Abstract
One reason for the popularity of the zebrafish (Danio rerio) as a model vertebrate is the ability to manipulate gene expression in this organism. A common method is to induce gene expression transiently under control of a heat-shock promoter (e.g., hsp70l). By making simple mechanical adjustments to small aquarium heaters (25–50W), we were able to produce consistent and reliable heat-shock conditions within a conventional zebrafish housing system. Up to two heat-shock intervals per day (>37°C) could be maintained under conditions of continuous flow (5–25 mL/min). Temperature logging every 30 s indicated rapid warm up times, consistent heat-shock lengths, and accurate and precise peak water temperatures (mean±SD=38°C±0.2°C). The biological effects of these heat-shock treatments were confirmed by observing inducible expression of enhanced green fluorescent protein (EGFP) and inhibition of caudal fin regeneration in a transgenic fish line expressing a dominant negative fibroblast growth factor receptor (Tg(hsp70l:dnfgfr1-EGFP)pd1). These devices are inexpensive, easily modified, and can be calibrated to accommodate a variety of experimental designs. After setup on a programmable timer, the heaters require no intervention to produce consistent daily heat shocks, and all other standard care protocols can be followed in the fish facility. The simplicity and stability of these devices make them suitable for long-term heat shocks at any stage of the zebrafish lifecycle (>7 days postfertilization), and useful for both laboratory and classroom experiments on transgenic zebrafish.
Introduction
The zebrafish is widely used to study genetic, cellular, and molecular aspects of vertebrate development and human disease.1,2 One reason the zebrafish has received so much attention is the ability to easily manipulate gene expression in this species by chemical mutagenesis,3 DNA or RNA injection,4 morpholino knockdown,5–7 or inducible transgenes.8–10 One popular form of inducing gene expression utilizes a heat shock promoter that is activated by elevated water temperatures at specific developmental stages.8 A powerful promoter used to drive transgene expression in zebrafish comes from the heat-shock cognate 70-kDa protein-like gene (hsp70l).11,12 This protein acts as a chaperone during cellular stress to ensure proper protein folding and avoid cell damage.13 The hsp70l promoter activates when cells are exposed to water temperatures of around 37°C. Most commonly, the whole fish or embryo is heated, producing a global heat shock.10 Recent studies, however, have focused the heat shock using modified tools such as a soldering iron,14 pulsed blue dye laser,15 infrared laser,16,17 or a laser fiber optic system.18 These local systems allow for more precise temperature control and micron-scale targeting of gene expression, but are expensive and require considerable engineering experience to build and calibrate.
Previous methods for global heat shock of adult zebrafish are rarely reported in detail, but have consisted of manually moving fish between a standard-temperature tank (typically 28°C) in a zebrafish housing rack and a separate tank of preheated water (>37°C).19 Other labs use timed electric heaters in a stand-alone aquarium.20 A heat-shock apparatus that would keep the advantages of a modular housing rack, eliminating the need to move fish or maintain separate heated tanks, might increase the usefulness of heat-shock technology as a method for inducing gene expression, especially over longer periods of time.
In this article, we present our experiences building small heat-shock systems within the context of a standard zebrafish rack, under conditions of continuous flow. By modifying two types of submersible aquarium heaters, we were able to consistently increase water temperature from 27°C to >37°C and maintain this for 1 to several hours. The onset, intensity and duration of the heat-shock cycles could be controlled using an outlet timer and adjusting the flow rate of incoming water. Monitoring of daily heat-shock cycles for 7–14 days with a water temperature data logger showed quick warm up times (30–45 min), consistent heat-shock lengths, and accurate and precise peak water temperatures. These heat-shock profiles allowed us to induce dominant negative gene expression and observe expected phenotypes (e.g., enhanced green fluorescent protein [EGFP] expression, inhibition of tail regeneration) in a known transgenic fish line. Such devices are inexpensive, easy to set up, and can be left unmonitored for weeks at a time. These qualities drastically reduce the labor necessary to establish and run heat-shock experiments, facilitating the use of multiple transgenic lines for both laboratory and teaching purposes.
Materials and Methods
Fish lines and husbandry
All animal protocols were approved by the IACUC of Children's Memorial Research Center and/or DePaul University. A wild-type strain (AB) and a dominant negative transgenic strain (Tg(hsp70l:dnfgfr1-EGFP)pd1; ZIRC #ZL1476) were used. Transgenic clutches were genotyped at 3 days postfertilization (dpf) by heat shocking a subsample of embryos (n=10–12) for 1 h at 37°C in a Eppendorf tube of embryo medium in a water bath. Three to 4 h later, transgenics could be identified by strong whole-body EGFP expression (not shown). All remaining embryos in the clutch were reared at ∼26.5°C using standard husbandry techniques and were not exposed to any elevated water temperatures until experimental heat-shock treatments 3–4 months later. All adult fish were kept at densities less than one fish/200 mL and were fed brine shrimp and commercial fish flakes twice a day.
