Abstract
Muramyl dipeptide (MDP) is a synthetic immunoreactive peptide consisting of N-acetyl muramic acid attached to a short amino acid chain of L-Ala-D-isoGln. It was first identified in bacterial cell wall peptidoglycan as an active component in Freund’s complete adjuvant. In the cell, MDP is detected by NOD2, a cytoplasmic receptor belonging to the human innate immune system. NOD2 mutations are frequently observed in patients with Crohn’s disease, an autoimmune disorder, suggesting the significance of the MDP-NOD2 pathway in activating immunity. For this reason, structural modifications of MDP and its derivatives have been extensively studied in an attempt to increase adjuvant activity and boost the immune response effectively for clinical use in the treatment of cancer and other diseases. This review summarizes the synthetic chemistry of MDP and its derivatives and discusses their pharmacological action and stereoselective synthesis.
Keywords: adjuvancy, anti-cancer, anti-inflammatory, MDP, MDP synthesis, medicinal application
1. Introduction
Peptidoglycan is found in the bacterial cell wall as a thin layer in Gram-negative and as a thick layer in Gram-positive bacteria. The presence of peptidoglycan serves not only to preserve cell integrity and to maintain a defined cell shape but also as an important scaffold for anchoring other components such as lipoproteins 1. Peptidoglycan consists of an N-acetylyglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) disaccharide chain and intercalating amino acid chains linked from the lactyl group of one N-acetylmuramic acid to the other. This chain is typically composed of four to five amino acids starting with L-Ala and D-Glu as the first and the second amino acids, respectively. L-Lys or DAP (diaminopimeric acid) often follows as the third amino acid (Fig. 1).
Fig. 1.
MDP is a component of bacterial cell wall peptidoglycan
Smaller products of peptidoglycan containing MurNAc are called muropeptides. The minimum component that remains biologically potent is muramyl dipeptide (MDP), which consists of MurNAc and two amino acids, D-Ala and D-isoGln (or D-Glu). While MDP is recognized by the NOD2 protein immune receptor, muropeptides containing DAP activate the related protein NOD1 2, 3. Synthetic immunoactive peptides that activate NOD1 include FK-156 (D-lactoyl-L-alanyl-gamma-D-glutamyl-(L)-meso-diaminopimelyl-(L)-glycine), which will be described later.
2. Discovery of MDP
In 1974, MDP was discovered to be the minimal structure required for the efficacy of Freund’s Complete Adjuvant (FCA), one of the most potent and widely used adjuvants in animal experimental models to date 2. FCA was developed in 1937 by Freund and colleagues 3. Composed of heat-killed mycobacterial components in an oil emulsion, FCA can strongly elicit both humoral and cellular immune responses. Unfortunately, its strong toxicity hampers the possibility of its use in a clinical setting. A search for smaller yet biologically active components in FCA resulted in the discovery of a tripeptide-monosaccharide by Lederer’s laboratory at the Université Paris-Sud 2. A series of similar peptide-monosaccharides were synthesized and tested in rabbits for adjuvant activity through their ability to elicit immunoglobulin production 4, 5. These peptides included MDP as well as DAP (diaminopimeric acid)-containing peptides, which we know today is a ligand for NOD1 6, 7. MDP was the smallest compound found to elicit adjuvant activity and could thus replace FCA for its ability to induce both humoral and cellular activity. However, it did not induce immunoglobulin production as it is a pure adjuvant lacking the antigens contained in the FCA complex 2, 5, 8.
3. Medical and research applications
3.1. Biological activity
3.1.1. Adjuvant activity of MDP
An adjuvant is an agent that enhances the stimulatory response elicited by compounds having few if any direct effects on their own. MDP and other muropeptides are effective adjuvants and may be used for boosting the potency of drugs and vaccines. They do so by enhancing the expression surface markers necessary for cell adhesion and antigen presentation, thereby increasing phagocytic and anti-microbial activity and facilitating antibody-mediated cytotoxicity 9–12, 13, 14. Moreover, MDP and other muropeptides (tripeptides and disaccharide tri- and tetrapeptides) induce immune responses by increasing IFN-γ and other cytokine production, stimulating the differentiation and proliferation of lymphocytes, a subset of white blood cells that play and integral role in the body’s defense against foreign intruders 15–17. MDP has also been shown in vitro and in vivo to be the minimal structure required for the priming of cells, where pre-exposure to the peptide augments immune responses to a later challenge 18, 19. Analogues where the D-isoglutamine residue is replaced by D-glutamine, D-glutamic acid, or D-isoasparagine have a reduced priming effect, whereas analogues replaced with L-glutamic acid, L-glutamine, or L-isoglutamine are inactive 15, 19. Furthermore, muropeptides express strong synergy with other ligands, where together they elicit a greater immune response than each alone would. For example, MDP has been shown to have a synergistic effect with LPS (lipopolysaccharides), found in the outer membrane of Gram-negative bacteria and recognized by the cell-surface receptor Toll-like receptor-4 (TLR4). This synergy was observed in vitro in human primary cells, including whole blood, peripheral blood mononuclear cells (PBMCs), purified monocytes, and various human monocytic and rodent cell lines, and in vivo in a rat model for anorexia 20–28.
3.1.2. MDP for therapies of cancer and other diseases
MDP and its derivatives have a variety of clinical uses and therapeutic potential. Murabutide (MB), for example, is a synthetic immunomodulator derived from MDP that enhances non-specific resistance to bacterial and viral infections without fever and decreases the lethality of LPS in mice 29–32. It has also been observed to synergize with antiviral and anti-inflammatory cytokines such as IFN-α as well as increase the anti-tumor effects of IFN-α and IL-2 in mouse models 33, 34. Most importantly, MB regulates cytokine production without dramatically inducing proinflammatory mediators 35. Studies have shown that injecting it in combination with IL-2 into Meth-A sarcoma-bearing mice resulted in significant tumor inhibition and complete tumor regression in 70% of the treated mice 33. MB has also been shown to significantly inhibit HIV-1 replication in acutely infected monocyte-derived macrophages and dendritic cells 36. Efforts have already been made to develop other similarly MDP-derived drugs. Macrophages activated by a liposome-encapsulated immunomodulator (MTP-PE, a MDP-derivative) or MDP conjugated by PolyG (a 10-mer polyguanylic acid), have resulted in tumoricidal activity 37, 38. Another reagent, Paclitaxel (Taxol®) conjugated to MDP, has not only antitumor activity but also immunoenhancement effects 39.
3.2. Mechanism of actions
3.2.1. Nod2: MDP receptor and its signaling
MDP and its derivatives are specifically recognized by the pathogen recognition receptor molecule NOD2 (CARD15) that plays a role in both adaptive and innate immune systems by regulating cytokine, chemokine, and antimicrobial peptide production 40–44. NOD2 belongs to the NLR (nucleotide binding domain-leucine rich repeats) protein family and is characterized by three motifs: (1) An N-terminal effector domain containing a caspase recruitment domain (CARD); (2) An NBD (nucleotide binding domain), which has a binding site for ATP and is required for oligomerization; and (3) A leucine rich repeats (LRR) domain 45–48. It is expressed in the cytoplasm of several cell types involved in host defense, including macrophages, dendritic cells, peripheral blood mononuclear cells, and intestinal epithelial cells (especially Paneth cells) 42, 49–52.
