Summary
The mammalian target of rapamycin (mTOR) is a key regulator of cell growth and metabolism. It associates with multiple proteins and forms two distinct signaling complexes, mTORC1 and mTORC2. Accumulating evidence has revealed critical roles for intact mTOR signaling during T cell activation and responses to microbial infection. However, the importance of mTOR regulation in T cells has yet to be explored. The TSC1/TSC2 complex has been shown to inhibit mTORC1 signaling in cell line models. We show here that deletion of TSC1 in the murine T cell lineage resulted in a dramatic reduction of the peripheral T cell pool, correlating with increased cell death. While mTORC1 is constitutively activated, mTORC2 signaling, reflected by Akt phosphorylation and activity, is decreased in TSC1-deficient T cells. Furthermore, TSC1-deficient T cells contain elevated reactive oxygen species and exhibit decreased mitochondrial content and membrane potential, which is correlated with the activation of the intrinsic death pathway. Together, our results demonstrate that TSC1 differentially regulates mTORC1 and mTORC2 activity, promotes T cell survival, and is critical for normal mitochondrial homeostasis in T cells.
Keywords: Cell survival, Signal transduction, T cells, Tuberous sclerosis complex, mammalian target of rapamyin
Introduction
The induction of the adaptive immune response is, in part, characterized by the aggressive expansion of an antigen specific T cell pool, coincident with the production of cytokines by said population. After antigen encounter, T cells must integrate and balance a plethora of extracellular signals, both cooperative and antagonistic, to ensure that an appropriate immune response is initiated. The mammalian target of rapamycin (mTOR) signaling is of central importance for the integration of environmental signals [1]. The mTOR protein is a member of two distinct signaling complexes, mTOR Complex 1 and 2 (mTORC1 and mTORC2), with each complex mediating unique and non-redundant signaling pathways. mTORC1 is comprised of mTOR, which directly interacts with GβL and Raptor, and is sensitive to rapamycin. Conversely, mTORC2 associates with Rictor to form a complex that is insensitive to acute rapamycin treatment [2, 3]. T cell receptor engagement activates both mTORC1 and mTORC2, which is dependent on the RasGRP1-Ras-Erk1/2 pathway and is inhibited by diacylglycerol kinases [4–6]. Inhibition of mTORC1 by rapamycin induces T cell anergy and promotes the generation of inducible regulatory T cells (iTreg) [7, 8]. In the absence of mTOR, T cells normally up-regulate of CD25 and CD69, and produce equivalent amounts of IL-2 after TCR stimulation. However, mTOR-deficient T cells exhibit defective Th1, Th2, and Th17 lineage differentiation, adopting instead the Treg fate [9]. Additional evidence indicates that mTORC2 is of central importance in the differentiation of T cells into Th1 and Th2 lineages by regulating Akt and PKC-θ, respectively [10]. Interestingly, and contrary to its perceived immunosuppressive properties, treating mice with rapamycin results in the generation of a larger and more effective memory CD8+ T cell pool against viral infection and regulates transcriptional programs that determine effector and/or memory cell fates in CD8+ T cells [11, 12]. Using rapamycin, it has also been demonstrated that mTOR signaling regulates the trafficking of T cells in vivo by modulating the expression of the chemokine receptor CCR7 [13]. While it is becoming clear that mTOR signaling is involved in many aspects of T cell biology, how the mTOR complexes are regulated, and the importance of their regulation in T cells remains poorly understood.
The tuberous sclerosis complex (TSC), a heterodimer of TSC1 and TSC2, is a potent upstream regulator of mTORC1 [14]. The TSC complex, by virtue of its GAP activity, inactivates Ras homolog enriched in brain (RheB) by decreasing the GTP bound active form of Rheb, subsequently inhibiting mTORC1 activation [15, 16]. Germ-line deletion of TSC1 in mice results in embryonic lethality [17]. Deletion of TSC1 in hematopoietic stem cells (HSCs) converts them from a normally quiescent state into a highly proliferative population correlated with increased mitochondrial content and reduced hematopoietic competency [18]. In this report, we demonstrate that TSC1 is critical for T cell survival and the maintenance of a normal peripheral T cell pool. Its deficiency causes constitutive activation of mTORC1, inhibition of mTORC2 and Akt activity, decreased mitochondrial content, and impaired mitochondrial membrane integrity in T cells. Furthermore, TSC1-deficient T cells display activation of the intrinsic death pathway.
Results
Effects of TSC1 deficiency on T cell numbers and activation
To investigate the role of TSC1 in T cells, we bred TSC1f/f mice to CD4-Cre transgenic mice to generate the TSC1f/f-CD4-Cre line (referred to as TSC1KO) to delete the TSC1 gene at CD4+CD8+ double positive stage of thymocyte development. In both thymocytes and purified peripheral T cells, TSC1 protein is present in wild-type (WT) T cells but was barely detectable in TSC1KO T cells, indicating efficient deletion of the TSC1 gene (Fig. 1A). In addition, TSC2 was also virtually undetectable in TSC1KO T cells, suggesting that TSC1 is crucial for the stability of TSC2 and confers a total functional loss of the TSC complex in TSC1KO T lymphocytes.