Modification and calibration of stealth aquarium heaters
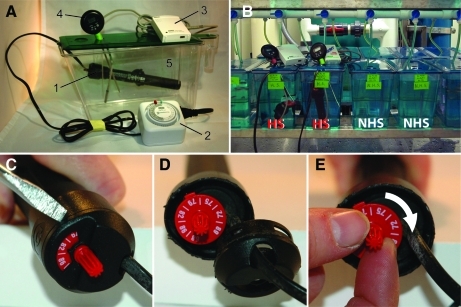
The first heat-shock system we developed is shown in Figure 1A and B. Table 1 lists the individual components. The core of the system (Fig. 1A.1) is a small 25-watt aquarium heater (Marineland Stealth: ETP25-25W) manually modified to produce temperatures outside its normal 20°C–32°C range. Modification of a 100-watt heater from the same manufacturer produced similar results (data not shown). To produce higher temperatures, the top of the heater was removed using a flat head screwdriver (Fig. 1C, D). The red temperature dial was then turned clockwise past the highest temperature setting (Fig. 1E) and the cap was snapped back into place. The effect of this modification is to tighten the bimetallic strip that controls voltage to the ceramic heating element (not shown). This type of strip, found in older aquarium heaters, bends when heated, breaking a magnetic contact between the strip and a wire. This turns the heating element off. When the strip cools down, it straightens, reconnecting the circuit and turning the heating element back on. By tightening the strip toward the “circuit closed” or “on” position, higher temperatures (a greater deflection force) are needed before the heating cycle ends.
FIG. 1.
A modified submersible aquarium heater for global heat shock (HS). (A) Parts include a Marineland 25W aquarium heater (1), an Intermatic outlet timer (2), a HOBO temperature data logger (3), a digital thermometer (4), and a 2.8 L zebrafish tank (5). For details, see Table 1. (B) Two HS tanks on the bottom shelf of an Aquaneering zebrafish housing rack. Two adjacent non-HS (NHS) tanks serve as controls. (C–E) Adjustment of the heater thermostat was performed by removing the temperature dial cover (C, D) and turning the dial clockwise past the highest temperature setting (E). Color images available online at www.liebertonline.com/zeb
Table 1.
List of Heat-Shock Equipment with Manufacturer and Model Descriptions
| Equipment | Manufacturer | Model |
|---|---|---|
| Submersible heater | Marineland | Stealth ETP25-25W |
| Outlet timer | Intermatic | TN111 |
| Two-channel temperature logger | Onset Computer | HOBO U12-013 |
| Min/max digital thermometer | General Tools | Digital Stem DPT392FC |
| Zebrafish housing tank | Aquaneering | 2.8 L volume |
| Heater-controller system | Won Brothers | Pro Heat IC 50W |
To calibrate the modified heaters to 38°C, each unit was plugged in and submerged in a 2.8 L tank filled with 26°C water. Water temperature was monitored using a digital min-max thermometer (Fig. 1A.4) and rotational adjustments to the heater temperature dial were made over several hours until the reading was stable at 38°C. To maintain this position, the dial was marked with a permanent marker and secured with laboratory tape. Lastly, the calibrated heater was set up in a second tank of 26°C system water and the warm up time (typically 30–40 min) from ambient to 38°C was noted.
In-rack heater calibration and effect of flow rate
We next tested the modified heaters to determine if they could produce high-temperature heat shocks in a standard zebrafish housing rack under continuous flow. The goal was to find the maximum flow rate that would not disturb peak temperatures (>37°C), which are essential for inducing transgene expression. A calibrated Stealth heater was completely submerged in a fish-free 2.8 L tank on the bottom shelf of an Aquaneering housing rack. An attached outlet timer (Fig. 1A.2) was set to power on for approximately 90 min (30 min warm up +1 h heat shock) once per day. The flow of system water into the tank was measured by allowing the inlet tube to drip into a clean, empty 50 mL conical tube for exactly 1 min. Flow rates were set at 5, 10, 20, 30, 50, and 70 mL/min on consecutive days; there were no adjustments to the heater or timer. Temperatures were recorded using a two-channel portable data logger with submersible temperature probe (Fig. 1A.3). This logger collected ambient (air) temperature and in-tank (water) temperature every 30 s throughout the experiment. Data were retrieved using GreenLine software (Onset Computer Corp.), exported to Microsoft Excel, and graphed using GraphPad Prism software.
Heat-shock induction of gene expression in adult zebrafish
To test if the in-system heat shocks were necessary and sufficient to induce transgene expression in a well-characterized zebrafish strain, we generated 50:50 clutches of wild-type:transgenic zebrafish from the Tg(hsp70l:dnfgfr1-EGFP)pd1 line. When exposed to heated water, the hsp70l promoter in these zebrafish drives global expression of a dominant negative fibroblast growth factor (FGF) receptor, inhibiting tail regeneration.20 50:50 clutches were genotyped by a subsample of embryos as described previously (Fish Lines and Husbandry) and the rest of the clutch was returned to the rearing system. Until the time of tissue collection (4 months later), the experimenter (R.J.D.) was blind to the genotypes of the adults.
