enables their more efficient migration on a CC-chemokine ligand 25 (CCL25) gradient and consequent access to the gut epithelium25,26. However, these markers do not clearly distinguish RTEs from bulk peripheral CD8+ T cells. In vitro studies demonstrate that the CD8+ RTE population contains a lower frequency of cytolytic precursors than the mature T cell compartment, suggesting that these young T cells may possess functional defects9. Indeed, CD8+ RTEs produce significantly less tumour necrosis factor (TNF) in response to CD3- and CD28-specific stimulation in vitro27. During bacterial or viral infections in vivo, activated CD8+ T cells derived from RTEs have lower levels of cytokine production than those derived from mature naive T cells and generate fewer IL-7RαhiKLRG1low memory precursor CD8+ T cells28,29. These cytokine production defects are found even 60 days after infection, well after the 3 week RTE maturation period has expired28. Thus, a T cell’s maturation status at the time of antigen encounter may determine cell fate decisions.
CD4+ RTEs are functionally distinct
In vitro studies have provided valuable insights into the functional differences between CD4+ RTEs and mature T cells. Under non-polarizing conditions (no exogenous cytokines), activated CD4+ RTEs exhibit lower proliferation rates, decreased production of IL-2, IL-4 and interferon-γ (IFNγ), and reduced expression of CD25 (the high-affinity IL-2 receptor α-chain) compared with mature CD4+ T cells9,30,31. Defective IL-2 production is exacerbated in CD4+ RTEs from aged mice32. These functional differences also extend to the ability of RTEs to differentiate into various T helper (TH) cell lineages. Compared with their mature T cell counterparts, CD4+ RTEs are defective in their commitment to the TH1 and TH17 cell lineages, in that they show reduced proliferation and cytokine production3,31. Analyses of cytokine production as a function of cell division indicate that lower proliferation rates are not solely responsible for the reduced cytokine production31. It should be emphasized that CD4+ RTE function is not globally defective, and is influenced by the cytokine and cellular environment. Proliferative defects are more pronounced under TH1 cell-polarizing than TH17 cell-polarizing conditions, and TH1 cell-associated cytokine production is restored when RTEs are cultured in the presence of mature naive T cells31. Most strikingly, despite dramatic proliferative and cytokine defects under non-polarizing conditions, CD4+ RTEs surpass their mature T cell counterparts under TH2 cell-polarizing conditions, as they have enhanced TH2 cell-associated cytokine production and effector function both in vitro and in vivo31,33,34.
What drives these functional differences?
That RTEs are a functionally distinct subset of the peripheral T cell pool has been firmly established only recently. Thus, there is a dearth of experimental evidence on the pathways or molecules that orchestrate the distinct functions of RTEs and mature T cells. It is unlikely, however, that any single factor is responsible. Analyses of transcription factor and cytokine receptor expression by cells taken directly ex vivo suggest that CD4+ RTEs are not inherently biased towards the TH2 cell lineage but, rather, are biased away from the TH1 cell lineage31. Indeed, unlike neonatal CD4+ T cells, RTEs from young adults do not produce TH2 cell-associated cytokines directly ex vivo33,34. Reduced steady-state expression by RTEs of TH1 cell-associated molecules, such as T-bet and IL-12R, probably drives the gradual acquisition of a bias towards a TH2 cell phenotype31.
Poor IL-2 secretion by activated CD4+ and CD8+ RTEs and differential secretion of IL-4 by CD4+ RTEs under non-polarizing and TH2 cell-polarizing conditions suggest that these cytokine loci may be subject to distinct epigenetic regulation in RTEs and mature naive T cells9,28,31. Indeed, preliminary DNA methylation studies from our laboratory on CD4+ RTEs and mature naive T cells show that the Il2 and Il4 promoter regions are hypermethylated in unstimulated adult RTEs (D.W.H. and P.J.F., unpublished observations).
It is tempting to speculate that antigen recognition and subsequent signalling through the TCR have a role in the distinct function of RTEs. In response to TCR stimulation, a number of factors — including signal strength and the presence of co-stimulatory molecules and cytokines — influence the balance between a full response and the induction of anergy or non-responsiveness35–37. Although RTEs express higher levels of CD3–TCR than mature T cells, they are characterized by lower levels of cell surface CD28 (REF. 9), suggesting that reduced co-stimulation in certain environments may play a part in RTE functional defects. Studies comparing the efficacy of signalling through the TCR and co-stimulatory pathways in RTEs and mature T cells are ongoing. In the presence of anergizing stimuli, CD4+ T cells initiate a gene expression programme that dampens proliferation and IL-2 secretion38. Anergy-associated genes include those encoding transcription factors — such as EGR2, EGR3 and Ikaros — and E3 ubiquitin ligases, such as gene related to anergy in lymphocytes (GRAIL) and CBLB. Of future interest would be a comparison of the expression of such key regulatory proteins in RTEs and mature T cells.