Three mutations within NOD2 have been identified in 30 – 40% of Crohn’s Disease patients in North American and European populations 52, 53. Inflammatory bowel diseases (IBDs) such as Crohn’s Disease (CD) are due to genetic, epigenetic, and environmental factors leading to the overproduction of cytokines from a chronically activated immune system 54–58. Mapping of the IBD1 locus has led to the discovery of NOD2 encoded on human chromosome 16q12 as the first gene linked to CD 52, 53. All three CD-associated mutations are restricted to or are in the vicinity of the LRR domain located in the C-terminus of the protein. While the 3020insC frameshift mutation results in a premature stop codon that partially truncates the LRR domain, the R702W and G908R mutants are single nucleotide polymorphisms (SNPs) 59, 60. The precise mechanism underlying how mutations in the NOD2 gene cause CD is not yet fully understood. Proposed hypotheses include an altered immune response by dysregulated Toll-like receptor signaling or a defective function of Paneth cells, which regulate commensal and pathogenic bacteria thorough antimicrobial compounds 42, 61–65.
3.2.2. Signaling cascades of MDP stimulation
Upon detection of MDP, NOD2 binds to the kinase RIP2 via CARD-CARD homophilic interactions, a step required in order for downstream signaling to proceed 66–68. Signaling to RIP2 leads to NF-kB transcriptional activity through the IKK (IkB kinase) complex as well as other cascades involving MAP kinases that result in the production of pro-inflammatory cytokines and chemokines such as interleukin-6 (IL-6), tumor necrosis factor-a (TNF-α), IL-12, and IL-8 (Fig. 2) 42, 69, 70.
Fig. 2.
A simplified schematic of the NOD2 signaling pathway
Several proteins are postulated to regulate NOD2 signaling, including Erbin 58, 71–74. Currently, it has been shown that Erbin serves as a negative regulator of NOD2 by binding to the protein via its CARDs and thus inhibiting its ability to induce NF-kB activity upon MDP stimulation 58, 73. NOD2 and RIP2 are also involved in the regulation of cell death and inflammation through the caspase-1-dependent maturation of IL-1β and IL-18. It has been shown in vitro and in vivo that upon MDP stimulation NOD2 and RIP2 are both required for Caspase-1 activation and IL-1β production 75.
4. Synthetic approach of MDP analogs
4.1. Background of MDP synthesis
The first synthesis of N-acetylmuramic acid was reported by Jeanloz and Flowers in 1963 76. Since then, several reviews have followed 77–81. In general, MDP (N-acetylmuramyl- L-alanyl-D-isoglutamine) can be synthesized by the coupling reaction of 3 subunits; N-acetyl-D-glucosamine, lactic acid or its equivalent and dipepide. In 4.1) and 4.2), a synthetic approach of the N-acetylmuramic acid (MurNAc) moiety is mainly discussed. MDP has (R)-configuration at the lactic acid moiety, while N-acetylisomuramyl-L-alanyl-D-isoglutamine has (S)-configuration. Treatment of the N-acetylisomuramyl-derivative ((S)-isomer) in acetic acid at 80 °C for 1 h caused decomposition to give the intramolecular ester and the dipeptide in about 50% yield through an N to O migration of the amide bond. In comparison, MDP ((R)-isomer) decomposed only 5%, suggesting that the (S)-isomer undergoes migration more easily than its (R)-isomer (Scheme 1). Furthermore, a biological assay for the induction of delayed-type hypersensitivity to N-acetyl-3-(4-arsonophenylazo)-L-tyrosine in guinea pigs revealed that the (S)-isomer dramatically reduced its adjuvant activity 82. It should be noted that this chiral center has an impact on chemical stability as well as biological activity. Therefore, it is important to obtain a diastereochemically pure MurNAc moiety.
Scheme 1.
Stability of N-acetylisomuramyl-L-alanyl-D-isoglutamine
4.2. Synthesis of MurNAc moiety
Two examples of synthetic procedures are described in Schemes 2 and 383–87. To achieve diastereomerically pure MurNAc derivatives, D-Glucosamine and its equivalents are often used as starting materials. Anomeric isomers are separable by chromatography. In the following introduction step of the lactic acid moiety to the masked-form of D-Glucosamine, the desirable (R) isomer 2 in Scheme 2 or 2′ in Scheme 3, is also separable respectively. In general, the syntheses of MurNAc in protected forms for the preparation of MDP analogues involve laborious multi-stage procedures. 2 is an important intermediate for modifying the C4 and C6 positions, whereas 2′ acts to more easily diversify the synthetic analogs.
Scheme 2.
Synthesis of MurNAc precursor (1)
Scheme 3.
Synthesis of MurNAc precursor (2)
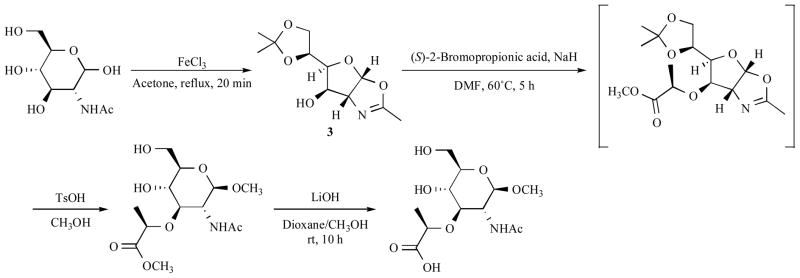
On the other hand, 2-methyl-(1,2-dideoxy-5,6-O-isopropylidene-α-D-glucofurano-[2, 1-d])-2-oxazolin 3 (Scheme 4) is accessible from 2-acetamido-2-deoxy-D-glucose in one step on a large scale 88. The oxazoline moiety already contains the NAc group of MurNAc in a masked form. Therefore, under the treatment of sodium hydride, a lactate side-chain can be introduced at the HO-3 selectively without further protection (Scheme 4) 89, 90.
Scheme 4.
Synthesis of methyl-β-glucoside of N-acetylmuramic acid
Protected MurNAc is coupled with dipeptide ester (L-Ala-D-Glu(OR)-NH2, R = Bzl, tBu, CH3) 85, followed by the de-protection to afford MDP (Scheme 5). A number of synthetic approaches to MDP analogs have been made to improve its pharmacological properties by changing the peptide chain or sugar parts.
Scheme 5.
Coupling of N-acetylmuramic acid with dipeptide
4.3. Peptide modifications and their biological activities
4.3.1. Peptide effect on adjuvant activity
Many peptide variations were introduced on N-acetyl muramic acid and their impact on adjuvant activity was evaluated in two different guinea pig models: Immunization with MDP-supplemented water in mineral oil emulsions containing (1) heterologous protein antigens or azobenzenearsonate-N-acetyl-L-tyrosine (induction of delayed type hyper sensitivity) or (2) encephalitogenic proteins and peptides (induction of experimental allergic encephalomyelitis). A study of the structure-activity relationship suggested that the L-configuration of an amino acid linked to the muramyl part and D-configuration of the glutamic acid residue was important in retaining or increasing biological activity. L-Alanine of N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP-L-D) could be replaced with another L amino acid such as L-serine, while replacement of L-alanine with D-alanine (MDP-D-D) dramatically decreased adjuvant activity. As for the replacement of D-isoglutamine, the functionality of D-glutamic acid is important and the α-amide is not essential. For example, the D-aspartic, D-norleucine as well as the L-isoglutamine analogs (MDP-D-L) were inactive, while the D-glutamic acid α,γ-dimethyl ester analogs showed high adjuvant activity 77, 91, 92.
4.3.2. Lipophilic peptides as prodrugs
Given that it can be modified to eliminate the drawbacks posed by poor macrophage penetration and rapid elimination, MDP has the potential of becoming a useful immunomodulator. From a synthetic standpoint, the peptide moiety is the easiest part of the MDP structure to modify.
One important parameter to consider in improving the pharmacological properties of MDP is lipophilicity. The lipophilic MDP analogs are described in Figure 3. MTP-Cholesterol contains a hydrolyzable ester, while MTP-octadecane and MTP-heptadecafluorooctadecane have non-hydrolyzable ethers. MTP-Cholesterol was active as free MDP in the stimulation of RAW264.7 cells, measured by nitrite production as an indication of NO-synthase activity, a major effector of macrophage-mediated cytostatic activity in rodent systems responsible for antimicrobial, antiparasitic and antitumoral effects. On the other hand, the lipophilic ether derivatives were not active, suggesting that lipophilic MDP analogs need to be hydrolyzed inside the cells to produce a hydrophilic metabolite in order to activate macrophages 85, 93.