Sixty mature fish (∼4 months postfertilization) were anesthetized using 0.015% μg/mL buffered Tricaine (Sigma-Aldrich; E10521) in system water. The distal half of each caudal fin was then removed with a razor blade. After recovery from anesthesia, the fish were split randomly into four tanks of system water (∼15 per tank); two tanks received heat-shock treatment, whereas two tanks did not (Fig. 1B). For the heat-shock tanks, two modified Stealth heaters were plugged into a single multi-outlet timer set to run for 1 h and 15 min (as determined from the heater calibrations) once a day for 7 days. To allow for adequate wound healing, the timer was set to begin the first heating cycle 24 h postamputation. The flow rate of all four tanks was set to <10 mL/min; all other fish husbandry procedures (e.g., feeding) were the same as for any other tank in the zebrafish room. Periodic temperature monitoring was performed with a min–max thermometer and continuous air/water temperature logs were recorded as previously described.
After 7 days of daily heat shock, the fish were euthanized in ice water and fixed in 4% paraformaldehyde/1×phosphate-buffered saline (PBS, pH 7.4) for 4 h at room temperature. The fish were rinsed extensively in 1×PBS and stored in this solution at 4°C for several days. Tail regeneration was observed under a fluorescent dissecting scope (Zeiss SteREO Discovery V8) and photographed on a Zeiss AxioCam at 2.25×magnification. A constant, manual exposure was used on both experimental and control fish to ensure accurate recording of relative levels of fluorescent signal. Frames were saved as TIFF files and processed in Photoshop Elements 4.0.
Modification and calibration of the Won Pro Heat heater controller
While this report was in revision, the manufacturer issued a voluntary recall of several Stealth heater models, including the ones used in our experiments (www.marineland.com/update.aspx). Seeking an alternative, we tested multiple models of small, submersible aquarium heaters to see if they could be recalibrated in a similar way. Most could not be mechanically modified, or automatically shut off at water temperatures above 33°C. Our most successful approach was to modify the electronics of an inexpensive aquarium heater controller (Won Brothers Pro Heat IC 50W) to drive higher temperatures than normal. This one-piece unit consists of a small plug-in dial thermostat (Fig. 2A.1) connected to a 50-watt submersible titanium heating element (Fig. 2A.2) and a separate temperature probe (Fig. 2A.3). The heating element and probe are placed in the tank, whereas the thermostat remains outside and regulates temperature. This model had an attractive low cost (<$40), a small-sized controller box (3′′×2′′), a relatively short (10′′) heating element that could be fully submerged in a 2.8 L zebrafish tank, and a red indicator light to show when the heating element was on.
FIG. 2.
Modification of the Won Pro Heat 50W heater controller. (A) Parts include the controller dial box (1) with integrated 50W titanium heating element (2), and temperature probe (3). A 15 cm ruler is included for scale. (B) Back of the controller dial box showing the four case screws. (C–E) The controller is modified by opening the case and turning an internal capacitor (arrow, C) by clockwise rotation. A small screwdriver (D) makes the adjustment to the final position (E). (F) Working position of the heating element and probe in a 2.8 L zebrafish tank. The horizontal line shows the approximate water level. For clarity, the heater controller, timer, and logger are not shown. Color images available online at www.liebertonline.com/zeb
The maximum temperature on the dial of this controller is 34°C. However, we recalibrated this by removing four small case screws (Fig. 2B) and using a medium screwdriver to turn clockwise a single variable capacitor or trimmer on the circuit board (Fig. 2C–E). This modification is not original to us, but was posted in 2009 on a discussion board of the Analog Photography Users Group (APUG) as a low-cost approach to warm color film developing solutions (www.apug.org/forums/forum40/61014-heating-h20-color-film-processing-2.html#post783041). We repeated this modification on two Won Pro Heat 50W devices, calibrated them to 38°C, and tested them in the context of zebrafish flow-through tanks. Briefly, an outlet timer was plugged into an extension cord, and the modified heater controller was plugged into the outlet timer. The heating element and probe were placed in a full, fish-free 2.8 L tank on the bottom shelf of an Aquaneering fish rack, with the heating element angled diagonally across the tank and the probe placed near the water surface above the lower end of this element (Fig. 2F). The timer was turned on and the controller was allowed to heat with the indicator light on until a min-max thermometer in the middle of the tank read 37°C. The controller dial was then turned down until the indicator light went off, then cycled periodically to maintain the proper temperature. This setting was marked on the dial and secured with laboratory tape. A low flow rate (∼5 mL/min) was set initially; faster flow rates were tested as previously described. Tank temperatures were logged every 30 s for 5–7 days at a time.
