Abstract
A new study in this issue (De Saint-Jean et al. 2011. J. Cell Biol. http://dx.doi.org/jcb.201104062) reveals that the sterol transfer protein Osh4p can also transport the signaling phospholipid phosphatidylinositol 4-phosphate (PI(4)P), which binds to the same site in Osh4p as sterol. This finding helps explain some previously published studies and also indicates that lipid/sterol exchange could contribute to establishing a sterol gradient in cells.
It had long been known that intracellular sterol traffic is rapid (t1/2 = 10 min), nonvesicular, and requires ATP (DeGrella and Simoni, 1982). Osh4p (also known as Kes1p) is in a large family of proteins (7 in yeast, 12 in humans) that share an oxysterol binding protein-related domain (ORD) of ∼360 amino acids. Osh4p transfers sterol from donor to acceptor liposomes in vitro (Raychaudhuri et al., 2006) and was previously crystallized in a 1:1 complex with a sterol or oxysterol in an internal hydrophobic pocket that is covered by a hinged lid (Im et al., 2005). These results suggested that ORD proteins could be the sterol trafficking machines inside cells. Biological evidence that sterol traffic is a shared function of ORD proteins was sought using yeast lacking all seven ORD genes. In these cells, sterol traffic was much reduced, but not eliminated (Raychaudhuri et al., 2006; Sullivan et al., 2006), leaving the question unresolved. Another possible role for ORDs is that they are sterol sensors (Wang et al., 2005), a functional category that has been proposed for phosphatidylinositol (PI)/phosphatidylcholine (PC) transfer proteins (PITPs) such as Sec14p, which may act as a lipid sensor at the TGN, not transferring lipids but conveying information about lipid status to components of the secretory pathway (McGee et al., 1994). Finally, ORDs may be involved in some aspect of sterol traffic other than bulk movement out of the ER (Georgiev et al., 2011).
ORD proteins were first connected to phosphoinositol phosphates (PIPs) because they can contain pleckstrin homology domains that bind to PI 4-phosphate (PI(4)P) and PI 4,5-bisphosphate (PI(4,5)P2) to mediate their targeting to the TGN or plasma membrane (PM). Osh4p has no pleckstrin homology domain, but it transfers sterol much faster if liposomes contain anionic lipids such as phosphatidylserine, PI(4)P, and PI(4,5)P2 (Raychaudhuri et al., 2006). This was attributed to two positively charged patches on the surface of Osh4p-binding anionic headgroups to increase access of Osh4p to the bilayer (Fig. 1). However, Schulz et al. (2009) then found that for Osh4p, not all PIPs are equal. Although PI(4,5)P2 stimulates sterol occupancy, under the same conditions, PI(4)P inhibited sterol occupancy (Schulz et al., 2009). The special relationship between Osh4p and PI(4)P was the starting point for De Saint-Jean et al. in this issue, who applied a new approach to study sterol transfer: monitoring protein–lipid interaction in real time using FRET between Osh4p and dehydroergosterol, a fluorescent cholesterol analogue. As before, they found that all anionic lipids stimulate sterol traffic. In most cases, sterol occupancy increases with traffic, but PI(4)P uniquely inhibits sterol occupancy, and even drives sterol out of Osh4p. This pointed to a simple mechanism: competitive binding of PI(4)P to the same internal site as sterol. They obtained powerful support for this with a new structure of Osh4p co-crystallized with PI(4)P. The pocket that binds sterol (headgroup down) also binds PI(4)P (headgroup up). The acyl chains of PI(4)P are accommodated in the sterol-binding pocket, and the PI(4)P headgroup is recognized by a secondary pocket under the lid. The Osh4p structure does not accept PI(3)P or PI(4,5)P2 (De Saint-Jean et al., 2011), but further work with other ORD proteins is needed to determine their PIP specificity. The new structure will also stimulate a fresh look at unsuspected cargoes for other lipid transfer proteins.
