Abstract
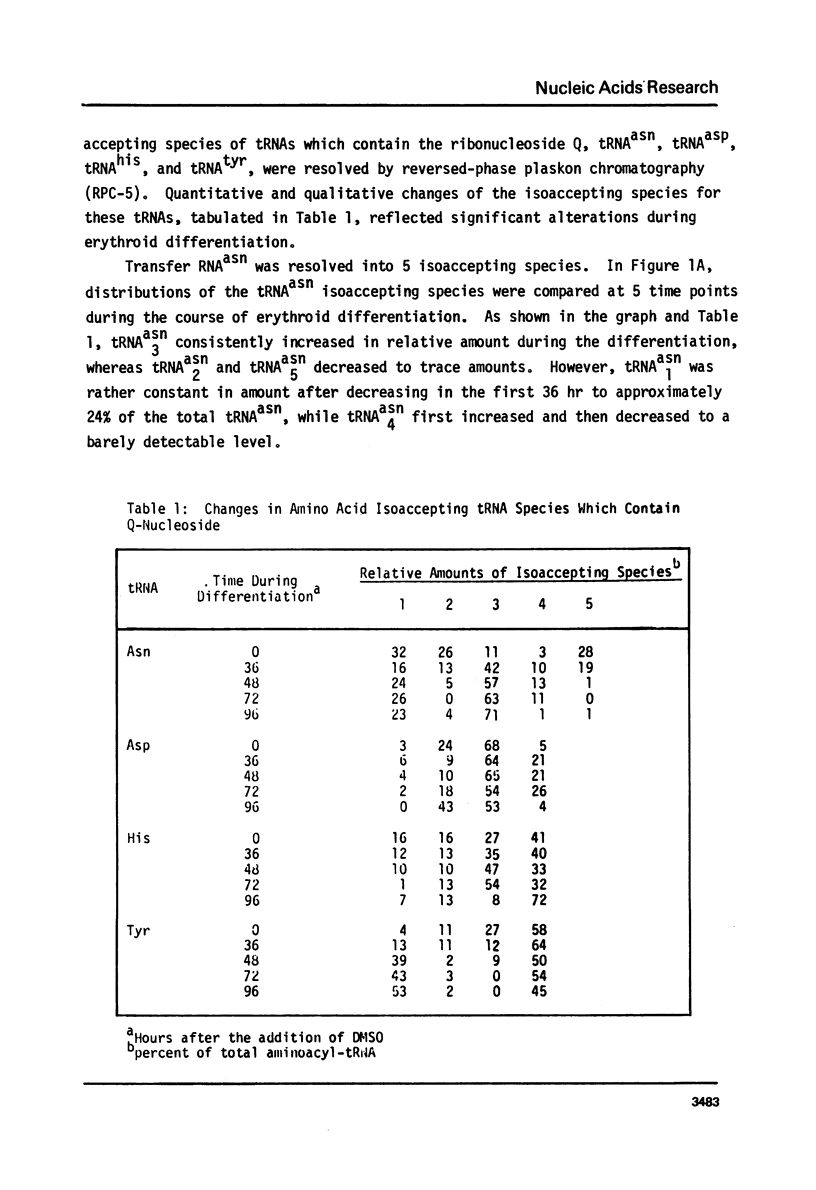
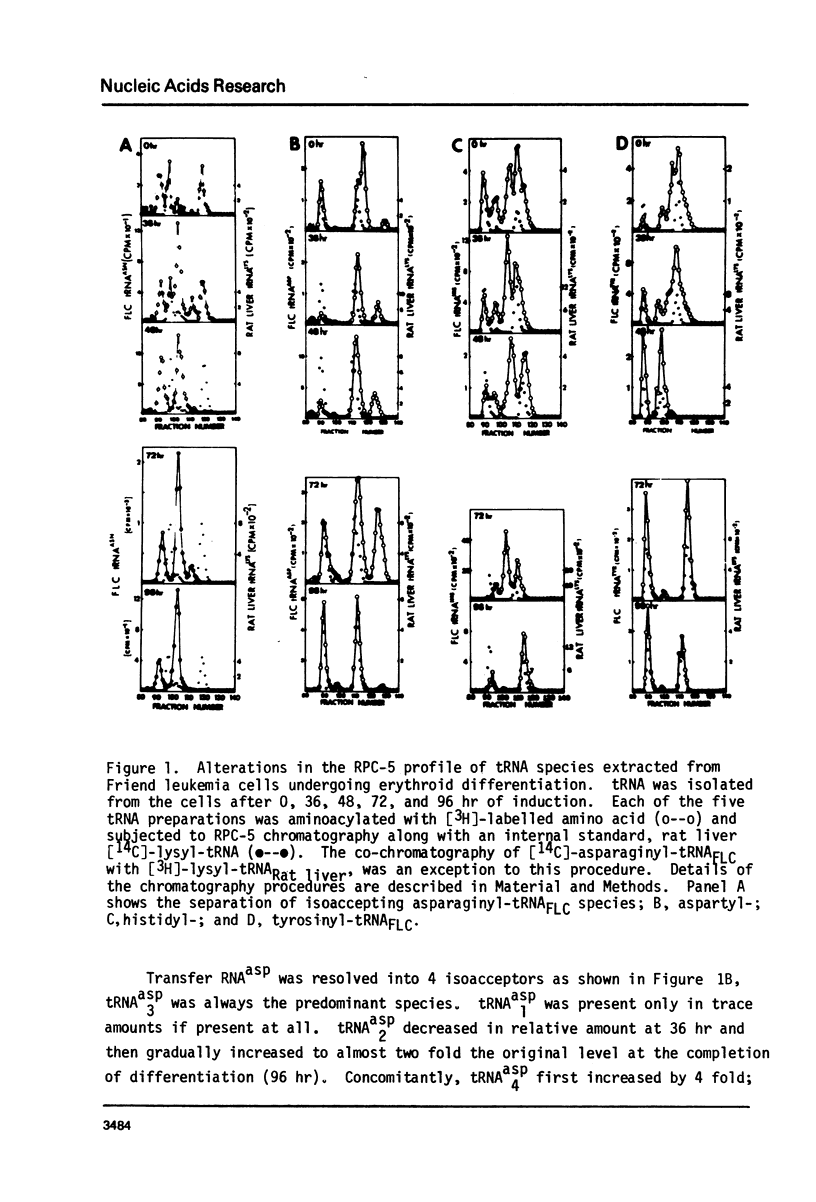
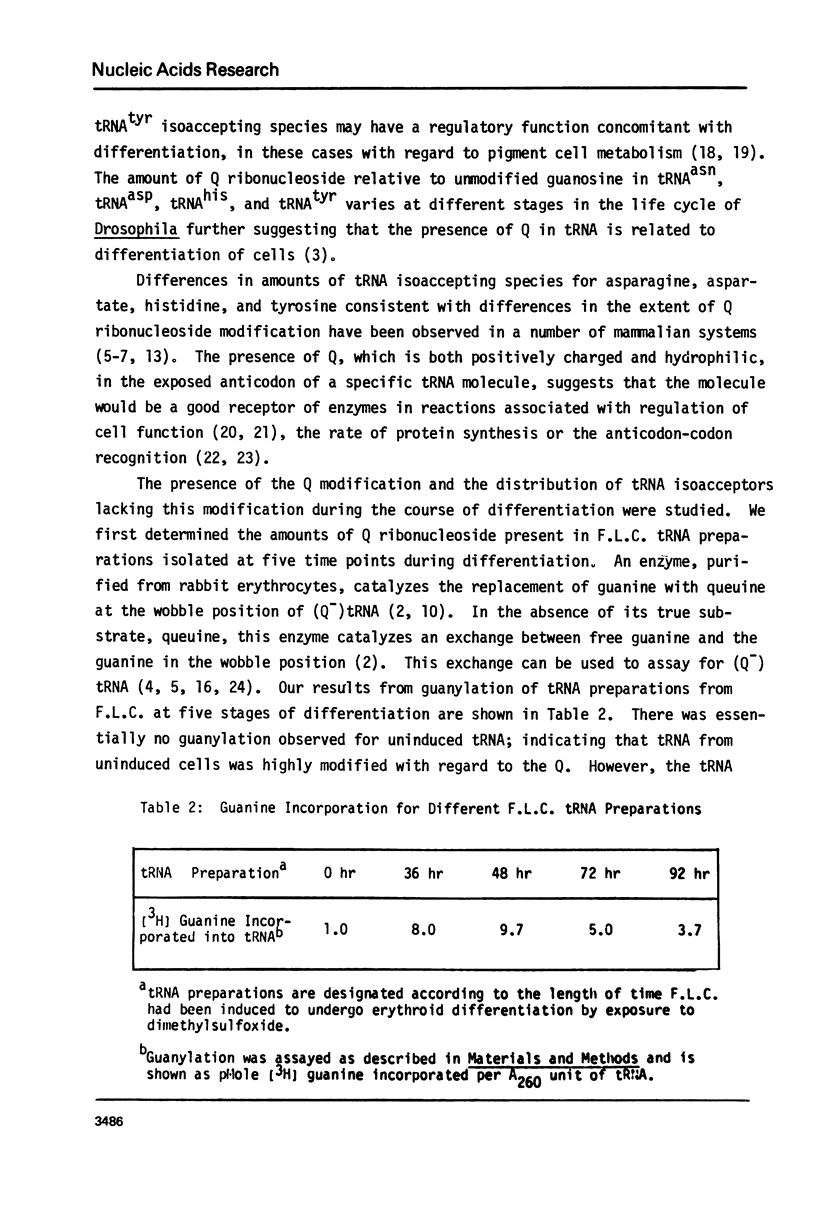
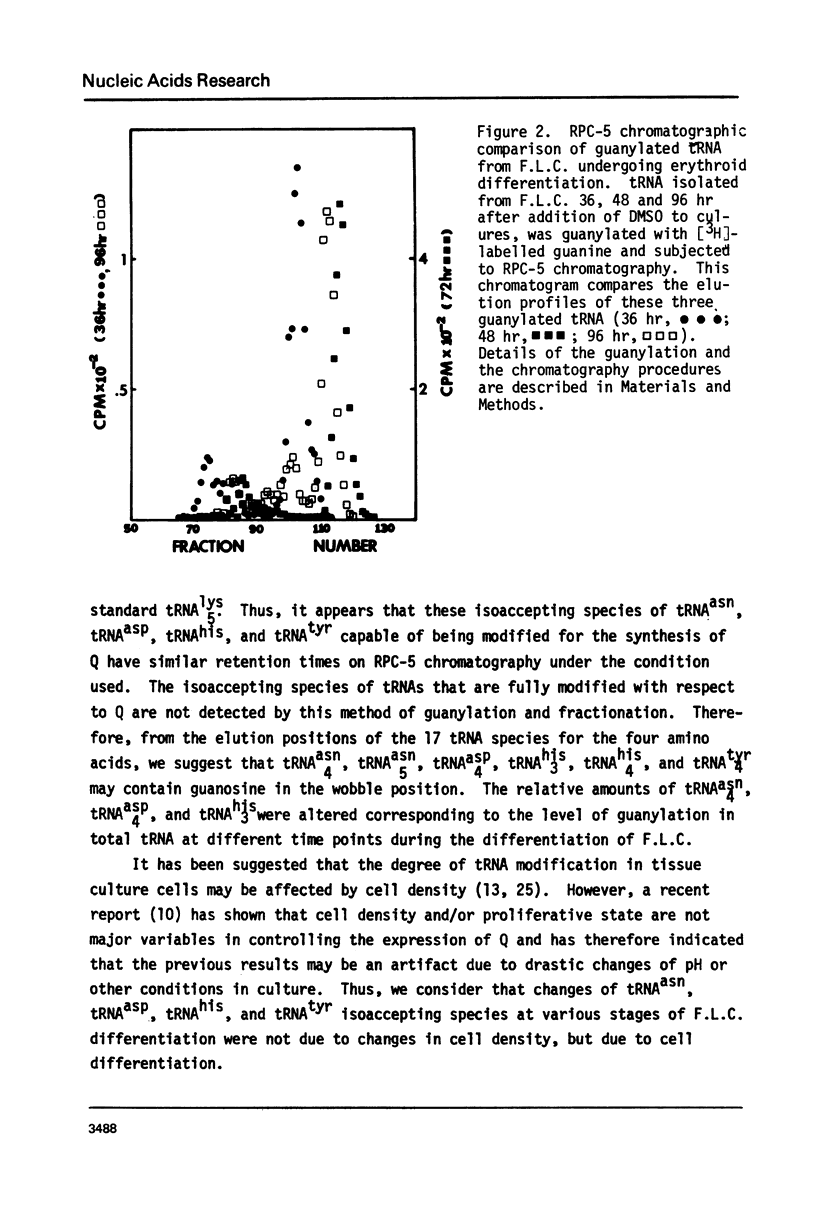
Changes in specific tRNA isoacceptors during Friend leukemia cell (F.L.C.) erythroid differentiation have been found to be concomitant with differences in the extent of the Q-base modification in certain species of tRNA. Transfer RNA was isolated from F.L.C. cultures after 0, 36, 48, 72, and 96 hr of DMSO induced differentiation. Changes in 17 isoacceptors of tRNAasn, tRNAasp, tRNAhis and tRNAtyr were compared by RPC-5 chromatography. Isoacceptors of these tRNA changed in relative amounts, following consistent trends throughout cell differentiation. The amount and distribution of Q-base containing tRNA isoacceptors was assayed by measuring the quanine-tRNA transferase catalyzed incorporation of [3H]-labeled guanine into tRNA species undermodified in Q-base followed by RPC-5 chormatography of the tRNA. The amount of Q-base containing tRNA species decreased in the first 48 hr after the induction, then increased again, indicating the level of Q-modification is correlated to the process of differentiation. Isoacceptors that lacked the Q-base were eluted late from