Abstract
Mammalian phagocytes control bacterial infections effectively through phagocytosis, the process by which particles engulfed at the cell surface are transported to lysosomes for destruction. However, intracellular pathogens have evolved mechanisms to avoid this fate. Many bacterial pathogens use specialized secretion systems to deliver proteins into host cells that subvert signaling pathways controlling membrane transport. These bacterial effectors modulate the function of proteins that regulate membrane transport and alter the phospholipid content of membranes. Elucidating the biochemical function of these effectors has provided a greater understanding of how bacteria control membrane transport to create a replicative niche within the host and provided insight into the regulation of membrane transport in eukaryotic cells.
Introduction
When phagocytic cells encounter bacteria, they have the ability to engulf them and transport them inside a vacuole called a phagosome (Fig. 1 A), a compartment that has similarities with early endosomes and will sequentially acquire host proteins that regulate biogenesis and transport of this organelle (Kinchen and Ravichandran, 2008). Rab5 is a GTPase critical for early membrane transport decisions after phagocytosis. Activated Rab5 is recruited at the phagosome shortly after phagocytosis and is necessary for maturation of the phagosome through the recruitment of a large number of effector proteins. Rab5 is replaced by Rab7 in a process called Rab conversion, which is required for the subsequent fusion of the phagosome with lysosomes to generate the phagolysosome (Rink et al., 2005). The phagolysosome is acidic and enriched in proteases, conditions that promote bacterial degradation.
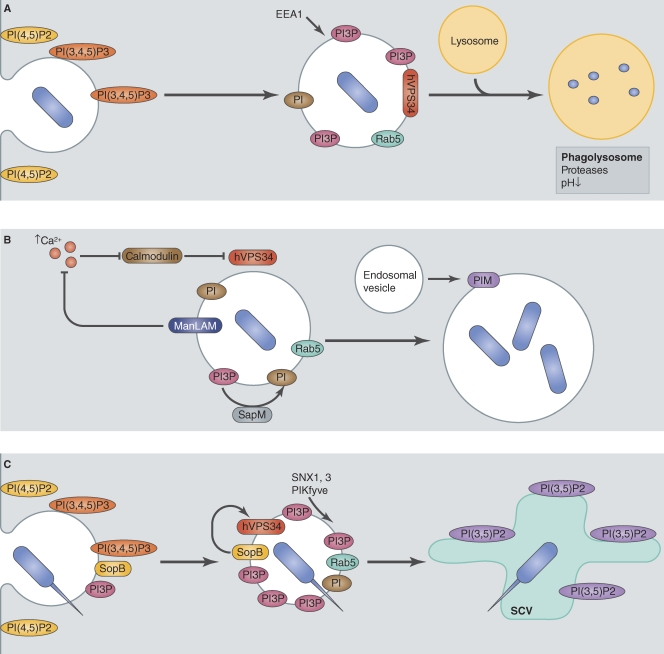
Figure 1.
Mtb and S. Typhimurium manipulate the fate of their vacuole through modification of phosphoinositide metabolism. (A) Normal maturation of a phagosome containing nonpathogenic bacteria. After phagocytosis, bacteria reside in a vacuole showing similarities with early endosomes, notably presenting the small GTPase Rab5. Rab5 recruits the PI3 kinase hVPS34 that produces PI3P at the phagosome surface. The presence of PI3P is required for the maturation of the phagosome to phagolysosome by the recruitment of a subset of proteins, including EEA1. (B) Mtb creates a replicative niche by manipulating PI3P metabolism. Mtb blocks the activation of hVPS34 at its vacuole by ManLAM, thereby preventing PI3P production. The mechanism for this block involves the inhibition of Ca2+ rise, which is necessary for hVPS34 activation through a cascade involving calmodulin. Moreover, Mtb secretes SapM, a phosphatase that could be involved in depleting the vacuole from any residual PI3P. Finally, Mtb can expand its vacuole by recruitment of endosome vesicles. This recruitment could be achieved by the Mtb lipid PIM, a phosphoinositide analogue. (C) The S. Typhimurium T4SS effector SopB generates PI3P at the vacuole membrane. A possible mechanism for PI3P enrichment at the early S. Typhimurium vacuole is an indirect modulation of hVPS34 recruitment by SopB. This results in a prolonged and increased presence of PI3P at the vacuole surface. The presence of a high amount of PI3P induces subsequent recruitment of PI3P-binding proteins, including SNX1, SNX3, and PIKfyve, which were shown to be required for the maturation of the S. Typhimurium–containing vacuole (SCV).
Pathogenic intracellular bacteria have evolved different strategies to counteract the endocytic pathway and avoid being degraded in lysosomes. Some bacteria, such as Coxiella burnetii, are able to survive in a compartment that is derived from fusion of the vacuole with lysosomes (Voth and Heinzen, 2007). Other bacteria have acquired mechanisms to escape the lysosomal pathway. Shigella flexneri and Listeria monocytogenes escape from the phagosome before lysosome fusion to reach the cytosol of the host, where they can replicate intracellularly (Goebel and Kuhn, 2000). Many bacteria, such as Salmonella enterica serovar Typhimurium or Legionella pneumophila, actively modify host vesicular transport pathways to prevent the fusion of the vacuole in which they reside with lysosomes, a process that is necessary for creating a specialized organelle favorable for replication. Most of these bacteria use a T3SS (type III secretion system) or T4SS (type IV secretion system) to modulate membrane transport and create specialized vacuoles. These secretion systems enable the bacteria to translocate proteins known as effectors across the vacuolar membrane where they can then manipulate host proteins residing in the cytoplasm or on other cellular organelles.
Studying the mechanisms used by pathogenic bacteria to subvert their host pathways has two main objectives. First, an understanding of how these effector proteins function provides important insight into the mechanism of host cell infection and could lead to the development of new therapeutic approaches to target these pathogens. Second, because bacteria disrupt eukaryotic processes, they can be used as tools to dissect eukaryotic pathways. Reports coming from studies on many different pathogens show that vesicular transport pathways are manipulated in diverse ways by intracellular bacteria, and understanding how bacteria manipulate these pathways could provide a deeper understanding of the homeostatic regulation of membrane transport in eukaryotic cells. Thus, this review will focus on general strategies used by different bacteria to create a specialized organelle that promotes survival and replication, with specific examples falling into the broad context of pathogen manipulation of the phosphoinositide composition of membranes, manipulation of the host cytoskeleton, and modulation of small GTPases.
Phosphoinositide subversion by vacuolar pathogens
Lipid subversion by pathogens is emerging as a process critical for microbial infection (van der Meer-Janssen et al., 2010). Bacteria are able to subvert host lipids for metabolism or for the formation of the vacuoles in which they reside. In many pathogens, effector proteins that can sense host lipids and modify the phosphoinositide signatures on membranes are being found to play critical roles in controlling vesicular transport in the cell.
