Abstract
This study reports the first production of offspring with vitrified sperm from a live-bearing fish Xiphophorus hellerii. The overall goal of this study was to develop streamlined protocols for integration into a standardized approach for vitrification of aquatic species germplasm. The objectives were to (1) estimate acute toxicity of cryoprotectants, (2) evaluate vitrification solutions, (3) compare different thawing methods, (4) evaluate membrane integrity of post-thaw sperm vitrified in different cryoprotectants, and (5) evaluate the fertility of vitrified sperm. Nine cryoprotectants and two commercial vitrification additives were tested for acute toxicity and glass forming ability, alone and in combination. Two vitrification solutions, 40% glycerol (Gly) and 20% Gly+20% ethylene glycol (EG) in 500 mOsmol/kg Hanks' balanced salt solution (HBSS), were selected for vitrification of 10 μL sperm samples using inoculating loops plunged into liquid nitrogen. Samples were thawed at 24°C (one loop in 5 μL of HBSS or three loops in 500 μL of HBSS). Samples thawed in 500 μL were concentrated by centrifugation (1000 g for 5 min at 4°C) into 5 μL for artificial insemination. Offspring were produced from virgin females inseminated with sperm vitrified with 20% Gly+20% EG and concentrated by centrifugation.
Introduction
Aquarium fish models such as zebrafish (Danio rerio), medaka (Oryzias latipes), and Xiphophorus have provided useful tools for the study of human diseases. Fishes are one of the few vertebrate model systems that can be used for high-throughput bioassays while, at the same time, providing physiologically relevant data derived from a whole organism.1 For example, the use of Xiphophorus fishes in cancer research dates back to the 1920s, when it was discovered that certain hybrids of platyfish (Xiphophorus maculatus) and green swordtails (Xiphophorus hellerii) develop melanomas spontaneously.2 Models of spontaneous and induced carcinogenesis for several tumor varieties can be produced by selective backcrossing among the 27 described Xiphophorus species. The Xiphophorus Genetic Stock Center (XGSC, www.xiphophorus.org) maintains >57 pedigreed lines. Several of these lines have been inbred in the XGSC for >50 years.3 In addition to cancer research, Xiphophorus fishes are also used in other fields of study, including evolution, behavioral ecology, sex determination, and for biogeographical systematic molecular events leading to speciation (recently reviewed by Walter 2011).4 Further, the genome (∼830 MB) of X. maculatus (Jp 163 A strain), in its 109th generation of inbreeding, has recently been sequenced and a draft assembly produced (R. Walter, pers. comm.). This allows study of genetic regulation at the molecular level.5
Because of the short generation time, high fecundity, and easy maintenance of large numbers in a relatively small space, several thousand mutants and transgenic lines of oviparous aquarium fish models have been produced.6,7 Similar to the husbandry space limitations encountered in mouse breeding facilities, the large number of strains increases the cost of labor and maintenance of these facilities and is becoming overwhelming.8 Thus, there is a risk that many valuable strains could become lost and presents an immediate need to improve preservation of genetic resources from aquarium fish models.9 Further, many wild populations of these fishes have become imperiled. For example, human activities have negatively impacted the natural habitat of Xiphophorus fishes leading to the decline of wild populations.10 Six species of this genus are imperiled, four of which are classified as severely endangered (X. couchianus, X. gordoni, X. meyeri, and X. milleri).11 Although population studies on these species in the wild are far from complete, conservation efforts need not be delayed while awaiting more thorough assessments. Cryopreservation is a technique that may be employed to address the need for preservation of these valuable research lines and for restoration or protection of imperiled species.12
Sperm cryopreservation efforts in Xiphophorus confront significant challenges. Xiphophorus are characterized by a small body size (2–4 cm), and fertilization is internal, so artificial insemination is needed for fertility estimation of cryopreserved sperm. In addition, sperm sample availability is limited. In X. hellerii the maximum sperm volume available was calculated to be 9.2 μL,13 whereas for X. couchianus the maximum volume was <5 μL.14 Small (μL) sample volume limits experimental replication and the numbers of treatments.12 Despite these limitations, live young have been produced from cryopreserved sperm in X. hellerii,15 X. couchianus,16 X. maculatus,17 and X. variatus (Yang et al., unpublished data) since the first research on sperm cryopreservation of Xiphophorus fishes in 2004.13
Vitrification is a form of cryopreservation that utilizes rapid cooling rates (>1000°C/min compared with <40°C/min for conventional cryopreservation) and high concentrations of cryoprotectants (40%–60% compared with 5%–15% for conventional cryopreservation) to form glass (noncrystalline ice). The ultra-rapid cooling is typically achieved by plunging samples directly into the liquid nitrogen.18,19 In general, the smaller the sample volume, the higher the cooling rate, and the higher the probability of vitrification. Vitrification is therefore suited to cryopreservation of small volumes, and offers advantages for use in laboratory and field environments. Previous attempts have been made to cryopreserve fish sperm by direct plunging into liquid nitrogen, but inconsistent results were obtained.20 In one study that focused on standard cryopreservation in the X. hellerii, minimal motility (<1%) was observed when 0.25-mL straws containing 80 μL of sperm in 14% glycerol (Gly) were plunged into liquid nitrogen,21 possibly because complete vitrification was not attained due to the low concentration of cryoprotectant and the relatively large sample volume used. Successful vitrification has previously been reported for sperm of mammals (human)22 and channel catfish (Ictalurus punctatus)20 by employment of loops designed to hold small sample volumes (20 μL). Very recently the success (post-thaw motility as high as 86%) of cryoprotectant-free vitrification in rainbow trout (Oncorhynchus mykiss) by use of the microdrop method (20 μL) has been published.23
The present study evaluated the effectiveness of loops for vitrification of sperm from X. hellerii. The goal was to develop streamlined protocols that could be integrated into a standardized approach for vitrification of aquatic species germplasm. The objectives were to (1) estimate acute toxicity of cryoprotectants, alone and in combination, at concentrations ranging from 10% to 40%; (2) evaluate vitrification solutions; (3) compare different thawing methods; (4) evaluate membrane integrity of post-thaw sperm vitrified in different cryoprotectants; and (5) evaluate the fertility of vitrified sperm by artificial insemination. This is the first report of sperm vitrification in a live-bearing fish with production of offspring. Vitrification offers an alternative to conventional cryopreservation and it can be applied to small body-sized fishes such as ornamentals, endangered species, and biomedical models.
Materials and Methods
Animals
Male X. hellerii used in this study were obtained from EkkWill Waterlife Resources, Crystal River Aquarium, Segrest Farms, and the XGSC (Texas State University, San Marcos, TX) for experiments performed between 2008 and 2009. Males used for the acute toxicity experiments were from EkkWill and Crystal River, and had an average (mean±standard deviation [SD]) body length of 4.3±0.78 cm, and body weight of 1.6±0.68 g. Males used for artificial insemination were from XGSC, and had a mean body length of 4.03±0.65 cm, and body weight of 0.62±0.20 g. Males used for the flow cytometry and acute toxicity studies were from Segrest Farms, and had a mean body length of 4.23±0.25 cm, and body weight of 1.55±0.21 g.
All males were maintained at the Aquaculture Research Station of the Louisiana State University Agricultural Center in a recirculating aquaculture system using a bead filter at a density of 1 fish per 10 L. Fish were fed twice daily with commercial flakes (Tropical Mix; Aquatic Eco-Systems Inc.) and live Artemia nauplii grown from cysts (INVE Aquaculture Inc.). The bead filter was backwashed weekly and water quality was monitored weekly. The water quality standards were as follows: alkalinity >100 mg/L, hardness >100 mg/L, and total ammonia nitrogen and nitrite <1 mg/L. Females used in 2008 for artificial insemination were X. maculatus of the strain Jp Wild and X. hellerii of the albino strain, whereas females used in 2009 were X. maculatus and X. hellerii of the strain BxII.24 Virgin females were selected by separation from mixed-sex broods before maturation (at around 6 weeks of age).3 Females were maintained at the XGSC and cultured following routine protocols (www.xiphophorus.org) which included feeding twice daily with Artemia and liver paste.25 Guidelines from the Institutional Animal Care and Use Committees (IACUC) of Louisiana State University Agricultural Center and Texas State University were followed for animal care in this study. These IACUC animal protocols and inspections are current (IACUC no. 05-05F7651F62), as is the National Institutes of Health Protection from Research Risks approval.
Sperm collection
Sperm were collected by crushing of dissected testis. Male fish were anesthetized on ice for 1 min, killed by decapitation, and blotted with a paper towel to dry the body. The testes (13.8±10.3 mg, n=101) were removed and separated from the surrounding lipid tissues while viewing with a dissection microscope (10×magnification) and transferred to 1.5-mL centrifuge tubes for weighing. Sperm were released by crushing of the testis in Hanks' balanced salt solution at an osmolality of 500 mOsmol/kg (HBSS500),16 and diluted to a final concentration of 1×108 cells/mL unless otherwise stated. Sperm concentration was estimated by use of a hemacytometer (Hausser Scientific), and osmolality was measured with a vapor pressure osmometer (Model 5520; Wescor Inc.).