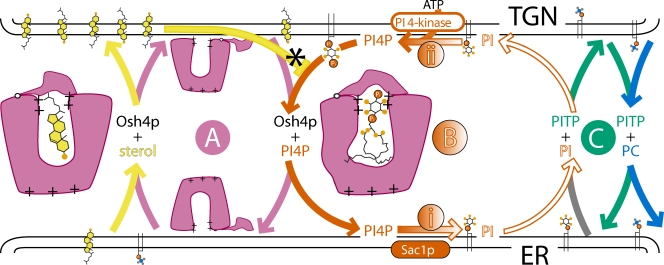
Figure 1.
Cycling by two lipid transfer proteins and a “futile cycle” of PI(4)P production can drive sterol traffic. Osh4p can exist in one of three states: empty, bound to sterol, or bound to phosphatidylinositol 4-phosphate PI(4)P, altering its structure slightly. PI(4)P synthesis potentially drives sterol up a concentration gradient. (A) Osh4p (pink) exchanges sterol forward (yellow) for PI(4)P back (red). (B, i) PI(4)P is dephosphorylated by Sac1p. (C) PITP (PI/PC transfer protein, green; detail omitted) exchanges PC (blue) into the ER and PI (white) forward. (B, ii) PI is phosphorylated. For the cost of one ATP, PI goes round an apparently futile cycle. A key point is that after bringing PI(4)P back to the ER, Osh4p will most likely fill up with sterol. However, extraction of PI(4)P at the TGN is limited because sterol competes for binding with PI(4)P (asterisk). An ER-TGN cycle is shown, but an ER-PM cycle would be similar, possibly using a different ORD protein.
The new finding explains several observations in the literature. Osh4p and other ORD proteins interact with the PIP-phosphatase Sac1p, stimulating its activity (Stefan et al., 2011). Here the salient fact is that Sac1p is anchored in the ER, even though most PI(4)P is elsewhere: on the TGN and PM. Therefore, Stefan et al. (2011) suggested that Sac1p cleaves PI(4)P in the PM in trans across a 20-nm gap, after recruitment by ORD proteins to cortical ER-to-PM contact sites. Though attractive, this idea fails to account for one observation: cells lacking Sac1p accumulate a pool of excess PI(4)P on the ER (Li et al., 2002). Because this PI(4)P is made by the PI 4-kinase II-α Stt4p (Foti et al., 2001), which is located at the PM, transfer of PI(4)P from the PM to the ER by ORD proteins might be part of the explanation. Certainly, the Osh4p–Sac1p interaction might help Osh4p deliver PI(4)P to the ER (Fig. 1, A and B).
Another aspect of Osh4p is its toxicity. Endogenous expression of Osh4p kills cells when PI(4)P in the TGN is limiting, which occurs with inactivation of either the PI 4-kinase III-β Pik1p (Fairn et al., 2007) or Sec14p (Li et al., 2002). This toxic effect is mitigated not only by deletion of Osh4p, but also by deleting Sac1p. One explanation for the toxicity is that Osh4p, which is present at very high copy number (30,000 copies per cell), simply binds to up to 30,000 PI(4)P molecules in the TGN, and titrates out other essential PI(4)P-binding proteins (Fairn et al., 2007). Until now, Osh4p was only known to bind PI(4)P by its headgroup, so this titration theory was incompatible with the localization of Osh4p, which targets membranes poorly, even though other highly expressed PI(4)P-binding proteins target the TGN tightly. Solubilization of PI(4)P addresses this inconsistency, as all the Osh4p can bind PI(4)P without remaining at the TGN. The new result also goes some way to explain the recent finding that the Y97F mutant of Osh4p is dominantly super-toxic (Alfaro et al., 2011). This mutation in Osh4p abolishes sterol occupancy, but it may not affect PI(4)P binding. Assuming that the mutant traffics PI(4)P but not sterol, it will be able to extract far more PI(4)P than normal from the TGN or PM. This is because extraction of PI(4)P from these sites would normally be limited by its low concentration compared with sterol, which competes with it for back-extraction (Fig. 1, asterisk).