Are there consequences to these functional differences?
Studies in which RTEs and mature naive T cells were mixed at varying ratios in vitro suggested that RTEs behave differently when they constitute the entire T cell compartment from when they comprise a small fraction of the population31. These data indicate the need to consider RTE function in both lymphopenic and lymphoreplete animals. The main evolutionary advantage for dampened cytokine production combined with efficient homeostatic proliferation in an empty periphery would be displayed in neonates, who are lymphopenic and whose TCR repertoires are cross-reactive and have an average avidity lower than that of adult repertoires39,40.
Driving CD8+ RTEs towards terminal effector differentiation at the expense of generating good memory T cells may benefit the host. In lymphopenic neonates and lymphoablated adults, mobilizing all available T cells, the majority of which are RTEs, may be essential to mount an effective primary immune response, without which a memory immune response would be useless. In the presence of a highly inflammatory secondary challenge, however, the response of CD8+ RTE-derived cells is similar to that of cells derived from mature naive CD8+ T cells, suggesting that the RTE-derived memory compartment can rise to the challenge28.
Similarly, dampened cytokine production by CD4+ RTEs, especially in response to antigen recognition under non-polarizing conditions, may ensure that RTEs do not respond inappropriately to self antigens expressed only in the periphery and not in the thymus41,42. Reducing production of highly pro-inflammatory TH1 and TH17 cell-associated cytokines and developing a gradual TH2 cell bias may be beneficial to the host during RTE maturation, particularly when RTEs constitute a large proportion of the T cell population. This is especially crucial in neonates, whose RTEs enter a lymphopenic environment that can promote autoimmune responses43. However, the TH2 cell response is not completely innocuous, and recent data demonstrate that antigen-specific CD4+ RTEs drive enhanced eosinophilia compared with mature T cells in a mouse model of induced allergic airway inflammation31.
What triggers RTE maturation?
The acquisition by RTEs of a mature phenotype and function is not solely a product of selective survival or proliferation44 but a result of maturation at the individual cell level. What, then, triggers this maturation?
Entry into secondary lymphoid organs is required
One question that arises is whether RTE maturation is on ‘autopilot’, or whether specific signals drive this process. Intrathymic sequestration of RTEs blocks their full maturation. In addition to the need for thymic egress, RTE phenotypic and functional maturation requires access to secondary lymphoid organs, such as the lymph nodes or the spleen44. The only structural requirement elucidated so far is the presence of a full dendritic cell compartment.
The usual suspects are not involved
T cell homeostasis is driven by IL-7 and TCR engagement with self peptide–MHC complexes, two factors that are concentrated in secondary lymphoid organs45. Work from our laboratory has ruled out self peptide–MHC complexes as candidates for driving RTE maturation46. Studies are ongoing to investigate the role of IL-7 in promoting RTE maturation, but preliminary data from our laboratory suggest that RTE maturation is IL-7 independent (E. G. Houston Jr and P.J.F., unpublished observations). Recent work shows that the transcriptional repressor NKAP may regulate post-thymic maturation, because T cells lacking NKAP do not undergo complete maturation47. As it is currently unclear how NKAP exerts its influence on RTEs, these data deepen the mystery behind the identity of the trigger for RTE maturation.
Why do RTEs need further maturation?
Understanding why post-thymic T cell maturation may be necessary requires a careful comparison between the phenotypes and functions of mature single-positive thymocytes and RTEs. Mature CD4+ thymocytes stimulated under non-polarizing conditions produce more IL-4 than similarly treated mature CD4+ peripheral T cells48,49, whereas CD4+ RTEs make less31. Given that CD4+ thymocytes are hypomethylated at the Il4 promoter compared with peripheral T cells49, studies on the methylation status of the Il4 locus in RTEs are clearly needed. The IL-4 expression levels and the unique gene and protein expression pattern in RTEs compared with single-positive thymocytes and mature naive T cells3,9,23,24,50 suggest that RTEs are not simply the cellular midpoint between progenitor thymocytes and mature peripheral T cells. What is gained by requiring that T cells transit through this distinct developmental stage?
Does maturation help to define homeostatic fitness?