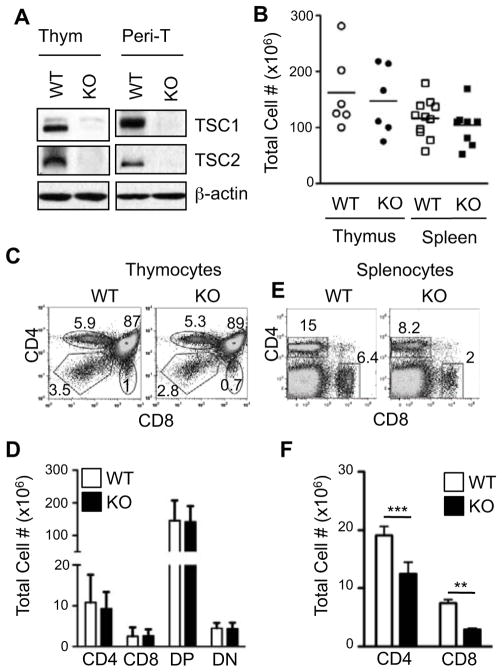
Figure 1. Decrease of Peripheral T cells in TSC1-deficient mice.
(A). TSC1 and TSC2 expression in WT and TSC1KO thymocytes (Thym) and peripheral T cells (Peri-T). Lysates from thymocytes and purified splenic T cells were subjected to immunoblot analysis with the indicated antibodies. (B) Total thymocyte and splenocyte numbers in WT and TSC1KO mice. (C, E) Flow cytometric analysis of WT and TSC1KO thymocytes (C) and splenocytes (E) stained with α-CD4 and CD8 antibodies. (D, F) Absolute numbers of thymocyte (D, n=6) and splenocyte (F, n=11) populations. P values are defined as follows: **p<0.01; ***p<0.001. Data shown are indicative of at least three experiments.
TSC1KO mice showed no significant perturbation in overall thymic cellularity in comparison to their WT counterparts (Fig. 1B). The percentage distribution and numbers of the CD4−CD8− double negative (DN), CD4+CD8+ DP, CD4+single positive (SP), and CD8+SP subsets appeared similar to their WT counterparts (Fig. 1C and 1D). The overall splenic cellularity in TSC1KO mice also appeared normal (Fig 1B). However, significant reductions in proportion and absolute cell number in both the CD4+ and CD8+ T cell compartments was observed (Fig. 1E and 1F), indicating that TSC1 is critical for normal homeostasis of peripheral T cells. While thymic T cell numbers are not grossly affected in the TSC1KO mice, we cannot rule out that more subtle abnormalities may occur in the TSC1KO thymus.
Constitutive activation of mTORC1 in TSC1-deficient thymocytes and peripheral T cells
We further investigated whether TSC1-deficiency may affect TCR signaling and mTOR activation in T cells. TCR stimulation induced phosphorylation of S6K1 and 4EBP1, both substrates of mTORC1 [19] in WT thymocytes. Elevated phosphorylation of these two proteins was observed in TSC1KO thymocytes before and after TCR stimulation. Such phosphorylation was inhibited in the presence of rapamycin, indicating constitutive activation of mTORC1 in TSC1KO thymocytes (Fig. 2A). Similar to thymocytes, TCR-induced S6K1 and 4EBP1 phosphorylation is enhanced in peripheral TSC1KO T cells (Fig. 2B). While the mTORC1 pathway is clearly hyper-activated in peripheral TSC1KO T cells, ERK1/2 phosphorylation is similar to WT T cells after TCR stimulation, suggesting that TSC1-deficiency does not globally affect T cell signaling. Consistent with elevated mTORC1 activity, and observations from drosophila to mammalian cells [20, 21], TSC1KO peripheral T cells were enlarged using forward scatter as a measurement for cell size (Fig. 2C). Clearly, TSC1 negatively regulates mTORC1 activity in T cells and its deficiency results in structurally enlarged peripheral T cells.
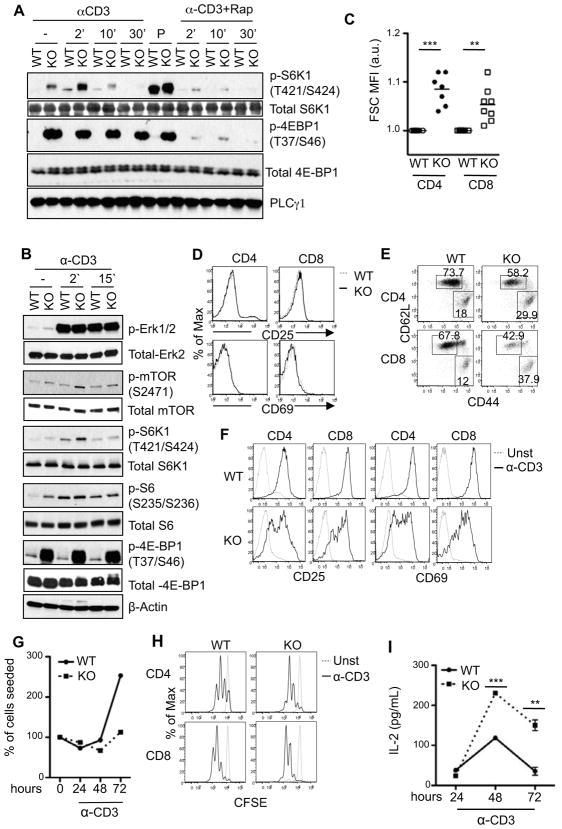
Figure 2. Altered mTORC1 signaling in and activation status of TSC1-deficient T cells.