Results
Effect of flow rate on peak heat-shock temperatures (25W modified heater)
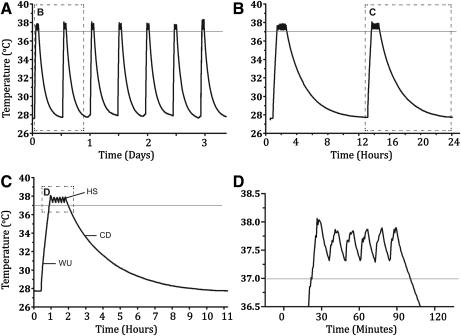
To determine the flow rate of incoming system water that would allow for the most efficient heat shocks, we recorded in-tank temperature profiles for our first heat-shock system (25W Stealth) for flow rates varying from 5 to 70 mL/min (Fig. 3A). A flow rate of 5 mL/min produced a strong heat-shock cycle, reaching the critical temperature (>37°C) within 40 min and achieving a peak temperature of 39.4°C. As flow rates increased to 70 mL/min, however, peak temperatures dropped below the 37°C optimum at a rate of approximately −0.07 °C for every +1 mL/min of flow. This indicated that only a very low flow rate (∼5–10 mL/min) would allow adequate heat-shock cycles using this type of low-powered heater (25W).
FIG. 3.
Temperature readings from a 2.8 L flow-through tank during heat shock (HS) experiments (25W Stealth heater). Boxes indicate the data magnified in successive panels. A horizontal line indicates the 37°C temperature threshold. (A) HS cycles decrease in intensity with increasing flow rates. (B) The first 7 days of a 14-day HS experiment show consistent cycles with peak temperatures above 37°C. (C) A single HS cycle consists of warm up (WU: ambient to 37°C), heat shock (HS: >37°C), and cool down (CD: 37°C to 29°C) stages. (D) HS stage of the first heating cycle.
Accuracy and precision of heat-shock cycles (25W modified heater)
Figure 3B shows tank temperatures recorded every 30 s (constant flow rate=5 mL/min) over 7 days using the same 25W heater. Summary data are given in Table 2. The timer was programmed for one heat-shock cycle per day for the duration of the experiment. Each cycle consistently raised water temperature from ∼27°C to above 37°C. A closer look at each daily cycle showed that the heating cycle could be broken down into three stages (Fig. 3C). The warm up (WU) stage lasted from the time the water temperature rapidly rose above the ambient temperature until the water temperature reached 37°C. The heat shock stage was the total time that the water temperature was above 37°C (Fig. 3C, D). The final stage was the cool down (CD) stage, which lasted from the time the temperature fell below 37°C until it reached 29°C (Fig. 3C). We did not measure cool down relative to ambient water temperature because the rate of change in the tail of the cycle was quite slow, making it difficult to distinguish a return to baseline given normal temperature fluctuations in the fish room (±1°C over the course of the experiment).
Table 2.
Times and Temperatures Taken from Seven Consecutive Heat-Shock Cycles of a Modified Stealth Heater in a 2.8 L Zebrafish Tank
| |
Cycle times |
Cycle temperatures |
|||
|---|---|---|---|---|---|
| Cycle | WU (min) | HS (min) | CD (min) | Peak (°C) | CDM (°C) |
| 1 | 43.0 | 61.0 | 276.0 | 39.1 | 69.6 |
| 2 | 49.0 | 55.0 | 281.0 | 38.7 | 52.4 |
| 3 | 50.0 | 62.0 | 284.0 | 39.0 | 66.8 |
| 4 | 48.0 | 53.0 | 294.0 | 38.6 | 47.2 |
| 5 | 47.0 | 61.0 | 294.0 | 38.9 | 64.4 |
| 6 | 48.0 | 55.0 | 289.0 | 38.6 | 52.1 |
| 7 | 43.0 | 52.0 | 259.0 | 39.1 | 55.0 |
| Avg. | 46.9 | 57.0 | 282.4 | 38.9 | 58.2 |
| SD | 2.8 | 4.2 | 12.3 | 0.2 | 8.6 |
| Min | 43.0 | 52.0 | 10.0 | 38.6 | 47.2 |
| Max | 50.0 | 62.0 | 22.2 | 39.2 | 69.6 |
Cycle times were analyzed in three stages: WU, HS, and CD. Temperatures during the HS stage were analyzed using peak temperature (°C) and CDM, a measure of heat dose. For definitions and calculations, see Materials and Methods section.
CDM, cumulative degree-minutes; CD, cool down; HS, heat shock; WU, warm up.
Continuous temperature logging showed that the heat shocks produced by the modified 25W devices were both accurate and precise (Table 2). Peak temperatures for each cycle were always above 37°C (mean±SD=38.9°C±0.2°C) and never passed 39.2°C, ensuring that the temperature for most efficient transgenic expression was reached, but that fish were not excessively overheated. The warm up stages were relatively quick, always lasting under an hour (46.9±2.8 min). The heat-shock stages were slightly shorter (57±4.2 min) than the desired 1-h heat-shock interval previously described for similar experiments,20 but this did not seem to affect the desired transgene expression (see below). This interval is easily adjusted by extending the “on” time of the outlet timer. Finally, the cool down stages lasted considerably longer than the other stages (282±12.3 min), due to the relatively low flow rates into the tank.