Motility estimation
Sperm motility was estimated using dark-field microscopy (Optiphot 2; Nikon, Inc.) at 200×magnification. The addition of 20 μL of HBSS at an osmolality of 300 mOsmol/kg (HBSS300) was used to activate 2 μL of sperm suspension placed on a glass slide; no coverslip was added to the sample. Motility was estimated subjectively based on observation of three to five different fields within 20 s after activation, and expressed as the percentage of sperm swimming progressively forward within the sample; sperm that vibrated in place were not considered to be motile. For consistency, motility was evaluated by a single skilled operator in a blind protocol (the examiner did not know the treatment given).
Fluorescent staining and flow cytometry
Sperm membrane integrity was evaluated with the fluorescent dyes SYBR-14 and propidium iodide (PI) (live/dead sperm viability kit; Molecular Probes). Duplicate aliquots of 250 μL of sperm sample at a concentration of 1×106 cells/mL were stained with 100 nm membrane-permanent nucleic acid stain (SYBR)-14® and 12 μM PI for 10 min. Membrane integrity was assessed by analyzing 10 μL of sperm sample at a flow rate of 35 μL/min using an Accuri C6 flow cytometer, equipped with a 488-nm, 50-mW solid-state laser (Accuri Cytometers Inc.), and CFlow® software (version 1.0.202.1; Accuri Cytometers Inc.). Green fluorescence (SYBR 14) was detected with a 530±15 nm bandpass filter (FL1), and red fluorescence (PI) was detected with a >670 nm longpass filter (FL3). Events were viewed on forward-scatter versus side-scatter plots with gating to exclude nonsperm events, and gated events were viewed on a scatter plot showing fluorescence detector 1 (FL1) versus FL3 with fluorescence compensation to reduce spectral overlap. The proportion of intact sperm was expressed as a percentage of the fluorescent population (i.e., sperm stained with SYBR 14, PI, or both) to exclude nonsperm particles from calculations.
Vitrification of sperm samples
Sperm samples were prepared by crushing of dissected testes in HBSS500 at an initial volume of five times the testes weight, and the concentration was adjusted to 2–5×108 cells/mL. Cryoprotectant solutions were prepared at double-strength in HBSS500. To vitrify, sperm samples were mixed with double-strength cryoprotectants at room temperature, immediately loaded (within 15 s) into 10-μL polystyrene loops (Nunc™) or 5-mm nichrome loops (∼15 μL) (Cole-Parmer) without equilibration, submerged in liquid nitrogen within 1 min (∼50 s after the mixing), and packed under liquid nitrogen into goblets for storage in a Dewar flask. After at least 24 h of storage in liquid nitrogen, the vitrified loops were thawed in 20 μL of HBSS300 at room temperature or other temperatures as noted, and the motility of thawed sperm was estimated within 30 s.
For membrane integrity assessment of vitrified sperm after thawing, sperm samples were vitrified at 1×107 cells/mL in 10-μL polystyrene loops, and thawed by warming four loops directly in 300 μL of HBSS500 at 40°C to yield a sperm concentration of around 1×106 cells/mL. Duplicate aliquots (250 μL) of the thawed samples were stained with 100 nM SYBR14 and 12 μM PI, and analyzed by flow cytometry within 10 min of thawing as described above.
Artificial insemination
The artificial insemination procedure used in this study was based on previously published protocols (see details in Yang et al.15 and Dong et al.26). In brief, females were anesthetized in 0.01% MS-222 (w/v), and transferred to a Petri dish with the abdomen facing up. The tip end of the insemination device (injector) was filled with sperm sample and gently pushed into the genital duct (viewed at 10×magnification), and the sperm sample (5 μL) from each male was injected into the genital duct. After insemination, the females were returned to fresh water for recovery and were maintained in aquaria (five females in each tank) in the XGSC for harvest of live young. These tanks contained live plants (java moss, Vesicularia dubyana) to provide refuge for newborn fish, thereby reducing the chances of cannibalism. At 90 days after insemination or when live young were collected (whichever came first), the inseminated females were dissected for examination of the reproductive tract.
Experiment I: acute toxicity of cryoprotectants
In the first trial, 49 males were dissected and testis samples from individual males or pooled samples from several males (2–8) were used depending on the volume collected. Three replicates were produced by pooling sperm from multiple males. Nine cryoprotectants, (1) 2-methyl-2,4-pentanediol (MPD; Acros Organics), (2) 1-methoxy-2-propanol (MP; Acros Organics), (3) methyl glycol (2-methoxyethanol, MG; Sigma-Aldrich), (4) polyethylene glycol (PEG molecular weight [MW] 200; Sigma-Aldrich), (5) ethylene glycol (EG; Mallinckrodt Baker), (6) 2,3-butanediol (BD; Acros Organics), (7) Gly (Mallinckrodt Baker), (8) 1,2-propanediol (PrOH; Sigma-Aldrich), and (9) dimethyl sulfoxide (DMSO; OmniSolv), were used at final concentrations of 5%, 10%, 15%, 20%, 25%, and 30% (v/v) with two exposure temperatures (24°C room temperature and 4°C on ice). In the second trial, EG, Gly and PrOH were evaluated at increased concentrations of 30%, 35%, and 40%. EG and Gly exposures were performed at room temperature (24°C) while PrOH were held on ice (4°C). Three replicates were produced for each treatment with different fishes (n=5). Cryoprotectant solutions were prepared in HBSS500 at double strength of the final concentrations, followed by mixing with sperm suspension at a ratio of 1:1 for toxicity estimation. Motility was estimated immediately (within 10 s) and at 5, 10, 15, 20, 25, 30, and 60 min.
Experiment II: acute toxicity of commercial vitrification solutions
Five commercial solutions, VitriFreeze™ Freezing Medium 1 and 2 (FertiPro N.V.), VEG, VM3, X-1000™, and Z-1000™ (21st Century Medicine), were used at final concentrations of 10%, 20%, 30%, 40%, and 50% (v/v). Vitrification solutions were prepared in HBSS500 at double strength of the final concentrations, followed by mixing with the sperm suspension at a ratio of 1:1. Motility was estimated immediately (within 10 s) and at 5-min intervals for 30 min, and finally at 60 min. Three replicates were produced for each treatment with different fishes (n=4).
Experiment III: acute toxicity of combined cryoprotectants
Fifteen combinations from different cryoprotectants were tested: (1) 20% EG+20% Gly, (2) 30% EG+10% DMSO+0.45 M trehalose dihydrate (Tre; Acros Organics), (3) 30% EG+10% PrOH, (4) 20% methanol (MeOH; Fisher Scientific)+20% MG, (5) 40% Gly+0.45 M Tre, (6) 30% EG+10% BD, (7) 30% EG+10% Gly, (8) 40% Gly, (9) 30% EG+15% Gly, (10) 15% EG+10% Gly+15% DMSO+1% X-1000+1% Z-1000, (11) 20% EG+20% Gly+0.45 M Tre, (12) 30% EG+10% MeOH, (13) 20% DMSO+10% PrOH+6% PEG+15% acetamide (Sigma-Aldrich), (14) 40% EG+0.45 M Tre, and (15) 40% EG. Double-strength cryoprotectant solutions were prepared in HBSS500 and mixed with sperm suspension at a ratio of 1:1 at 24°C. Motility was estimated immediately (within 10 s) and at 5 and 10 min. Sperm from four males were used in this experiment, and three replicates were produced with two replicates from individual males and one from pooling of two males.
Experiment IV: effect of thawing temperatures
Six vitrification solutions were tested: (1) 20% EG+20% Gly, (2) 40% Gly, (3) 40% Gly+0.45 M Tre, (4) 30% EG+10% DMSO+Tre, (5) 15% EG+10% Gly+15% DMSO+1% X-1000+1% Z-1000, and (6) 10% EG+20% Gly+5% DMSO+1% X-1000+1% Z-1000. Double-strength cryoprotectant solutions were prepared in HBSS500 and diluted at 24°C with sperm suspension at a ratio of 1:1 (final sperm concentration 5×107 cells/mL). Samples were immediately loaded (within 15 s) into 5-mm nichrome loops (∼15 μL) (Cole-Parmer) without equilibration, and submerged in liquid nitrogen within 1 min (∼50 s) after the addition of the vitrification solutions. Glass formation was assessed by observing the appearance of the vitrified sample (a milky appearance indicated ice crystal formation). Loops were thawed directly onto a microscope slide containing a 20-μL drop of HBSS300 at two temperatures (24°C and 37°C). The motility of each sample was estimated immediately after thawing. Sperm from five males were used in this experiment.
Experiment V: effect of cryoprotectant on membrane integrity by flow cytometry
Sperm samples from three males were used to evaluate the toxicity of three treatments: (1) 30% EG, (2) 35% Gly, and (3) 20% EG+20% Gly. Sperm samples (2×106 cells/mL) were stained with 100 nM SYBR-14 in duplicate for 10 min, and mixed with the same volume (125 μL) of double-strength vitrification solution containing PI at a final concentration of 12 μM. The mixture was analyzed by flow cytometry as described above at 1 min and 5 min after the addition of the vitrification/PI solution. Post-thaw motility after vitrification for each treatment was evaluated.