(A) Enhanced mTORC1 signaling in TSC1KO thymocytes. Thymocytes were rested in PBS with or without Rapamycin at 37° C for 30 minutes and were then left un-stimulated or stimulated with 5 μg/mL α-CD3 or PMA for the indicated times. Lysates were subjected to immunoblot analysis with indicated antibodies. (B) Enhanced mTORC1 signaling in peripheral T cells. Purified peripheral T cells were used for immunoblot analysis as in (A). (C) Increased sizes of TSC1KO T cells. Mean fluorescence intensity (MFI) of forward scatter of TSC1KO CD4+ and CD8+ T cells relative to WT controls are shown. (D) CD69 and CD25 expression in TSC1KO T cells. WT and TSC1KO splenocytes were stained with α-CD4, α-CD8, α-CD25, and α-CD69 antibodies ex vivo and were analyzed by flow cytometry. (E) Relative increase of CD44hi T cells in TSC1KO mice. Dot plots show CD44 and CD62L expression on gated CD4 and CD8 T cells from freshly isolated WT and TSC1KO splenocytes. (F) Impaired up regulation of early activation markers in TSC1KO T cells. WT and TSC1KO splenocytes were left un-stimulated or stimulated overnight with α-CD3 antibody. Overlaid histograms show CD69 and CD25 expression on gated CD4 and CD8 cells are shown. (G) Blunted expansion of TSC1KO CD4 T cells following in vitro anti-CD3 stimulation. Purified peripheral CD4 T cells from WT and TSC1KO mice were stimulated with plate-bound 1 μg/mL α-CD3 for the indicated times. T cell numbers were determined via Trypan-blue exclusion. (H) CFSE dilution assay to assess T cell proliferation. WT and TSC1KO splenocytes were labeled with CFSE and left un-stimulated or stimulated with an α-CD3 antibody for 48 hours. Cultured cells were stained with CD4 and CD8 and analyzed by flow cytometry. CFSE intensity on CD4 and CD8 cells are shown. (I) Production of IL-2 by TSC1KO T cells. CD4 T cells were stimulated similar to (G) and IL-2 in culture supernatants were determined by ELISA. Data shown represent at least three experiments. P values are defined as follows: **p<0.01; ***p<0.001.
While mTORC1 was constitutively active, TSC1KO T cells did not show obvious up-regulation of CD25 or CD69 (markers of T cell activation) ex vivo (Fig 2D). However, the percentages of CD44hiCD62Llow effector/memory T cells and CD44lowCD62Lhi naïve T cells were consistently higher and lower, respectively, in TSC1KO mice than in WT T cells (Fig 2E). At present, it is unclear whether the increase of the CD44hiCD62Llow population in TSC1KO mice represents a population of homeostatically proliferating T cells due to the T cell lymphopenia in these mice. After overnight α-CD3 stimulation, both TSC1KO CD4 and CD8 T cells up-regulated CD25 and CD69 in a heterogeneous manner. A portion of TSC1KO T cells showed decreased CD25 and CD69 up-regulation as compared to WT T cells (Fig. 2F), suggesting impaired early activation of T cells in the absence of TSC1. α-CD3 stimulation resulted in expansion of WT CD4+ T cells in vitro. Such expansion appeared blunted in the absence of TSC1 (Fig. 2G). However, TSC1KO CD4+ as well as CD8+ T cells underwent similar or even more divisions than WT T cells during the same time of α-CD3 stimulation (Fig 2H). Although decrease in CD4+ T cell expansion was observed, elevated levels of IL-2 were detected in the supernatants of TSC1KO CD4+ T cells compared to that of WT CD4+ T cells after 48 or 72 hours of stimulation with α-CD3 (Fig. 2I), suggesting increased IL-2 production by TSC1KO T cells on a per cell basis. These results indicate that TSC1 deficiency results in constitutive activation of mTORC1 in thymocytes and peripheral T cells, and has complex effects on T cell activation manifested by decreased early activation and blunted expansion, but increased IL-2 production and slightly enhanced proliferation.
Increased T cell death in TSC1-deficient mice
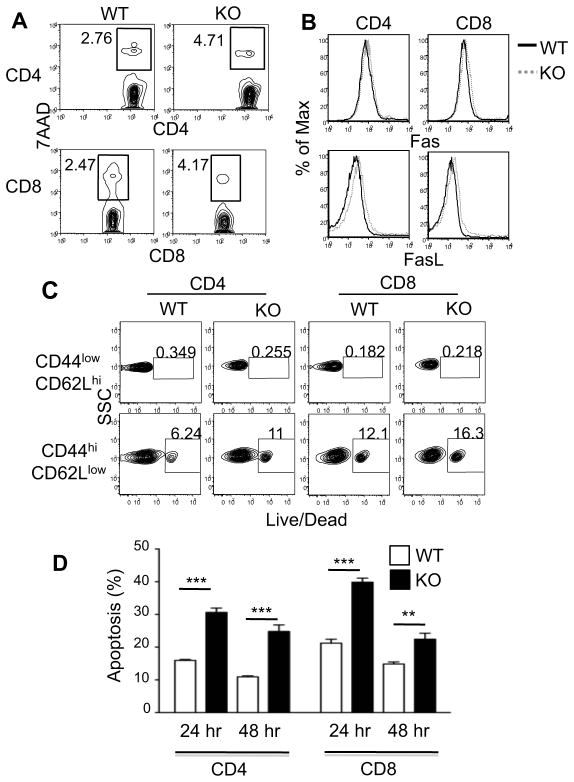
The decreases in both CD4+ and CD8+ peripheral T cell compartments in TSC1-deficient mice, and the blunted expansion concordant with normal or enhanced proliferation of TSC1KO T cells in vitro led us to hypothesize that TSC1 might control T cell survival. Indeed, an increased proportion of freshly isolated TSC1KO CD4+ and CD8+ T cells stained positive for 7-AAD ex vivo (Fig. 3A). The increase in cell death of TSC1KO T cells was not associated with the up-regulation of Fas or FasL (Fig. 3B). The vast majority of cell death within the T cell subsets is confined to the CD44hiCD62Llow population in both WT and TSC1KO T cells, and the death occurring in this particular subset is noticeably pronounced in TSC1KO T cells (Fig. 3C). The amount of cell death seen in TSC1KO T cells was intensified after α-CD3 stimulation (Fig. 3D). Collectively, these observations demonstrate that that the absence of TSC1 in T cells renders them less fit for survival in the periphery, particularly during T cell activating conditions.