In addition to measuring cycle times and temperature peaks, we determined the total dose of heat delivered in each cycle by calculating the sum of degrees above 37°C at each minute of the heat-shock stage. This variable is called cumulative degree-minutes (CDM) and approximates the area under the heat-shock curve but above the 37°C threshold (e.g., Fig. 3D). Because CDM depends on both time and temperature, CDM had a predictably larger range (47.2°C–69.6°C) and greater variance (58.2°C±8.6°C) than each variable alone.
Confirmation of transgene expression (25W modified heater)
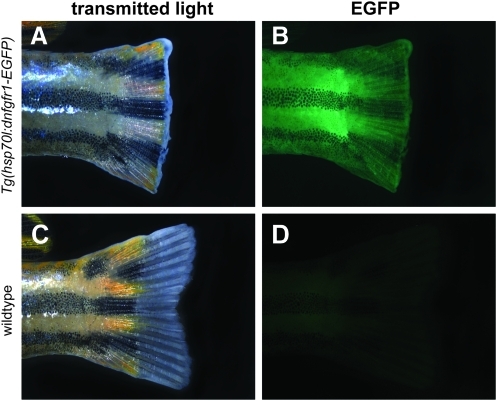
To confirm that these heat-shock conditions were capable of inducing transgene expression, we performed a simple 7-day tail regeneration experiment (Fig. 4). Transgenic zebrafish expressing a dominant negative FGF receptor (Tg(hsp70l:dnfgfr1-EGFP)pd1) show a decrease in FGF signaling and partial inhibition of tail regeneration when exposed to heat-shock temperatures of 36°C–37°C, but a blockade of signaling and complete inhibition of regeneration when exposed to temperatures above 37°C.20 In our experiments, transgenic zebrafish housed in heat-shock tanks for 7 days showed both a complete lack of tail regeneration and whole-body EGFP expression (Fig. 4A, B). Wild-type siblings housed in the same heat-shock tanks had normal tail regeneration and lacked EGFP expression (Fig. 4C, D). All wild-type and transgenic fish that did not receive heat shock had normal tail regeneration (data not shown). One out of 29 fish that received heat shock died. The heat-shock conditions within the flow-through tank thus replicate the biological effects previously seen in the hsp70l:dnfgfr1 transgenic fish line, suggesting an effective blockade of FGF signaling.
FIG. 4.
Confirmation of heat shock phenotypes in Tg(hsp70l:dnfgfr1-EGFP)pd1 zebrafish. White light (A) and green fluorescent (B) images showing inhibited tail regeneration and whole-body enhanced green fluorescent protein (EGFP) expression of an adult transgenic dnfgfr1 fish that received six cycles of HS treatment. White light (C) and green fluorescent (D) images showing normal regeneration and absent EGFP expression in wild-type fish that received the same 6 heating cycles. Color images available online at www.liebertonline.com/zeb
Accuracy and precision of heat-shock cycles (50W modified heater-controller)
We repeated our temperature sensing experiments for the second type of heat-shock apparatus, the modified Pro Heat heater-controller. This system differs from the first in several ways. It has an electronic (not mechanical) thermostat, a more powerful heating element (50W vs. 25W), and a separate submersible temperature probe. This unit was somewhat more difficult to install, as the thermostat had to be mounted outside the tank and two separate wires had to go inside the tank (Fig. 2F). However, in temperature performance this system was superior. Figure 5A shows tank temperatures recorded every 30 s under a constant, low flow rate (∼6 mL/min) for 3.5 days. Summary data are shown in Table 3. In this experiment, the outlet timer was set for two 1-h heat-shock cycles 12 h apart, demonstrating the capacity for more rapid cycling if desired. Unsurprisingly for a stronger heater (50W), the warm up stages were shorter, but they were also less variable (50W: 29.4±1.0 min vs. 25W: 46.9±2.8 min). Peak temperatures of the 50W heater (38.0°C±0.2°C) were nearly identical to the 25W mechanical model, but the temperature range within the heat-shock stage was more tightly regulated, keeping water temperature within half a degree Celsius (Fig. 5D). In contrast, the mechanical thermostat produced swings up to 1°C in the same interval (Fig. 3D). Interestingly, the variation in CDM was similar between the two systems (25W: SD=8.6 CDM; 50W: SD=7.4 CDM).
FIG. 5.
Temperature readings from a 2.8 L flow-through tank using the modified 50W Pro Heat at a steady, low flow rate (∼6 mL/min). Boxes indicate the data magnified in successive panels. Horizontal lines indicate 37°C. (A) Four days of twice-daily heat shock show accurate, repeatable temperature cycles. (B) Magnification of the first two cycles shown in (A). (C) Magnification of the second cycle shown in (B), showing typical warm up (WU), heat shock (HS) and cool down (CD) stages. Compare to Figure 3C. (D) HS stage of the heating cycle shown in (C). Compare to Figure 3D.