Phosphatidylinositol (PI) phosphates (PIPs) have important functions in regulating pathways involved in signal transduction and in vesicular transport (Di Paolo and De Camilli, 2006). PIPs phosphoinositol ring can be reversibly phosphorylated at positions 3, 4, and 5, which can generate up to seven different PIP species. The PIP signatures on the membrane mediate organelle-specific recruitment of proteins that regulate vesicular transport. Specific kinases and phosphatases act in concert to tightly regulate the phosphorylation state of the PIPs, so the regulation of the localization of these enzymes is critical for controlling vesicular transport in the cell. Pathogens have evolved mechanisms to modify membrane signatures both directly and indirectly by controlling the phosphorylation state of PIPs on the vacuoles in which they reside, which is critical for modulating the transport of this pathogen-occupied organelle (Weber et al., 2009).
The species PI(4,5)P2 and PI(3,4,5)P3 are mainly found at the plasma membrane and are critical for recruiting the cellular machinery important for phagocytosis (Fig. 1 A). Once phagosomes have been formed, the major PIP species found on the organelle is PI3P (Kinchen and Ravichandran, 2008). Proteins containing PI3P recognition motifs are recruited to the endosome, inducing maturation to the late endosomal and phagolysosomal stages. Bacteria have evolved different mechanisms to manipulate the levels of PI3P on vacuole membranes. Mycobacterium tuberculosis (Mtb) aims to decrease PI3P level on the vacuole to prevent maturation of the phagosome at an early stage in endocytic transport, whereas S. Typhimurium increases PI3P levels on the vacuole to stimulate biogenesis of a unique compartment with properties of late endosomes.
PI3P depletion by Mtb.
Mtb survives in host cells by delaying phagosome maturation at an early stage (Fig. 1 B). Vacuoles containing Mtb are enriched for Rab5 but largely devoid of Rab7, suggesting that endocytic maturation of this compartment is stalled shortly after fusion with early endosomes in the cell (Via et al., 1997). Mechanisms by which Mtb controls membrane transport appear to be diverse and are still not fully understood. Many bacterial factors, proteins as well as lipids, have been associated with the impairment of the vacuole to mature (Philips, 2008). Interestingly, even in the presence of Rab5, it was shown that the Rab5 effectors EEA1 (Fratti et al., 2001) and Hrs (Vieira et al., 2004), both PI3P-binding regulatory proteins, fail to accumulate on the Mtb-containing vacuole (MCV) and that the absence of these factors impairs endocytic maturation of this vacuole. Consistent with PI3P being important for the localization of proteins involved in the maturation of early endosomal compartments, levels of PI3P on vacuoles containing live Mtb were shown to be reduced compared with vacuoles containing beads or dead bacteria (Purdy et al., 2005; Vergne et al., 2005), indicating that Mtb can actively prevent the acquisition or deplete PI3P from the vacuole in which it resides.
Two complementary strategies appear to be used by Mtb to reduce PI3P levels on the MCV. The mycobacteria-derived lipid mannose-capped lipoarabinomannan (ManLAM) arrests maturation of the MCV by a mechanism that involves suppression of the PI3 kinase hVPS34, the kinase involved in PI3P production in early endosomes (Fig. 1 B; Fratti et al., 2003). ManLAM suppresses hVPS34 by interfering with Ca2+ fluxes, which induce a signaling cascade that activates hVPS34 (and therefore PI3P production) at the phagosome membrane (Vergne et al., 2003). Nevertheless, increasing intracellular Ca2+ in the cell is not sufficient to restore PI3P level on the phagosome, suggesting that Mtb has additional activities that suppress endosomal maturation (Vergne et al., 2005). SapM is a phosphatase produced by Mtb that can dephosphorylate PI3P in vitro (Vergne et al., 2005). SapM is secreted by Mtb and is sufficient to block phagosome–late endosome fusion in vitro in a PI3P-dependent mechanism. Phosphate consumption by a bacterial protein may then represent another mechanism used by Mtb to decrease PI3P levels on the vacuole.
It is intriguing that the Mtb vacuole recruits early endosomal vesicles while blocking fusion with late endosomes and lysosomes. Mtb produces a surface lipid called PI mannoside (PIM) that is very similar to PIPs. PIM has been shown to induce fusion of the phagosome with early endosomes in vitro and in vivo (Vergne et al., 2004), and it is thought that PIM-stimulated fusion of early endosomes helps maintain vacuole integrity to balance the inhibitory activities that prevent acquisition of additional membranes through fusion with late endosomes and lysosomes.
Although the SapM protein is secreted by Mtb (Vergne et al., 2005), and the lipids ManLAM and PIM have been reported to cross the vacuole membrane (Beatty et al., 2000), whether these products exert their function at the cytosolic face of the vacuole membrane remains unclear. Another possibility is that SapM and the lipids ManLAM and PIM alter the biophysical properties of the vacuole membrane from the luminal side of the compartment (Vergne et al., 2004, 2005).
PI metabolism by the S. Typhimurium SopB protein.
S. Typhimurium is also able to subvert normal trafficking to the phagolysosome compartment, even though a study indicates at least a partial fusion with lysosomes (Drecktrah et al., 2007). Effectors delivered by two different pathogenicity island-encoded T3SSs are critical for the development of the S. Typhimurium–containing vacuole (SCV) and for creating an organelle that permits intracellular survival (Bakowski et al., 2008).
SopB is a T3SS effector with homology to eukaryotic inositol phosphatases and was shown to have a phosphatase activity against different PIPs in vitro (Norris et al., 1998). Vacuoles containing S. Typhimurium acquire PI3P very rapidly after phagocytosis, and PI3P remains associated with the vacuole for an extended period (Fig. 1 C). In contrast, a ΔsopB strain resides in a vacuole that contains PI3P only transiently (Hernandez et al., 2004). SopB seems sufficient for generating PI3P on vacuoles, given that expression of the SopB protein in mammalian cells will induce the formation of large macropinocytic vesicles on which PI3P is present (Hernandez et al., 2004).
The mechanism of PI3P production by SopB is unclear. It has been suggested that PI3P results from the phosphatase activity of SopB on PI(3,5)P2 and PI(3,4,5)P3. However, it has also been shown that generation of PI3P on the SCV is sensitive to wortmannin, which blocks host PI3P kinase activity, suggesting PI3P originates from host phosphorylation of PI. This may indicate that SopB indirectly recruits the PI3P kinase hVPS34 at the SCV (Mallo et al., 2008), perhaps by creating PIP signatures that enhance the recruitment of hVPS34 regulators, including Rab5. Recent data may provide insight into why PI3P generation would benefit maturation of the SCV. Sorting nexin 1 (Bujny et al., 2008) and sorting nexin 3 (Braun et al., 2010) are recruited to the SCV in a PI3P- and SopB-dependent manner (Fig. 1 C). These host regulators of membrane transport are required for normal replication of S. Typhimurium in cells. It has also been shown that the recruitment of the PtdIns(5) kinase PIKfyve, an effector of PI3P, to the SCV is required for the formation of the SCV (Kerr et al., 2010). Lastly, it has been suggested that the change in electrostatic charge at the surface of the SCV resulting from activities mediated by SopB could be sufficient to alter the recruitment of specific effectors (Bakowski et al., 2010).