Experiment VI: artificial insemination with vitrified sperm
In July 2008, testes from 10 X. hellerii males were vitrified at a final concentration of 5×108 cells/mL with Gly (final concentration 40% v/v) in 5-mm nichrome loops. For artificial insemination, the vitrified loops were thawed in 20 μL of HBSS300 at 24°C after 24 h of storage in liquid nitrogen. Females of X. maculatus (n=20) and albino females of X. hellerii (n=5) were used for artificial insemination. Each loop was thawed in a 5-μL drop of HBSS300 at 24°C, and 5 μL of the thawed sperm sample (∼7×105 cells) were injected into the female within 3 min after thawing. To evaluate artificial insemination success, fresh sperm samples were collected from males of X. maculatus (n=15) and used to inseminate females of X. hellerii (n=15) and X. maculatus (n=15).
In July 2009, testes from 20 X. hellerii males were vitrified at final concentrations of 5×108 cells/mL (5 males) or 2×108 cells/mL (15 males) with 20% EG+20% Gly in 10-μL polystyrene loops. Artificial insemination was performed in four groups. For the first group, each loop from individual males (n=10) was thawed in a 5-μL drop of HBSS300 at 24°C, and 5 μL of the thawed sperm sample (∼1×105 cells) were injected into females of X. hellerii (n=10) and X. maculatus (n=10) within 3 min after thawing. For group 2, three loops of vitrified samples from each individual male (n=10) were thawed into 1.5-mL microcentrifuge tubes containing 500 μL of HBSS500 at 24°C. For concentrating and washing, the thawed sperm suspensions were centrifuged (1000 g) for 5 min at 4°C, the supernatant was decanted, and the sperm pellet was re-suspended by adding 100 μL of fresh HBSS500. Centrifugation was repeated (1000 g for 5 min at 4°C), and the supernatant decanted again. For artificial insemination, the sperm pellet (1.5×106 to 2.5×106 cells) was suspended into a total volume of 5 μL of fresh HBSS500 before injection. Females of X. hellerii (n=5) and X. maculatus (n=5) were inseminated with samples from 10 males. For group 3 (designated the “chemical control”), fresh sperm from X. hellerii (n=5) were exposed to 20% EG+20% Gly and used for artificial insemination of females of X. hellerii (n=5) and X. maculatus (n=5). For each female, 5 μL of the sample (2.5×106 cells) were injected within 3 min after addition of cryoprotectant. For group 4, female X. hellerii (n=15) from the same batch as the females used in the other three groups were used for artificial insemination with fresh sperm from X. variatus (concentration range 2×108 to 1×109 cells/mL). This group was used as a control to evaluate the success of the artificial insemination procedure.
Data analysis
Data were analyzed as a factorial randomized block design. Analysis was conducted using a mixed analysis of variance (ANOVA) procedure for all interactions. For acute toxicity experiments, the fixed treatment variables were cryoprotectant, concentration, temperature, and incubation time. The green swordtail males were grouped in a block to remove variation among individual motility from the error term. The dependant variable was sperm motility (percent). The control (fresh sperm) was excluded from the model, but was used as a reference to ensure sperm viability. For the thawing experiment, the fixed treatments were temperature and vitrification solution, and the dependant variable was post-thaw motility (percent). Membrane integrity data were analyzed using a mixed ANOVA procedure with cryoprotectants as fixed treatments and membrane intact (percent) as a dependent variable. The control (fresh sperm) was included in the model. Statistical differences were determined at an α=0.05 level using Tukey's adjustment. Statistical analyses were performed using SAS software (Statistical Analysis System Inc., version 9.1; SAS Institute).
Results
Experiment I: acute toxicity of single cryoprotectants
The motility of fresh sperm before incubation with cryoprotectants was 60%±12% (mean±SD). In the first trial, the addition of cryoprotectants at two temperatures (24°C and 4°C) was significantly different for the cryoprotectants EG (p=0.004), BD (p=0.014), Gly (p<0.001), and DMSO (p=0.004), where toxicity was less evident at room temperature except for BD. The least toxic cryoprotectant was Gly, which showed no significant differences in motility among concentrations as high as 25% in all time intervals (p=0.112) (Fig. 1). EG showed no significant differences among concentrations as high at 25% and for as long as 30 min (p=0.466). The highest concentrations without significant differences for the rest of the cryoprotectants were 20% DMSO (p=0.773), 15% PrOH (p=0.530), 15% BD (p=0.490), 10% MG (p=0.109), and 10% MP (p=0.107) (Fig. 1). The highest concentration that could be used for MPD and PEG was 5%. The longest time without significant motility reduction for these cryoprotectants at 5% was 60 min for PEG (p=0.174), and 30 min for MPD (p=0.055).
FIG. 1.
Percent sperm motility of green swordtail Xiphophorus hellerii in samples incubated with different cryoprotectants. The cryoprotectants used were glycerol (Gly), ethylene glycol (EG), dimethyl sulfoxide (DMSO), polyethylene glycol (PEG), propanediol (PrOH), butanediol (BD), methyl pentanediol (MPD), methyl glycol (MG), and methoxy propanol (MP). Each point represents the mean of three replicates; error bars were omitted for clarity.
In the second trial with higher concentrations of cryoprotectants (30%–40%), EG was not significantly different from Gly (p=0.837), but it was significantly different from PrOH (p=0.037). Gly and PrOH were significantly different from each other (p=0.025). EG at concentrations of 30% and 35% were not significantly different (p=0.083). Glass formation was observed for concentrations of 35% PrOH, 35% Gly, and 45% EG (Fig. 2 and Table 1), although only partial glass formation could be observed at 40% EG.
FIG. 2.
Sperm motility of green swordtail X. hellerii when suspended at different concentrations of cryoprotectants at 1, 5, 15, and 30 min. As concentration increased, exposure time played a major role in toxicity with the highest motility observed just after the addition (<1 min) of cryoprotectants. Some glass formations appeared in the polystyrene loops (inset pictures) at 35% and 40% for each cryoprotectant (identified by a transparent state, while crystallization appeared milky). Each point represents the mean of three replicates.
Table 1.
Sperm Motility of Green Swordtail (n=3) After Exposure to Vitrifying Solutions
| Time (min) | Control | 40% EG | 35% Gly | 40% Gly | 20% EG+20% Gly | 35% PrOH | 40% VM3 |
|---|---|---|---|---|---|---|---|
| <1 | 61±9 | 18±10 | 37±6 | 18±11 | 20±21 | 18±13 | 12±8 |
| 5 | 58±10 | 11±15 | 18±18 | 13±8 | 4±1 | 5±9 | 0 |
| 10 | 61±9 | 4±8 | 13±15 | 24±12 | 0 | 1±2 | 0 |
EG, ethylene glycol; Gly, glycerol; PrOH, 1,2 propanediol; VM3, vitrification solution.
Experiment II: acute toxicity of commercial vitrification solutions
The motility of fresh sperm before incubation with vitrification solutions was 50%±0%. The least toxic commercial vitrification solution was VitriFreeze Freezing Medium 1, which showed no significant effect on motility among all the concentrations tested (as high as 50%). For VitriFreeze Freezing Medium 2, there was no difference within the first 5 min of exposure at concentrations as high as 30%. When VitriFreeze Freezing Medium 2 was used for <1 min, there was no difference at concentrations as high as 50%. When samples were exposed to VM3 for <1 min, there was no difference in motility at concentrations as high as 30% (p=0.572). For the polymer X-1000 there was no significant difference in motility at concentrations as high as 30% and at all time intervals (p=0.347). There was no difference between VEG and Z-1000 (p=0.803). The highest concentration that could be used for VEG and Z-1000 was 10% for <1 min. Glass formation was observed only in 40% VM3 (Table 1).
Experiment III: acute toxicity of combined cryoprotectants
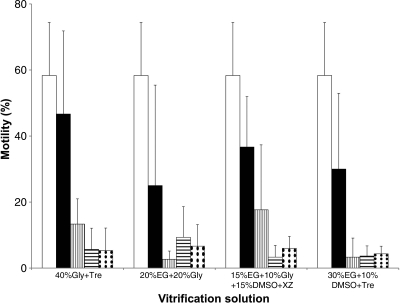
The motility of fresh sperm before incubation with cryoprotectants was 65%±10%. Time was an important factor in the toxicity of combined cryoprotectants, which could be separated into three groups at <1 min. In the first group (20% MeOH+20% MG, 30% EG+10% BD, 30% EG+15% Gly, 20% EG+20% Gly+Tre, 30% EG+10% MeOH, and 20% DMSO+10% PrOH+6% PG+Ace) motility estimates were close to zero at <1 min and remained the same afterward. In the second group there were no significant differences in motility at any time interval. The least toxic cryoprotectant from this group and from this experiment was 40% Gly, for which the average motility at <1 min was 18% and was not significantly different through time (p=0.454). The other combined cryoprotectants from the second group were 30% EG+10% PrOH (15% motility at <1 min; no difference through time p=0.137), 30% EG+10% Gly (12% motility at <1 min; no difference through time p=0.708), and 40%EG (12% motility at <1 min; no difference through time p=0.851). In the third group, motility was highest at <1 min but declined at subsequent time intervals. Combined cryoprotectants from this group were 20% EG+20% Gly, 30% EG+10% DMSO+Tre, 40% Gly+Tre, 15% EG+10% Gly+15% DMSO+XZ (Fig. 3), and 40% EG+Tre (motility at <1 min was 37%, and at 5 min was 7%).