If the maturation process is required to test the ability of RTEs to respond to homeostatic signals, then these signals probably do not include IL-7 or MHC molecules17,46. Alternatively, maturation may help to ensure metabolic fitness in RTEs, given that homeostasis, migration and activation all entail metabolic demands. In support of this notion, RTEs express lower levels of CD28 (REF. 9), and CD28 signals are required for the regulation of glucose uptake by T cells51. Maturation may also test the ability of a young cell to transmigrate through endothelial cells, a notion bolstered by the differences in integrin expression between RTEs and mature naive T cells26. Perhaps movement from the cloistered environment of the thymus out into the peripheral lymphoid system requires time and suspension in a somewhat quiescent state.
Does post-thymic maturation influence self tolerance?
Although central tolerance culls a considerable proportion of self-reactive T cells52, the inevitable escape of autoreactive T cells must be adequately dealt with. Recent evidence shows that tissue-restricted antigens are expressed in secondary lymphoid organs and mediate peripheral tolerance by driving the deletion of autoreactive T cells41,42. That RTE maturation requires access to secondary lymphoid organs with an intact dendritic cell compartment offers circumstantial evidence for the influence of post-thymic maturation on self tolerance44. More concrete evidence comes from studies indicating that RTEs do not elicit lethal acute graft-versus-host disease, even when the number injected is eight times the dose of splenic T cells required to mediate acute lethality24. These data suggest that RTEs become tolerized on antigen encounter, but their function may also be kept in check by inhibitory molecule expression50. The preference of RTEs to home to the gut26 — the tissue with the greatest extrathymic antigenic load53 — may promote exposure to gut-associated antigens at a tolerance-prone maturational stage. With chronic exposure to environmental antigens from the gut, there is an apparent loss of TCR specificities in the responding RTE pool54, and this suggests a tolerance mechanism. Collectively, these data support a role for post-thymic maturation in maintaining peripheral T cell tolerance, although further experiments to interrogate this role for RTE maturation are clearly needed.
Conclusions
RTEs are a key T cell population that help to maintain TCR repertoire diversity throughout life and comprise the majority of the T cell population in neonates and in adults recovering from lymphopenia. Recent tools for identifying and analysing RTE phenotype and function have revealed that RTEs represent a distinct T cell subset undergoing a recently discovered phase of post-thymic maturation. Many questions about RTE biology remain, and future studies must elucidate additional factors that drive RTE maturation, as well as those that trigger the distinct phenotype and function of RTEs. The continued expansion in our understanding of post-thymic maturation is likely to help us to implement improvements in neonatal vaccine immunity and to accelerate the recovery of the peripheral T cell compartment following lymphoablation.
Acknowledgements
This work was supported by US National Institutes of Health grants AI064318 (to P.J.F. with a supplement to D.W.H.) and DK091953 (to P.J.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institute of Allergy and Infectious Diseases or the US National Institutes of Health.
Glossary
- Recent thymic emigrants
T cells that have completed thymic development and have recently entered the lymphoid periphery. These young T cells undergo a maturation process that includes changes in function and cell surface phenotype.
- Lymphoreplete
A lymphoid periphery that is relatively full of lymphocytes.
- Lymphopenic
A lymphoid periphery that is depleted of lymphocytes.
- Homeostasis
The controlled turnover of cell populations in which the balance between cell proliferation and cell death maintains constancy in the size of the lymphocyte pool.
- Memory precursor CD8+ T cells
A subset of effector CD8+ T cells, defined as IL-7RαhiKLRG1low that have an enhanced potential to become long-lived memory CD8+ T cells.
- DNA methylation
A repressive epigenetic modification in which methyl groups are present on cytosine bases that are followed by guanine bases (CpGs). CpG-rich areas are typically found in gene promoter and other regulatory regions.
- Anergy
A state of immune unresponsiveness. Anergic B and T cells do not respond fully to their cognate antigens.
- Secondary lymphoid organs
Organs, including the spleen and lymph nodes, that support lymphocyte homeostasis, maturation and activation-induced differentiation.