Figure 3. Increased death in TSC1KO T cells.
(A) 7-AAD staining of freshly isolated T cells. Freshly isolated, splenocytes were harvested from WT and TSC1KO mice and stained with α-CD4, α-CD8, and 7-AAD. Plots show 7-AAD staining in gated CD4 and CD8 cells. (B) Freshly isolated splenocytes were harvested and stained with α-CD4, α-CD8, α-Fas, and α-FasL. Fas and FasL intensity on CD4 and CD8 cells are shown. (C) Freshly isolated splenocytes were harvested and stained with α-CD4, α-CD8, α-CD44, α-CD62L, and Live/Dead marker. Live/Dead staining is showed on populations pre-gated on CD4, CD8, CD44, and CD62L. (D) Splenocytes were stimulated with α-CD3 for 24 and 48 hours in triplicates and then stained with α-CD4, α-CD8 antibodies, 7-AAD, and annexin V. Data show apoptosis rates of gated CD4+ and CD8+ T cells. P values are defined as follows *p<0.05; ***p<0.01.
Abnormal mitochondrial homeostasis and activation of the intrinsic death pathway in TSC1KO T cells
The mitochondrion plays a central role in apoptosis [22]. In HSCs, TSC1-deficiency results in increased mitochondrial content and the production of harmful reactive oxygen species (ROS) [18]. To our surprise, TSC1KO T cells exhibited decreased mitochondrial content compared to WT T cells based on MitoTracker Green staining (Fig. 4A). Also, the ratio of mitochondrial DNA to nuclear DNA using the 12S rRNA gene and 18S rRNA as mitochondrial and nuclear DNA markers, respectively, was significantly decreased in TSC1KO T cells (Fig. 4B). A decrease in the amount of mitochondrial membrane content and absolute number of mitochondria in TSC1KO T cells suggests that TSC1 plays an important role for normal mitochondrial homeostasis in T cells.
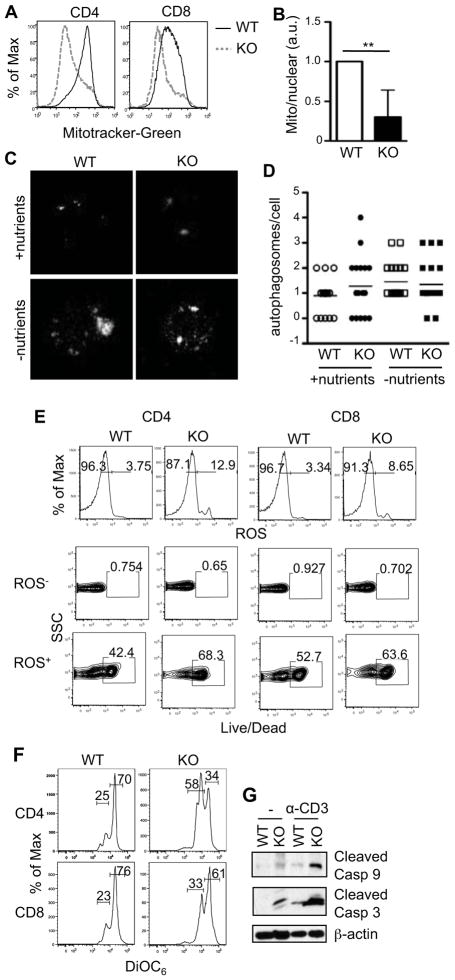
Figure 4. Abnormal mitochondrial homeostasis and activation of intrinsic cell death pathway in TSC1KO T cells.