Figure 3.
Lipophilic MDP analogs
4.3.3. Methods to derivatize MDP structures: Solid-phase synthesis
Solid-phase is conventionally used for peptide, oligosaccharide, DNA and RNA syntheses. The advantages of using solid phase are easy handling and simple product separation from the reaction mixture, although there are several drawbacks such as difficulty in monitoring the reactions or the requirement of an excess amount of reagents. Solid-phase synthesis would be the best way to make a diverse MDP derivative library with potential application for drug screening. The use of macro crowns with a loading capacity of 5–8 mmol/pin from Chiron Mimotopes for MDP analog synthesis is described in Scheme 694, 95.
Scheme 6.
MDP analog synthesis on solid support (1)
N2-[α-O-benzyl-N-(acetylmuramyl)-L-alanyl-D-isoglutamyl]-N6-trans-(m-nitrocynnamoyl)-L-lysine; MDP-C could be synthesized in a similar way on an MBHA amide resin, and it induced strong cytolytic activity by macrophages on P388 leukemia cells and cytotoxic activity by cytotoxic T lymphocytes on P815 mastocytoma cells (Scheme 7) 96. Furthermore, a hyper acid-sensitive Sieber amide resin was used for the synthesis of MDP and spacer modified MDP 4. The neoglycopeptide polymers 5 could be prepared from 4 and pre-activated poly(N- acryloyloxysuccnimide) (pNAS). 5 increased the production of NF-α compared to monomeric MDP (Scheme 8) 97.
Scheme 7.
MDP analog synthesis on solid support (2)
Scheme 8.
MDP analog synthesis on solid support (3)
4.4. Sugar modifications
4.4.1. Effect of lactic acid moiety (C3)
As described in 4.1), the chirality of MDP at the lactic acid moiety has an impact on stability as well as activity. In contrast, nor-MDP, which does not have a methyl group at the same position, is known to exhibit comparable biological activity and less toxicity 98.
4.4.2. D-Glucosamine with various aglycones (C1)
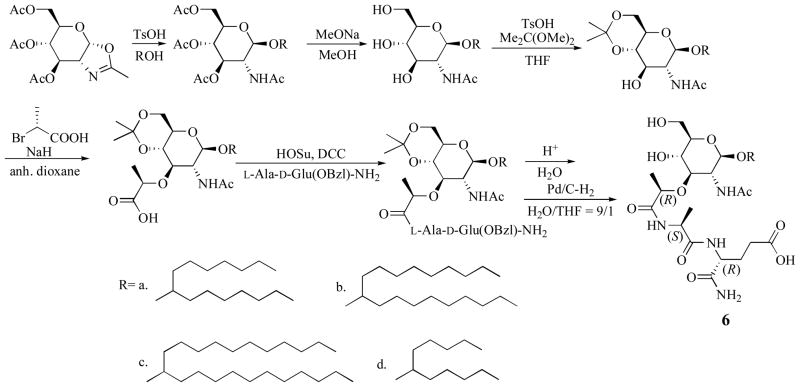
The hydroxy group at the C1 position can be removed 99 and replaced by thiol or substituted by α- or β-benzyl-glycoside. Lipophilic 1-O-acyl and 1-S-acyl groups do change MDP adjuvant activity 100. A recent synthetic approach of O-glycoside is described in Scheme 9101–105. It was reported that as the aglycone carbon number increased (R= 6d<6a<6b<6c), the ability of MDP derivatives to stimulate NK cytotoxic activity also increased.
Scheme 9.
An example of the synthesis of O-glycoside MDP analogs
MDP containing S-glycoside could be synthesized in a similar way (Scheme 10). 1-O-aryl and 1-S-aryl analogs stimulated antibacterial resistance. The substitution on the aromaic ring may change the cytolytic activity on E-562 cells, since non-substituted phenyl thioglycoside did not display significant cytolytic effect toward K-562, whereas substituted analogs did 106.
Scheme 10.
An example of the synthesis of S-glycoside MDP analogs
The methyl β-glycoside of MDP was reported to be more adjuvant-active than the corresponding methyl α-glycoside 107. The size and orientation of the aglycon in MDP also influence its biological activities. For example, loss of activity occurred when a p-aminophenyl group was introduced at the anomeric center (Figure 4). However, when the inert p-aminophenyl β-glycoside was cross-linked with glutaldehyde, several biological activities could be recovered and the cross-linked oligomer was more active than MDP in protecting mice nonspecifically against bacterial challenge 108.
Figure 4.
p-Aminophenyl β-glycoside of MDP
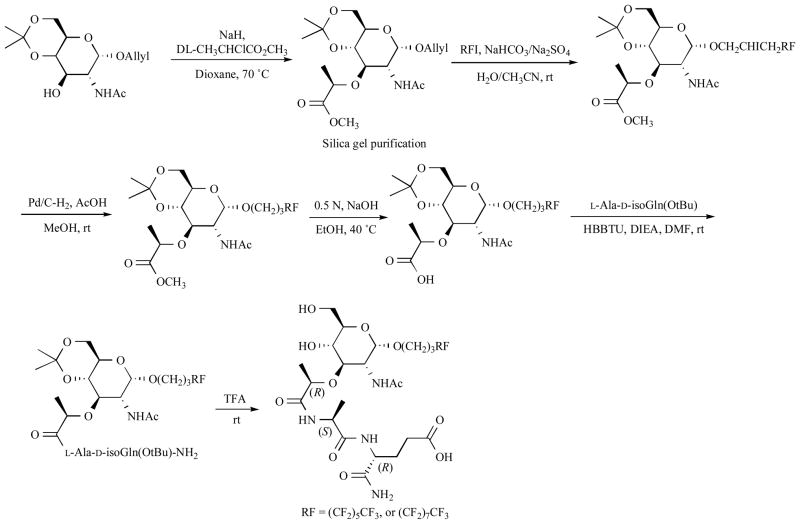
Biological evaluation of MDP analogs indicated that lipophilicity of the molecule caused various important effects on biological activity by increasing adjuvant activity and decreasing pyrogenicity, which is one of the major side effects of MDP 93, 109. As an example, introduction of a perfluoroalkyl group at the anomeric position of the sugar moiety is described (Scheme 11) 110.
Scheme 11.
Perfluoroalkylated MDP
4.4.3. Effect of N-acetyl moiety (C2)
The acetoamide group could be replaced by an OH, NH2 or N-methylacetoamide group, but deamino/deoxy compounds lost the ability to induce delayed-type hypersensitivity to azobenzenearsonate-N-acetyl-L-tyrosine as examined in guinea pigs. In contrast, the introduction of a lipophilic acylamide group increased adjuvant activity 111,112,
4.4.4. 4, 6-O-Substitution of D-glucosamine (C4, C6)
N-Acetyl-β-D-glucosaminyl-(1–4)-N-acetylmuramyl-L-alanyl-D-isoglutamine was more potent than MDP for the induction of delayed-type hypersensitivity and circulating antibodies to ovalbumin in guinea pigs 113, 114.
At C-6, the hydroxyl could be replaced by a thiol, amino, or a non-acylated amino function 112. In contrast to lipophilic 6-S-acyl-MDP derivatives, lipophilic 6-O-acyl-MDP derivatives were potent compounds 115. Acylation at 6-position of the carbohydrate with several mycolic acids, hydroxy fatty acids and quinonylalkanoic acids enhanced anti-HIV-1 and antitumor activity 116, 117. B30-MDP displayed adjuvant activity on the induction of antibody response antigens and vaccines (Scheme 12) 114, 118. Acridine N-substituted w-aminoalkanocarboxylic acid derived MDP (Scheme 13) showed an immunostimulating effect on the cytotoxic activity of the NK cells obtained from the spleen of healthy and Abmelanoma bearing animals 119, 120.