FIG. 3.
Sperm motility (mean±standard deviation [SD]) of green swordtail X. hellerii (n=3 males) in relation to exposure to vitrification solutions for 1 min (black bars) and 5 min (vertical stripes), and thawed at 24°C (horizontal stripes) or at 37°C (dotted bars). Motility of control samples was assessed before the addition of cryoprotectants (white bars). There was a significant difference in sperm motility between exposure at 1 and 5 min. There was no significant difference in post-thaw sperm motility due to thawing at 24°C or at 37°C in all treatments.
Experiment IV: effect of thawing temperatures
The motility of fresh sperm before vitrification was 64%±13%. There were no significant differences (p=0.945) in post-thaw motility of sperm thawed at 24°C or at 37°C in all the treatments (Fig. 3). In all treatments, the only significant differences found were between 20% EG+20% Gly and 30% EG+10% DMSO+Tre (p=0.033). The highest post-thaw motility was for samples vitrified in 20% EG+20% Gly (ranging from 3% to 20%) followed by 40% Gly+Tre (ranging from 1% to 15%). In general, the sperm in most vitrification solutions had a tendency toward vibration rather than what we considered to be progressive motility. This could be due to low inherent motility (perhaps due to cellular damage), or could be in part due to the high viscosity of the solutions which impeded sperm movement. The highest post-thaw sperm vibration was for 20% EG+20% Gly (vibration 54%±14%) followed by 40% Gly (vibration 53%±12%).
Experiment V: effect of cryoprotectant on sperm membrane integrity
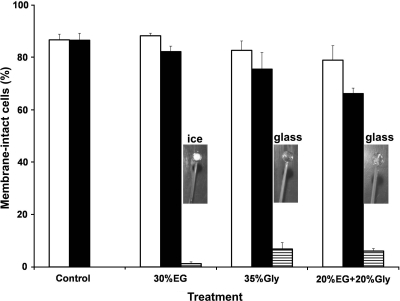
Although, the fresh sperm motility was 47%±6%, the percentage of membrane-intact sperm cells before vitrification was 87%±2%. For the cryoprotectant toxicity, the percentage membrane-intact sperm in the 30% EG treatment did not differ significantly from the control (p=0.490), but membrane integrity in the 35% Gly (p=0.002) and 20% EG+20% Gly (p<0.001) treatments was significantly lower than in the control. There was a significant difference in sperm membrane integrity between <1 min and at 5 min for the treatments 30% Gly (p=0.048), 35% Gly (p=0.022), and 20% EG+20% Gly (p<0.001) but not for the control (p=0.961). After vitrification, the percentage of membrane-intact post-thaw sperm was low (<12%) which corresponded with the post-thaw motility (<7%) (Fig. 4). There was no significant difference in the percentage of membrane-intact sperm between 35% Gly and 20% EG+20% Gly (p=0.590).
FIG. 4.
Sperm membrane integrity (mean±SD) of green swordtail Xiphophorus hellerii (n=3 males) in relation to exposure to cryoprotectants for 1 min (white bars) and 5 min (black bars), and post-thaw (horizontal stripes). The control values (fresh sperm) were not significantly different from 30% EG but were significantly different from 35% Gly and 20% EG + 20% Gly. There were <10% membrane-intact cells after vitrification. Polystyrene loops were evaluated for glass formation for each treatment. Vitrification produced a clear, transparent glass rather than an opaque, milky solid, caused by the appearance of ice crystals.
Experiment VI: artificial insemination with vitrified sperm
The motility of fresh sperm used for the artificial insemination was 71%±11%. For the first insemination trial in 2008 using sperm from one loop of vitrified samples in 40% Gly, none of the 25 females inseminated yielded offspring. In the control group, which consisted of 30 females that were inseminated with fresh sperm, 14 females contained embryos or oocytes after dissection. Of the 15 X. hellerii females from the control group, 14 live offspring were observed and 3 females contained secondary growth oocytes at advanced vitellogenesis (abundant oil droplets and yolk globules) (∼6 oocytes per female) after dissection, and of the 15 X. maculatus females, 2 live offspring were observed and 11 females contained secondary growth oocytes (∼7 oocytes per female) after dissection. In the second insemination trial in 2009, none of the 20 females in group 1 that were inseminated with vitrified sperm from a single loop yielded offspring. From group 2 that consisted of 10 females inseminated with concentrated and washed sperm pooled from three loops (pellet 1.5×106 cells), five X. hellerii females contained secondary growth oocytes. Four live offspring (3 females and 1 male) and 31 oocytes were collected from these five females. Each female contained an average of six oocytes after dissection at 130 days post-insemination. The other five X. maculatus females from group 2 that were inseminated with the concentrated and washed sperm from three loops (pellet containing 2.5×106 cells) did not yield offspring. In group 3 (chemical control), which consisted of 10 females that were inseminated with fresh sperm with 20% EG+20% Gly, none of the females yielded offspring. In group 4 (control), which consisted of 15 females that were inseminated with fresh sperm, 3 live offspring were observed and none of the females contained embryos or oocytes after dissection.
Discussion
Aquarium fish such as zebrafish, medaka, and Xiphophorus have proven to be valuable as disease models for molecular genetic studies. As vertebrates, fish and humans share most developmental processes, physiological mechanisms, and organ systems. By generating fish mutants, human diseases can be modeled and can provide experimental systems to aid pathological investigations or for use in screening of therapeutics.1 Certain fish species possess novel attributes making them valuable models for a specific diseases; for example, Xiphophorus provides a long-standing model for melanoma. Genetic control of tumor susceptibility in Xiphophorus has been investigated in pure strains and in interspecific hybrids for a variety of spontaneous and induced neoplasias.27 Inbred lines have been available for research since 1939 from the XGSC.3 Despite the significant costs to maintain and generate these pedigreed fish lines, few alternatives currently exist to safely preserve important individuals, strains, and lines that may include endangered species. Of the 27 species of Xiphophorus, conventional cryopreservation of germplasm yielding offspring has been reported in only 3 species (X. hellerii, X. couchianus, and X. maculatus).15–17 Cryopreservation and artificial insemination efforts in Xiphophorus are faced with four major challenges: (1) limited volume of sperm samples (<10 μL); (2) a low percentage (20%–30%) of females producing offspring by artificial insemination for control (fresh) sperm; (3) the need for virgin females because sperm can be stored in the female reproductive tract; and (4) low control over the fertilization process due to difficulties selecting spawning-capable females.16
Conventional cryopreservation has been applied for the protection of genetic resources in fish and shellfish, but is not always practical for use in remote locations or in developing countries (often the places where preservation is most necessary). Vitrification is considered to provide an alternative to standard cryopreservation and has been used successfully for cryopreservation of spermatozoa, embryos, oocytes, stem cells, and organs from several mammalian species.19,28 The advantages of vitrification are that it does not require expensive equipment, it is simple, fast, and can be used to preserve samples in the field especially in remote, inaccessible areas. In addition, it offers perhaps the greatest potential for success in overcoming the challenges for preservation of fish embryos. At present, there are three studies that have reported vitrification of fish sperm. In one study the investigators observed sperm vitrification in rainbow trout (listed in the article as O. mikiss) and Russian sturgeon Acipenser gueldenstaedtii (listed in the article as A. guldenshtadti) by use of cryomicroscopy.29 The second study specifically addressed fish sperm vitrification and development of a streamlined protocol for sperm vitrification in channel catfish,20 and a recent study reported sperm vitrification in rainbow trout without the use of cryoprotectants but no production of offspring was reported.23 These are all large-bodied (e.g., >2.5 kg) externally fertilizing species. In the present study, we report the first successful production of offspring from vitrified sperm in a live-bearing fish.
