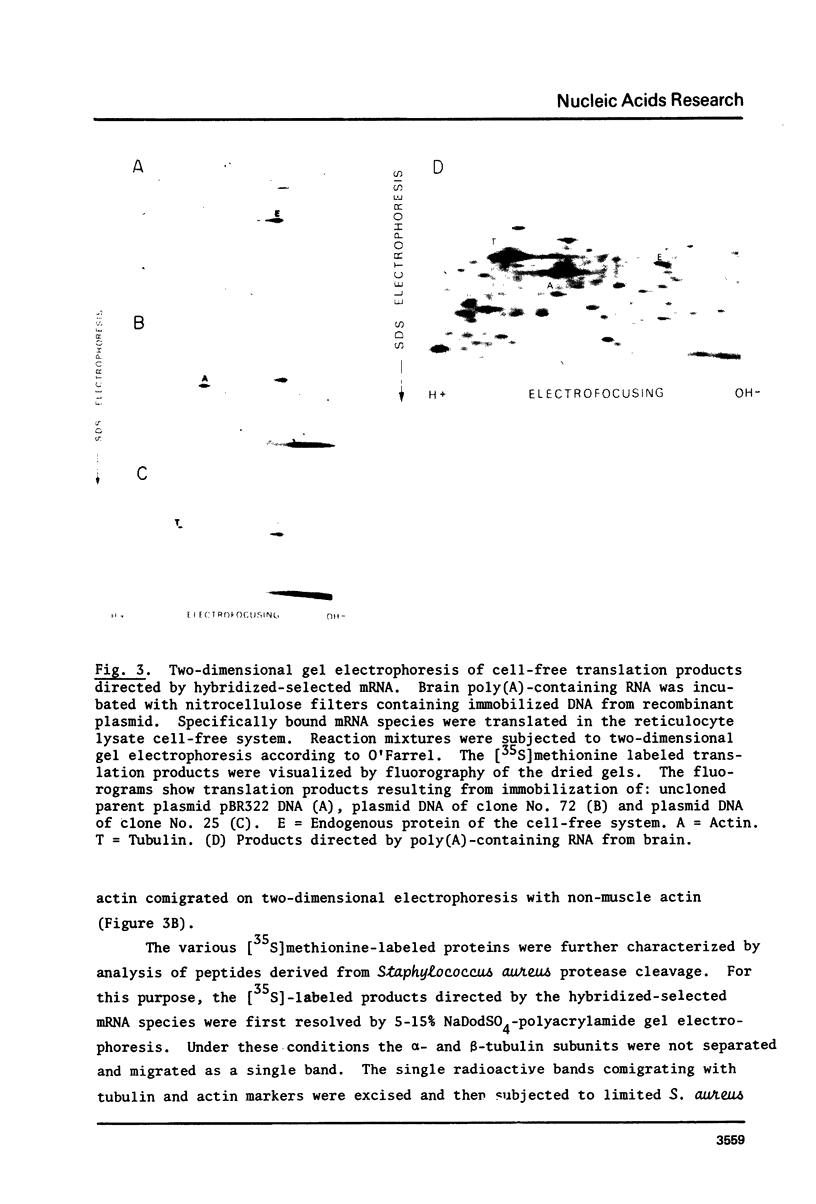
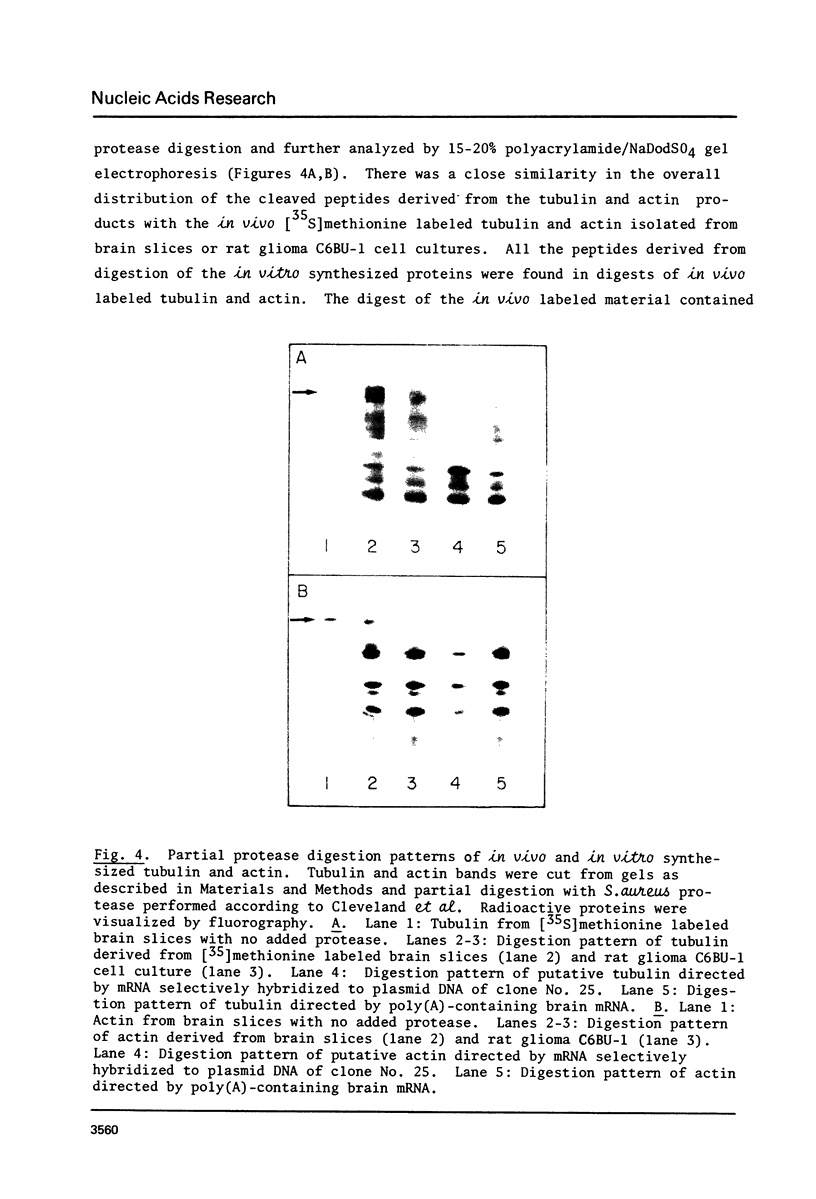
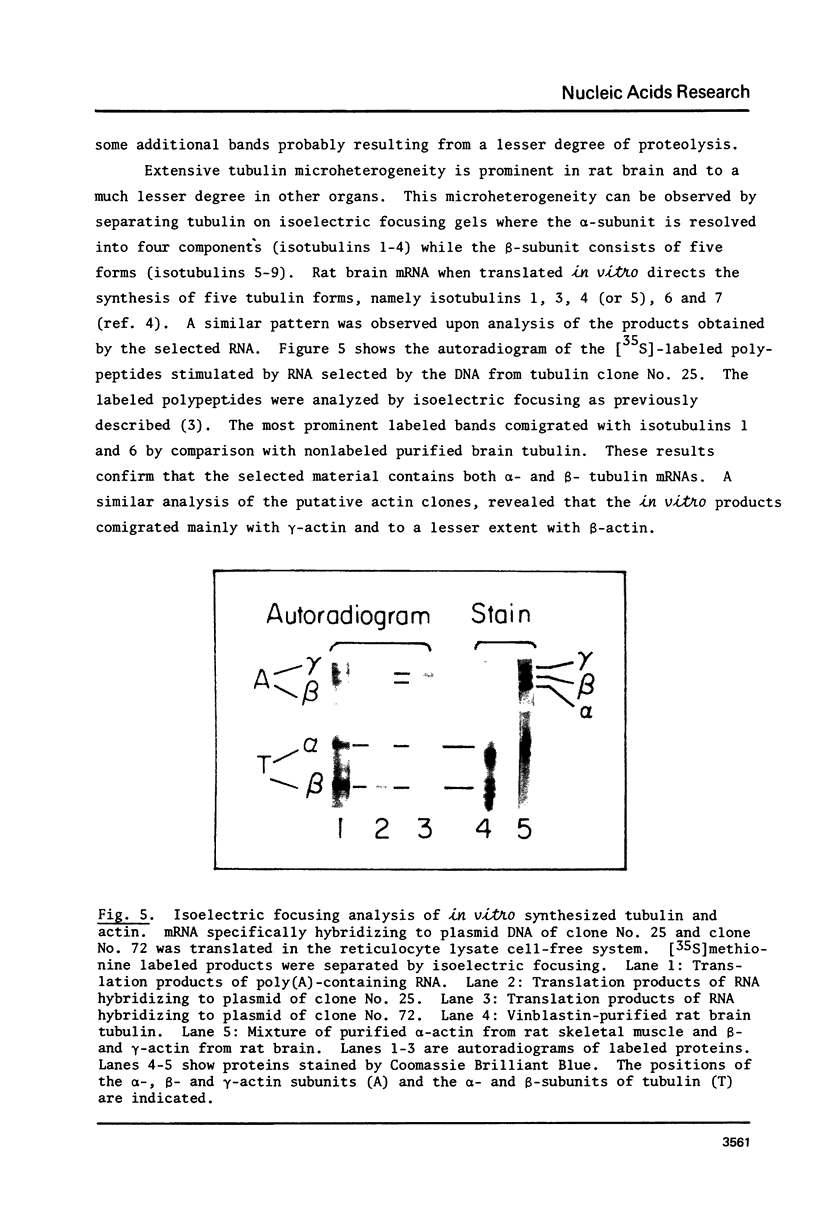
Abstract
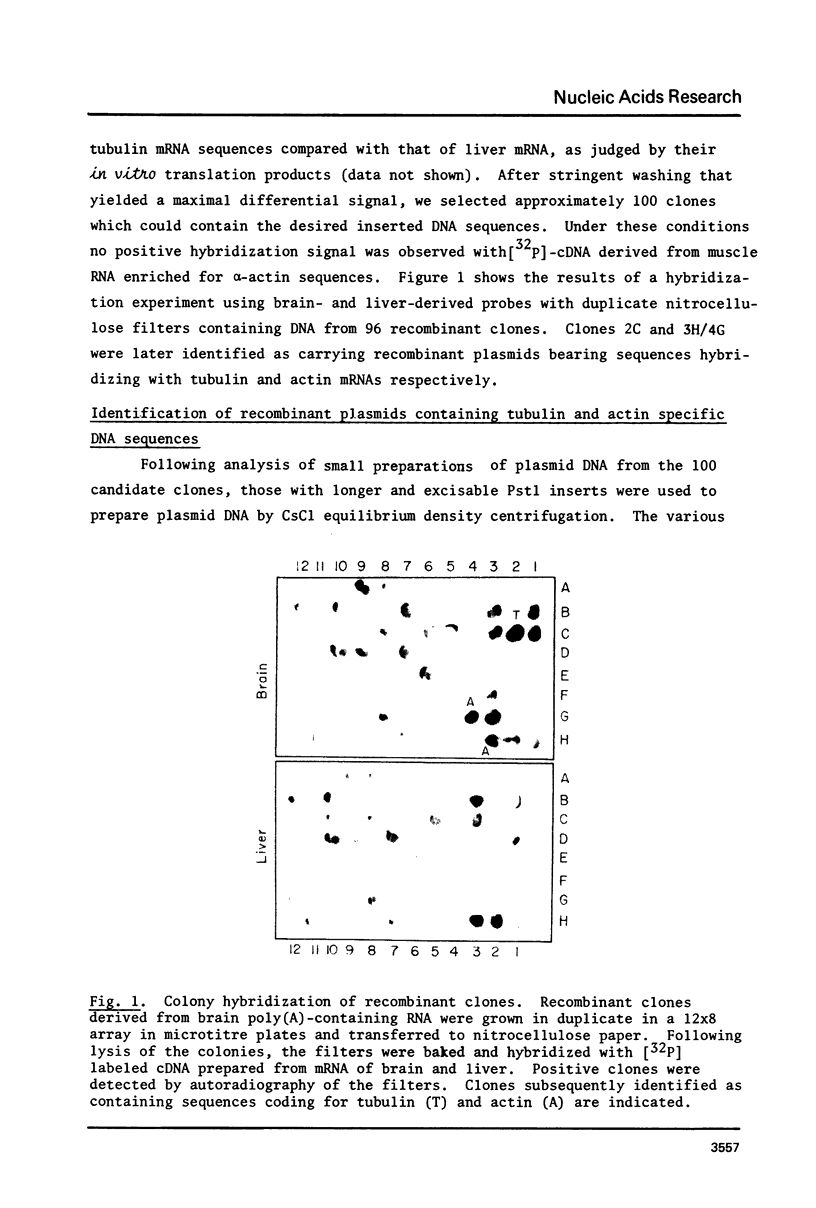
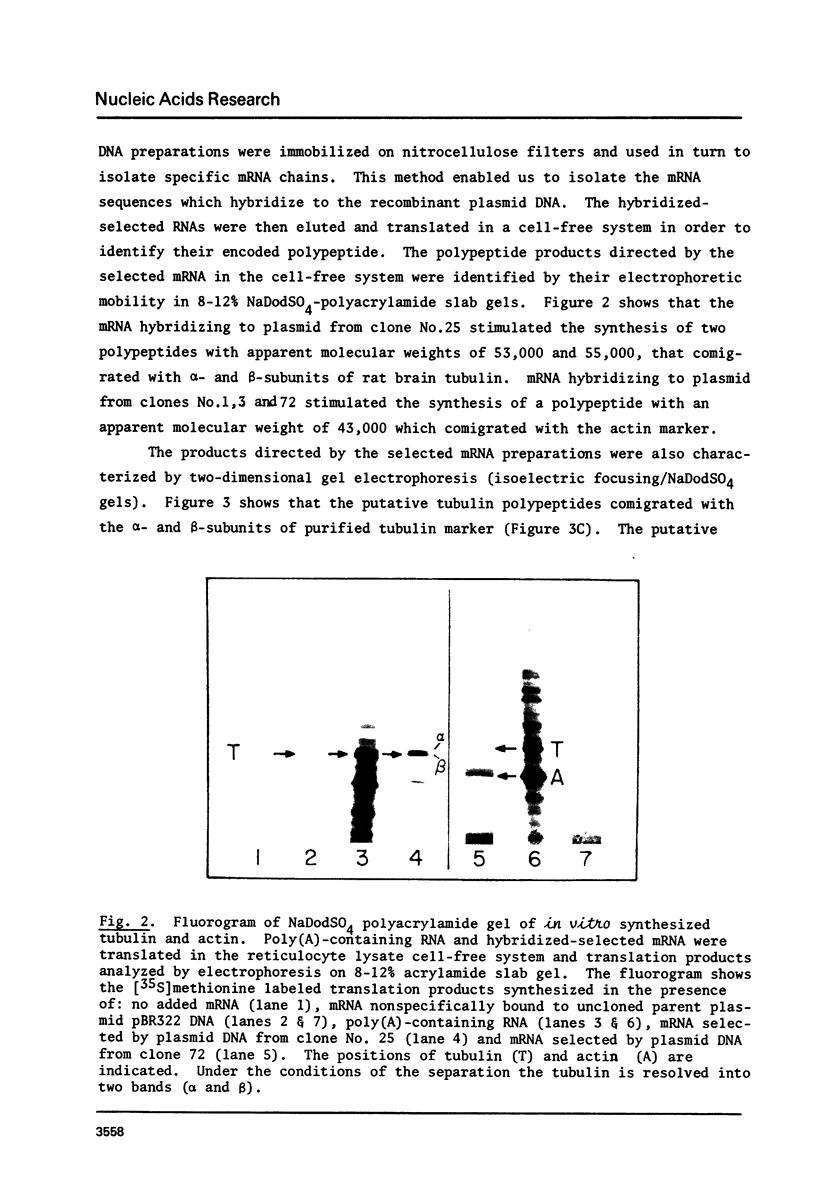
Rat brain mRNA enriched for tubulin and actin sequences was used to prepare double stranded cDNA. A library of recombinant clones was constructed by inserting the dsDNA into the Pst1 site of pBR322 plasmid and transformation of E. coli chi 1776 host. Clones bearing sequences coding for tubulin and actin were identified and characterized.