Scheme 12.
Synthesis of long chain fatty acid esters of MDP
Scheme 13.
Acridine N-substituted w-aminoalkanocarboxylic acid derived MDP
4.4.5. Desmuramylpeptides
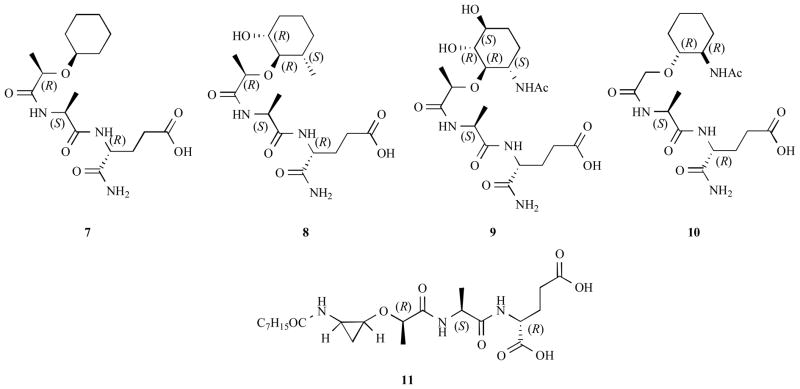
A carbocyclic MDP analog (7) and (8) (Figure 5), which have cyclohexanol moieties instead of N-acetylmuramic acid, were inactive as adjuvants for the induction of delayed-type hypersensitiviy to azobenzenearsonate N-acetyl-L-tyrosine in guinea pigs 112. A carbocyclic MDP analog (9) was synthesized via Ferrier rearrangement as a key step 121, 122 and its derivatives exhibit the common activity of MDP, especially the stimulation of unspecific resistance against bacterial and viral infections, liberation of colony-stimulating factors, induction of IL-1 production in macrophages and antitumor activity (Scheme 14) 123.
Figure 5.
Carbocyclic MDP analogs
Scheme 14.
Synthesis of 9
A carbocyclic nor-MDP analog (10) in which the N-acetylmuramyl moiety was replaced by a trans-2-[[2′-(acetylamino)cyclohexyl]oxy]acetyl group and D-isoglutamine by D-glutamic acid retained the immunostimulating properties of MDP and also displayed antitumor activity. In contrast to MDP, compound 10 was neither pyrogenic nor toxic 124. Moreover, cyclopropanoid analog 11 and its analogs have been reported, although no biological data is available 125.
In addition to NOD2, the related protein NOD1 is also activated by muropeptides. FK-156 activates NOD1 but not NOD2 and is a potent stimulant of antibody production. It is free of pyrogenicity. Its analog FK-565 is a strong anticancer reagent (Figure 6) 126, 127.
Figure 6.
Desmuramylpeptides (1)
N-Acetylmuramic acid can also be replaced by various N-phthaloylated amino acids or phthalimido substituted aminoethoxyacetic acid (Figure 7) 128, 129.
Figure 7.
Desmuramylpeptides (2)
LK423 augments the capacity to produce IL-10 in the spleen cells of cyclophosphoramide treated mice and alleviates dextran sulfate sodium-induced colitis in rodents. Thus, LK423 is a candidate substance for the development of an anti-inflammatory pharmaceutical agent. Furthermore, LK423 stimulated the production of tumor necrosis factor in vitro phorol 12-myristate 13-acetate and ionomycin-stimulated cultures of human peripheral blood mononuclear cells 130–134. Adamantane substituted analogs LK 415 and LK 517 as well as LK 423 are strong regulators of IL-12 synthesis and IFN-γ synthesis. The phosphonate moiety introduced in LK 415 plays a key role for augmented T-cell cytokine production (Schemes 15) 134, 135, 136.
Scheme 15.
Synthesis of LK 423 related analogs
5. Concluding remarks
MDP derivatives have a variety of clinical uses and therapeutic potential. Murabutide, for example, has been used to boost immune responses as a form of cancer therapy. MDP is the smallest compound found to elicit adjuvant activity and its multiple functional groups provide a platform to vary its structure, as each functional group can be synthetically modified to improve chemical as well as biological properties. Most studies thus far have been focused on generating derivatives with a higher level of adjuvant activity, but the development of derivatives that suppress rather than enhance immune responses is also a promising area of study. We recently discovered DFK1012, an anti-inflammatory MDP derivative that acts to suppress proinflammatory cytokine production in macrophages upon stimulation of innate immune receptors such as TLR (Toll-like receptor) or NLR (Nucleotide binding domain, Leucine rich repeats) proteins137. Together with these recent findings, the synthetic approaches outlined in this paper will help us diversify the chemical structure of MDP and study the relationship between its structure and function in an effort to optimize its desirable biological activity.
Acknowledgments
The authors thank Lynn D. Hawkins for helpful discussions; Catherine Leimkuhler Grimes, Andrea Dearth and Kyoung-Hee Lee for critical reading. This work was supported by grants from the NIH (K.S.K. R01DK074738). K.S.K. is a recipient of the Investigator Award from the Cancer Research Institute and the Claudia Adams Barr Award.
Abbreviation
- MDP
muramyl dipeptide
- TLR
Toll-like receptor
- NLR
Nucleotide-binding domain, leucine-rich repeat protein
- LDH
lactose dehydrogenase
- IKK
IκB kinase
- HBBTU
O-benzotriazol-1-yl-N, N, N′, N′-bis(tetramethylene)uronium hexafluorophosphate
- BOP
benzotriazoloxy-tris-(dimethylamino)phosphonium hexafluorophosphate
- DCC
N,N′-dicyclohexylcarbodiimide
- DIEA
N, N-diisopropylethylamine
- DMF
N, N-dimethyl formamide
- NMM
N-methylmorpholine
- HOSu
N-hydroxysuccinimide
- HOBt
1-hydroxybenzotriazole hydrate
- TFA
trifluoroacetic acid
- AcOH
acetic acid
- TfOH
trifluoromethanesulfonic acid
- Boc-Ala-NCA
Boc-alanine N-caboxyanhydride
- Fmoc
9-fluoromethoxycarbonyl
- Boc
tert-butoxycarbonyl
- Dde
1-(4,4-dimethyl-2,6-dioxocyclohexyidene)ethyl
- Pin
macro crown with a loading capacity of 5–8 mmol/pin from Chiron Mimotopes
- Bzl
Bn, benzyl
- Bz
benzoyl
- Ts
p-toluenesulfonyl
- AIBN
2,2′-azoisobutyronitrile
Footnotes
The authors have no conflicting financial interests.