The activities of SopB also assist in the process of S. Typhimurium uptake by nonphagocytic cells (Terebiznik et al., 2002; Dai et al., 2007). Interestingly, SopB possesses multiple ubiquitination sites, and a recent study indicates that the ubiquitination state of the protein allows the protein to have distinct functions by affecting the localization of SopB to membranes (Patel et al., 2009). Ubiquitination of SopB has been shown to be important for localization of the protein to the SCV and for promoting the recruitment of Rab5 to the vacuole, but ubiquitination of SopB does not appear to be needed for the activities that promote bacterial entry. Thus, this S. Typhimurium effector utilizes the host posttranslational modification machinery to spatially regulate functions that affect different host cell processes.
Subversion of the cytoskeleton by vacuolar pathogens
Central to most membrane transport processes are the movement of vesicles and tubules along the actin and microtubule cytoskeleton (Soldati and Schliwa, 2006). There are now several documented examples in which pathogenic bacteria target the cytoskeleton to modulate vacuole biogenesis and maintain an organelle that supports their intracellular survival. These strategies involve control over host processes that are central to the assembly of cytoskeletal networks and modulation of the motor proteins that transport cargo along these structures.
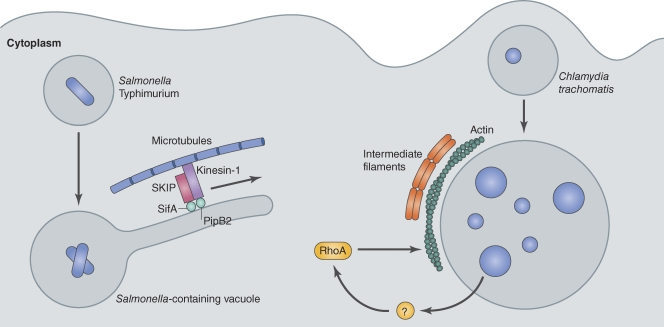
In certain host cells, the vacuoles containing S. Typhimurium will display long membrane tubules called S. Typhimurium–induced filaments (SIFs; Fig. 2; Garcia-del Portillo et al., 1993). Although several S. Typhimurium T3SS effectors are present on the SIFs, one effector called SifA is necessary for the formation of these structures (Schroeder et al., 2011). The SifA protein was found to interact directly with the human protein SKIP (Boucrot et al., 2005; Ohlson et al., 2008), which is a host protein that binds to the motor protein kinesin-1 (Boucrot et al., 2005). SKIP appears to function as a linker that regulates kinesin-1 on the Golgi apparatus and late endosomal and lysosomal compartments (Dumont et al., 2010). Structural studies have shown that SifA also has a domain that mediates interactions with the Rho family GTPases, and this interaction may activate these proteins by stimulating the exchange of GDP for GTP (Ohlson et al., 2008). Thus, SifA may generate membrane filaments by coordinating both cytoskeleton dynamics and transport of membranes by host motor proteins along these structures, which could provide insight into how host proteins control vesicular transport processes that involve membrane tubules.
Figure 2.
Intracellular bacteria subvert the eukaryotic cytoskeleton to create a replicative niche. Two representative examples are depicted here. S. Typhimurium uses microtubules to form SIFs. SIFs are typical structures of the SCV. The mechanism of their formation, which involves microtubules, is not completely understood. At least two T3SS effectors, SifA and PipB2, play a role in SIF formation. They target the mammalian proteins SKIP and kinesin-1 and altogether induce protrusion and expansion of tubules from the SCV. C. trachomatis actively induces polymerization of actin and intermediate filaments to consolidate its large vacuole. This relies on an unknown bacterial effector and the host GTPase RhoA.
A second T3SS effector called PipB2 was also found to be important for generating SIFs during infection, as an S. Typhimurium mutant deficient for PipB2 was found to occupy vacuoles with shorter SIFs (Knodler and Steele-Mortimer, 2005). PipB2 also interacts with kinesin-1 (Henry et al., 2006) and may participate in the recruitment of this motor to the SCV. Thus, it is thought that SifA and PipB2 assist in the positioning of the SCV and can regulate the transport of membranes between the SCV and host organelles by spatially controlling the activity of kinesin-1 and modulating cytoskeletal dynamics by regulating Rho GTPase functions. However, the mechanisms that underlie SIF dynamics are probably more complex as genetic experiments indicate that several other effectors are involved in SCV biogenesis and transport (Schroeder et al., 2011). Further characterization of S. Typhimurium effectors and their targets in eukaryotic cells could then lead to a better understanding of specific cytoskeleton-driven membrane transport in eukaryotes.
Chlamydia trachomatis occupies a vacuole that diverges from the endocytic pathway shortly after these bacteria are internalized and creates a replicative organelle intimately associated with the Golgi apparatus that is called an inclusion. Subversion of the cytoskeleton is central to the transport and maintenance of the C. trachomatis inclusion, and several mechanisms for C. trachomatis subversion of the cytoskeleton have been revealed. During entry, C. trachomatis induces actin polymerization using a type III effector protein called TARP, which contains actin-binding regions that facilitate actin polymerization and are important for bacterial uptake (Elwell et al., 2008; Jewett et al., 2008; Lane et al., 2008). Shortly after uptake, the vacuoles containing C. trachomatis are transported on microtubules by a dynein-dependent, but dynactin-independent, mechanism (Grieshaber et al., 2003). C. trachomatis then utilizes actin and intermediate filaments to form a scaffold that surrounds the mature inclusion in a process that is dependent on RhoA (Fig. 2; Kumar and Valdivia, 2008). Disruption of the cytoskeletal framework that surrounds the inclusion leads to destabilization of the vacuole and fragmentation of this organelle. Lastly, C. trachomatis egress from cells also involves subversion of host actin regulators, namely, Wiskott–Aldrich syndrome protein, myosin, and Rho GTPases (Hybiske and Stephens, 2007). Thus, manipulation of the host cytoskeleton is essential for the biogenesis, maintenance, and eventual egress of C. trachomatis from the specialized vacuole it creates in host cells.
GTPase manipulation by pathogens
Small GTPases are critical regulators of most host cellular processes, including the sorting and transport of cargo inside vesicles. The Rab and ADP ribosylation factor (ARF) families of small GTPases are highly conserved regulators of membrane transport, and the Rho family of GTPases is a critical regulator of the cytoskeleton. These GTPases have several common features. They are lipidated to facilitate their association with membranes. They are activated by guanine nucleotide exchange factors (GEFs) that stimulate GDP for GTP exchange and deactivated by GTPase-activating proteins (GAPs) that stimulate GTP hydrolysis. Activated GTPases recruit cellular proteins to membranes and create the protein complexes that promote membrane transport and changes to the cytoskeleton at the sites of activation. Activated GTPases can also recruit effectors that have signaling functions and modulate gene expression. Thus, GTPases provide an attractive target for pathogen manipulation, and there are now several well-documented examples of how intracellular pathogens have evolved specific mechanisms to manipulate the function of host GTPases. The two general themes that are observed are strategies for pathogens to subvert the function of GTPases by using effectors that function as activators (Fig. 3 A) and those that interfere with the signaling capacity of GTPases (Fig. 3 B).
