Abstract
Chromatin core particles containing 146 base pairs of DNA have been found to undergo a single defined transition below 10 mM ionic strength as studied by both sedimentation velocity and tyrosine fluorescence anisotropy. A method is described for the preparation of such core particles from chicken erythrocytes with greater than 50% yield.
Full text
PDF






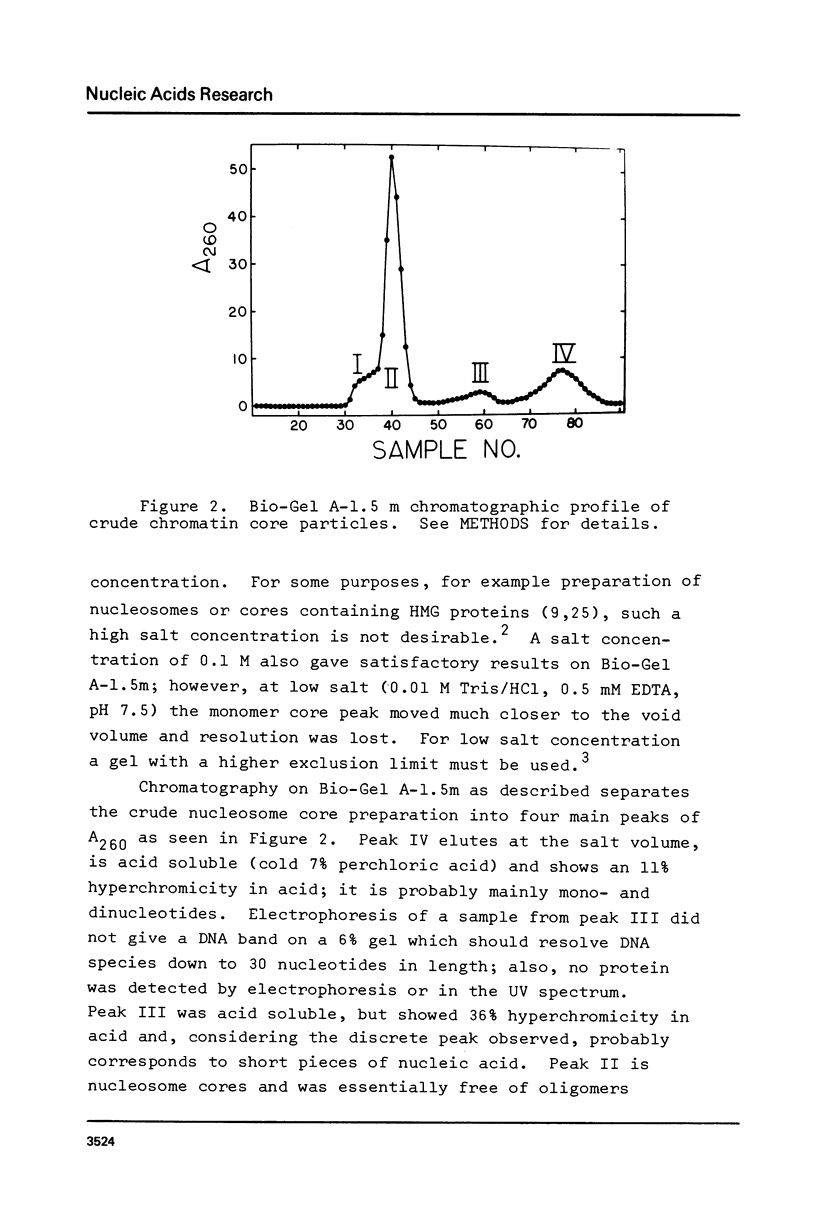
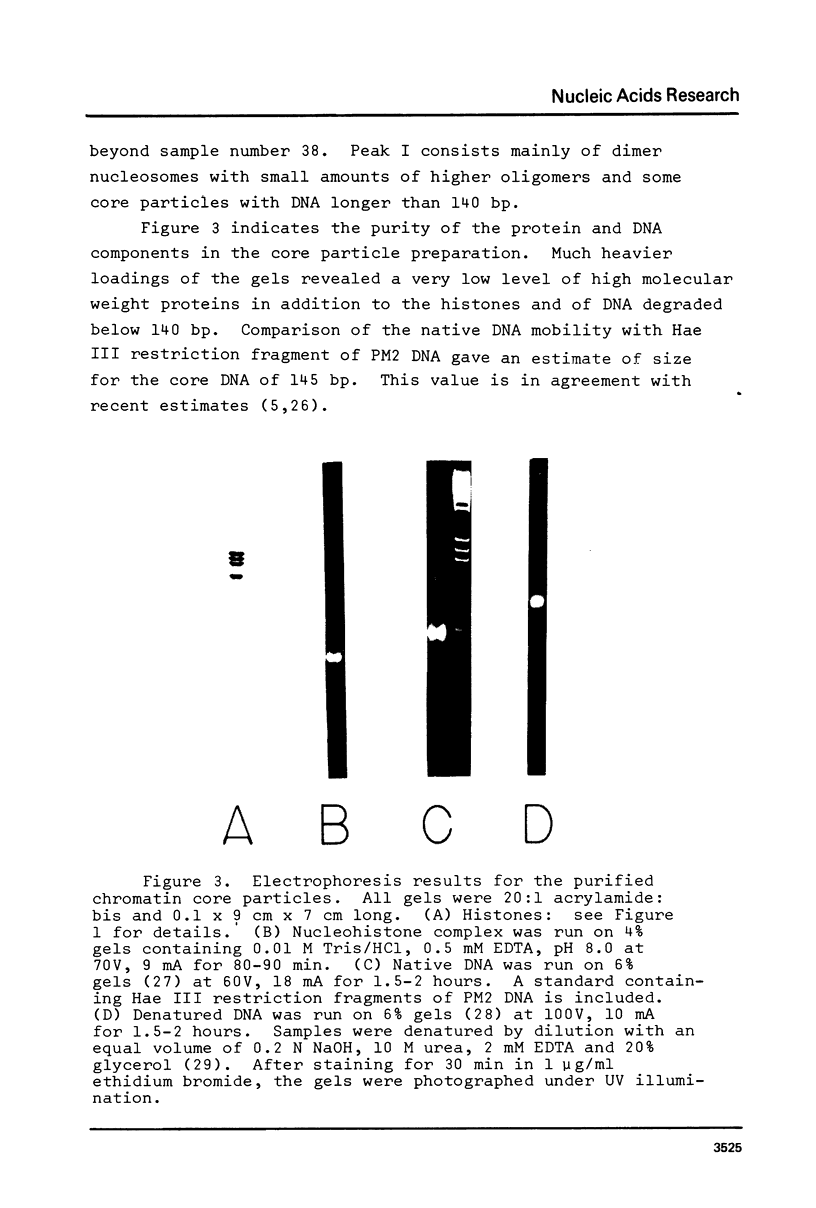
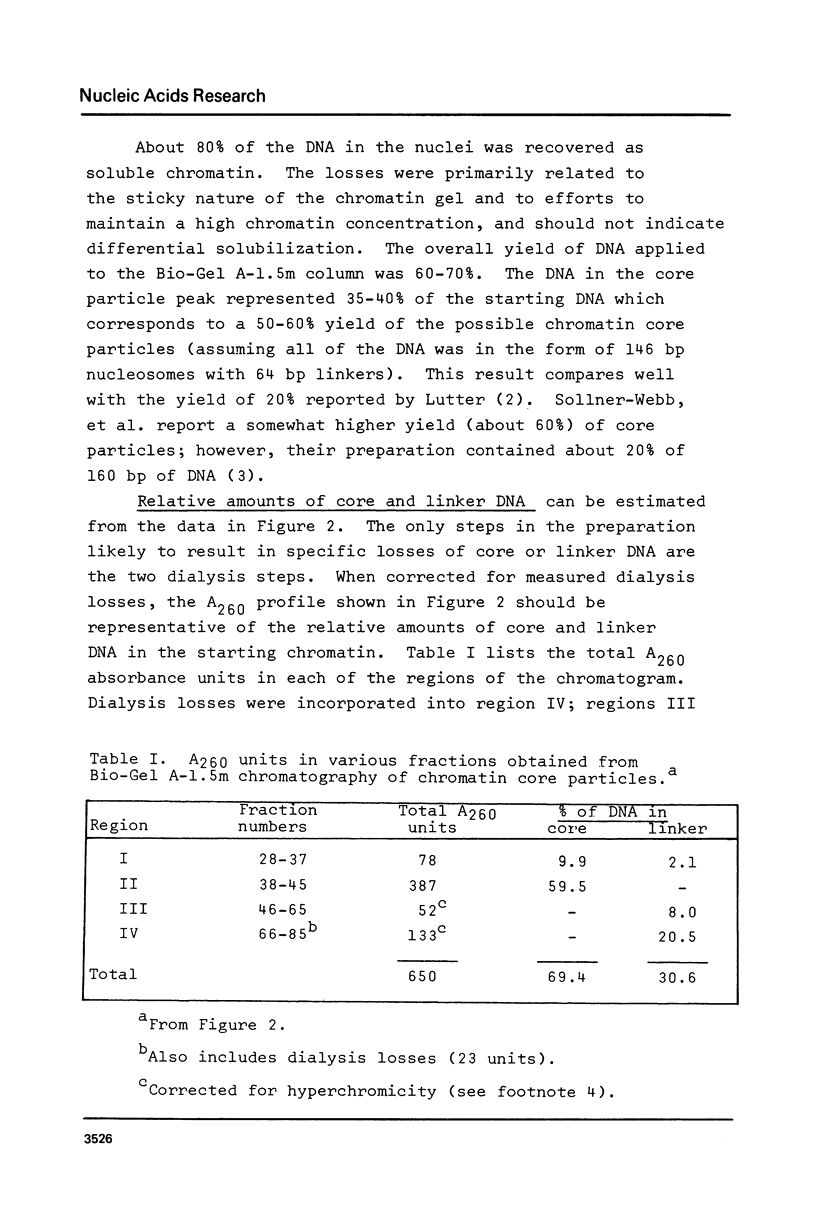

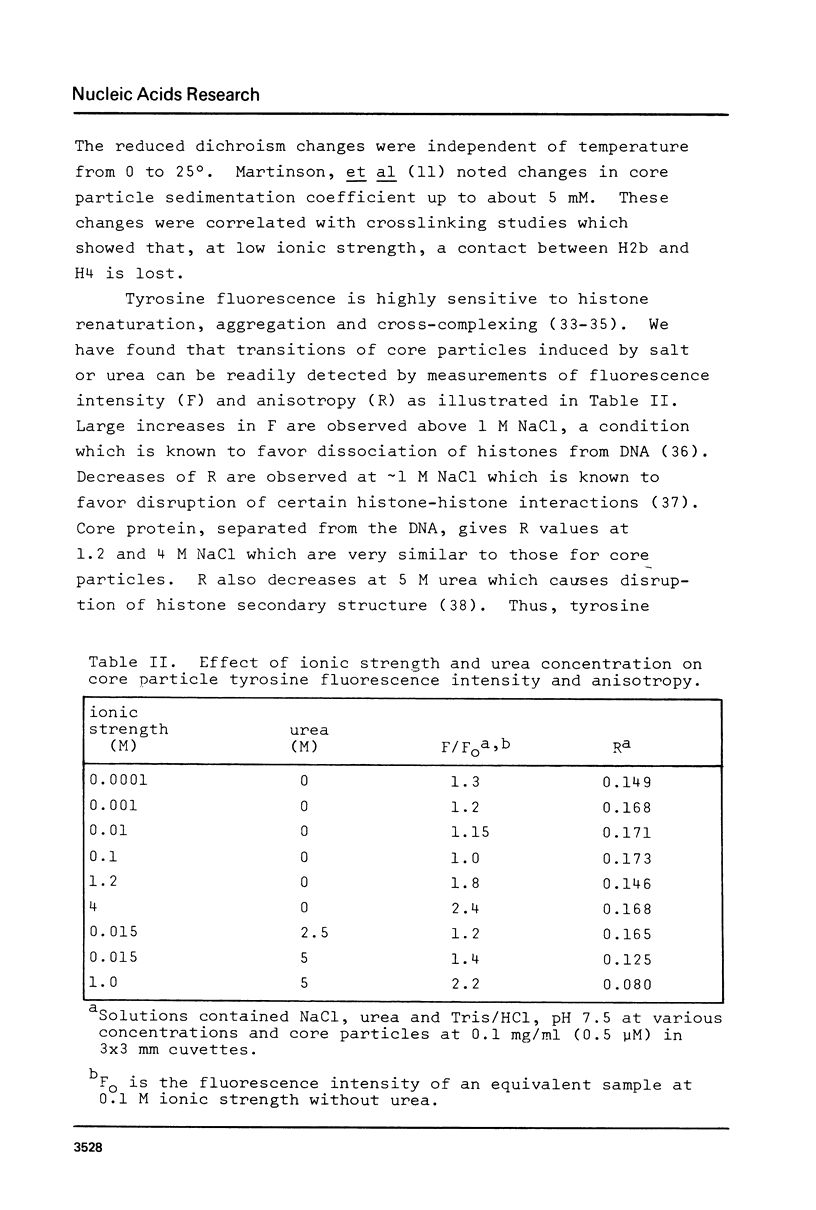
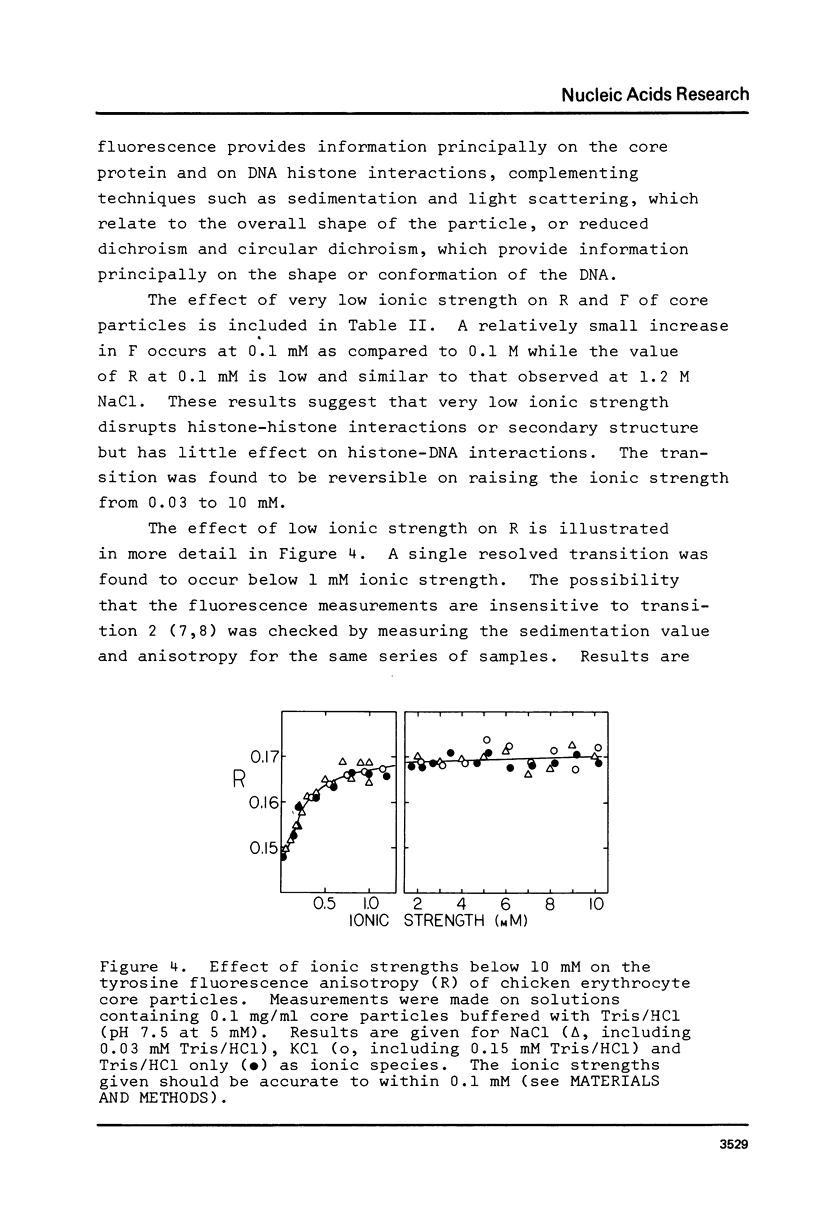




Images in this article
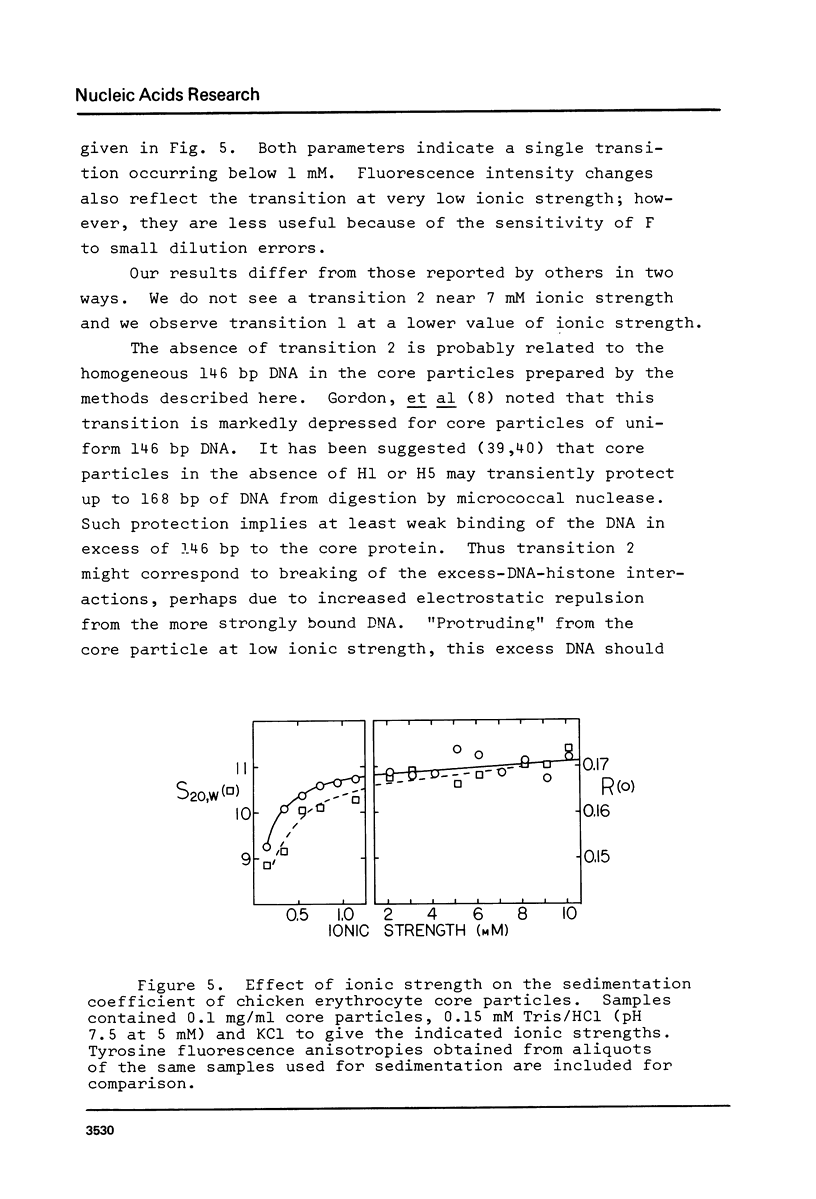
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres W. A., Small E. W., Isenberg I. A computerized fluorescence anisotropy spectrometer. Anal Biochem. 1974 Apr;58(2):361–367. doi: 10.1016/0003-2697(74)90203-6. [DOI] [PubMed] [Google Scholar]
- Billett M. A., Hall T. J. Cations and the accessibility of chromatin to nucleases. Nucleic Acids Res. 1979 Jun 25;6(8):2929–2945. doi: 10.1093/nar/6.8.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Molgaard H. V., Stephens R. M., Bolund L. A., Johns E. W. X-ray studies of nucleoproteins depleted of lysine-rich histone. Eur J Biochem. 1972 Dec 18;31(3):474–482. doi: 10.1111/j.1432-1033.1972.tb02555.x. [DOI] [PubMed] [Google Scholar]