Table 3.
Times and Temperatures Taken from Seven Consecutive Heat-Shock Cycles of the Modified Won Pro Heat in a 2.8 L Zebrafish Tank
| |
Cycle times |
Cycle temperatures |
|||
|---|---|---|---|---|---|
| Cycle | WU (min) | HS (min) | CD (min) | Peak (°C) | CDM (°C) |
| 1 | 31.0 | 78.0 | 274.0 | 37.9 | 76.4 |
| 2 | 29.0 | 66.0 | 270.0 | 38.1 | 77.8 |
| 3 | 29.0 | 66.0 | 288.0 | 38.1 | 74.2 |
| 4 | 28.0 | 67.0 | 287.0 | 38.0 | 73.0 |
| 5 | 29.0 | 67.0 | 284.0 | 37.9 | 69.6 |
| 6 | 30.0 | 66.0 | 260.0 | 37.8 | 64.5 |
| 7 | 30.0 | 64.0 | 278.0 | 38.3 | 88.2 |
| Avg. | 29.4 | 67.7 | 277.3 | 38.0 | 74.8 |
| SD | 1.0 | 4.6 | 10.1 | 0.2 | 7.4 |
| Min | 28.0 | 64.0 | 260.0 | 37.8 | 64.5 |
| Max | 31.0 | 78.0 | 288.0 | 38.3 | 88.2 |
For explanation, see Table 2.
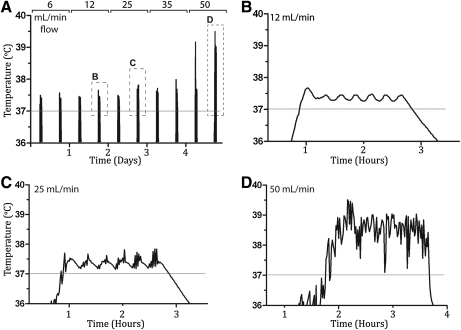
Effect of flow rate on peak heat-shock temperatures (50W modified heater)
Finally, we tested the more powerful 50W heating element at a range of incoming flow rates to determine at what level consistent heat-shock cycles could be maintained. Figure 6 shows a 5-day experiment with the outlet timer set to two 2-h heat-shock cycles per day, with the flow rate modified once per day. Unlike the 25W mechanical model, the 50W electronically controlled element was able to maintain water temperatures at >37°C for all the flow rates tested (Fig. 6A; 6–50 mL/min). Surprisingly, the fastest flow rates produced the highest water temperatures (Fig. 6A), with increased variability and spikes up to 39.5°C (Fig 5B–D; compare to Fig. 3D). We speculate that rapid flow in a small aquarium interferes with the feedback between the heating element and separate temperature probe in this system, causing more erratic heater behavior.
FIG. 6.
Temperature readings from a 2.8 L flow-through tank using the modified 50W Pro Heat at increasing flow rates (6–50 mL/min). Horizontal lines indicate the 37°C temperature threshold. (A) Four days of twice daily HS, with increasing flow rates each day. For clarity, these traces are shown in column form. (B–D) Magnification of selected HS stages at flow rates of 12 mL/min (B), 25 mL/min (C) and 50 mL/min (D). All three traces are shown at the same time and temperature scales.
Discussion
Currently, there are more than 40 transgenic heat-shock lines available from the Zebrafish International Resource Center (ZIRC, zebrafish.org). Many more are housed in individual laboratories. These valuable organisms express a variety of genes important to vertebrate development and disease. As the use of this technology increases, it is important to develop and characterize heat-shock methods that can reliably induce gene expression at different stages of the zebrafish life cycle for variable periods of time. Unfortunately, most off-the-shelf aquarium heaters are not designed to heat water to temperatures greater than 37°C, and the laboratory methods for achieving, calibrating and monitoring these water temperatures are rarely reported.
For the past few years, we have experimented with several inexpensive systems to produce weeks or months of heat-shock cycles within the context of a standard zebrafish housing rack. Under conditions of continuous flow, we were able to achieve highly accurate and repeatable temperatures in excess of 37°C with a peak temperature variance of±0.5%. This environment was suitable for inducing the hsp70l promoter in a known transgenic line (hsp70l:dnfgfr1). Induction of target gene expression was confirmed by robust EGFP signal and complete inhibition of tail regeneration in fish living in heat-shock tanks for 7 days. Using the same devices to heat-shock regenerating dnfgfr1 adults for up to 14 days has produced similar results (not shown), demonstrating that heat-shock conditions can be maintained in flow-through systems for even longer periods of time.