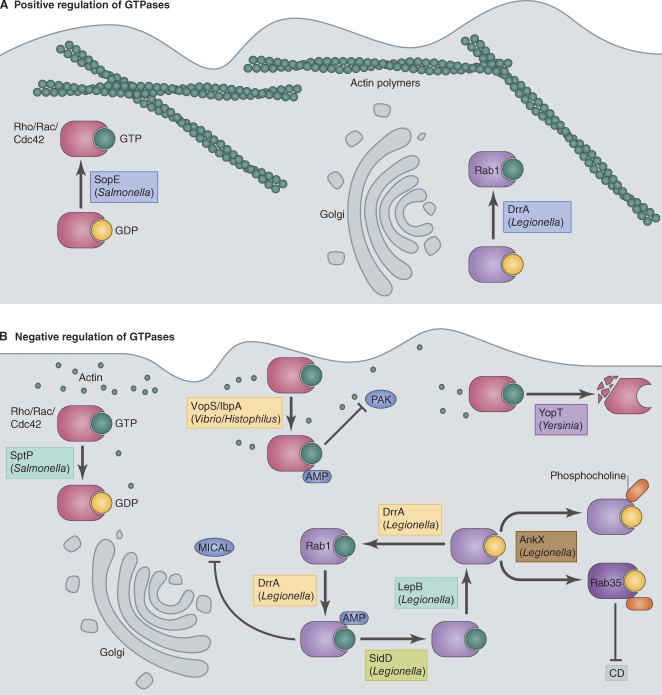
Figure 3.
Pathogens manipulate host GTPases. (A) Positive regulation of host GTPases. The S. Typhimurium effector SopE binds and activates Rho/Rac/Cdc42 GTPases, thereby stimulating assembly of an actin-based network at the plasma membrane. SopE acts as a GEF and catalyzes the exchange of GTP for GDP. Similarly, the L. pneumophila effector DrrA functions as a GEF for the small GTPase Rab1 (a key GTPase involved in ER to Golgi and intra-Golgi vesicular transport) and activates it on the LCV. (B) Negative regulation of host GTPases. Bacteria have evolved several ways to negatively regulate GTPases. First, bacteria encode GAPs, such as SptP and LepB, to catalyze the hydrolysis of GTP to GDP to inactivate GTPases. Second, bacteria encode enzymes, such as VopS, IbpA, and DrrA, that inhibit downstream signaling of GTPases by posttranslationally modifying them with AMP. AMPylated Rac failed to interact with its effector p21-activated kinase (PAK). Similarly, AMPylated Rab1 was unable to bind to its effector MICAL-3 (microtubule-associated monoxygenase, calponin, and LIM domain containing 3). Recently, the L. pneumophila effector SidD has been shown to act on Rab1 to remove this AMP modification. Third, a novel modification on Rab1 and Rab35 was reported whereby the L. pneumophila effector AnkX modified the class II switch region of the Rabs with a phosphocholine moiety. This modification was observed in Rabs bound to both GDP and GTP. Phosphocholination of Rab35-GDP prevented its binding to its GEF connecdenn (CD). Finally, effectors such as YopT function as proteases to cleave Rho GTPases to inhibit their function.
Activation of GTPases by bacterial effectors.
Intracellular pathogens activate host GTPases as a strategy to promote internalization into a membrane-bound compartment and to alter the biochemical properties of the vacuole to modulate transport and fusion. L. pneumophila is an intracellular pathogen that uses a T4SS called Dot/Icm to create a vacuole that avoids fusion with late endosome and lysosome and is remodeled into an ER-derived organelle through subversion of membranes in the early secretory pathway of the host. A unique feature of the L. pneumophila–containing vacuole (LCV) is the localization of several small GTPases found on early secretory vesicles, including Rab1 and ARF1, which typically are not enriched on phagosomes (Nagai et al., 2002; Kagan et al., 2004). Expression of dominant-negative Rab1 or inactivation of ARF1 by brefeldin A inhibits formation of a vacuole that supports intracellular replication of L. pneumophila, indicating that these GTPases are functioning in the transport and fusion of the pathogen-occupied vacuole (Kagan and Roy, 2002; Derré and Isberg, 2004).
Consistent with the function of these GTPases being important for biogenesis of the LCV, two different T4SS effector proteins from L. pneumophila have been shown to have GEF activity. The protein RalF activates ARF GTPases and is required for the recruitment of ARF1 to the LCV (Nagai et al., 2002). Similar to host proteins that function as GEFs for ARF, the RalF protein has a Sec7 domain that mediates the nucleotide exchange reaction, indicating that this effector was likely acquired recently by the pathogen through a horizontal gene transfer mechanism, and through divergent evolution, this protein can now function to subvert ARF function during infection of host cells by L. pneumophila. The protein DrrA (also known as SidM) is a Rab1 GEF (Fig. 3 A) that is required for the recruitment of this GTPase to the LCV (Machner and Isberg, 2006; Murata et al., 2006). Unlike RalF, which has a GEF domain that is highly similar to the GEF domain found in host activators of ARF, the DrrA GEF domain has no sequence or structural similarities to any known eukaryotic Rab GEFs but interacts precisely with the switch regions of Rab1 to function as a highly specific GEF for this GTPase (Machner and Isberg, 2006; Ingmundson et al., 2007; Schoebel et al., 2009; Suh et al., 2010; Zhu et al., 2010). DrrA also contains a structurally defined PI4P-binding domain that mediates association of the effector protein with the vacuole in which L. pneumophila resides (Brombacher et al., 2009; Schoebel et al., 2009; Zhu et al., 2010). Thus, convergent evolution has resulted in a bacterial protein that subverts the PI signature on the LCV membrane to specifically recruit and activate Rab1 on the vacuole in which the pathogen resides.
The S. Typhimurium T3SS effector SopE was one of the first bacterial proteins shown to function directly as an activator of small GTPases (Hardt et al., 1998). SopE is important for stimulating membrane ruffling during S. Typhimurium contact with epithelial cells. S. Typhimurium caught in membrane ruffles are engulfed and localize inside of macropinocytic vesicles inside the host cell. Biochemical analysis of SopE revealed that this bacterial protein could bind and activate the GTPases Rac1 and Cdc42 by inducing a conformational change in their switch region to catalyze the exchange of GDP for GTP (Fig. 3 A). Activation of the GTPase at the site of bacterial contact would then promote the membrane ruffling process by stimulating assembly of an actin-based network at the plasma membrane, mimicking signals that lead to the formation of filopodial extensions. Additional biochemical studies found that SopE has a close homologue called SopE2 that is specific for Cdc42 (Friebel et al., 2001). It has also been reported that SopE can act as a GEF for Rab5 (Mukherjee et al., 2001), although Rab5 activation by S. Typhimurium is likely to be controlled indirectly though the activities of other effectors, including SopB, as previously described (Mallo et al., 2008). Regardless, it is clear that signaling pathways initiated through the activation of Rac1 and Cdc42 by SopE are important for biogenesis of the early vacuole compartment in which S. Typhimurium resides, demonstrating how a bacterial GEF can be used to create a unique pathogen-occupied vacuole in cells that are normally nonphagocytic.
