Abstract
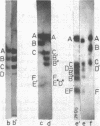
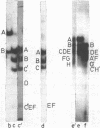
Double-stranded RNA (dsRNA) sequences were isolated from either total RNA or cytoplasmic poly(A)+RNA of D. melanogaster culture cells using a method described previously /1,2/. Virtually all dsRNA was found to be of high molecular weight (> 200 base pairs) and unable to snap back after RNA melting. Thus, it corresponds to one of dsRNA classes found in mouse cells, namely, to dsRNA-A /3/. Three different cloned DNA fragments of D melanogaster which hybridized to melted dsRNa were selected among 100 randomly taken. All of them efficiently bound poly(A)+RNA and high percentage of total cellular DNA. According to these and other properties, they were assigned to a group of mobile dispersed genes of D. melanogaster. DsRNA hybridizes to all subfragments of the two mobile dispersed genes tested (mdg 1 and mdg 3). Thus, complete transcripts of mobile dispersed genes are present in dsRNA. In total RNA, transcripts from one strand are more abundant than those from another one. DsRNA is heavily enriched in the transcripts from mobile dispersed genes as compared to total or poly(A)+RNA of the cytoplasm. It has been suggested that dsRNA in D. melanogaster is formed as the result of symmetric transcription of mobile dispersed genes. At least in the cytoplasmic fraction, two complementary strands are separated in vivo and may combine during the isolation of RNA.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananiev E. V., Gvozdev V. A., Ilyin Yu V., Tchurikov N. A., Georgiev G. P. Reiterated genes with varying location in intercalary heterochromatin regions of Drosophila melanogaster polytene chromosomes. Chromosoma. 1978 Dec 21;70(1):1–17. doi: 10.1007/BF00292211. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Secondary structure of heterogeneous nuclear RNA: two classes of double-stranded RNA in native ribonucleoprotein. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3705–3709. doi: 10.1073/pnas.74.9.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Panasenko S. M., Lehman I. R., Davis R. W. In vitro construction of bacteriophage lambda carrying segments of the Escherichia coli chromosome: selection of hybrids containing the gene for DNA ligase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3416–3420. doi: 10.1073/pnas.72.9.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Rubin G. M., Young M. W., Hogness D. S. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. doi: 10.1101/sqb.1978.042.01.106. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ilyin Y. V., Ryskov A. P., Tchurikov N. A., Yenikolopov G. N., Gvozdev V. A., Ananiev E. V. Isolation of eukaryotic DNA fragments containing structural genes and the adjacent sequences. Science. 1977 Jan 28;195(4276):394–397. doi: 10.1126/science.401545. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Varshavsky A. J., Ryskov A. P., Church R. B. On the structural organization of the transcriptional unit in animal chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:869–884. doi: 10.1101/sqb.1974.038.01.089. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Tchurikov N. A., Ananiev E. V., Ryskov A. P., Yenikolopov G. N., Limborska S. A., Maleeva N. E., Gvozdev V. A., Georgiev G. P. Studies on the DNA fragments of mammals and Drosophila containing structural genes and adjacent sequences. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):959–969. doi: 10.1101/sqb.1978.042.01.097. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Tchurikov N. A., Georgiev Selection and some properties of recombinant clones of lambda bacteriophage containing genes of Drosophila melanogaster. Nucleic Acids Res. 1976 Aug;3(8):2115–2127. doi: 10.1093/nar/3.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R. Inverted repeated DNA from Chinese hamster ovary cells studied with cloned DNA fragments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2679–2683. doi: 10.1073/pnas.75.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R. Specific nucleotide sequences in HeLa cell inverted repeated DNA: enrichment for sequences found in double-stranded regions of heterogeneous nuclear RNA. J Mol Biol. 1977 Oct 5;115(4):591–601. doi: 10.1016/0022-2836(77)90104-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov D. A., Grigoryan A. A., Ryskov A. P., Georgiev G. P. Long double-stranded sequences (dsRNA-B) of nuclear pre-mRNA consist of a few highly abundant classes of sequences: evidence from DNA cloning experiments. Nucleic Acids Res. 1979 Feb;6(2):697–713. doi: 10.1093/nar/6.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov D. A., Ryskov A. P., Georgiev G. P. The structural organization of nuclear pre-mRNA. II. Very long double-stranded structures in nuclear pre-mRNA. Biochim Biophys Acta. 1977 Apr 4;475(3):461–475. doi: 10.1016/0005-2787(77)90062-4. [DOI] [PubMed] [Google Scholar]
- Kramerov D. A., Ryskov A. P. Posledovatel'nosti mPNK, komplementarnye dvuspiral'nym uchastkam pro-nRNK. Gibridizatsiia s ispol'zovaniem merkurirovaniia RNK i khromatografiia na SH-sefaroze. Mol Biol (Mosk) 1979 May-Jun;13(3):601–612. [PubMed] [Google Scholar]
- Kramerov D. A., Ryskov A. P. Struktura iadernoi pre-mRNK. X. Novyi tip dvuspiral'nykh struktur v pre-mRNK. Mol Biol (Mosk) 1978 Mar-Apr;12(2):343–355. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár J., Besson J., Samarina O. P. Secondary structure of pre-mRNA in nuclear ribonucleoprotein particles. Mol Biol Rep. 1975 Mar;2(1):11–17. doi: 10.1007/BF00357292. [DOI] [PubMed] [Google Scholar]
- Naora H., Whitelam J. M. Presence of sequences hybridisable to dsRNA in cytoplasmic mRNA molecules. Nature. 1975 Aug 28;256(5520):756–759. doi: 10.1038/256756a0. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Brorein W. J., Jr, Dunsmuir P., Rubin G. M. Transposition of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):415–427. doi: 10.1016/0092-8674(79)90168-5. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Farashyan V. R., Georgiev G. P. Ribonuclease-stable base sequences specific exclusively for giant dRNA. Biochim Biophys Acta. 1972 Apr 12;262(4):568–572. doi: 10.1016/0005-2787(72)90502-3. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Saunders G. F., Farashyan V. R., Georgiev G. P. Double-helical regions in nuclear precursor of mRNA (pre-mRNA). Biochim Biophys Acta. 1973 Jun 8;312(1):152–164. doi: 10.1016/0005-2787(73)90060-9. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Tchurikov N. A., Ilyin Y. V., Ananiev E. V., Georgiev G. P. The properties of gene Dm 225, a representative of dispersed repetitive genes in Drosophila melanogaster. Nucleic Acids Res. 1978 Jun;5(6):2169–2187. doi: 10.1093/nar/5.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]