Identifying suitable cryoprotectants
One of the first steps in protocol development is the measurement of acute toxicity and selection of the least toxic vitrification solutions. Sperm vitrification is new in fish and there is little knowledge of cryoprotectants that vitrify at nontoxic concentrations. There have been no published studies that specifically address acute toxicity of cryoprotectants in X. hellerii. A previous study in X. hellerii estimated the toxicity of cryoprotectants after thawing.30 The cryoprotectants used in that study were DMSO, dimethyl formamide, dimethyl acetamide, PrOH, MeOH, and sucrose at concentrations of 6% and 10%, and with an equilibration time of 10 min. Unfortunately, that study did not report sperm motility after the equilibration time and before freezing, so it is difficult to differentiate the effect of the cryoprotectants from the effects of freezing and thawing.31 In addition, sperm concentration was estimated and no attempts were made to adjust the concentration to an established value.30 In the present study, we tested the effect of nine cryoprotectants at six concentrations (5%–30%) and at two exposure temperatures on sperm at one standardized concentration (1×108 cells/mL). We evaluated motility immediately (within 10 s) after the addition of the cryoprotectant, and at 7 time intervals (as long as 60 min). This first screening identified three potential cryoprotectants (EG, Gly, and PrOH) that presented the least toxicity at concentrations suitable for vitrification. Because vitrification requires high concentrations of cryoprotectants, we evaluated concentrations as high as 40% of these cryoprotectants. Temperature influenced the toxicity of some cryoprotectants. For example, Gly was least toxic at room temperature (24°C) compared to cold (4°C), which is different from human sperm where lower temperatures reduced the toxicity of Gly.32 Temperature plays an important role in the addition of cryoprotectants because it interacts with factors such as chilling sensitivity of the gametes, toxic properties of the cryoprotectant, and permeation rate (lower temperatures require a longer period of equilibration).33,34 Because cryoprotectant exposure time is kept to a minimum in vitrification, high concentrations of cryoprotectants are usually added at room temperature.35,36 One aim in vitrification (nonequilibrium cooling) protocols is to dehydrate the cells before cooling begins by using high concentrations of cryoprotectants.37 Even so, it is possible to attain vitrification in the absence of cryoprotectants as was discovered for human sperm38,39 and recently in rainbow trout.23
Evaluating vitrification solutions
Most cryoprotectants tend to have toxic and hypertonic effects when used at concentrations that are effective for vitrification.40 There are a number of ways of varying practicality that can be used to reduce the concentration of individual cryoprotectants required for vitrification, including applying high hydrostatic pressure, stepwise addition of cryoprotectants, combination of cryoprotectants, addition of nonpermeating polymers or sugars, and reducing exposure time at high concentrations to a minimum.41 In addition, the toxicity of cryoprotectants can be counteracted by the use of “toxicity neutralizers” such as formamide or urea.42
Commercial vitrification solutions are not widely used in fish cryopreservation. This could be due to the cost, because most of the commercial solutions are designed for use with mammals. A previous study in fish embryo vitrification used the polymer X-1000 to inhibit ice formation.43 In this study the commercial polymers (X-1000 and Z-1000) were not toxic at the recommended concentration (1%).44 Another nonpermeating cryoprotectant that has been used to enhance glass formation and cell biostabilization is trehalose. This disaccharide has been used to cryopreserve sperm from fishes such as rainbow trout,45 longtooth grouper (Epinephelus bruneus, formerly E. moara46), humpback grouper (Cromileptes altivelis47), and orange-spotted grouper (Epinephelus coioides48). In the present study, we decided to mix cryoprotectants to combine the additive actions of each, such as permeability and glass formation. The combination of cryoprotectants has been reported to reduce toxicity compared to high concentrations of individual cryoprotectants.49 In the present study, the motility of sperm exposed to the combination of Gly and trehalose immediately after addition (<1 min) was higher than for sperm exposed to Gly alone, but Gly alone yielded higher motilities over time. This is probably because at the time of cryoprotectant addition there was a rapid osmotic shock. This resulted in sperm activation and motility, which declined during the equilibration period. A similar reduction of motility was observed in X. hellerii when the thawed sperm were immediately diluted in HBSS creating a rapid change in osmolality (from as high as 2608 to as low as 599 mOsmol/kg) which caused sperm volume to change.50
In this study, the combination of acetamide and DMSO42 did not reduce damaging effects. Overall the combinations of cryoprotectants (vitrification solutions) were less toxic than individual cryoprotectants. This is in agreement with the standard mammalian procedures for vitrification in which a combination is often used to increase viscosity, increase the glass transition temperature, and reduce the level of toxicity.28 In the present study exposure time played a major role in toxicity with the highest motility observed just after the addition (<1 min) of cryoprotectants. We decided to use vitrification solutions containing Gly because it was the cryoprotectant of choice for conventional cryopreservation for Xiphophorus, and because it was the least toxic in the present study at high concentrations (i.e., 25%). It has been suggested that as the concentration of Gly increases, the post-thaw motility of green swordtail sperm increases.30 In addition to Gly, we decided to use DMSO because in previous studies it was used for conventional cryopreservation in green swordtail.13,30 Post-thaw motilities in a previous study of conventional cryopreservation using 20% DMSO were around 15%, whereas they were 50% for 20% Gly.30
Effect of thawing temperatures
Warming has been a topic of interest in recent studies in vitrification.51,52 Previous studies have focused on achieving the highest possible cooling rates (e.g., ultravitrification53). This is because there is an inverse relationship between the rates of cooling and warming, and the concentration of cryoprotectant (i.e., the faster the cooling and warming, the lower the concentration of cryoprotectant needed and vice versa54). Overall, the high concentrations of cryoprotectants required are near the maximum tolerable limits of cells. This is one of the reasons that previous studies focused on minimum volume methods to attain high cooling rates and prevent ice formation.18 Even so, neither high cryoprotectant concentration nor increased cooling rates are essential for vitrification to occur. Partial (usually) or total intracellular vitrification can occur incidentally during conventional cryopreservation, and may be responsible for some degree of survival of cryopreserved samples.55 A recent publication found that a wide range of cooling rates (160°C–250°C/min) could produce vitrification of human sperm.56 In addition, cryopreservation in rhesus monkey (Macaca mulatta) was attained at cooling rates of 220°C/min with the absence of permeable cryoprotectants.57
Previous studies in X. hellerii achieved cooling rates of 200°C/min by the use of a differential scanning calorimeter (DSC) but caused the sperm cells to lyse and become osmotically inactive.58 In the same study, the optimal cooling rate predicted for samples without cryoprotectant was 90°C/min. In a different study, minimal post-thaw motility (<1%) was reported when 0.25-mL straws containing 80 μL samples of X. hellerii sperm with 14% Gly were plunged into liquid nitrogen.30 The cooling rate for a 0.25-mL straw was estimated to range from 1700°C/min40 to 2500°C/min.59 In the present study, we used loops (5 mm; 15 μL) similar to those used previously for human sperm vitrification (5 mm; 20 μL).22 The cooling rate estimated for the loops could be as fast as 720,000°C/min,38 whereas the warming rate at 37°C could be as fast as 200,000°C/min.60 In the present study, there was no significant difference in motility between the two thawing temperatures (24°C and 37°C) tested. This means that the combination of the small volumes used and the two temperatures yielded warming rates fast enough to avoid ice crystal formation (devitrification) or recrystallization of small intracellular ice crystals produced during cooling. This result is in agreement with a recent publication that found that high warming rates (118,000°C/min) yielded high survival of mouse oocytes (Mus musculus) (70%–85%) regardless of the cooling rate used (95°C to 69,250°C/min).52 This fast warming rate could explain results from a previous report in X. hellerii in which samples were cooled at a relatively fast rate (200°C/min) but the sperm were destroyed due to the slow warming rate used (20°C/min).58 In addition, X. hellerii sperm in 0.25-mL straws that were plunged into liquid nitrogen (cooling of 1700°C to 2500°C/min) and thawed for 7 s in a 40°C water bath (thawing of around 1300°C to 2500°C/min) yielded minimal motility (<1%).30 The reason for this could be the use of a slow warming rate compared with the rate suggested recently (118,000°C/min52). Another reason could be the use of low cryoprotectant concentrations (14% Gly), which means that the glass transition temperature was low (below −120°C).
However, the big question that remains is: What is the source of sperm damage during vitrification? There is controversy over whether intracellular ice is formed in the sperm during rapid rates of cooling. While some studies suggest that at cooling rates as high as 3000°C/min there is no formation of intracellular ice,61,62 other studies suggest that intracellular ice starts forming at a cooling rate of 2000°C/min but not in cells that are cooled at 250°C–1000°C/min.63 The intracellular nucleation temperature inferred by DSC for X. hellerii was −30°C.58 In a similar live-bearing fish (Poecilia reticulata) the nucleation temperature was calculated to be in the range of −25°C to −32°C at cooling rates from 5°C/min to 100°C/min.64 It has been suggested that survival of vitrified sperm could be due to the presence of large amounts of osmotically inactive water bound to macromolecular structures, such as DNA and histones, or the presence of high molecular weight components in sperm that affect the viscosity and glass transition temperature of the intracellular cytosol.56,65 To help identify if sperm were damaged by cryoprotectant toxicity, osmotic shock, or intracellular ice formation, in the present study we evaluated membrane integrity before and after the addition of the cryoprotectants, and after vitrification.