Interestingly, several type III effectors that contain a WxxxE motif were found to regulate cellular dynamics controlled by host GTPases (Alto et al., 2006). Ectopic expression of S. flexneri effectors IpgB1 and IpgB2 led to formation of stress fibers and membrane ruffles (Alto et al., 2006), structures that are regulated by RhoA, suggesting that these effectors could either mimic or activate Rho-dependent processes. Likewise, coexpression of the WxxxE effector SifA from S. Typhimurium with the effector SseJ induced tubulation of endosomes, similar to that induced by constitutively active RhoA expressed together with SseJ, indicating that SifA either mimics or activates a RhoA family GTPase (Ohlson et al., 2008). Clarity on a possible mechanism underlying the function of these effectors was provided when the SifA structure revealed that the C terminus has a fold comparable with the GEF domain in SopE, suggesting that SifA may also have GEF activity. This could explain how this effector promotes changes in the host cytoskeleton that are similar to those observed upon expression of an active RhoA protein (Ohlson et al., 2008). In addition, SifA interacted with GDP-bound RhoA, similar to SopE and other GEFs. This GEF paradigm was further supported by data showing that the enteropathogenic Escherichia coli WxxxE motif protein Map could modulate actin dynamics by functioning as a GEF for Cdc42, and structural studies revealed similarities in the GTPase-binding mechanisms used by Map and SifA (Huang et al., 2009). Thus, these effectors appear to stimulate host GTPases by acting as GEFs and by possibly stabilizing GTPases in an active conformation. Lastly, the activities of type III effectors do not appear to be restricted to Rho family GTPases, as recent structural studies showed that the E. coli type III effector EspG binds ARF GTPases and p21-activated kinases, providing evidence for a unique GTPase–kinase signaling complex (Selyunin et al., 2011).
Bacterial effectors that interfere with GTPase functions.
Although activation of host GTPases can stimulate bacterial internalization and promote transport of vacuoles along novel pathways, deactivation of GTPases that promote internalization and transport of pathogens to lysosomes is an effective strategy to avoid destruction by phagocytes. Whereas direct activation of host GTPases by a bacterial effector is usually mediated by a GEF mimic, strategies available for deactivation of host GTPases are more diverse.
Similar to the mechanism used by eukaryotic cells to deactivate GTPases, many bacterial pathogens have been shown to encode effector proteins that function as GAPs. The SptP protein in S. Typhimurium and the LepB protein in L. pneumophila provide two examples (Fu and Galán, 1999; Ingmundson et al., 2007). SptP has an N-terminal GAP domain that deactivates Rho GTPases (Fu and Galán, 1999; Stebbins and Galán, 2000; Humphreys et al., 2009). SptP counteracts the GEF functions provided by the effectors SopE and SopE2, thereby reestablishing the actin cytoskeleton after uptake of S. Typhimurium (Fig. 3 B). Cytoskeletal stabilization provided by SptP is thought to maintain host cell integrity so that the vacuole containing S. Typhimurium can mature and support replication. The paradigm of a pathogen encoding a GAP that can counteract the activity of a bacterial GEF is also observed for LepB, which is a GAP that deactivates Rab1 (Fig. 3 B; Ingmundson et al., 2007). It is thought that Rab1 deactivation by the L. pneumophila LepB protein might promote the removal of Rab1 from the LCV, which may facilitate maturation of this organelle into an ER-like structure and prevent recruitment of Golgi matrix proteins. An L. pneumophila mutant deficient in LepB, however, has no dramatic defect in the dynamics of Rab1 cycling on the vacuole membrane, suggesting that there could be other effectors produced by L. pneumophila that influence the kinetics of Rab1 association with the vacuole.
A new mechanism by which bacteria can perturb the normal function of GTPases was revealed through studies on a type III effector from Vibrio parahaemolyticus called VopS (Fig. 3 B). This protein was shown to disrupt actin dynamics by interfering with the function of Rho GTPases (Yarbrough et al., 2009). The VopS protein sequence was found to harbor a conserved region of unknown function called a Fic domain. Genetic analysis showed that the VopS Fic region was essential for disrupting actin dynamics. The Fic region was determined to have an adenylyl transferase activity that enabled the VopS protein to use ATP as a substrate to posttranslationally modify host Rho family proteins through the covalent addition of the AMP moiety on a threonine residue in the switch I region of the GTPase, a process that was termed AMPylation. Similar results were obtained for a protein from Histophilus somni called IbpA (Worby et al., 2009), which also has a Fic domain required for disrupting actin dynamics.
Recent structural studies on the L. pneumophila protein DrrA revealed a domain in this effector that was similar to the catalytic domain of glutamine synthetase adenylyl transferase, which is another enzyme that uses ATP as a substrate in an adenylylation reaction (Müller et al., 2010). The DrrA adenylyl transferase domain was outside of the GEF domain in an N-terminal region of the protein shown to be extremely toxic to mammalian cells when produced ectopically (Murata et al., 2006). Consistent with the structural predictions, DrrA was found to function as an adenylyl transferase and mediated the covalent attachment of AMP to a tyrosine residue in the switch II region of Rab1 (Fig. 3 B). Collectively, studies on VopS, IbpA, and DrrA reveal that AMPylation of GTPases represents a general strategy bacteria use to modulate signaling by host GTPases and can be mediated by structurally distinct effectors.
Two recent studies indicate that Rab1 AMPylation by DrrA can be reversed by an effector called SidD, which functions as a de-AMPylating enzyme (Fig. 3 B; Neunuebel et al., 2011; Tan and Luo, 2011). These data suggest that L. pneumophila temporally regulate Rab1 AMPylation, which may be important for controlling the dynamics of Rab1 cycling on the vacuole. AMPylated Rab1 was found to be insensitive to deactivation by the GAP protein LepB, whereas de-AMPylated Rab1 regained sensitivity to GTP hydrolysis stimulated by LepB. Finding a de-AMPylating enzyme, in conjunction with the discovery that eukaryotic organisms also encode proteins with Fic domains, suggests that AMPylation could be a general mechanism to modulate GTPase functions endogenously.