Our labs have been using these small heaters in a medium-sized (500+tank) shared zebrafish facility, where we find that they have multiple advantages. The systems are very inexpensive (<U.S. $40 for each heater), and the necessary mechanical alterations can be made within minutes. After calibration, adjusting the flow rate, and setting the timer for the desired number and length of heat shocks per day, one heater can be kept in the same location for months running continuously. Fish are added at the start of the experiment and removed at the end. During this time, all other variables such as tank volume, light cycles, ambient noise and feeding schedules remain the same. Stress and damage to the fish are minimized because they do not have to be relocated daily to separate tanks of heated water. Because there are no separate heat-shock tanks to maintain, the labor of fish maintenance workers is decreased. Finally, because of the very small components, the systems are modular and can be expanded to as many tanks as there are heaters and available outlets. A multi-outlet timer, for example, could be used to control up to 8 separate heaters on the same daily schedule.
Although we chose certain devices to modify for our experiments, other combinations of equipment are possible. More sophisticated controllers will regulate submersible aquarium heaters to >37°C (e.g., the Won D-58 or Jehmco ETCI-R1), but most of these are meant to deliver high wattages (500W and up) into large volumes of water (tens or hundreds of gallons). These devices are more expensive (>U.S. $70 for the controller alone), and have bulkier control boxes and heavier power cords, making them less attractive to install in tight spaces. The essential elements of a heat-shock system for multiple small aquaria such as zebrafish tanks would be low cost per tank, small size of both in-tank and out-of-tank hardware, no large electrical transformers or adapters, at least 50W of power, a precise thermostat that can be easily adjusted, and a configuration that minimizes wires. In the future, such features could be engineered into specialized zebrafish heat-shock tanks, or built as a modular insert for existing tanks in zebrafish housing systems.
We strongly recommend that every lab doing heat-shock experiments obtain a continuous temperature logger (Fig. 1A.3). Although the most expensive component (∼U.S. $150), this device allows a complete readout of the heating history of each tank, and is useful for both calibration and quality control. Smaller than a pack of cards, these loggers are lightweight, durable, and can run for several years on one battery. Between experiments, the logger is connected to a PC with a USB cable and read out using simple software. The data are compatible with any spreadsheet program. While connected, the logger can be programmed to record data at any interval (seconds, minutes, and hours) until its memory is full. A measurement of ambient air and water temperature every 30 s will yield 2 weeks of data; measurements every minute will accumulate for 30 days. Other models are available with extra memory, double submersible probes (to measure two tanks at the same time), and remote sensing capabilities. Using this method, any heater system can be tested to ensure that the temperature profiles are safe for fish and appropriate to the experiment, before any valuable transgenic animals are used.
Drawbacks to the heaters we describe include the constraint of low flow rates (caused by the relatively low wattage of the heating elements), and subtle variability among multiple heat-shock cycles over time. Flow rates <10 mL/min are more similar to a drip than a flow, and were initially a concern when planning multiweek experiments. Flows in the 5 to 8 mL/min range, however, accomplish a complete change of system water within a 2.8 L tank approximately 2.5 to 4 times a day. For the more powerful 50W heater, reasonably stable heating cycles were maintained at up to 25 mL/min, equivalent to a complete water exchange every 2 h. Given the general hardiness of zebrafish, this seems sufficient to maintain acceptable water quality at reasonable stocking densities. Long-term water clarity in our low-flow heat-shock tanks was similar to other tanks in our system; despite the tanks receiving similar amounts of food (commercial fish flakes and live brine shrimp) we saw no extra accumulation of waste under low flow. Aquarium dip-strips to test pH, ammonia, nitrite, and nitrate showed no consistent water quality differences between densely stocked tanks held at 10 mL/min and similarly stocked tanks held at 100 mL/min for several weeks (not shown). Although food debris and nitrogenous wastes seemed well controlled under low flow conditions, we had no way to measure dissolved oxygen, which at high temperatures might become limiting. Elevated temperatures make oxygen less soluble in water, yet simultaneously increases fish metabolic demand. If many large adult zebrafish were to be heat shocked for long periods of time, decreasing the density of fish per tank and/or increasing oxygenation using an air pump linked to the outlet timer would be advisable.
We also observed small variations among consecutive cycles in peak temperatures and the timing of each cycle's stages. The most variability was observed in the CDM, or heat-shock dose (±14%), which could affect the efficiency of transgene expression. According to the manufacturers, the temperature precision of the heaters used is approximately±0.6°C; smaller variations are therefore not well controlled. Although we confirmed that our heat-shock cycles could induce phenotypic effects (EGFP expression and inhibition of tail regeneration) in a strong transgenic line, the exact timing of gene expression and/or protein production over multiple days of heat shock was not measured. During our experiments, we also made no attempt to control the number, movement, position, or flow rates of any of the other tanks in the zebrafish housing rack, any of which might influence the variability of the intended cycles and thus the level of promoter activity. Researchers wishing to use such devices should test each transgenic line empirically to find the optimal heating profiles for inducing gene expression.