RPC-5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briscoe W. T., Syrewicz J. J., Marshall M. V., Griffin A. C. Regulation of an aspartyl-tRNA species in BHK cells in culture and in solid tumor form. Biochim Biophys Acta. 1975 Apr 2;383(4):441–445. doi: 10.1016/0005-2787(75)90314-7. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Taylor W., Griffin A. C., Duff R., Rapp F. Aspartyl transfer RNA profiles in normal and cancer cells. Cancer Res. 1972 Aug;32(8):1753–1755. [PubMed] [Google Scholar]
- Dubrul E. F., Farkas W. R. Partial purification and properties of the reticulocyte guanylating enzyme. Biochim Biophys Acta. 1976 Sep 6;442(3):379–390. doi: 10.1016/0005-2787(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Chernoff D. Identification of the minor guanylated tRNA of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2521–2528. doi: 10.1093/nar/3.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Ting R. C., Gallo R. C. A common change of aspartyl-tRNA in polyoma- and SV40 -transformed cells. Biochim Biophys Acta. 1972 Jul 31;272(4):568–582. doi: 10.1016/0005-2787(72)90512-6. [DOI] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- Howes N. K., Farkas W. R. Studies with a homogeneous enzyme from rabbit erythrocytes catalyzing the insertion of guanine into tRNA. J Biol Chem. 1978 Dec 25;253(24):9082–9087. [PubMed] [Google Scholar]