Mass spectrometry was used to investigate modifications to Rab1 that occur during L. pneumophila infection of host cells, and this analysis revealed two different posttranslational modifications to Rab1 that are mediated by bacterial effector proteins (Mukherjee et al., 2011). In addition to Rab1 AMPylation mediated by DrrA, it was found that L. pneumophila also promotes the addition of a phosphocholine moiety onto the serine 79 residue in the switch II region of Rab1A, which is immediately adjacent to the AMPylated tyrosine 80 residue (Fig. 3 B). Interestingly, the L. pneumophila effector protein AnkX was found to be the enzyme directly responsible for Rab1 phosphocholination in a reaction that used cytidine diphosphate–choline as a substrate. This was unexpected because AnkX is a Fic domain–containing protein and was therefore predicted to function as an AMPylating toxin. Similar to the VopS adenylylation reaction, the phosphocholination reaction centers on Fic domain–dependent hydrolysis of the anhydrous bond in a diphosphoryl 5-ribose structure in the donor substrate. In addition to Rab1, AnkX was found to modify Rab35, which is a Rab1 subfamily member that is involved in membrane transport in the early endocytic pathway, suggesting how AnkX could modulate membrane transport in both the secretory and endocytic pathway. Protein phosphocholination had been demonstrated previously for the type IV pili protein of Neisseria gonorrhoeae (Hegge et al., 2004); however, studies on AnkX provide the first demonstration of direct protein phosphocholination by an effector protein and suggest that other Fic domain–containing effectors may function as phosphocholine transferases.
There are other examples of bacterial proteins that alter the covalent structure of GTPases to modulate their function. For example, large clostridial toxins, such as Clostridium difficile toxin A and toxin B and Clostridium novyi α toxin, are glycosyltransferases that modify the class I switch region of Rho, Rac, and Cdc42 (Just et al., 1995). A Yersinia pestis T3SS effector called YopT is a protease that cleaves a C-terminal residue in Rho GTPases to interfere with their function by removing the lipidation motif needed for association with membranes (Fig. 3 B; Shao et al., 2002). Given the additional paradigms by which microbial factors can modulate Rho GTPases, it is likely that these and other strategies will be revealed for modulation of Rab GTPases and other membrane traffic regulators.
Conclusions and future perspectives
In addition to providing important details on the mechanism that pathogens use to infect host cells, the study of these organisms can also lead to a better understanding of normal cellular processes. Because sequential transport of membrane and cargo through eukaryotic cells is essential for the biogenesis and maintenance of distinct organelles, disruptions in membrane transport at a specific stage can indirectly affect biogenesis of multiple organelles in the cell, making it difficult to address the contribution of a host factor in a specific membrane transport process. In this regard, studying the mechanisms by which pathogens can manipulate membrane transport can be very insightful. For instance, the fact that the L. pneumophila DrrA protein is sufficient to recruit Rab1 to the vacuole in which the bacterium resides provides proof of principle that localization of a GEF is sufficient to mediate the accumulation of a specific Rab GTPase to an organelle. This is also true for localization of ARF mediated by RalF. Interestingly, these examples also show that localization of the active GTPase is not sufficient for the recruitment of cognate host effectors specific for the GTPase. In the case of RalF-mediated recruitment of ARF to vacuoles, it was initially surprising to find an organelle in the cell that was highly enriched for this GTPase that did not demonstrate the recruitment of coat proteins such as COPI, which are typically found on organelles displaying high levels of ARF. A similar example of a pathogen creating membrane domains enriched for ARF that were devoid of COPI was reported during infection of cells by coxsackievirus, and it was found that ARF was recruiting host PI4 kinase IIIβ to these membranes to generate a novel pool of PI4P that was recognized by viral proteins mediating genome replication (Hsu et al., 2010). Thus, studying pathogen manipulation of host membrane transport provides a unique perspective on how GTPases and their cognate effectors confer distinct properties to organelles.
The modulation of phosphoinositides, the cytoskeleton, and small GTPases represent common strategies for microbial manipulation of membrane transport; however, there are certainly additional strategies shared by other pathogens. Bacterial manipulation of tethering proteins, vesicular coats, and fusion factors, such as SNAREs, have emerged as other mechanisms to control membrane transport that have not been discussed but are equally interesting and important. Indeed, it is likely that bacteria have evolved mechanisms to mimic and control aspects of membrane transport that remain to be discovered by cell biologists. In this regard, studies that investigate bacterial manipulation of membrane transport will not only continue to advance our basic understanding of how intracellular pathogens create organelles that support replication but will also continue to advance our understanding of how normal cellular activities are regulated. In addition to discovering new activities that allow pathogens to subvert host cell functions, it is also important to understand how these proteins function in unity to generate the organelles in which microbes prosper. Determining how proteins with potentially antagonistic activities, such as DrrA and LepB, are regulated spatially and temporally is key to determining the stages in vacuole maturation in which their functions are most important. Along these same lines, it is important to more fully understand the function and eventual fate of proteins that are posttranslationally modified by microbial effectors. Thus, many interesting questions remain, and studies on microbial manipulation of host membrane transport should remain a fertile and exciting field for years to come.
Acknowledgments
Illustrations were provided by Neil Smith, neil@neilsmithillustration.co.uk.
This work was supported by National Institutes of Health grants AI41699 and AI64559 (to C.R. Roy), an Anna Fuller Postdoctoral Fellowship Award (to S. Mukherjee), and an European Molecular Biology Organization Postdoctoral Fellowship Award (to E. Alix).
Footnotes
Abbreviations used in this paper:
- ARF
- ADP ribosylation factor
- GAP
- GTPase-activating protein
- GEF
- guanine nucleotide exchange factor
- LCV
- L. pneumophila–containing vacuole
- ManLAM
- mannose-capped lipoarabinomannan
- MCV
- Mtb-containing vacuole
- Mtb
- Mycobacterium tuberculosis
- PI
- phosphatidylinositol
- PIP
- PI phosphate
- PIM
- PI mannoside
- SCV
- S. Typhimurium–containing vacuole
- SIF
- S. Typhimurium–induced filaments
References
- Alto N.M., Shao F., Lazar C.S., Brost R.L., Chua G., Mattoo S., McMahon S.A., Ghosh P., Hughes T.R., Boone C., Dixon J.E. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell. 124:133–145 10.1016/j.cell.2005.10.031 [DOI] [PubMed] [Google Scholar]
- Bakowski M.A., Braun V., Brumell J.H. 2008. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 9:2022–2031 10.1111/j.1600-0854.2008.00827.x [DOI] [PubMed] [Google Scholar]