- Brevet A., Kellermann O., Tonetti H., Waller J. P. Macromolecular complexes of aminoacyl-tRNA synthetases from eukaryotes. 2. Agarose gel-filtration behaviour of the extensively purified high-molecular-weight complex(es) of seven aminoacyl-tRNA synthetases from sheep liver. Eur J Biochem. 1979 Sep;99(3):551–558. doi: 10.1111/j.1432-1033.1979.tb13287.x. [DOI] [PubMed] [Google Scholar]
- Bryan P. N., Wright E. B., Olins D. E. Core nucleosomes by digestion of reconstructed histone-DNA complexes. Nucleic Acids Res. 1979 Apr;6(4):1449–1465. doi: 10.1093/nar/6.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne L. A., Wagar M. A., Atkinson M. R. Calcium-dependent priming of DNA synthesis in isolated rat liver nuclei. Biochem Biophys Res Commun. 1970 Apr 24;39(2):254–259. doi: 10.1016/0006-291x(70)90786-2. [DOI] [PubMed] [Google Scholar]
- Compton J. L., Bellard M., Chambon P. Biochemical evidence of variability in the DNA repeat length in the chromatin of higher eukaryotes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4382–4386. doi: 10.1073/pnas.73.12.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Fuchs S., Anfinsen C. B. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1967 Apr 10;242(7):1541–1547. [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Conformational changes of histone ARE(F3, III). Biochemistry. 1974 Nov 19;13(24):4987–4992. doi: 10.1021/bi00721a018. [DOI] [PubMed] [Google Scholar]
- Dieterich A. E., Axel R., Cantor C. R. Salt-induced structural changes of nucleosome core particles. J Mol Biol. 1979 Apr 25;129(4):587–602. doi: 10.1016/0022-2836(79)90470-4. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The histone core complex: an octamer assembled by two sets of protein-protein interactions. Biochemistry. 1978 Nov 14;17(23):4955–4964. doi: 10.1021/bi00616a016. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Frank J. J., Hawk I. A., Levy C. C. Polyamine activation of staphylococcal nuclease. Biochim Biophys Acta. 1975 Apr 16;390(1):117–124. doi: 10.1016/0005-2787(75)90014-3. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Cuatrecasas P., Anfinsen C. B. An improved method for the purification of staphylococcal nuclease. J Biol Chem. 1967 Oct 25;242(20):4768–4770. [PubMed] [Google Scholar]
- Gordon V. C., Knobler C. M., Olins D. E., Schumaker V. N. Conformational changes of the chromatin subunit. Proc Natl Acad Sci U S A. 1978 Feb;75(2):660–663. doi: 10.1073/pnas.75.2.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. C., Schumaker V. N., Olins D. E., Knobler C. M., Horwitz J. The temperature and pH dependence of conformational transitions of the chromatin subunit. Nucleic Acids Res. 1979 Aug 24;6(12):3845–3858. doi: 10.1093/nar/6.12.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin Y. V., Varshavsky A. Y., Mickelsaar U. N., Georgiev G. P. Studies on deoxyribonucleoprotein structure. Redistribution of proteins in mixtures of deoxyribonucleoproteins, DNA and RNA. Eur J Biochem. 1971 Sep 24;22(2):235–245. doi: 10.1111/j.1432-1033.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Jorcano J. L., Ruiz-Carrillo A. H3.H4 tetramer directs DNA and core histone octamer assembly in the nucleosome core particle. Biochemistry. 1979 Mar 6;18(5):768–774. doi: 10.1021/bi00572a005. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li H. J., Wickett R., Craig A. M., Isenberg I. Conformational changes in histone IV. Biopolymers. 1972 Feb;11(2):375–397. doi: 10.1002/bip.1972.360110206. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R. J., Burch J. B. Specific histone-histone contacts are ruptured when nucleosomes unfold at low ionic strength. Biochemistry. 1979 Mar 20;18(6):1082–1089. doi: 10.1021/bi00573a023. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Johns E. W. Studies on the association of the high mobility group non-histone chromatin proteins with isolated nucleosomes. Nucleic Acids Res. 1979 Jan;6(1):167–179. doi: 10.1093/nar/6.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R. A comparison of the structure of chicken erythrocyte and chicken liver chromatin. Cell. 1976 Dec;9(4 Pt 1):627–632. doi: 10.1016/0092-8674(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Bryan P. N., Harrington R. E., Hill W. E., Olins A. L. Conformational states of chromatin nu bodies induced by urea. Nucleic Acids Res. 1977 Jun;4(6):1911–1931. doi: 10.1093/nar/4.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani A., Goodwin G. H., Johns E. W. High mobility group non-histone chromosomal proteins from chicken erythrocytes. Biochem Biophys Res Commun. 1978 Mar 30;81(2):351–358. doi: 10.1016/0006-291x(78)91540-1. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Jorcano J. L., Eder G., Lurz R. In vitro core particle and nucleosome assembly at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3284–3288. doi: 10.1073/pnas.76.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw B. R., Herman T. M., Kovacic R. T., Beaudreau G. S., Van Holde K. E. Analysis of subunit organization in chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):505–509. doi: 10.1073/pnas.73.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Isenberg I. Conformational changes in subfractions of calf thymus histone H1. Biochemistry. 1976 Sep 21;15(19):4233–4242. doi: 10.1021/bi00664a016. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Melchior W., Jr, Felsenfeld G. DNAase I, DNAase II and staphylococcal nuclease cut at different, yet symmetrically located, sites in the nucleosome core. Cell. 1978 Jul;14(3):611–627. doi: 10.1016/0092-8674(78)90246-5. [DOI] [PubMed] [Google Scholar]
- Spadafora C., Oudet P., Chambon P. Rearrangement of chromatin structure induced by increasing ionic strength and temperature. Eur J Biochem. 1979 Oct;100(1):225–235. doi: 10.1111/j.1432-1033.1979.tb02053.x. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Streeck R. E., Zachau H. G. Closely spaced nucleosome cores in reconstituted histone.DNA complexes and histone-H1-depleted chromatin. Eur J Biochem. 1978 Feb;83(2):615–628. doi: 10.1111/j.1432-1033.1978.tb12131.x. [DOI] [PubMed] [Google Scholar]