References
- 1.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32(2):149–67. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59(4):1317–25. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 3.Freund J, Casals J, Hismer EP. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc Soc Exp Bioi Med. 1937;37:509. [Google Scholar]
- 4.Kotani S, Watanabe Y, Kinoshita F, Shimono T, Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975;18(2):105–11. [PubMed] [Google Scholar]
- 5.Merser C, Sinay P, Adam A. Total synthesis and adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1975;66(4):1316–22. doi: 10.1016/0006-291x(75)90503-3. [DOI] [PubMed] [Google Scholar]
- 6.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4(7):702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 7.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zathringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300(5625):1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 8.Warren HS, Vogel FR, Chedid LA. Current status of immunological adjuvants. Annu Rev Immunol. 1986;4:369–88. doi: 10.1146/annurev.iy.04.040186.002101. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly T, Zak O. Enhancement of the effectiveness of antimicrobial therapy by muramyl peptide immunomodulators. Clin Infect Dis. 1992;14(5):1100–9. doi: 10.1093/clinids/14.5.1100. [DOI] [PubMed] [Google Scholar]
- 10.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30(Suppl 3):S266–70. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 11.Darcissac EC, Bahr GM, Parant MA, Chedid LA, Riveau GJ. Selective induction of CD11a,b,c/CD18 and CD54 expression at the cell surface of human leukocytes by muramyl peptides. Cell Immunol. 1996;169(2):294–301. doi: 10.1006/cimm.1996.0121. [DOI] [PubMed] [Google Scholar]
- 12.Heinzelmann M, Polk HC, Jr, Chernobelsky A, Stites TP, Gordon LE. Endotoxin and muramyl dipeptide modulate surface receptor expression on human mononuclear cells. Immunopharmacology. 2000;48(2):117–28. doi: 10.1016/s0162-3109(00)00195-8. [DOI] [PubMed] [Google Scholar]
- 13.Dreesman GR, Sanchez Y, Ionescu-Matiu I, Sparrow JT, Six HR, Peterson DL, Hollinger FB, Melnick JL. Antibody to hepatitis B surface antigen after a single inoculation of uncoupled synthetic HBsAg peptides. Nature. 1982;295(5845):158–60. doi: 10.1038/295158a0. [DOI] [PubMed] [Google Scholar]
- 14.Morisaki I, Michalek SM, Harmon CC, Torii M, Hamada S, McGhee JR. Effective immunity to dental caries: enhancement of salivary anti-Streptococcus mutans antibody responses with oral adjuvants. Infect Immun. 1983;40(2):577–91. doi: 10.1128/iai.40.2.577-591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traub S, von Aulock S, Hartung T, Hermann C. MDP and other muropeptides--direct and synergistic effects on the immune system. J Endotoxin Res. 2006;12(2):69–85. doi: 10.1179/096805106X89044. [DOI] [PubMed] [Google Scholar]
- 16.Saiki I, Fidler IJ. Synergistic activation by recombinant mouse interferon-gamma and muramyl dipeptide of tumoricidal properties in mouse macrophages. J Immunol. 1985;135(1):684–8. [PubMed] [Google Scholar]
- 17.Souvannavong V, Brown S, Adam A. Muramyl dipeptide (MDP) synergizes with interleukin 2 and interleukin 4 to stimulate, respectively, the differentiation and proliferation of B cells. Cell Immunol. 1990;126(1):106–16. doi: 10.1016/0008-8749(90)90304-a. [DOI] [PubMed] [Google Scholar]
- 18.Takada H, Yokoyama S, Yang S. Enhancement of endotoxin activity by muramyldipeptide. J Endotoxin Res. 2002;8(5):337–342. doi: 10.1179/096805102125000669. [DOI] [PubMed] [Google Scholar]
- 19.Takada H, Kawabata Y, Kawata S, Kusumoto S. Structural characteristics of peptidoglycan fragments required to prime mice for induction of anaphylactoid reactions by lipopolysaccharides. Infect Immun. 1996;64(2):657–9. doi: 10.1128/iai.64.2.657-659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JE, Jorgensen PF, Ellingsen EA, Almiof M, Thiemermann C, Foster SJ, Aasen AO, Solberg R. Peptidoglycan primes for LPS-induced release of proinflammatory cytokines in whole human blood. Shock. 2001;16(3):178–82. doi: 10.1097/00024382-200116030-00002. [DOI] [PubMed] [Google Scholar]
- 21.Traub S, Kubasch N, Morath S, Kresse M, Hartung T, Schmidt RR, Hermann C. Structural requirements of synthetic muropeptides to synergize with lipopolysaccharide in cytokine induction. J Biol Chem. 2004;279(10):8694–700. doi: 10.1074/jbc.M310556200. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen PF, Wang JE, Almlof M, Thiemermann C, Foster SJ, Solberg R, Aasen AO. Peptidoglycan and lipoteichoic acid modify monocyte phenotype in human whole blood. Clin Diagn Lab Immunol. 2001;8(3):515–21. doi: 10.1128/CDLI.8.3.515-521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchida K, Takemoto Y, Yamagami S, Edney H, Niwa M, Tsuchiya M, Kishimoto T, Shaldon S. Detection of peptidoglycan and endotoxin in dialysate, using silkworm larvae plasma and limulus amebocyte lysate methods. Nephron. 1997;75(4):438–43. doi: 10.1159/000189582. [DOI] [PubMed] [Google Scholar]
- 24.Netea MG, Ferwerda G, de Jong DJ, Jansen T, Jacobs L, Kramer M, Naber TH, Drenth JP, Girardin SE, Kullberg BJ, Adema GJ, Van der Meer JW. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174(10):6518–23. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 25.Vermeulen MW, David JR, Remold HG. Differential mRNA responses in human macrophages activated by interferon-gamma and muramyl dipeptide. J Immunol. 1987;139(1):7–9. [PubMed] [Google Scholar]
- 26.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69(4):2045–53. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daemen T, Veninga A, Roerdink FH, Scherphof GL. In vitro activation of rat liver macrophages to tumoricidal activity by free or liposome-encapsulated muramyl dipeptide. Cancer Res. 1986;46(9):4330–5. [PubMed] [Google Scholar]
- 28.Langhans W, Balkowski G, Savoldelli D. Differential feeding responses to bacterial lipopolysaccharide and muramyl dipeptide. Am J Physiol. 1991;261(3 Pt 2):R659–64. doi: 10.1152/ajpregu.1991.261.3.R659. [DOI] [PubMed] [Google Scholar]
- 29.Chedid LA, Parant MA, Audibert FM, Riveau GJ, Parant FJ, Lederer E, Choay JP, Lefrancier PL. Biological activity of a new synthetic muramyl peptide adjuvant devoid of pyrogenicity. Infect Immun. 1982;35(2):417–24. doi: 10.1128/iai.35.2.417-424.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parant M, Chedid L. Stimulation of non-specific resistance to infections by synthetic immunoregulatory agents. Infection. 1984;12(3):230–4. doi: 10.1007/BF01640913. [DOI] [PubMed] [Google Scholar]
- 31.Parant MA, Pouillart P, Le Contel C, Parant FJ, Chedid LA, Bahr GM. Selective modulation of lipopolysaccharide-induced death and cytokine production by various muramyl peptides. Infect Immun. 1995;63(1):110–5. doi: 10.1128/iai.63.1.110-115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goasduff T, Darcissac EC, Vidal V, Capron A, Bahr GM. The transcriptional response of human macrophages to murabutide reflects a spectrum of biological effects for the synthetic immunomodulator. Clin Exp Immunol. 2002;128(3):474–82. doi: 10.1046/j.1365-2249.2002.01872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bahr GM, Darcissac E, Pouillart PR, Chedid LA. Synergistic effects between recombinant interleukin-2 and the synthetic immunomodulator murabutide: selective enhancement of cytokine release and potentiation of antitumor activity. J Interferon Cytokine Res. 1996;16(2):169–78. doi: 10.1089/jir.1996.16.169. [DOI] [PubMed] [Google Scholar]