- Graft-versus-host disease
(GVHD). A disease that results from the immunological attack on target recipient organs or tissues (such as the skin or gut) by donor allogeneic T cells that are transferred along with the allograft (such as bone marrow, liver or gut allografts). GVHD occurs in graft recipients that are unable to eliminate the host-reactive donor T cells owing to immunosuppression, immunological immaturity or tolerance.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc. Natl Acad. Sci. USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vrisekoop N, et al. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc. Natl Acad. Sci. USA. 2008;105:6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haines CJ, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J. Exp. Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yager EJ, et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackall CL, Hakim FT, Gress RE. Restoration of T-cell homeostasis after T-cell depletion. Semin. Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 6.Elgbratt K, Kurlberg G, Hahn-Zohric M, Hornquist EH. Rapid migration of thymic emigrants to the colonic mucosa in ulcerative colitis patients. Clin. Exp. Immunol. 2010;162:325–336. doi: 10.1111/j.1365-2249.2010.04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, et al. Decreased level of recent thymic emigrants in CD4+ and CD8+T cells from CML patients. J. Transl. Med. 2010;8:47. doi: 10.1186/1479-5876-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armengol MP, et al. Influx of recent thymic emigrants into autoimmune thyroid disease glands in humans. Clin. Exp. Immunol. 2008;153:338–350. doi: 10.1111/j.1365-2249.2008.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boursalian TE, Golub J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nature Immunol. 2004;5:418–425. doi: 10.1038/ni1049. This study provided the first definitive evidence that RTEs undergo progressive phenotypic and functional maturation in secondary lymphoid organs.
- 10.Lewis DB, Haines C, Ross D. Protein tyrosine kinase 7: a novel surface marker for human recent thymic emigrants with potential clinical utility. J. Perinatol. 2011;31:S72–S81. doi: 10.1038/jp.2010.187. [DOI] [PubMed] [Google Scholar]
- 11.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PrabhuDas M, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nature Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 13.Di Rosa F, Ramaswamy S, Ridge JP, Matzinger P. On the lifespan of virgin T lymphocytes. J. Immunol. 1999;163:1253–1257. [PubMed] [Google Scholar]
- 14.Miller NE, Bonczyk JR, Nakayama Y, Suresh M. Role of thymic output in regulating CD8 T-cell homeostasis during acute and chronic viral infection. J. Virol. 2005;79:9419–9429. doi: 10.1128/JVI.79.15.9419-9429.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vezys V, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J. Exp. Med. 2006;203:2263–2269. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Houston EG, Jr, Higdon LE, Fink PJ. Recent thymic emigrants are preferentially incorporated only into the depleted T-cell pool. Proc. Natl Acad. Sci. USA. 2011;108:5366–5371. doi: 10.1073/pnas.1015286108. This study established that the incorporation of RTEs into the peripheral T cell pool is dependent on existing lymphocyte occupancy in secondary lymphoid organs.
- 17.Schnell FJ, Kersh GJ. Control of recent thymic emigrant survival by positive selection signals and early growth response gene 1. J. Immunol. 2005;175:2270–2277. doi: 10.4049/jimmunol.175.4.2270. [DOI] [PubMed] [Google Scholar]
- 18.Berzins SP, Boyd RL, Miller JFAP. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J. Exp. Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J. Exp. Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stutman O. Intrathymic and extrathymic T cell maturation. Immunol. Rev. 1978;42:138–184. doi: 10.1111/j.1600-065x.1978.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 21.Scollay R. Thymus cell migration: cells migrating from thymus to peripheral lymphoid organs have a “mature” phenotype. J. Immunol. 1982;128:1566–1570. [PubMed] [Google Scholar]
- 22.Scollay R, Chen WF, Shortman K. The functional capabilities of cells leaving the thymus. J. Immunol. 1984;132:25–30. [PubMed] [Google Scholar]
- 23.Kelly KA, Scollay R. Analysis of recent thymic emigrants with subset- and maturity-related markers. Int. Immunol. 1990;2:419–425. doi: 10.1093/intimm/2.5.419. [DOI] [PubMed] [Google Scholar]
- 24. Lee CK, et al. Thymic emigrants isolated by a new method possess unique phenotypic and functional properties. Blood. 2001;97:1360–1369. doi: 10.1182/blood.v97.5.1360. This study provided evidence that RTEs are functionally distinct from mature naive T cells, and that this characteristic may play a part in peripheral T cell tolerance.
- 25.Staton TL, Johnston B, Butcher EC, Campbell DJ. Murine CD8+ recent thymic emigrants are αE integrin-positive and CC chemokine ligand 25 responsive. J. Immunol. 2004;172:7282–7288. doi: 10.4049/jimmunol.172.12.7282. [DOI] [PubMed] [Google Scholar]
- 26. Staton TL, et al. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nature Immunol. 2006;7:482–488. doi: 10.1038/ni1319. This study provided definitive evidence that, despite their naive phenotype, CD8+ RTEs efficiently traffic to the gut epithelium.
- 27. Priyadharshini B, Welsh RM, Greiner DL, Gerstein RM, Brehm MA. Maturation-dependent licensing of naive T cells for rapid TNF production. PLoS ONE. 2010;5:e15038. doi: 10.1371/journal.pone.0015038. This study provided evidence that post-thymic maturation is crucial for rapid and optimal production of TNF by mature naive T cells.