- Sterner R., Boffa L. C., Vidali G. Comparative structural analysis of high mobility group proteins from a variety of sources. Evidence for a high mobility group protein unique to avian erythrocyte nuclei. J Biol Chem. 1978 Jun 10;253(11):3830–3836. [PubMed] [Google Scholar]
- Tatchell K., Van Holde K. E. Compact oligomers and nucleosome phasing. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3583–3587. doi: 10.1073/pnas.75.8.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischet W. O., Allen J. R., Riedel G., Van Holde K. E. The effects of salt concentration and H-1 depletion on the digestion of calf thymus chromatin by micrococcal nuclease. Nucleic Acids Res. 1979;6(5):1843–1862. doi: 10.1093/nar/6.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M. L., Mazen A., Wilhelm F. X. Comparison of the DNA repeat length in H1- and H3-containing chromatin. FEBS Lett. 1977 Jul 15;79(2):404–408. doi: 10.1016/0014-5793(77)80831-4. [DOI] [PubMed] [Google Scholar]
- Woodhead L., Johns E. W. The isolation of nucleosomes from saline-washed chromatin. FEBS Lett. 1976 Feb 15;62(2):115–117. doi: 10.1016/0014-5793(76)80031-2. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Dattagupta N., Hogan M., Crothers D. M. Structural changes of nucleosomes in low-salt concentrations. Biochemistry. 1979 Sep 4;18(18):3960–3965. doi: 10.1021/bi00585a018. [DOI] [PubMed] [Google Scholar]