- 34.Pouillart PR, Audibert FM, Chedid LA, Lefrancier PL, Bahr GM. Enhancement by muramyl peptides of the protective response of interferon-alpha/beta against encephalomyocarditis virus infection. Int J Immunopharmacol. 1996;18(3):183–92. doi: 10.1016/0192-0561(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 35.Bahr GM, Darcissac E, Bevec D, Dukor P, Chedid L. Immunopharmacological activities and clinical development of muramyl peptides with particular emphasis on murabutide. Int J Immunopharmacol. 1995;17(2):117–31. doi: 10.1016/0192-0561(94)00094-5. [DOI] [PubMed] [Google Scholar]
- 36.Darcissac EC, Truong MJ, Dewulf J, Mouton Y, Capron A, Bahr GM. The synthetic immunomodulator murabutide controls human immunodeficiency virus type 1 replication at multiple levels in macrophages and dendritic cells. J Virol. 2000;74(17):7794–802. doi: 10.1128/jvi.74.17.7794-7802.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killion JJ, Fidler IJ. Therapy of cancer metastasis by tumoricidal activation of tissue macrophages using liposome-encapsulated immunomodulators. Pharmacol Ther. 1998;78(3):141–54. doi: 10.1016/s0163-7258(98)00004-7. [DOI] [PubMed] [Google Scholar]
- 38.Srividya S, Roy RP, Basu SK, Mukhopadhyay A. Selective activation of antitumor activity of macrophages by the delivery of muramyl dipeptide using a novel polynucleotide-based carrier recognized by scavenger receptors. Biochem Biophys Res Commun. 2000;268(3):772–7. doi: 10.1006/bbrc.2000.2216. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Yu J, Xu S, Wang N, Yang H, Yan Z, Cheng G, Liu G. Chemical conjugation of muramyl dipeptide and paclitaxel to explore the combination of immunotherapy and chemotherapy for cancer. Glycoconj J. 2008;25(5):415–25. doi: 10.1007/s10719-007-9095-3. [DOI] [PubMed] [Google Scholar]
- 40.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003 doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 41.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278(8):5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307(5710):731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 43.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23(21):7531–9. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008;83(1):13–30. doi: 10.1189/jlb.0607402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fairbrother WJ, Gordon NC, Humke EW, O’Rourke KM, Starovasnik MA, Yin JP, Dixit VM. The PYRIN domain: a member of the death domain-fold superfamily. Protein Sci. 2001;10(9):1911–8. doi: 10.1110/ps.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inohara N, Nunez G. The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene. 2001;20(44):6473–81. doi: 10.1038/sj.onc.1204787. [DOI] [PubMed] [Google Scholar]
- 47.Inohara N, Nunez G. NODs: intracellular proteins involved in inflammation and apoptosis. Nat Rev Immunol. 2003;3(5):371–82. doi: 10.1038/nri1086. [DOI] [PubMed] [Google Scholar]
- 48.Martinon F, Hofmann K, Tschopp J. The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr Biol. 2001;11(4):R118–20. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 49.Gutierrez O, Pipaon C, Inohara N, Fontalba A, Ogura Y, Prosper F, Nunez G, Fernandez-Luna JL. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277(44):41701–5. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 50.Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125(1):47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 51.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276(7):4812–8. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 52.Ogura Y, Saab L, Chen FF, Benito A, Inohara N, Nunez G. Genetic variation and activity of mouse Nod2, a susceptibility gene for Crohn’s disease. Genomics. 2003;81(4):369–77. doi: 10.1016/s0888-7543(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 53.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 54.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 55.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115(1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 56.Lecine P, Esmiol S, Metais JY, Nicoletti C, Nourry C, McDonald C, Nunez G, Hugot JP, Borg JP, Ollendorff V. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282(20):15197–207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 57.MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51(1):2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 58.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307(5717):1920–5. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad T, Armuzzi A, Bunce M, Mulcahy-Hawes K, Marshall SE, Orchard TR, Crawshaw J, Large O, de Silva A, Cook JT, Barnardo M, Cullen S, Welsh KI, Jewell DP. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology. 2002;122(4):854–66. doi: 10.1053/gast.2002.32413. [DOI] [PubMed] [Google Scholar]
- 60.Kelsall B. Getting to the guts of NOD2. Nat Med. 2005;11(4):383–4. doi: 10.1038/nm0405-383. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 negative regulator of Toll-like receptor 2 is a-mediated T helper type 1 responses. Nat Immunol. 2004;5(8):800–8. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 62.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25(3):473–85. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H, Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A. 2005;102(50):18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn’s disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58(6):882–3. discussion 883–4. [PubMed] [Google Scholar]
- 65.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci U S A. 2009;106(37):15813–8. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416(6877):194–9. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 67.Bertin J, Nir WJ, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GH, Glucksmann MA, DiStefano PS. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kappaB. J Biol Chem. 1999;274(19):12955–8. doi: 10.1074/jbc.274.19.12955. [DOI] [PubMed] [Google Scholar]
- 68.Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, Nunez G. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274(21):14560–7. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110(2):191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 70.Li J, Moran T, Swanson E, Julian C, Harris J, Bonen DK, Hedl M, Nicolae DL, Abraham C, Cho JH. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13(16):1715–25. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 71.Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor-{kappa}B activation in muramyl dipeptide recognition. J Cell Biol. 2005;170(1):21–6. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen CM, Gong Y, Zhang M, Chen JJ. Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J Biol Chem. 2004;279(24):25876–82. doi: 10.1074/jbc.M400682200. [DOI] [PubMed] [Google Scholar]