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. N., Cutter G. A., Hayashi M. Separate mRNAs code for tubulin subunits. Nature. 1978 Mar 2;272(5648):81–83. doi: 10.1038/272081a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cleveland D. W., Kirschner M. W., Cowan N. J. Isolation of separate mRNAs for alpha- and beta-tubulin and characterization of the corresponding in vitro translation products. Cell. 1978 Nov;15(3):1021–1031. doi: 10.1016/0092-8674(78)90286-6. [DOI] [PubMed] [Google Scholar]
- Fulton C., Kane R. E., Stephens R. E. Serological similarity of flagellar and mitotic microtubules. J Cell Biol. 1971 Sep;50(3):762–773. doi: 10.1083/jcb.50.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., de Baetselier A., Littauer U. Z. Translation in vitro of rat brain mRNA coding for a variety of tubulin forms. Eur J Biochem. 1980 Jan;103(1):13–20. doi: 10.1111/j.1432-1033.1980.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1088–1095. doi: 10.1016/s0006-291x(75)80497-9. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H., Gozes I., Littauer U. Z. Decrease in levels and rates of synthesis of tubulin and actin in developing rat brain. Brain Res. 1977 Feb;121(2):327–342. doi: 10.1016/0006-8993(77)90155-x. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Vegetative Dictyostelium cells containing 17 actin genes express a single major actin. Nature. 1980 Apr 3;284(5755):475–477. doi: 10.1038/284475a0. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Yaffe D., Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]