(A) Decreased mitochondrial content TSC1KO CD4+ and CD8+ T cells measured by MitoTracker Green staining. Freshly harvested splenocytes were stained with α-CD4 and α-CD8 antibodies as well as MitoTracker Green, and were analyzed by flow cytometry. Histograms show overlay of MitoTracker Green intensity in gated WT and TSC1KO CD4 and CD8 T cells. (B) Decreased mitochondria numbers in TSC1KO T cells. The relative mitochondrial to nuclear genome copy numbers were determined by real-time PCR to quantify the levels of 12S rRNA and 18S rRNA genes in purified T cells. (C–D) Assessment of autophagy in TSC1KO T cells. Purified T cells were cultured in nutrient-sufficient or nutrient-deficient medium overnight, permeabilized, and stained with anti-LC3. Images of LC3 colocalization in individual cells were captured via fluorescent microscopy. (C). Representative microscopic images; (D). Number of distinct LC3 punctae per cell enumerated from multiple cells. (E) Increased ROS production in TSC1KO T cells. Splenocytes were harvested from WT and TSC1KO mice. Cells were stained ex vivo with α-CD4, α-CD8, dihydroethidium (ROS dye), and LiveDead marker. Histograms show ROS staining in CD4+ and CD8+ T cells. Contour plots show Live/Death staining of gated ROS− or ROS+ CD4+ and CD8+ T cells. (F) Decreased mitochondrial membrane potential in TSC1KO T cells. Freshly isolated splenocytes were stained with α-CD4, α-CD8 antibodies, and the fluorescent dye DiOC6, followed by flow cytometry. Histograms show DiOC66 intensity in gated CD4 and CD8 T cells. (G) Activation of the intrinsic death pathway in TSC1KO T cells. Freshly purified WT and TSC1KO T cells were lysed immediately or following stimulation with a plated bound α-CD3 antibody for six hours. Cleaved caspase 3 and 9 were detected by immunoblotting with indicated antibodies. Data shown represent at least three experiments. P values are defined as follows: **p<0.01
Recently, several reports have suggested that the amount of mitochondria in mature cells may be, in part, controlled by autophagy, a process usually inhibited by mTOR activity [23–25]. Because of the altered mTOR activity in TSC1KO T cells, we sought to determine whether TSC1-deficiency in T cells might deregulate the normal induction of autophagy. Using the colocalization of LC3 molecules within a cell as a readout of the induction of autophagy [26], we observed a slight increase of autophagy in TSC1KO T cells in a nutrient-sufficient environment compared to WT T cells. When starved, autophagy in both WT and TSC1KO T cells was increased. However, there was no obvious difference between these two types of cells (Fig. 4C and 4D). Thus, in the TSC1 deficiency setting, increased mTORC1 activity does not inhibit autophagy. Further studies are needed to understand mechanisms that may counter-balance with mTORC1 signaling to regulate autophagy in TSC1KO T cells.
ROS is a byproduct of mitochondrial energy production and is toxic to T cells in excess amounts [27]. Although mitochondrial content is reduced in TSC1KO T cells, they produced elevated amounts of ROS, which correlated to their positive staining for dead cells (Fig. 4E). The fluorescent dye DiOC6 has been utilized to measure mitochondrial potential. Its dilution is indicative of loss of mitochondrial membrane potential, a precursor to membrane permeabilization [28]. Both CD4+ and CD8 + TSC1KO T cells displayed diluted DiOC6 staining indicating decreased mitochondrial membrane potential and increased mitochondrial membrane permeabilization in these cells (Fig. 4F).
An increase of mitochondrial membrane permeability can result in the release of cytochrome C to the cytosol to trigger the activation of the intrinsic cell death pathway [22]. Increased cleaved caspase-9 (initiator caspase) and casapse-3 (effector caspase) were detected in TSC1KO T cells before and after anti-CD3 stimulation as compared to WT T cells, demonstrating activation of the intrinsic cell death pathway in TSC1KO T cells (Fig. 4G). Thus, TSC1 has a pro-survival function in T cells by maintaining mitochondrial membrane integrity and preventing the activation of the intrinsic death pathway.
Decreased mTORC2-Akt activity in TSC1KO T cells
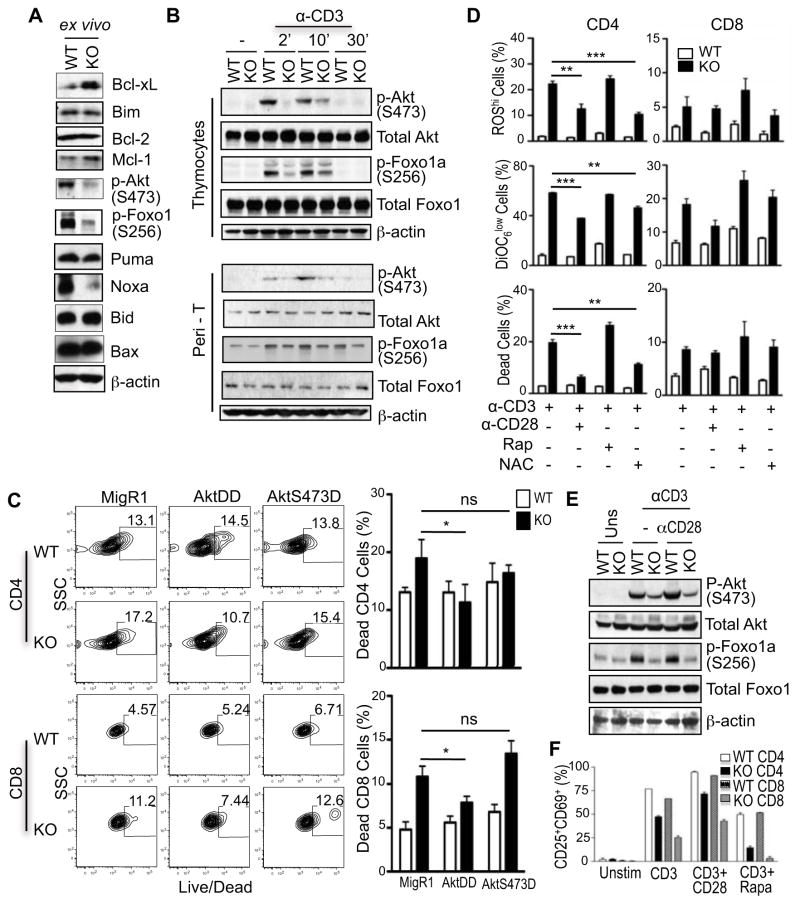
To investigate the mechanisms that promote death in TSC1KO T cells, we measured expression of several key pro-apoptotic and pro-survival proteins. No obvious decreases of pro-survival molecules, Bcl-2, Bcl-XL, Mcl-1, or increases of pro-apoptotic proteins, Bim, Puma, Bid, or Bax were observed in TSC1KO T cells (Fig. 5A). Noxa, another pro-apoptotic molecule was actually decreased in TSC1KO T cells. Whether the decreased Noxa expression contributes to TSC1KO T cell death remains to be investigated. Akt is downstream of both PI3K and mTORC2, and plays critical roles for cell survival. mTORC2 phosphorylates Akt at serine 473 (S473) to promote Akt activation [29]. In freshly isolated TSC1KO T cells, Akt phosphorylation at S473 and Foxo1a phosphorylation at S256, an Akt-mediated reaction [30], were decreased (Fig 5A). Following resting, TCR stimulation can induce phosphorylation of Akt at S473 and Foxo1a at S256 in WT T cells. Such phosphorylation was decreased in TSC1KO thymocytes and peripheral T cells (Fig. 5B). However, TCR-induced Akt phophorylation at T308 was similar between WT and TSC1KO T cells (data not shown). Thus, while mTORC1 signaling is enhanced, mTORC2 signaling and Akt activities are impaired in TSC1-deficient T cells.