- 73.Kufer TA, Kremmer E, Banks DJ, Philpott DJ. Role for erbin in bacterial activation of Nod2. Infect Immun. 2006;74(6):3115–24. doi: 10.1128/IAI.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamamoto-Furusho JK, Barnich N, Xavier R, Hisamatsu T, Podolsky DK. Centaurin beta1 down-regulates nucleotide-binding oligomerization domains 1- and 2-dependent NF-kappaB activation. J Biol Chem. 2006;281(47):36060–70. doi: 10.1074/jbc.M602383200. [DOI] [PubMed] [Google Scholar]
- 75.Pan Q, Mathison J, Fearns C, Kravchenko VV, Da Silva Correia J, Hoffman HM, Kobayashi KS, Bertin J, Grant EP, Coyle AJ, Sutterwala FS, Ogura Y, Flavell RA, Ulevitch RJ. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82(1):177–83. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 76.Flowers HMJRW. The synthesis of 2-acetamido-3-O-(D-1-caroboxymethyl)-2-deoxy-a-D-glucose (N-acetylmuramic acid) and of benzyl glycoside derivatives of 2-amino-2-O-(D-)1-carboxymethyl)-2-deoxy-D-glucose (muramic acid) J Org Chem. 1963;28:2983–86. [Google Scholar]
- 77.Dukor PTL, Baschang G. Immunostimulants. Ann Rep Med Chem. 1979;14:146–67. [Google Scholar]
- 78.Lefrancier P, Lederer E. Muramyl-peptides. Pure Appl Chem. 1987;59(3):449–54. [Google Scholar]
- 79.Baschang G. Muramyl peptides and lipopeptides: studies towards immunostimulants. Tetrahedron. 1989;45(20):6331–60. [Google Scholar]
- 80.Dzierzbicka KKAM. Muramyl peptid-synthesis and biological activity. Polish J Chem. 2003;77:373–95. [Google Scholar]
- 81.Szilagyi LPP. Structual aspects of peptides with immunomodulating activity. Mini Rev Med Chem. 2007;7:861–70. doi: 10.2174/138955707781387957. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi SFT, Yukimasa H, Fujino M, Azuma I, Yamamura Y. Synthesis of muramyl dipeptide analogs with enchanced adjuvant activity. Bull Chem Soc Jpn. 1980;53:2570–77. [Google Scholar]
- 83.Gigg RCPM, Warren CD. A synthesis of muramic acid. J Chem Soc. 1965;46:2975–77. doi: 10.1039/jr9650002975. [DOI] [PubMed] [Google Scholar]
- 84.Gross PHRM. Stereochemically pure derivatives of muramic and isomuramic Acids. Liebigs Ann Chem. 1986:37–45. [Google Scholar]
- 85.Kubasch NSRR. Synthesis of muramyl peptides containing meso-diaminopimelic acid. Eur J Org Chem. 2002:2710–26. [Google Scholar]
- 86.Kinzy WSRR. Glycosyl imidates. 25. Muramic acid as glycosyl donor and glycosyl acceptor. Liebigs Ann Chem. 1987:407–15. [Google Scholar]
- 87.Babič APS. An improved total synthesis of UDP-N-acetyl-muramic acid. Tetrahedron Lett. 2007;48:4403–05. [Google Scholar]
- 88.Cai YLC-C, Bundle D. Facile approach to 2-acetoamido-2-deoxy-b-D-pyranoside via a furanosyl oxazoline. Org Lett. 2005;7:4021–24. doi: 10.1021/ol051523k. [DOI] [PubMed] [Google Scholar]
- 89.Merten HBR. A facile two-steps synthesis of N-acetylmuramic acid by selective functionalization of HO-3 of 2-acetoamido-2-deoxyglucose. Carbohydr Res. 1989;191:144–49. [Google Scholar]
- 90.Cai YLC-C, Bundle D. Concise and efficient synthesis of 2-acetoamido-2-deoxy- b -D-hexopyranosides of diverse aminosugars from 2-acetoamido-2-deoxy-b-D-glucose. J Org Chem. 2009;74:580–89. doi: 10.1021/jo801927k. [DOI] [PubMed] [Google Scholar]
- 91.Adam A, Petit JF, Lefrancier P, Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- 92.Adam ALE. Muramyl peptides as immunomodulators. Immunology. 1988;1:205–244. [Google Scholar]
- 93.Zhang MZXJC. Synthesis of F-alkylated MDP analogs. Chin Chem Lett. 1996;7:993–94. [Google Scholar]
- 94.Liu GZS-D, Xia S-Q, Ding Z-K. Solid-phase synthesis of muramyl dipeptide (MDP) derivatives using a multipin method. Bioorg Med Chem. 2000;10:1361–63. doi: 10.1016/s0960-894x(00)00241-9. [DOI] [PubMed] [Google Scholar]
- 95.Zhang S-DLG, Xia S-Q, Wu P, Zhang L. “Meshes-bag gathered-bunch” method for solid-phase synthesis of small molecular diverse compounds. J Comb Chem. 2002;4:131–37. doi: 10.1021/cc010023p. [DOI] [PubMed] [Google Scholar]
- 96.Yang H-ZXS, Liao X-Y, Zhang S-D, Liang Z-L, Liu B-H, Bai J-Y, Jiang C, Ding J, Cheng G-F, Liu G. A novel immunostimulator, N2-[a-O-benzyl-N-(acetylmuramyl)-L-alanyl-D-isoglutamyl]-N6-trans-(m-nitrocynnamoyl)-L-lysine, and its adjuvancy on the hepatitis B surface antigen. J Med Chem. 2005;48:5112–22. doi: 10.1021/jm0493313. [DOI] [PubMed] [Google Scholar]
- 97.Siriwardena AJMR, Wolfert MA, Vandenplas ML, Moore JN, Boons G-J. Synthesis and proinflammatory effect of peptidoglycan-derived neoglycopeptide polymers. J Am Chem Soc. 2001;123:8145–46. doi: 10.1021/ja0104655. [DOI] [PubMed] [Google Scholar]
- 98.Danklmaier JHH. Synthesis of acyclic analog of N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP) Liebigs Ann Chem. 1990:145–50. [Google Scholar]
- 99.Hasegawa ASE, Kigawa K, Kiso M, Azuma I. Studies on immunoadjuvant active compounds. Part XXX. Synthesis and immunoadjuvant activity of N-[2-O-(1,5-anhydro-2,3-dideoxy-2-octadecanoylamino-D-lactoyl]-L-alanyl-D-isoglutamine and its derivatives. Agric Biol Chem. 1986;50:2133–35. [Google Scholar]
- 100.Hasegawa AHY, Kiso M, Okumura H, Azuma I. Synthesis and biological activities of N-acetyl-1-thiomuramoyl-L-alanyl-D-isoglutamine and some of its lipophilic derivatives. Carbohydr Res. 1983;123:183–99. doi: 10.1016/0008-6215(83)88476-6. [DOI] [PubMed] [Google Scholar]
- 101.Zemlyakov AEKyVO, Tsikalov VV, Aksenova EA, Chirva VYa. Synthesis of hydrophobic derivatives of muramyldipeptides. Chem Nat Comp. 1997;31:576–82. [Google Scholar]
- 102.Zemlyakov AETVN, Tsikalov VV, Chirva VYa, Mulik EL, Kalyuzhin OV. Synthesis and protective activity of b-glycosides of N-acetylmuramyl-L-alanyl-D-isoglutamine with alkyl-alicyclic and aryl-aliphatic aglycons. Russ J Bioorg Chem. 2005;31:576–82. [PubMed] [Google Scholar]
- 103.Zemlyakov AETVN, Tsikalov VV, Chirva VYa, Mulik EL, Kalyuzhin OV. Synthesis and protective anti-infective action of aromatic lipophilic glycosides of N-acetylmuramyl-L-alanyl-D-isogluyamine. Russ J Bioorg Chem. 2006;32:382–88. [PubMed] [Google Scholar]
- 104.Zemlyakov AETVN, Tsikalov VV, Chirva VYa, Mulik EL, Kuzovlev FN, Kalyuzhin OV, Kiselevsky MV. Dialkylmethyl b-glcosides of N-acetylmuramyl-L-alanyl-D-isoglutamie: Synthesis and protective antiinfection and cyotoxic activities. Russ J Bioorg Chem. 2008;34:103–9. [PubMed] [Google Scholar]
- 105.Kalyuzhin OVZAE, Kalina NG, Mulik EL, Kuzovlev FN, Makarova OV. Biological acivity of anomeric pairs of lipophilic glycosides of N-acetylmuramyl-L-alanyl-D-isoglutamie. Bull Exp Biol Med. 2008;145(5):623–25. doi: 10.1007/s10517-008-0157-8. [DOI] [PubMed] [Google Scholar]
- 106.Zemlyakov AETVN, Azizova LR, Chirva VYa, Mulik EL, Shkalev MV, Kalyuzhin OV, Kiselevsky MV. Synthesis and biological activity of aryl S-b-glycosides of 1-thio-N-acetylmuramyl-L-alanyl-D-isoglutamie. Russ J Bioorg Chem. 2008;34:223–29. [PubMed] [Google Scholar]
- 107.Nagai YAK, Kotani S, Watanabe Y, Shimono T, Shiba T, Kusumoto S. Structural specificity of synthetic peptide adjuvant for induction of experimental allergic encephalomyelitis. Cell Immunol. 1989;35:168–72. doi: 10.1016/0008-8749(78)90136-3. [DOI] [PubMed] [Google Scholar]