Although our goal was to drive gene expression in adult transgenic zebrafish in a laboratory setting, the capabilities of these systems allow other uses. The same cycles could be used to induce gene expression at any stage of the zebrafish life cycle after fry return to the recirculating system (>7 days postfertilization). Recently, we have heat shocked large clutches of dnfgfr1 larvae starting at 3 weeks postfertilization and continuing once a day for 21 days (=50% of their lifespan) with excellent rates of survival and growth (not shown). These experiments required no staff monitoring besides regularly scheduled fish feedings. Although we describe here the induction of genes involved in tissue regeneration, many other transgenic phenotypes could be studied, including genes that affect zebrafish behavior, physiology, reproduction, aging, and senescence.21,22 Lastly, due to the simplicity, low cost and low maintenance of components, similar devices could be used in an educational setting to demonstrate transgene activation, reporter gene expression, and genotype-phenotype interactions in zebrafish.
Acknowledgments
We thank Alyssa Hoekstra and J.D. Davis for zebrafish care and facilities maintenance, and Kevin Schwalbach for assistance in heater calibrations. This project was undertaken in partial fulfillment of the requirements for the degree of Master of Science at DePaul University (R.J.D.). Financial support was provided by the National Institutes of Health (R15HD064169 to E.E.L., R01DE016678 to J.T.), the DePaul University Research Council and Department of Biological Sciences (to E.E.L.), and the DePaul Graduate Research Fund (to R.J.D.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Grunwald DJ. Eisen JS. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 2.Ward AC. Lieschke GJ. The zebrafish as a model system for human disease. Front Biosci. 2002;7:d827–d833. doi: 10.2741/A814. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam A. Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Xu Q. Stemple D. Joubin K. Microinjection and cell transplantation in zebrafish embryos. Methods Mol Biol. 2008;461:513–520. doi: 10.1007/978-1-60327-483-8_35. [DOI] [PubMed] [Google Scholar]
- 5.Thummel R. Bai S. Sarras MP, Jr., et al. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dyn. 2006;235:336–346. doi: 10.1002/dvdy.20630. [DOI] [PubMed] [Google Scholar]
- 6.Cerda GA. Thomas JE. Allende ML. Karlstrom RO. Palma V. Electroporation of DNA, RNA, and morpholinos into zebrafish embryos. Methods. 2006;39:207–211. doi: 10.1016/j.ymeth.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Bill BR. Petzold AM. Clark KJ. Schimmenti LA. Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoji W. Sato-Maeda M. Application of heat shock promoter in transgenic zebrafish. Dev Growth Differ. 2008;50:401–406. doi: 10.1111/j.1440-169X.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 9.Langenau DM. Feng H. Berghmans S. Kanki JP. Kutok JL. Look AT. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le X. Langenau DM. Keefe MD. Kutok JL. Neuberg DS. Zon LI. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci U S A. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krone PH. Lele Z. Sass JB. Heat shock genes and the heat shock response in zebrafish embryos. Biochem Cell Biol. 1997;75:487–497. [PubMed] [Google Scholar]
- 12.Santacruz H. Vriz S. Angelier N. Molecular characterization of a heat shock cognate cDNA of zebrafish, hsc70, and developmental expression of the corresponding transcripts. Dev Genet. 1997;21:223–233. doi: 10.1002/(SICI)1520-6408(1997)21:3<223::AID-DVG5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Basu N. Todgham AE. Ackerman PA, et al. Heat shock protein genes and their functional significance in fish. Gene. 2002;295:173–183. doi: 10.1016/s0378-1119(02)00687-x. [DOI] [PubMed] [Google Scholar]
- 14.Hardy ME. Ross LV. Chien C-B. Focal gene misexpression in zebrafish embryos induced by local heat shock using a modified soldering iron. Dev Dyn. 2007;236:3071–3076. doi: 10.1002/dvdy.21318. [DOI] [PubMed] [Google Scholar]
- 15.Halloran MC. Sato-Maeda M. Warren JT, et al. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- 16.Kamei Y. Suzuki M. Watanabe K, et al. Infrared laser-mediated gene induction in targeted single cells in vivo. Nat Methods. 2009;6:79–81. doi: 10.1038/nmeth.1278. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi T. Itoh M. Urawa H, et al. Infrared laser-mediated local gene induction in medaka, zebrafish and Arabidopsis thaliana. Dev Growth Differ. 2009;51:769–775. doi: 10.1111/j.1440-169X.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- 18.Placinta M. Shen MC. Achermann M. Karlstrom RO. A laser pointer driven microheater for precise local heating and conditional gene regulation in vivo. Microheater driven gene regulation in zebrafish. BMC Dev Biol. 2009;9:73. doi: 10.1186/1471-213X-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoick-Cooper CL. Weidinger G. Riehle KJ, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y. Grill S. Sanchez A. Murphy-Ryan M. Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- 21.Stoletov K. Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 22.Sager J. Bai Q. Burton E. Transgenic zebrafish models of neurodegenerative diseases. Brain Struct Funct. 2010;214:285–302. doi: 10.1007/s00429-009-0237-1. [DOI] [PubMed] [Google Scholar]