Figure 5. Impaired mTORC2 and AKT activities in TSC1KO T cells.
(A) Expression of pro-apoptosis and pro-survival proteins in purified TSC1KO T cells determined by immunoblot analysis. (B) Impairment of TCR-induced mTORC2 signaling and Akt activation in TSC1KO T cells. WT and TSC1KO thymocytes and purified peripheral T cells were rested at 37°C for 30 minutes and were then left un-stimulated or stimulated with α-CD3 antibody for the indicated times. Phosphorylation of Akt and Foxo1a was determined by immunoblotting with the indicated antibodies. Data shown represent three experiments. (C) Differential effects of AKT-DD and AKT-S473D on activation induced cell death of TSC1KO T cells. WT and TSC1KO T cells were stimulated overnight and then retrovirally infected with a control construct (MigR1-GFP), Akt-DD, or Akt-S473D. Cells were stimulated with α-CD3 for an additional 48 hours and then stained with α-CD4, α-CD8, and Live/Dead marker. Percentages collected via flow cytometry were enumerated for CD4 and CD8 cells in graphs. (D) Rescue of TSC1KO abnormalities with CD28 stimulation. Splenocytes were harvested from WT and TSC1KO mice. Cells were then stimulated overnight with conditions identified in figure. Cells were then stained with α-CD4, α-CD8, and ROS (top panel), or DiOC6 (middle panel), or Live/Dead marker (bottom panel). Percentages are enumerated in graphs. (E). Effects of CD28 costimulation on mTORC2 activation. Cell lysates from purified T cells stimulated or unstimulated with plate-bound α-CD3 or α-CD3 + α-CD28 for 6 hours were used for immunoblotting analysis. P values are defined as follows: *p<0.05, **p<0.01, ***p<0.001
Akt is activated by phosphorylation at T308 and S473 by PI3K/PDK1 and mTORC2 respectively [29, 31, 32]. To determine if the decreased Akt activity observed in TSC1KO T cells may contribute to the increased death subsequent to T cell receptor stimulation, we transduced these cells with retrovirus expressing either the constitutively active (ca) form of Akt (Akt-DD) or Akt-S374D mutant. Death of the GFP+ Akt-DD expressing TSC1KO T cells was significantly reduced in comparison to the MigR1-GFP+ vector control cells in both CD4+ and CD8+ T cell subsets after TCR stimulation (Fig. 5C). However, Akt-S473D manifested minimal effects in preventing death of TSC1KO T cells. Thus, although enhanced Akt activity can promote TSC1KO T cell survival, relief of the requirement of mTORC2-mediated Akt activation is not sufficient to rescue TSC1KO T cells from death, suggesting complex regulation of T cell survival by TSC1.
Contribution of increased ROS to the death of TSC1KO T cells
CD28 costimulatory receptor promotes PI3K/Akt activation during T cell activation. Stimulation of TSC1KO CD4+ T cells through the TCR and CD28 reduced TSC1KO CD4+ T cell death, correlated with decreased ROS production, and improved mitochondrial integrity as compared with stimulated by TCR alone. However, the protective effect of CD28 was not observed in TSC1KO CD8+ T cells (Fig. 5D). In addition, CD28 costimulation was not able to restore Akt phosphorylation at S473 in TSC1KO T cells (Fig. 5E), suggesting that CD28 promotes TSC1KO T cell survival through an mTORC2-independent mechanism. We asked further whether the increased in ROS production may contribute to the death of TSC1KO T cells. Treatment with N-acetylcysteine (NAC), a ROS scavenger, resulted in decreased death of TSC1KO CD4+ T cells but not CD8+ T cells, suggesting that increased ROS production contributes to increased death of TSC1KO CD4+ T cells. Inhibition of mTOR activity has been reported to enhance survival and reduce contraction of viral specific CD8 T cells [10]. However, rapamycin treatment could not prevent increased ROS production, restore mitochondrial membrane integrity, and rescue the cells from death of TSC1KO T cells. In fact, it made TSC1KO T cells more prone to death (Fig. 5D). Similarly, rapamycin treatment could not restore early activation of TSC1KO T cells, although CD28 co-stimulation can slightly increase CD25 and CD69 expression (Fig. 5F). Together, these results suggest that increased ROS production contributes to the elevated death of TSC1KO T cells, particular CD4+ T cells.