With all these results, is it resolved whether Osh4p is a transporter or a sensor? Subtle differences in Osh4p structure depending on its internal content would allow ORDs to act as a dual-specific sensor. Both models (transporter and sensor) can accommodate the idea that an imbalance between Osh4p-PI(4)P and Osh4p-sterol is toxic. However, where they differ is in the final localizations of lipid. Sterol might be hard to follow, but PI(4)P is readily detected in live cells. If Osh4p transports PI(4)P, one might predict that accumulation of PI(4)P at the ER in Δsac1 cells requires ORD proteins. Likewise, the toxic Osh4p(Y97F) mutant might be expected to extract PI(4)P out of the TGN, post-Golgi carriers, and maybe even the PM (Alfaro et al., 2011). Such predictions will have to be tested experimentally, and then expanded to other ORDs.
Considering just the transporter model, De Saint-Jean et al. (2011) attempt to integrate other lipid traffic in the secretory pathway with the reciprocal relationship they have discovered between sterol and PI(4)P. They suggest that sterol/PITP exchange by Osh4p may be coupled to PI/PC exchange by a PITP such as Sec14p, leading to net traffic of sterol out of the ER to the TGN or PM (Fig. 1, A and C). Such cycles of lipid traffic could be driven by the conversion of PI(4)P to PI in the ER, and resynthesis of PI(4)P in the late secretory pathway (Fig. 1 B). This would explain how energy from ATP is used to transfer sterols out of the ER up a concentration gradient (analogous to antiporter transport across biological membranes). For now, this scheme remains highly speculative, in particular because it is not certain that ORD proteins are involved in sterol traffic (Georgiev et al., 2011).
References
- Alfaro G., Johansen J., Dighe S.A., Duamel G., Kozminski K.G., Beh C.T. 2011. The sterol-binding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic. 12:1521–1536 10.1111/j.1600-0854.2011.01265.x [DOI] [PubMed] [Google Scholar]
- DeGrella R.F., Simoni R.D. 1982. Intracellular transport of cholesterol to the plasma membrane. J. Biol. Chem. 257:14256–14262 [PubMed] [Google Scholar]
- De Saint-Jean M., Delfosse V., Douguet D., Chicanne G., Payrastre B., Bourguet W., Antonny B., Drin G. 2011. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J. Cell Biol. 195:965–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairn G.D., Curwin A.J., Stefan C.J., McMaster C.R. 2007. The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc. Natl. Acad. Sci. USA. 104:15352–15357 10.1073/pnas.0705571104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti M., Audhya A., Emr S.D. 2001. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol. Biol. Cell. 12:2396–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev A.G., Sullivan D.P., Kersting M.C., Dittman J.S., Beh C.T., Menon A.K. 2011. Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic. 12:1341–1355 10.1111/j.1600-0854.2011.01234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im Y.J., Raychaudhuri S., Prinz W.A., Hurley J.H. 2005. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 437:154–158 10.1038/nature03923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Rivas M.P., Fang M., Marchena J., Mehrotra B., Chaudhary A., Feng L., Prestwich G.D., Bankaitis V.A. 2002. Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J. Cell Biol. 157:63–77 10.1083/jcb.200201037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T.P., Skinner H.B., Whitters E.A., Henry S.A., Bankaitis V.A. 1994. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J. Cell Biol. 124:273–287 10.1083/jcb.124.3.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S., Im Y.J., Hurley J.H., Prinz W.A. 2006. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J. Cell Biol. 173:107–119 10.1083/jcb.200510084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T.A., Choi M.G., Raychaudhuri S., Mears J.A., Ghirlando R., Hinshaw J.E., Prinz W.A. 2009. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J. Cell Biol. 187:889–903 10.1083/jcb.200905007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan C.J., Manford A.G., Baird D., Yamada-Hanff J., Mao Y., Emr S.D. 2011. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 144:389–401 10.1016/j.cell.2010.12.034 [DOI] [PubMed] [Google Scholar]
- Sullivan D.P., Ohvo-Rekilä H., Baumann N.A., Beh C.T., Menon A.K. 2006. Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem. Soc. Trans. 34:356–358 10.1042/BST0340356 [DOI] [PubMed] [Google Scholar]
- Wang P.Y., Weng J., Anderson R.G. 2005. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 307:1472–1476 10.1126/science.1107710 [DOI] [PubMed] [Google Scholar]