- Bakowski M.A., Braun V., Lam G.Y., Yeung T., Heo W.D., Meyer T., Finlay B.B., Grinstein S., Brumell J.H. 2010. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe. 7:453–462 10.1016/j.chom.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Beatty W.L., Rhoades E.R., Ullrich H.J., Chatterjee D., Heuser J.E., Russell D.G. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 1:235–247 10.1034/j.1600-0854.2000.010306.x [DOI] [PubMed] [Google Scholar]
- Boucrot E., Henry T., Borg J.P., Gorvel J.P., Méresse S. 2005. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 308:1174–1178 10.1126/science.1110225 [DOI] [PubMed] [Google Scholar]
- Braun V., Wong A., Landekic M., Hong W.J., Grinstein S., Brumell J.H. 2010. Sorting nexin 3 (SNX3) is a component of a tubular endosomal network induced by Salmonella and involved in maturation of the Salmonella-containing vacuole. Cell. Microbiol. 12:1352–1367 10.1111/j.1462-5822.2010.01476.x [DOI] [PubMed] [Google Scholar]
- Brombacher E., Urwyler S., Ragaz C., Weber S.S., Kami K., Overduin M., Hilbi H. 2009. Rab1 guanine nucleotide exchange factor SidM is a major phosphatidylinositol 4-phosphate-binding effector protein of Legionella pneumophila. J. Biol. Chem. 284:4846–4856 10.1074/jbc.M807505200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujny M.V., Ewels P.A., Humphrey S., Attar N., Jepson M.A., Cullen P.J. 2008. Sorting nexin-1 defines an early phase of Salmonella-containing vacuole-remodeling during Salmonella infection. J. Cell Sci. 121:2027–2036 10.1242/jcs.018432 [DOI] [PubMed] [Google Scholar]
- Dai S., Zhang Y., Weimbs T., Yaffe M.B., Zhou D. 2007. Bacteria-generated PtdIns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic. 8:1365–1374 10.1111/j.1600-0854.2007.00613.x [DOI] [PubMed] [Google Scholar]
- Derré I., Isberg R.R. 2004. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:3048–3053 10.1128/IAI.72.5.3048-3053.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G., De Camilli P. 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature. 443:651–657 10.1038/nature05185 [DOI] [PubMed] [Google Scholar]
- Drecktrah D., Knodler L.A., Howe D., Steele-Mortimer O. 2007. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic. 8:212–225 10.1111/j.1600-0854.2006.00529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A., Boucrot E., Drevensek S., Daire V., Gorvel J.P., Poüs C., Holden D.W., Méresse S. 2010. SKIP, the host target of the Salmonella virulence factor SifA, promotes kinesin-1-dependent vacuolar membrane exchanges. Traffic. 11:899–911 10.1111/j.1600-0854.2010.01069.x [DOI] [PubMed] [Google Scholar]
- Elwell C.A., Ceesay A., Kim J.H., Kalman D., Engel J.N. 2008. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 4:e1000021 10.1371/journal.ppat.1000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti R.A., Backer J.M., Gruenberg J., Corvera S., Deretic V. 2001. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154:631–644 10.1083/jcb.200106049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti R.A., Chua J., Vergne I., Deretic V. 2003. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA. 100:5437–5442 10.1073/pnas.0737613100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebel A., Ilchmann H., Aepfelbacher M., Ehrbar K., Machleidt W., Hardt W.D. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035–34040 10.1074/jbc.M100609200 [DOI] [PubMed] [Google Scholar]
- Fu Y., Galán J.E. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 401:293–297 10.1038/45829 [DOI] [PubMed] [Google Scholar]
- Garcia-del Portillo F., Zwick M.B., Leung K.Y., Finlay B.B. 1993. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc. Natl. Acad. Sci. USA. 90:10544–10548 10.1073/pnas.90.22.10544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Kuhn M. 2000. Bacterial replication in the host cell cytosol. Curr. Opin. Microbiol. 3:49–53 10.1016/S1369-5274(99)00050-8 [DOI] [PubMed] [Google Scholar]
- Grieshaber S.S., Grieshaber N.A., Hackstadt T. 2003. Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J. Cell Sci. 116:3793–3802 10.1242/jcs.00695 [DOI] [PubMed] [Google Scholar]
- Hardt W.D., Chen L.M., Schuebel K.E., Bustelo X.R., Galán J.E. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 93:815–826 10.1016/S0092-8674(00)81442-7 [DOI] [PubMed] [Google Scholar]
- Hegge F.T., Hitchen P.G., Aas F.E., Kristiansen H., Løvold C., Egge-Jacobsen W., Panico M., Leong W.Y., Bull V., Virji M., et al. 2004. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc. Natl. Acad. Sci. USA. 101:10798–10803 10.1073/pnas.0402397101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T., Couillault C., Rockenfeller P., Boucrot E., Dumont A., Schroeder N., Hermant A., Knodler L.A., Lecine P., Steele-Mortimer O., et al. 2006. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc. Natl. Acad. Sci. USA. 103:13497–13502 10.1073/pnas.0605443103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L.D., Hueffer K., Wenk M.R., Galán J.E. 2004. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 304:1805–1807 10.1126/science.1098188 [DOI] [PubMed] [Google Scholar]
- Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T., et al. 2010. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 141:799–811 10.1016/j.cell.2010.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Sutton S.E., Wallenfang A.J., Orchard R.C., Wu X., Feng Y., Chai J., Alto N.M. 2009. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat. Struct. Mol. Biol. 16:853–860 10.1038/nsmb.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys D., Hume P.J., Koronakis V. 2009. The Salmonella effector SptP dephosphorylates host AAA+ ATPase VCP to promote development of its intracellular replicative niche. Cell Host Microbe. 5:225–233 10.1016/j.chom.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybiske K., Stephens R.S. 2007. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc. Natl. Acad. Sci. USA. 104:11430–11435 10.1073/pnas.0703218104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmundson A., Delprato A., Lambright D.G., Roy C.R. 2007. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 450:365–369 10.1038/nature06336 [DOI] [PubMed] [Google Scholar]
- Jewett T.J., Dooley C.A., Mead D.J., Hackstadt T. 2008. Chlamydia trachomatis tarp is phosphorylated by src family tyrosine kinases. Biochem. Biophys. Res. Commun. 371:339–344 10.1016/j.bbrc.2008.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just I., Selzer J., Wilm M., von Eichel-Streiber C., Mann M., Aktories K. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 375:500–503 10.1038/375500a0 [DOI] [PubMed] [Google Scholar]
- Kagan J.C., Roy C.R. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945–954 10.1038/ncb883 [DOI] [PubMed] [Google Scholar]