Discussion
Mechanisms that control normal T cell homeostasis have not been well understood. In this study, we demonstrate that TSC1 plays a critical role for the maintenance of peripheral T cell numbers by promoting T cell survival through the maintenance of normal mitochondrial homeostasis.
We have shown that TSC1 inhibits mTORC1 activity, but promotes mTORC2 signaling in T cells. TSC1 may affect mTORC2 signaling through several potential mechanisms. In cell line models, the TSC1/TSC2 complex can associate with mTORC2 to promote mTORC2 signaling [33]. In HEK293 cells, it has been demonstrated that Rictor, a component of mTORC2, is directly phosphorylated at Thr1135 by S6K1 after growth factor stimulation, and that this phosphorylation is sensitive to rapamycin. In cells that express a Rictor T1135A mutant, which cannot be phosphorylated by S6K1, mTORC2 dependant Akt phosphorylation was markedly increased, strongly suggesting that mTORC1 activation can directly suppress mTORC2 activity [34]. In our model, TSC1-deficient T cells exhibit highly phosphorylated S6K1 and decreased phosphorylation of Akt and its downstream targets. Whether or not the regulation of mTORC2 by mTORC1, through Rictor, is true during the activation of primary T cells remains to be determined. It has also been reported the elevated S6K1 activity can trigger a negative feedback mechanism to inhibit growth factor induced mTOR activation. For example, S6K1 can phosphorylate IRS-1 to inhibit insulin receptor signaling [35]. Elevated S6K1 activity in TSC1 T cells may illicit similar negative feedback inhibition on mTOR dependant signaling. The exact mechanism how TSC1-deficiency leads to mTORC2 inhibition in T cells will require further examination.
Our studies indicate that the TSC1/TSC2 complex is paramount for mature T cell survival. mTORC2 and Akt activities are decreased in TSC1KO T cells. Since only expression of Akt-DD but not Akt-S473D can rescue these cells from death, it suggests that the increased death of TSC1KO T cells could not be solely due to decreased mTORC2 activity. The lack of survival defects in Rictor-deficient T cells also supports that mTORC2 is not essential for T cell survival [10]. Increased mTORC1 signaling has been reported to promote cell death. In hepatocyte cell lines, S6K1-deficiency led to down-regulation of caspase-8, caspase-3 activation, cytochrome c release, and protected against the onset of apoptosis [36]. S6K1 may promote cell death by inhibiting BAD phosphorylation [37]. Since rapamycin cannot rescue TSC1KO T cells from death, enhanced mTORC1 may not directly cause death of these cells. However, this result does not completely rule out a role of increased mTORC1 activity in the death of TSC1KO T cells. The death prone property of TSC1KO T cells could be caused by a chronic increase of mTORC1 signaling, which could program these cell to a pro-death fate that could not be reverted by acute rapamycin treatment.
It is known that ROS causes mitochondrial damage and plays an important role for death of activated T cells [27]. TSC1KO T cells display increased ROS production but decreased mitochondrial content, number, and membrane potential. Since the ROS scavenger NAC can reduce the death of TSC1KO T cells and can increase mitochondrial membrane potential, it suggests that TSC1 may promote T cell survival mainly through the inhibition of ROS production to maintain mitochondrial integrity. Of note, CD28 mediated co-stimulation, but not rapamycin treatment, can reduce TSC1KO T cell death correlated with reduced ROS production and increased mitochondrial potential, but without obvious increase of Akt activity. Thus, TSC1 may inhibit ROS production in T cells and promote T cell survival through mTOR-independent mechanisms. Further studies are needed to determine the mechanisms by which TSC1 regulates ROS production and mitochondrial homeostasis.
Materials and Methods
Mice and cells
The TSC1flox/flox and CD4-Cre transgenic mice were purchased from Jackson laboratory and Taconic Farm, Inc respectively [38, 39]. All experiments were performed in accordance with protocols approved by the Duke University Institutional Animal Care and Use Committee. Single cell suspension of thymocytes, splenocytes, and lymph node cells in IMDM medium supplemented with 10% FBS, penicillin/streptomycin, and 2-Mercaptoethanol (IMDM-10) were made according to standard protocols. Purification of T cells was achieved using either the Mouse T Cell Enrichement Kit (STEMCELL Technologies) or the LD depletion columns (Miltenyi Biotech) and purities of ≥90% were achieved.
Immunoblot
Thymocytes, splenocytes and purified T cells (5–20 × 106 cells/ml in PBS) were left un-stimulated or stimulated with 5 μg/ml of anti-CD3ε (500A2; BD Pharmingen) for different times. Cells were lysed in 1% Nonidet P-40 Lysis solution (1% Nonidet-40, 150 mM NaCl, and 50 mM Tris, pH 7.4) with freshly added protease and phosphatase inhibitors. Proteins were resolved by SDS-PAGE and were transferred to a Trans-Blot Nitrocellulose membrane (Bio-Rad Laboratories). The blots were probed with specified antibodies and detected by ECL. Antibodies for TSC1 (#4906), TSC2 (#3612), p-Foxo1 (#9461S), p-ERK1/2 (#91015), p-p70 S6K (#9204S), p70 S6K (#9202), p-4EBP1 (#2855S), 4EBP1 (#9644), Cleaved Caspase-3 (#9661), Cleaved Caspase-9 (#9509), p-Akt T308 (#9275S), p-Akt S473 (#9271S), Puma (#4976), Bid (#2003), Bax (#2772), Bim (#4582), Bcl-xL (#2762), Mcl-1 (#4572), Akt (#2938), Foxo1a (#94545), S6K1(#9202) were purchased from Cell Signaling Technology. Bcl-2 (#554087) antibody was purchased from BD. Noxa (#2437) was purchased from ProSci Inc. Anti-β-actin antibody was from Sigma-Aldrich (A1978).