Effect of cryoprotectant on membrane integrity
Flow cytometry is a useful technique to evaluate sperm quality parameters such as membrane integrity, mitochondrial status, and DNA damage.66,67 A previous study in Xiphophorus used flow cytometry to measure nuclear DNA content in nine species, and was able to differentiate sex based on sex chromosomes heteromorphism in four species.68 In the present study we used flow cytometry to evaluate membrane integrity in response to cryoprotectant toxicity and after vitrification. Because vitrification uses high cryoprotectant concentrations, we evaluated membrane integrity at two time intervals, just after the addition of cryoprotectant solution (1 min) and after 5 min of exposure. We decided to use three treatments, based on our toxicity study and on the glass formation characteristics of these solutions. Although 30% EG (5.4 M) did not form glass, we tested it because previous work in mammals had success with a similar vitrification solution (VS14=5.5 M EG+1 M sucrose69). Gly at 35% was the minimum concentration needed to form glass in loops in the present study. The mixture of EG and Gly is a common combination used in the development of vitrification solutions.69 We used 20% EG (3.6 M)+20% Gly (2.7 M) which corresponded to 80% of the vitrification solution VS5 (100% VS5 contains 4.5 M EG+3.5 M Gly69). After 5 min of the addition of these solutions, the percentage of sperm with intact membranes remained relatively high (>66%). However, after vitrification, damage to the membrane was significant (highest value for membrane-intact sperm was 12% for 35% Gly). The lowest percentage of membrane-intact sperm (1%) was for 30% EG, which was the solution that did not form glass. The highest value for membrane-intact sperm was for 35% Gly (7%) and 20% EG+20% Gly (6%) which corresponded with post-thaw motility (<8%). Although we used high cooling and warming rates, glass formation needs to be attained for the sperm to survive, as low cryoprotectant concentrations that did not form glass had minimum motility (<1%) after thawing. This finding is similar to a previous study in channel catfish where higher concentrations of cryoprotectants that led to glass formation had higher sperm viability,20 but this finding is different from previous studies where human sperm was vitrified without cryoprotectant.22
Human sperm contains large amounts of proteins, sugars, and other components that make the cytosol highly viscous and this may provide a degree of natural protection.70 Fish sperm, in contrast are characterized by low protein concentrations, containing mainly mineral compounds and low concentrations of other organic substances such as sugars.71 Xiphophorus sperm58 are similar in size,72 with a head length of 3.6 μm (human 4–5 μm), midpiece length of 6.8 μm (human 7–8 μm), and flagellum length of 43 μm (human 45 μm). However Xiphophorus sperm have a higher surface area-to-volume ratio than human sperm (6.81 for Xiphophorus and 4.8 for humans) and a higher osmotically inactive cell volume than humans (0.6 for Xiphophorus and 0.5 for humans).58,73 These characteristics should allow the sperm from Xiphophorus to have higher hydraulic conductivity (water loss), higher cryoprotectant permeability, and in theory better cryopreservation success. Based on the membrane integrity results in the present study, we suggest that the plasma membrane is damaged either by intracellular ice formation58 or by changes in the physical properties of the extracellular environment.61 In contrast, a recent publication reported post-thaw motilities as high as 86% and membrane integrity of 90% of sperm from rainbow trout that was vitrified without the use of cryoprotectants.23 Because of the numerous biological differences in gametes between these species and technical differences between these studies, more research is required to make conclusive comparisons.
Artificial insemination of vitrified sperm
One of the most important sperm quality tests for cryopreserved sperm is the ability to fertilize eggs and produce offspring. Artificial insemination of live-bearing fishes is required to achieve fertilization. Studies on artificial insemination date back to 1914 when hybrids were produced from X. hellerii and X. maculatus74 and since then several artificial insemination protocols have been published using fresh sperm75–77 and cryopreserved sperm.15,26 Artificial insemination involves the injection of sperm (2–5 μL) into the female reproductive tract and pregnancy monitoring for as long as 90 days because females can store sperm and delay fertilization.25,78 Success rates for artificial insemination using fresh sperm are usually 20%–30%,16 although one study reported 50% success by insemination of at least 40 sperm bundles (spermatozeugmata) into the female (a single male can provide as many as 3000 bundles per stripping79).80 Previously reported success rates for artificial insemination using cryopreserved sperm ranged from 10% to 20%,15,16 although one study reported as high as 40% fertilization.17 Thawed sperm requires washing by centrifugation and resuspension of the pellet to remove the cryoprotectant solution and concentrate the sperm into a small volume.
One of the constraints of artificial insemination, in addition to the need to use virgin females, is the uncontrolled variable of selection of spawning-capable females.17 In the present study, we decided in the first trial not to remove the cryoprotectant because the volume held by the loop (15 μL) was small enough for artificial insemination, but of the 25 artificial inseminations with unwashed vitrified sperm none yielded offspring. To determine whether cryoprotectants played a major role in the lack of fertilization, in the second trial we decided to add a chemical control that included fresh sperm with the cryoprotectant. In addition, in the second trial we inseminated small volumes with higher sperm concentration by centrifugation of vitrified sperm from three loops. From the 20 females inseminated with vitrified sperm without centrifugation, none yielded offspring, and neither did the 10 females inseminated with the chemical control. Five of ten X. hellerii females yielded offspring from the concentrated and washed sperm after centrifugation, whereas the five X. maculatus females did not yield offspring. Four live young were collected from the aquarium, and 31 oocytes were collected from these five X. hellerii females after dissection.
Previous studies demonstrated the production of offspring from cryopreserved sperm, and in this study we demonstrated fertilization and production of live young from vitrified sperm. Because there was not an evaluation of the fecundity of the females before insemination and there was no control for the selection of spawning-capable females, it is difficult to make a strong conclusion of why only females from the same species as the males were the ones that yielded offspring. While some studies support the concept of sperm competition,80,81 in this study we standardized the sperm concentration, and the characteristics of the males such as body size and sword length were similar. This is not the only report of fertilization after artificial insemination in X. hellerii females. In a previous study that used cryopreserved sperm from X. maculatus, none of the 20 X. maculatus females yielded offspring, but live young were produced from X. hellerii females.17 Highly inbred X. maculatus lines such as Coatzacoalcos were reported to have a high percentage of infertile females due to an ovarian regression syndrome,82 but previous studies that used X. maculatus females from other lines (e.g., Jp) produced offspring from cryopreserved sperm from X. hellerii15 and X. couchianus.16 Further research is needed to study the fecundity and ovarian maturation of female Xiphophorus as recently suggested.17
Of the 95 described species of live-bearing fishes (family Poeciliidae) in North America, 33% are imperiled.11 A generalized protocol using conventional cryopreservation for live-bearing fishes such as mollies, guppies and Xiphophorus has been described.83 In this study, we demonstrated a different technique that could be used to cryopreserve sperm samples from live-bearing Xiphophorus fishes (Table 2). Vitrification is a simple technique that is well suited for use with small-bodied species, does not require specialized equipment, and offers advantages for use in the field. Further, vitrification can be used to reconstitute lines from valuable biomedical models, conserve mutants for development of novel lines for ornamental aquaculture, and transport frozen sperm from the field to the laboratory to expand genetic resources. Further research is needed to evaluate whether vitrification can be applied with other species such as marine fishes.
Table 2.