- 28. Makaroff LE, Hendricks DW, Niec RE, Fink PJ. Postthymic maturation influences the CD8 T cell response to antigen. Proc. Natl Acad. Sci. USA. 2009;106:4799–4804. doi: 10.1073/pnas.0812354106. This study provided the first observation that T cell maturation status at the time of antigen encounter can influence future cell fate decisions.
- 29.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 30.Chang JF, Thomas CA, Kung JT. Induction of high level IL-2 production in CD4+8− T helper lymphocytes requires post-thymic development. J. Immunol. 1991;147:851–859. [PubMed] [Google Scholar]
- 31. Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood. 2011;117:1239–1249. doi: 10.1182/blood-2010-07-299263. This study provided the first observation that post-thymic maturation influences CD4+ T cell lineage commitment and function in RTEs from young adult mice.
- 32.Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4+ recent thymic emigrants from aged mice. J. Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- 33.Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants (RTE) are phenotypically and functionally distinct from adult RTE. Blood. 2009;113:5635–5643. doi: 10.1182/blood-2008-08-173658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J. Immunol. 2007;178:2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu. Rev. Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J. Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 37.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signaling co-stimulates murine T cells and prevents induction of anergy of T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 38.Macian F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 39.Gavin MA, Bevan MJ. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity. 1995;3:793–800. doi: 10.1016/1074-7613(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 40.Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol. Res. 2000;22:177–190. doi: 10.1385/IR:22:2-3:177. [DOI] [PubMed] [Google Scholar]
- 41.Lukacs-Kornek V, Turley SJ. Self-antigen presentation by dendritic cells and lymphoid stroma and its implications for autoimmunity. Curr. Opin. Immunol. 2011;23:138–145. doi: 10.1016/j.coi.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner JM, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 44.Houston EG, Jr, Nechanitzky R, Fink PJ. Cutting edge: contact with secondary lymphoid organs drives postthymic T cell maturation. J. Immunol. 2008;181:5213–5217. doi: 10.4049/jimmunol.181.8.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Houston EG, Jr, Fink PJ. MHC drives TCR repertoire shaping, but not maturation, in recent thymic emigrants. J. Immunol. 2009;183:7244–7249. doi: 10.4049/jimmunol.0902313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hsu FC, Pajerowski AG, Nelson-Holte M, Sundsbak R, Shapiro VS. NKAP is required for T cell maturation and acquisition of functional competency. J. Exp. Med. 2011;208:1291–1304. doi: 10.1084/jem.20101874. This study provided evidence that RTE maturation depends on the transcriptional repressor NKAP.
- 48.Chen YT, Chen FL, Kung JT. Age-associated rapid and Stat6-independent IL-4 production by NK1−CD4+CD8− thymus T lymphocytes. J. Immunol. 1999;163:4747–4753. [PubMed] [Google Scholar]
- 49.Makar KW, et al. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nature Immunol. 2003;4:1183–1190. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 50.Thangavelu G, et al. Programmed death-1 is required for systemic self-tolerance in newly generated T cells during the establishment of immune homeostasis. J. Autoimmun. 2011;36:301–312. doi: 10.1016/j.jaut.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyewski B, Klein L. A central role for central tolerance. Annu. Rev. Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 53.Elson CO, Cong Y, Iqbal N, Weaver CT. Immuno-bacterial homeostasis in the gut: new insights into an old enigma. Semin. Immunol. 2001;13:187–194. doi: 10.1006/smim.2001.0312. [DOI] [PubMed] [Google Scholar]
- 54.Bourgeois C, Hao Z, Rajewsky K, Potocnik AJ, Stockinger B. Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen. Proc. Natl Acad. Sci. USA. 2008;105:8691–8696. doi: 10.1073/pnas.0803732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butcher EC, Weissman IL. Direct fluorescent labeling of cells with fluorescein or rhodamine isothiocyanate. I. Technical aspects. J. Immunol. Methods. 1980;37:97–108. doi: 10.1016/0022-1759(80)90195-7. [DOI] [PubMed] [Google Scholar]
- 56.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 57.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kong FK, Chen CL, Six A, Hockett RD, Cooper CJ. T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc. Natl Acad. Sci. USA. 1999;96:1536–1540. doi: 10.1073/pnas.96.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Douek DC, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 60.Berzins SP, Godfrey DI, Miller JFAP, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc. Natl Acad. Sci. USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCaughtry TM, Wilden MS, Hogquist KA. Thymic emigration revisited. J. Exp. Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]