- 108.Parant MDC, Audibert F, Parant F, Chedid L, Sache E, Lefrancier P, Choay J, Lederer E. In vivo and in vitro stimulation of nonspecific immunity by the b-D-p-aminophenyl glycoside of N-acetylmuramyl-L-alanyl-D-isoglitamine and an oligomer prepared by cross-linking with glutaraldehyde. J Infect Dis. 1978;138:378–86. doi: 10.1093/infdis/138.3.378. [DOI] [PubMed] [Google Scholar]
- 109.Kamisango KSI, Tanio Y, Kobayashi S, Fukuda T, Sekikawa I, Azuma I, Yamamura Y. Chemical synthesis and adjuvant activity of N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP) analogs. Chem Pharm Bull. 1981;29:1644–54. doi: 10.1248/cpb.29.1644. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z-FXJ-C. Synthesis of fluoro-containing muramyl dipeptide analogs. Tetrahedron. 1998;54:12597–608. [Google Scholar]
- 111.Hasegawa AOH, Nishibori K, Kaneda Y, Kiso M, Azuma I. Studies on immunoadjuvant active compounds. Part XIV. The chemical modification of the C-2 substituent in the sugar moiety of N-acetylmuramoyl-L-alanyl-D-isoglutamine, and the immunoadjuvamt activities. Carbohydr Res. 1981;97(2):337–45. [Google Scholar]
- 112.Azuma IOH, Saiki I, Kiso M, Hasegawa A, Tanio Y, Yamamura Y. Adjuvant activity of carbohydrate analogs of N-acetylmuramyl-L-alanyl-D-isogutamine on the induction of delayed-type hypersensitivity to azobenzeneasonate-N-acetyl-L-tyrosine in guinea pigs. Infect Immun. 1981;33:834–39. doi: 10.1128/iai.33.3.834-839.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasegawa AKY, Amano M, Kiso M, Azuma I. Studies on immunoadjuvant active compounds. Part II. A facile synthesis of N-acetylmuramyl-L-alanyl-D-isoglutamine and its carbohydrate analogs, and their immunoadjuvant activities. Agric Biol Chem. 1978;42:2187–89. [Google Scholar]
- 114.Kusumoto SIM, Shiba T. Synthesis of long chain fatty acid esters of N-acetylmuramyl-L-alanyl-D-isoglutamine in relation to antitumor activity. Tetrahedron Lett. 1978;49:4899–902. [Google Scholar]
- 115.Hasegawa ASE, Hioki Y, Kiso M, Azuma I. Synthesis and immunoadjuvant activity of N-[2-O-(2-acetamido-2,3,di-deoxy-6-thio-D-glucopyranose-3-yl)-D-lactoyl]-L-alanyl-D-isoglutamine derivatives. Carbohydr Res. 1984;129:271–77. doi: 10.1016/0008-6215(84)85318-5. [DOI] [PubMed] [Google Scholar]
- 116.Fukuda TKS, Yukimasa H, Imada I, Fujino M, Azuma I, Yamamura Y. Synthesis of immunologically active muramyl dipeptide derivatives containing a quinonyl moiety via aminoacyl intermediates. Chem Pharm Bull. 1981;29:2215–21. doi: 10.1248/cpb.29.2215. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi SFT, Yukimasa F, Fujino M, Azuma I, Yamamura Y. Synthesis, and the adjuvant and tumor-suppressive activities of quinonyl muramyl dipeptides. Bull Chem Soc Jpn. 1984;57:3182–96. [Google Scholar]
- 118.Shiba TOS, Kusumoto S. Synthesis of 6-O-mycoloyl- N-acetylmuramyl-L-alanyl-D-isoglutamine with antitumor activity. Bull Chem Soc Jpn. 1978;51:3307–11. [Google Scholar]
- 119.Dzierzbicka KKAM, Wysocka-Skrzela B, Kolodziejczyk AS. Synthesis of muramyl dipeptide conjugated with acridine derivatives, showing anti-HIV-1 and anticancer activity. Pol J Chem. 1994;68:37–45. [Google Scholar]
- 120.Dzierzbicka KKAM. Synthesis and antitumor activity of conjugates of muramyldipeptide, normuramyldipeptide, and desmuramylpeptides with acridine/acridone derivatives. J Med Chem. 2001;44:3606–15. doi: 10.1021/jm001115g. [DOI] [PubMed] [Google Scholar]
- 121.Ferrier R. Unsaturated carbohydrates. Part 21. A carbocyclic rong closure of a hex-5-enopyrasnoside derivative. J Chem Soc, Perkin Trans. 1979;1:1455–58. [Google Scholar]
- 122.Barton DHRCJ, Dalco P, Géro SD, Quiclet-Sire B, Stütz P. Synthesis of biologically active carbocyclic analogs of N-acetylmuramyl-}-L-alanyl-D-isoglutamine (MDP) J Org Chem. 1989;54:3764–66. [Google Scholar]
- 123.Gero SSB, Dalko P, Philippe M. Dipeptide und deren Herstellung. German Pat Appl, DE. 1986 [Google Scholar]
- 124.Kikelj D, Pecar S, Kotnik V, Stalc A, Wraber-Herzog B, Simcic S, Ihan A, Klamfer L, Povsic L, Grahek R, Suhadolc E, Hocevar M, Honig H, Rogi-Kohlenprath R. N-[trans-2-[[2′-(acetylamino)cyclohexyl]oxy]acetyl]-L-alanyl-D-glutamic acid: a novel immunologically active carbocyclic muramyl dipeptide analogue. J Med Chem. 1998;41(4):530–9. doi: 10.1021/jm970509d. [DOI] [PubMed] [Google Scholar]
- 125.Cduk RGG. Synthesis of cyclopropanoid analogues of N-acyl-muramyldipeptides as potential immunostimulants. Tetrahedron. 2004;60:2201–11. [Google Scholar]
- 126.Hemmi KTH, Okada S, Nakaguchi O, Kitamura Y, Hashimoto M. Total synthesis of FK-156 isolated from a Streptomyces as an immnostimulating peptide: application of a novel copper chelate amino protection. J Am Chem Soc. 1981;103:7026–28. [Google Scholar]
- 127.Izumi SNK, Gotoh T, Hashimoto S, Kino T, Okuhara M. Antitumor effects of novel immunoactive peptides, FK-156 and its synthetic derivatives. J Antibiotics. 1983;36:566–74. doi: 10.7164/antibiotics.36.566. [DOI] [PubMed] [Google Scholar]
- 128.Urleb UGS, Prelog D. Synthesis and activity of phosphono desmuramyldipeptide analogs. Lett Pept Sci. 1995;2:193–97. [Google Scholar]
- 129.Gobec SUU. Synthesis of new phosphonoamidate and phosphinamide desmuramyldipeptide analogs. Lett Pept Sci. 1998;5:109–14. [Google Scholar]
- 130.Ochi CNN, Norisada N, Moriguchi M, Stalc A, Urleb U, Murooka S. Interleukin-10 inducing activity of LK 423, a Phthalimido-desmuramyldipeptide compound. Arzneim Forsch. 1999;49:72–79. doi: 10.1055/s-0031-1300363. [DOI] [PubMed] [Google Scholar]
- 131.Moriguchi MUK, Norisada N, Ochi C, Stalc A, Urleb U, Murooka S. Therapeutic Effects of LK 423, a Phthalimido-desmuramyldipeptide compound, on dextran sulfate sodium induced colitis in rodents through restoring their interleukin-10 producing capacity. Arzneim Forsch. 1999;49:184–92. doi: 10.1055/s-0031-1300400. [DOI] [PubMed] [Google Scholar]
- 132.Simcic SWB, Sollner M, Urleb U, Gobec S. Modulation of tumor necrosis factor production with desmuramyldipeptide analogs. Pflug Arch Eur J Phy. 2000;440:R64–66. [PubMed] [Google Scholar]
- 133.Gobec SUU. Synthesis of Phosphonophthalimido-desmuramyldipeptide analogs. Phosphorous, Sulfur and Silicon Relat Elem. 2000;156:125–33. [Google Scholar]
- 134.Gobec S, Urleb U, Auger G, Blanot D. Synthesis and biochemical evaluation of some novel N-acyl phosphono- and phosphinoalanine derivatives as potential inhibitors of the D-glutamic acid-adding enzyme. Pharmazie. 2001;56(4):295–7. [PubMed] [Google Scholar]
- 135.Urleb UGS. Synthesis of new adamantine substituted acyclic MDP analogs related to LK415. Acta Pharm. 2000;50:173–84. [Google Scholar]
- 136.Gobec S, Urleb U. Synthesis of new lipophilic phosphonate and phosphonamidate analogues of N-acetyl muramyl-L-alanul-D-isoglutamine related to LK423. Molecules. 2002;7:394–404. [Google Scholar]
- 137.Lee K-H, Liu Y-J, Biswas A, Ogawa C, Kobayashi KS. A novel aminosaccharide compound blocks immune responses by Toll-like receptors and nucleotide-binding domain, leucine-rich repeat proteins. J Biol Chem. 2011;286(7):5727–35. doi: 10.1074/jbc.M110.108001. [DOI] [PMC free article] [PubMed] [Google Scholar]