Comparison of Protocols for Sperm Cryopreservation of Green Swordtail
Acknowledgments
This work was supported in part by funding from the NIH National Center for Research Resources (R24RR023998 and R24RR024790), the Louisiana Sea Grant College Program, and the ACRES-LSU Collaborative Research Program. We thank S.P. Leibo for discussion, L. Hazlewood for assistance in the first artificial insemination trial, N. Novelo for article review, P. Lang for assistance with the statistical analysis, and J. Atilano, M. Hunt, and E. Tan for technical assistance. We are grateful to the staff of the XGSC for taking care of the fish, and to Crystal River Aquarium for donating some fish for this research. This article has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 2011-244-5969.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lam SH. Gong Z. Fish as a model for human disease. In: Speicher MR, editor; Motulsky AG, editor; Antonarakis SE, editor. Vogel and Motulsky's Human Genetics. Heidelberg, Germany: Springer; 2010. pp. 827–843. [Google Scholar]
- 2.Gordon M. The genetics of a viviparous top-minnow Platypoecilus; the inheritance of two kinds of melanophores. Genetics. 1927;12:253–283. doi: 10.1093/genetics/12.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter RB. Ju Z. Martinez A. Amemiya C. Samollow PB. Genomic resources for Xiphophorus research. Zebrafish. 2006;3:11–22. doi: 10.1089/zeb.2006.3.11. [DOI] [PubMed] [Google Scholar]
- 4.Walter RB. Xiphophorus fishes: varieties and genetic resources. In: Tiersch TR, editor; Green CC, editor. Cryopreservation in Aquatic Species. 2nd. Baton Rouge, LA: World Aquaculture Society; 2011. pp. 796–808. [Google Scholar]
- 5.Shen Y. Catchen J. Garcia T. Amores A. Beldroth I. Wagner JR, et al. Identification of transcriptome SNPs between Xiphophorus lines and species for assessing allele specific gene expression within F1 interspecies hybrids. Comp Biochem Phys C. 2011 doi: 10.1016/j.cbpc.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter RB. Introduction: aquaria fish models of human disease. Mar Biotechnol. 2001;3:S001–S002. doi: 10.1007/s10126-001-0020-7. [DOI] [PubMed] [Google Scholar]
- 7.Hagedorn M. Ricker J. McCarthy M. Meyers SA. Tiersch TR. Varga ZM, et al. Biophysics of zebrafish (Danio rerio) sperm. Cryobiology. 2009;58:12–19. doi: 10.1016/j.cryobiol.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight J. Abbott A. Mouse genetics: full house. Nature. 2002;417:785–786. doi: 10.1038/417785a. [DOI] [PubMed] [Google Scholar]
- 9.Yang H. Tiersch TR. Current status of sperm cryopreservation in biomedical research fish models: zebrafish, medaka, and Xiphophorus. Comp Biochem Phys C. 2009;149:224–232. doi: 10.1016/j.cbpc.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borowsky RL. Kallman KD. Loss of polymorphism in a declining population of Xiphophorus-variatus. J Hered. 1991;82:387–390. [Google Scholar]
- 11.Jelks HL. Walsh SJ. Burkhead NM. Contreras-Balderas S. Diaz-Pardo E. Hendrickson DA, et al. Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries. 2008;33:372–407. [Google Scholar]
- 12.Tiersch TR. Cryopreservation in aquarium fishes. Mar Biotechnol. 2001;3:S212–S223. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- 13.Huang C. Dong Q. Walter RB. Tiersch TR. Initial studies on sperm cryopreservation of a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. 2004;62:179–194. doi: 10.1016/j.theriogenology.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C. Dong Q. Tiersch TR. Sperm cryopreservation of a live-bearing fish, the platyfish Xiphophorus couchianus. Theriogenology. 2004;62:971–989. doi: 10.1016/j.theriogenology.2003.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H. Hazlewood L. Heater SJ. Guerrero PA. Walter RB. Tiersch TR. Production of F1 interspecies hybrid offspring with cryopreserved sperm from a live-bearing fish, the swordtail Xiphophorus helleri. Biol Reprod. 2007;76:401–406. doi: 10.1095/biolreprod.106.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H. Hazlewood L. Walter RB. Tiersch TR. Sperm cryopreservation of a live-bearing fish, Xiphophorus couchianus: male-to-male variation in post-thaw motility and production of F1 hybrid offspring. Comp Biochem Phys C. 2009;149:233–239. doi: 10.1016/j.cbpc.2008.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H. Savage MG. Hazlewood L. Walter RB. Tiersch TR. Offspring production with cryopreserved sperm from a live-bearing fish Xiphophorus maculatus and implications with females. Comp Biochem Phys C. 2011 doi: 10.1016/j.cbpc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajta G. Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12:779–796. doi: 10.1016/s1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 19.Tucker MJ. Liebermann J. Vitrification in Assisted Reproduction: A User's Manual and Trouble-Shooting Guide. London, United Kingdom: Informa Healthcare; 2007. [Google Scholar]
- 20.Cuevas-Uribe R. Leibo SP. Daly J. Tiersch TR. Production of channel catfish with sperm cryopreserved by rapid non-equilibrium cooling. Cryobiology. 2011 doi: 10.1016/j.cryobiol.2011.06.004. (In press). [DOI] [PubMed] [Google Scholar]
- 21.Huang CJ. Dong QX. Walter RB. Tiersch TR. Sperm cryopreservation of green swordtail Xiphophorus helleri, a fish with internal fertilization. Cryobiology. 2004;48:295–308. doi: 10.1016/j.cryobiol.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawroth F. Isachenko V. Dessole S. Rahimi G. Farina M. Vargiu N, et al. Vitrification of human spermatozoa without cryoprotectants. Cryo Lett. 2002;23:93–102. [PubMed] [Google Scholar]
- 23.Merino O. Risopatrón J. Sánchez R. Isachenko E. Figueroa E. Valdebenito I, et al. Fish (Oncorhynchus mykiss) spermatozoa cryoprotectant-free vitrification: stability of mitochondrion as criterion of effectiveness. Anim Reprod Sci. 2011;124:125–131. doi: 10.1016/j.anireprosci.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Walter RB. Hazlewood L. Kazianis S. Kallman KD. Schartl M. The Xiphophorus Genetic Stock Center Manual. San Marcos, TX: Texas State University; 2006. [Google Scholar]
- 25.Kazianis S. Walter RB. Use of platyfishes and swordtails in biological research. Lab Anim. 2002;31:46–52. doi: 10.1038/5000142. [DOI] [PubMed] [Google Scholar]
- 26.Dong Q. Huang C. Hazlewood L. Walter R. Tiersch T. Sperm cryopreservation for live-bearing fishes of the genus Xiphophorus. In: Cabrita E, editor; Robles V, editor; Herraez P, editor. Methods in Reproductive Aquaculture. Boca Raton, FL: CRC Press; 2009. pp. 339–344. [Google Scholar]
- 27.Hawkins WE. Clark MS. Shima A. Walter RB. Winn RN. Westerfield M. Four resource centers for fishes: specifies, stocks, and services. Mar Biotechnol. 2001;3:S239–S248. doi: 10.1007/s10126-001-0046-x. [DOI] [PubMed] [Google Scholar]
- 28.Saragusty J. Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction. 2011;141:1–19. doi: 10.1530/REP-10-0236. [DOI] [PubMed] [Google Scholar]
- 29.Andreev A. Sadikova D. Gakhova E. Pashovkin T. Tikhomirov A. Congelation of cryoprotective solutions and cryopreservation of fish sperm. Biophysics. 2009;54:612–616. [PubMed] [Google Scholar]
- 30.Huang C. Dong Q. Walter RB. Tiersch TR. Sperm cryopreservation of green swordtail Xiphophorus helleri, a fish with internal fertilization. Cryobiology. 2004;48:295–308. doi: 10.1016/j.cryobiol.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiersch TR. Process pathways for cryopreservation research application and commercialization. In: Tiersch TR, editor; Green CC, editor. Cryopreservation in Aquatic Species. 2nd. Baton Rouge, LA: World Aquaculture Society; 2011. pp. 646–671. [Google Scholar]
- 32.Clarke GN. Liu DY. Baker HWG. Improved sperm cryopreservation using cold cryoprotectant. Reprod Fertil Dev. 2004;15:377–381. doi: 10.1071/RD03007. [DOI] [PubMed] [Google Scholar]
- 33.Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett. 2004;25:375–388. [PubMed] [Google Scholar]
- 34.Lawson A. Ahmad H. Sambanis A. Cytotoxicity effects of cryoprotectants as single-component and cocktail vitrification solutions. Cryobiology. 2011;62:115–122. doi: 10.1016/j.cryobiol.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain JK. Paulson RJ. Oocyte cryopreservation. Fertil Steril. 2006;86:1037–1046. doi: 10.1016/j.fertnstert.2006.07.1478. [DOI] [PubMed] [Google Scholar]
- 36.Kuleshova LL. Gouk SS. Hutmacher DW. Vitrification as a prospect for cryopreservation of tissue-engineered constructs. Biomaterials. 2007;28:1585–1596. doi: 10.1016/j.biomaterials.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 37.Cuevas-Uribe R. Tiersch TR. Non-equilibrium vitrification: an introduction and review of studies done in fish. In: Tiersch TR, editor; Green CC, editor. Cryopreservation in Aquatic Species. 2nd. Baton Rouge, LA: World Aquaculture Society; 2011. pp. 309–324. [Google Scholar]
- 38.Isachenko E. Isachenko V. Katkov II. Dessole S. Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod Biomed Online. 2003;6:191–200. doi: 10.1016/s1472-6483(10)61710-5. [DOI] [PubMed] [Google Scholar]
- 39.Isachenko V. Isachenko E. Montag M. Zaeva V. Krivokharchenko I. Nawroth F, et al. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod Biomed Online. 2005;10:350–354. doi: 10.1016/s1472-6483(10)61795-6. [DOI] [PubMed] [Google Scholar]
- 40.Yavin S. Aroyo A. Roth Z. Arav A. Embryo cryopreservation in the presence of low concentration of vitrification solution with sealed pulled straws in liquid nitrogen slush. Hum Reprod. 2009;24:797–804. doi: 10.1093/humrep/den397. [DOI] [PubMed] [Google Scholar]