- Kagan J.C., Stein M.P., Pypaert M., Roy C.R. 2004. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J. Exp. Med. 199:1201–1211 10.1084/jem.20031706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M.C., Wang J.T., Castro N.A., Hamilton N.A., Town L., Brown D.L., Meunier F.A., Brown N.F., Stow J.L., Teasdale R.D. 2010. Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J. 29:1331–1347 10.1038/emboj.2010.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen J.M., Ravichandran K.S. 2008. Phagosome maturation: going through the acid test. Nat. Rev. Mol. Cell Biol. 9:781–795 10.1038/nrm2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler L.A., Steele-Mortimer O. 2005. The Salmonella effector PipB2 affects late endosome/lysosome distribution to mediate Sif extension. Mol. Biol. Cell. 16:4108–4123 10.1091/mbc.E05-04-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Y., Valdivia R.H. 2008. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 4:159–169 10.1016/j.chom.2008.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane B.J., Mutchler C., Al Khodor S., Grieshaber S.S., Carabeo R.A. 2008. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog. 4:e1000014 10.1371/journal.ppat.1000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner M.P., Isberg R.R. 2006. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev. Cell. 11:47–56 10.1016/j.devcel.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Mallo G.V., Espina M., Smith A.C., Terebiznik M.R., Alemán A., Finlay B.B., Rameh L.E., Grinstein S., Brumell J.H. 2008. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J. Cell Biol. 182:741–752 10.1083/jcb.200804131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K., Parashuraman S., Raje M., Mukhopadhyay A. 2001. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J. Biol. Chem. 276:23607–23615 10.1074/jbc.M101034200 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Liu X., Arasaki K., McDonough J., Galán J.E., Roy C.R. 2011. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature. 477:103–106 10.1038/nature10335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.P., Peters H., Blümer J., Blankenfeldt W., Goody R.S., Itzen A. 2010. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science. 329:946–949 10.1126/science.1192276 [DOI] [PubMed] [Google Scholar]
- Murata T., Delprato A., Ingmundson A., Toomre D.K., Lambright D.G., Roy C.R. 2006. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat. Cell Biol. 8:971–977 10.1038/ncb1463 [DOI] [PubMed] [Google Scholar]
- Nagai H., Kagan J.C., Zhu X., Kahn R.A., Roy C.R. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 295:679–682 10.1126/science.1067025 [DOI] [PubMed] [Google Scholar]
- Neunuebel M.R., Chen Y., Gaspar A.H., Backlund P.S., Jr, Yergey A., Machner M.P. 2011. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science. 333:453–456 10.1126/science.1207193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris F.A., Wilson M.P., Wallis T.S., Galyov E.E., Majerus P.W. 1998. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA. 95:14057–14059 10.1073/pnas.95.24.14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlson M.B., Huang Z., Alto N.M., Blanc M.P., Dixon J.E., Chai J., Miller S.I. 2008. Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe. 4:434–446 10.1016/j.chom.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.C., Hueffer K., Lam T.T., Galán J.E. 2009. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell. 137:283–294 10.1016/j.cell.2009.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips J.A. 2008. Mycobacterial manipulation of vacuolar sorting. Cell. Microbiol. 10:2408–2415 10.1111/j.1462-5822.2008.01239.x [DOI] [PubMed] [Google Scholar]
- Purdy G.E., Owens R.M., Bennett L., Russell D.G., Butcher B.A. 2005. Kinetics of phosphatidylinositol-3-phosphate acquisition differ between IgG bead-containing phagosomes and Mycobacterium tuberculosis-containing phagosomes. Cell. Microbiol. 7:1627–1634 10.1111/j.1462-5822.2005.00580.x [DOI] [PubMed] [Google Scholar]
- Rink J., Ghigo E., Kalaidzidis Y., Zerial M. 2005. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 122:735–749 10.1016/j.cell.2005.06.043 [DOI] [PubMed] [Google Scholar]
- Schoebel S., Oesterlin L.K., Blankenfeldt W., Goody R.S., Itzen A. 2009. RabGDI displacement by DrrA from Legionella is a consequence of its guanine nucleotide exchange activity. Mol. Cell. 36:1060–1072 10.1016/j.molcel.2009.11.014 [DOI] [PubMed] [Google Scholar]
- Schroeder N., Mota L.J., Méresse S. 2011. Salmonella-induced tubular networks. Trends Microbiol. 19:268–277 10.1016/j.tim.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Selyunin A.S., Sutton S.E., Weigele B.A., Reddick L.E., Orchard R.C., Bresson S.M., Tomchick D.R., Alto N.M. 2011. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature. 469:107–111 10.1038/nature09593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F., Merritt P.M., Bao Z., Innes R.W., Dixon J.E. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell. 109:575–588 10.1016/S0092-8674(02)00766-3 [DOI] [PubMed] [Google Scholar]
- Soldati T., Schliwa M. 2006. Powering membrane traffic in endocytosis and recycling. Nat. Rev. Mol. Cell Biol. 7:897–908 10.1038/nrm2060 [DOI] [PubMed] [Google Scholar]
- Stebbins C.E., Galán J.E. 2000. Modulation of host signaling by a bacterial mimic: structure of the Salmonella effector SptP bound to Rac1. Mol. Cell. 6:1449–1460 10.1016/S1097-2765(00)00141-6 [DOI] [PubMed] [Google Scholar]
- Suh H.Y., Lee D.W., Lee K.H., Ku B., Choi S.J., Woo J.S., Kim Y.G., Oh B.H. 2010. Structural insights into the dual nucleotide exchange and GDI displacement activity of SidM/DrrA. EMBO J. 29:496–504 10.1038/emboj.2009.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y., Luo Z.Q. 2011. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature. 475:506–509 10.1038/nature10307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terebiznik M.R., Vieira O.V., Marcus S.L., Slade A., Yip C.M., Trimble W.S., Meyer T., Finlay B.B., Grinstein S. 2002. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 4:766–773 10.1038/ncb854 [DOI] [PubMed] [Google Scholar]
- van der Meer-Janssen Y.P., van Galen J., Batenburg J.J., Helms J.B. 2010. Lipids in host-pathogen interactions: pathogens exploit the complexity of the host cell lipidome. Prog. Lipid Res. 49:1–26 10.1016/j.plipres.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I., Chua J., Deretic V. 2003. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198:653–659 10.1084/jem.20030527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I., Fratti R.A., Hill P.J., Chua J., Belisle J., Deretic V. 2004. Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell. 15:751–760 10.1091/mbc.E03-05-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I., Chua J., Lee H.H., Lucas M., Belisle J., Deretic V. 2005. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 102:4033–4038 10.1073/pnas.0409716102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via L.E., Deretic D., Ulmer R.J., Hibler N.S., Huber L.A., Deretic V. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326–13331 10.1074/jbc.272.20.13326 [DOI] [PubMed] [Google Scholar]
- Vieira O.V., Harrison R.E., Scott C.C., Stenmark H., Alexander D., Liu J., Gruenberg J., Schreiber A.D., Grinstein S. 2004. Acquisition of Hrs, an essential component of phagosomal maturation, is impaired by Mycobacteria. Mol. Cell. Biol. 24:4593–4604 10.1128/MCB.24.10.4593-4604.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth D.E., Heinzen R.A. 2007. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 9:829–840 10.1111/j.1462-5822.2007.00901.x [DOI] [PubMed] [Google Scholar]
- Weber S.S., Ragaz C., Hilbi H. 2009. Pathogen trafficking pathways and host phosphoinositide metabolism. Mol. Microbiol. 71:1341–1352 10.1111/j.1365-2958.2009.06608.x [DOI] [PubMed] [Google Scholar]
- Worby C.A., Mattoo S., Kruger R.P., Corbeil L.B., Koller A., Mendez J.C., Zekarias B., Lazar C., Dixon J.E. 2009. The fic domain: regulation of cell signaling by adenylylation. Mol. Cell. 34:93–103 10.1016/j.molcel.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough M.L., Li Y., Kinch L.N., Grishin N.V., Ball H.L., Orth K. 2009. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science. 323:269–272 10.1126/science.1166382 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Hu L., Zhou Y., Yao Q., Liu L., Shao F. 2010. Structural mechanism of host Rab1 activation by the bifunctional Legionella type IV effector SidM/DrrA. Proc. Natl. Acad. Sci. USA. 107:4699–4704 10.1073/pnas.0914231107 [DOI] [PMC free article] [PubMed] [Google Scholar]