- Jacobson K. B. Mechanism of suppression in Drosophila. VII. Correlation between disappearance of an isoacceptor of tyrosine tRNA and activation of the vermilion locus. Nucleic Acids Res. 1978 Jul;5(7):2391–2404. doi: 10.1093/nar/5.7.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Farkas W. R. A factor in serum and amniotic fluid is a substrate for the tRNA-modifying enzyme tRNA-guanine transferase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3271–3275. doi: 10.1073/pnas.76.7.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density in tissue culture to the isoaccepting spectra of the nucleoside Q containing tRNAs: tRNATyr, tRNAHis, tRNAAsn and tRNAAsp. Nucleic Acids Res. 1978 Jul;5(7):2513–2524. doi: 10.1093/nar/5.7.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density to the degree of post-transcriptional modification of tRNALys and tRNAPhe. Biochim Biophys Acta. 1975 Nov 4;407(4):392–398. doi: 10.1016/0005-2787(75)90291-9. [DOI] [PubMed] [Google Scholar]
- Kovacs S. H., Rodi C., Lin V. K., Ortwerth B. J., Agris P. F. Transfer RNATyr of melanoma tissues and cells: relevance to melanin synthesis? Nucleic Acids Res. 1979;6(6):2275–2288. doi: 10.1093/nar/6.6.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon R. D., Wosnick M. A., White B. N. The role of the guanine insertion enzyme in O-biosynthesis in Drosophila melanogaster. Nucleic Acids Res. 1978 Dec;5(12):4865–4876. doi: 10.1093/nar/5.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara A. L., Smith D. W. The function of the histidine tRNA isoaccepting species in hemoglobin synthesis. J Biol Chem. 1978 Sep 10;253(17):5964–5970. [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Sato S., Itoh Y. H., Oda K., Nishimura S. Detection of unique tRNA species in tumor tissues by Escherichia coli guanine insertion enzyme. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4247–4251. doi: 10.1073/pnas.75.9.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C. E., Penhoet E. E. Chromatographic and functional comparison of human placenta and HeLa cell tyrosine transfer ribonucleic acids. Biochemistry. 1976 Oct 19;15(21):4649–4654. doi: 10.1021/bi00666a016. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Rizi H. L., Weisz C., DiLauro M. N., Pike D., Chen C. Y., Chen E. Y. Comparison of rat liver and Walker 256 carcinosarcoma tRNAs. Nucleic Acids Res. 1979 Feb;6(2):673–688. doi: 10.1093/nar/6.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]