- 41.Fahy GM. MacFarlane DR. Angell CA. Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 42.Fahy GM. Cryoprotectant toxicity neutralization. Cryobiology. 2010;60:S45–S53. doi: 10.1016/j.cryobiol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Cabrita E. Robles V. Wallace JC. Sarasquete MC. Herraez MP. Preliminary studies on the cryopreservation of gilthead seabream (Sparus aurata) embryos. Aquaculture. 2006;251:245–255. [Google Scholar]
- 44.Wowk B. Fahy GM. Inhibition of bacterial ice nucleation by polyglycerol polymers. Cryobiology. 2002;44:14–23. doi: 10.1016/S0011-2240(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 45.Maisse G. Comparaison de l'effet cryoprotecteur de différents glucides sur le sperme de truite arc-en-ciel (Oncorhynchus mykiss) Aquat Living Resour. 1994;7:217–219. [Google Scholar]
- 46.Miyaki K. Nakano S. Ohta H. Kurokura H. Cryopreservation of kelp grouper Epinephelus moara sperm using only a trehalose solution. Fish Sci. 2005;71:457–458. [Google Scholar]
- 47.Sean-in N. Yahsiro R. Tunkijjanukij S. Ponchunchoovong S. Efficient cryopreservation of humpback grouper, Cromileptes altivelis (Valenciennes, 1828) spermatozoa. Kasetsart Univ Fisheries Res Bull. 2009;33:12–23. [Google Scholar]
- 48.Peatpisut T. Bart AN. Cryopreservation of sperm from natural and sex-reversed orange-spotted grouper (Epinephelus coioides) Aquacult Res. 2010;42:22–30. [Google Scholar]
- 49.Weiss ADH. Fraser Forbes J. Scheuerman A. Law GK. Elliott JAW. McGann LE, et al. Statistical prediction of the vitrifiability and glass stability of multi-component cryoprotective agent solutions. Cryobiology. 2010;61:123–127. doi: 10.1016/j.cryobiol.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Yang H. Hazlewood L. Walter RB. Tiersch TR. Effect of osmotic immobilization on refrigerated storage and cryopreservation of sperm from a viviparous fish, the green swordtail Xiphophorus helleri. Cryobiology. 2006;52:209–218. doi: 10.1016/j.cryobiol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seki S. Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59:75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazur P. Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to −196 °C at 95° to 70,000 °C/min and warmed at 610° to 118,000 °C/min: a new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62:1–7. doi: 10.1016/j.cryobiol.2010.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Criado E. Albani E. Novara PV. Smeraldi A. Cesana A. Parini V, et al. Human oocyte ultravitrification with a low concentration of cryoprotectants by ultrafast cooling: a new protocol. Fertil Steril. 2011;95:1101–1103. doi: 10.1016/j.fertnstert.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Mazur P. Leibo SP. Seidel GE. Cryopreservation of the germplasm of animals used in biological and medical research: importance, impact, status, and future directions. Biol Reprod. 2008;78:2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- 55.Vajta G. Nagy ZP. Cobo A. Conceicao J. Yovich J. Vitrification in assisted reproduction: myths, mistakes, disbeliefs and confusion. Reprod Biomed Online. 2009;19:1–7. doi: 10.1016/s1472-6483(10)60278-7. [DOI] [PubMed] [Google Scholar]
- 56.Isachenko V. Isachenko E. Katkov II. Montag M. Dessole S. Nawroth F, et al. Cryoprotectant-free cryopreservation of human spermatozoa by vitrification and freezing in vapor: effecy on motility, DNA integrity, and fertilization ability. Biol Reprod. 2004;71:1167–1173. doi: 10.1095/biolreprod.104.028811. [DOI] [PubMed] [Google Scholar]
- 57.Dong QX. Correa LM. VandeVoort CA. Rhesus monkey sperm cryopreservation with TEST-yolk extender in the absence of permeable cryoprotectant. Cryobiology. 2009;58:20–27. doi: 10.1016/j.cryobiol.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thirumala S. Huang C. Dong Q. Tiersch TR. Devireddy RV. A theoretically estimated optimal cooling rate for the cryopreservation of sperm cells from a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. 2005;63:2395–2415. doi: 10.1016/j.theriogenology.2004.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rall WF. Fahy GM. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 60.Katkov II. Isachenko V. Isachenko E. Nawroth F. Why can we vitrify mammalian spermatozoa without cryoprotectants? Physicochemical considerations. Cryobiology. 2003;47:267. [Google Scholar]
- 61.Morris GJ. Rapidly cooled human sperm: no evidence of intracellular ice formation. Hum Reprod. 2006;21:2075–2083. doi: 10.1093/humrep/del116. [DOI] [PubMed] [Google Scholar]
- 62.Morris GJ. Faszer K. Green JE. Draper D. Grout BWW. Fonseca F. Rapidly cooled horse spermatozoa: loss of viability is due to osmotic imbalance during thawing, not intracellular ice formation. Theriogenology. 2007;68:804–812. doi: 10.1016/j.theriogenology.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Mazur P. Koshimoto C. Is intracellular ice formation the cause of death of mouse sperm frozen at high cooling rates? Biol Reprod. 2002;66:1485–1490. doi: 10.1095/biolreprod66.5.1485. [DOI] [PubMed] [Google Scholar]
- 64.Wang X. Wang F. Wu X. Zhao X. Liu J. Huang C, et al. The use of cryomicroscopy in guppy sperm freezing. Cryobiology. 2010;61:182–188. doi: 10.1016/j.cryobiol.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Rama Raju GA. Murali Krishna K. Prakash GJ. Madan K. Vitrification: an emerging technique for cryopreservation in assisted reproduction programmes. Embryo Talk. 2006;1:210–227. [Google Scholar]
- 66.Graham JK. Assessment of sperm quality: a flow cytometric approach. Anim Reprod Sci. 2001;68:239–247. doi: 10.1016/s0378-4320(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 67.Martínez-Pastor F. Mata-Campuzano M. Álvarez-Rodríguez M. Álvarez M. Anel L. Paz PD. Probes and techniques for sperm evaluation by flow cytometry. Reprod Domest Anim. 2010;45:67–78. doi: 10.1111/j.1439-0531.2010.01622.x. [DOI] [PubMed] [Google Scholar]
- 68.Tiersch TR. Chandler RW. Kallman KD. Wachtel SS. Estimation of nuclear DNA content by flow cytometry in fishes of the genus Xiphophorus. Comp Biochem Phys B. 1989;94:465–468. doi: 10.1016/0305-0491(89)90182-x. [DOI] [PubMed] [Google Scholar]
- 69.Ali J. Shelton J. Development of vitrification solutions. In: Tucker MJ, editor; Liebermann J, editor. Vitrification in Assisted Reproduction. London, United Kingdom: Informa Healthcare; 2007. pp. 45–63. [Google Scholar]
- 70.Isachenko E. Isachenko V. Katkov II. Sanchez R. van der Ven H. Nawroth F. Cryoprotectant-free vitrification of spermatozoa. In: Tucker MJ, editor; Liebermann J, editor. Vitrification in Assisted Reproduction. London, United Kingdom: Informa Healthcare; 2007. pp. 87–105. [Google Scholar]
- 71.Ciereszko A. Chemical composition of seminal plasma and its physiological relationship with sperm motility, fertilizing capacity and cryopreservation success in fish. In: Alavi SMH, editor; Cosson JJ, editor; Coward K, editor; Rafiee G, editor. Fish Spermatology. Oxford, United Kingdom: Alpha Science International; 2008. pp. 215–240. [Google Scholar]
- 72.Mortimer D. Menkveld R. Sperm morphology assessment—historical perspectives and current opinions. J Androl. 2001;22:192–205. [PubMed] [Google Scholar]
- 73.Petrunkina AM. Fundamental aspects of gamete cryobiology. J Reprod Med Endocrinol. 2007;4:78–91. [Google Scholar]
- 74.Koßwig C. Über Bastarde der Teleostier Platypoecilus und Xiphophorus. Mol Gen Genet MGG. 1927;44:253. (Our translation from German). [Google Scholar]
- 75.Clark E. A method for artificial insemination in viviparous fishes. Science. 1950;112:722–723. doi: 10.1126/science.112.2920.722. [DOI] [PubMed] [Google Scholar]
- 76.Kazianis S. Trono D. Woodhead A. Artificial insemination in Xiphophorus. In the on-line Xiphophorus web. Xiphophorus.org. xgscwebchemistrytxstateedu/aihtm. 2002. Xiphophorus.orgxgscwebchemistrytxstateedu/aihtm
- 77.McGovern-Hopkins K. Tamaru CS. Takeshita G. Yamamoto M. Procedural Guide for the Artificial Insemination of the Lyretail Swordtail, Xiphophorus helleri. University of Hawai'i Sea Grant College Program, School of Ocean and Earth Science and Technology; Honolulu, HI: 2003. [Google Scholar]
- 78.Uribe MC. Grier HJ. De la Rosa Cruz G. Garcia Alarcon A. Modifications in ovarian and testicular morphology associated with viviparity in teleosts. In: Jamieson B, editor. Reproductive Biology and Phylogeny of Fish (Agnatha and Osteichthyes) 8A. Enfield, NH: Science Publishers; 2009. pp. 85–117. [Google Scholar]
- 79.Greven H. Structural and behavioral traits associated with sperm transfer in Poeciliinae. In: Uribe MC, editor; Grier HJ, editor. Viviparous Fishes. Homestead, FL: New Life Publications; 2005. pp. 145–163. [Google Scholar]
- 80.Gasparini C. Simmons LW. Beveridge M. Evans JP. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS ONE. 2010;5:e12146. doi: 10.1371/journal.pone.0012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith CC. Ryan MJ. Evolution of sperm quality but not quantity in the internally fertilized fish Xiphophorus nigrensis. J Evol Biol. 2010;23:1759–1771. doi: 10.1111/j.1420-9101.2010.02041.x. [DOI] [PubMed] [Google Scholar]
- 82.Burns JR. Kallman KD. An ovarian regression syndrome in the platyfish, Xiphophorus maculatus. J Exp Zool. 1985;233:301–316. doi: 10.1002/jez.1402330219. [DOI] [PubMed] [Google Scholar]
- 83.Huang C. Sun C. Su X. Zhao X. Miao M. Liu Y, et al. Sperm cryopreservation in guppies and black mollies—a generalized freezing protocol for livebearers in Poeciliidae. Cryobiology. 2009;59:351–356. doi: 10.1016/j.cryobiol.2009.09.011. [DOI] [PubMed] [Google Scholar]