Flow Cytometry
Cells were stained with fluorescence conjugated antibodies specific for CD4, CD8, CD25, CD44, and CD69 (eBioscience and BioLegend) at 4°C for 30 minutes. Dying cells were identified using 7AAD, annexin V, or the Violet Live/Dead cell kit (Invitrogen). Mitochondria were stained with MitoTracker Green (M7514; Molecular Probes) in IMDM-10 for 30 minutes at room temperature prior to surface staining. Mitochondrial potential was assessed via DiOC6 staining at a concentration of 50 nM for15 minutes prior to reading. Data were collected using a BD Canto II and analyzed with FlowJo (Treestar).
Real-time PCR
Mitochondrial genome copies were measured by using 1 μg total DNA from purified T cells as template. The PCR reaction was performed using the RealMasterMix (Eppendorf) solution on a MasterCycler RealPlex2 detection platform. DNA encoding mitochondrial 12S rRNA and nuclear18S rRNA was detected using the following primer set: 5’-ACCGCGGTCATACGATTAAC-3’ and 5’-CCCAGTTTGGGTCTTAGCTG-3’, and 5’-CGCGGTTCTATTTTGTTGGT-3’ and 5’-AGTCGGCATCGTTTATGGTC-3’, respectively.
Proliferation and activation Assays
CFSE proliferation assay was done as previously described [40]. CFSE-labeled or unlabeled WT and TSC1KO splenocytes were stimulated with α-CD3 (1 μg/mL; 2C-11) in the presence or absence of anti-CD28 (1 μg/mL; 37.51), rapamycin (20 nM), and NAC (2 mM) at 37°C for 72 hours for proliferation or overnight for CD25 and CD69 expression.
Retroviral infection
Akt S473D mutant was generated by converting D308 of Akt DD in Migr1 to T308 using site-directed mutagenesis with forward primer (5’-GGTGCCACCATGAAGACCTTTTGCGGCACACCT-3’) and reverse primer (5’-AGGTGTGCCGCAAAAGGTCTTCATGGTGGCACC-3’) and Pfu-Turbo DNA polymerase. The construct was sequenced and confirmed correct. Retrovirus was made using the Phenix-eco package cell line. For infection, one million purified CD4+ and CD8+ T cells were seeded in 1 mL IMDM-10 in 24-well plate and stimulated with plate-bound α-CD3 (1 μg/mL) overnight. The cells were then spin-infected (2000 rpm for 2 hours at 22°C with retrovirus (MigR1-GFP, Akt DD-GFP, and Akt S473D-GFP). Cells were left in culture for 48 more hours before staining and FACS analysis. Infected cells were gated on GFP+.
Fluorescence microscopy to assess LC3 localization
Purified T cells were cultured overnight in IMDM-10 (+nutrient) or Hank’s Balanced Salt solution (–nutrient). Cells were then permeabilized with 0.1% saponin, stained with rabbit anti-LC3 (MBL International), washed, and stained with FITC-labeled anti-rabbit IgG. Images were captured using a Zeiss Observer D1 platform furnished with Photometrics CoolSNAPHQ (Roper Scientific). A 40× objective lens was used and 25 individual z-stacks (vertical) were captured. 3D image deconvolution was performed and individual LC3 punctae (defined as > 10 pixels) were analyzed and enumerated with the aid of Metamorph (Molecular Probes) and Autoquant X2 (Media Cybernetics) software platforms.
Statistical Analysis
Statistical significance was determined using the Student’s t-Test. P values are defined as follows: *p<0.05;**p<0.01; ***p<0.001.
Acknowledgments
We thank Dr. Jeff Rathmell for providing the Akt expression vectors and reagents and helpful discussions. The study is supported by funding from the National Institutes of Health (R01AI076357, R01AI079088, and R21AI079873), the American Cancer Society, the American Heart Association, and the Food Allergy and Anaphylaxis Network (X.-P.Z.)
Abbreviations
- TSC
tuberous sclerosis complex
- mTOR
mammalian target of rapamycin
- mTORC1
mammalian target of rapamycin, complex 1
- mTORC2
mammalian target of rapamycin, complex 2
Footnotes
Author contributions
T.O.B designed and performed experiments, analyzed data, and prepared the manuscript. B.K.G., D.X., I.X.M., and Y.H. designed and performed experiments, and analyzed data. S.S. contributed critical reagents. X.-P.Z. supervised the study, designed the experiments, analyzed data, and prepared the manuscript.
Competing Interests Statement
The authors declare no competing financial interests.
References
- 1.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J Immunol. 2009;183:7388–7397. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- 6.Zhong XP, Shin J, Gorentla BK, O'Brien T, Srivatsan S, Xu L, Chen Y, Xie D, Pan H. Receptor signaling in immune cell development and function. Immunol Res. 2011;49:109–123. doi: 10.1007/s12026-010-8175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 9.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 15.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 16.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T, Minowa O, Sugitani Y, Takai S, Mitani H, Kobayashi E, Noda T, Hino O. A germ-line TSC1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by TSC2 mutation in mice. Proc Natl Acad Sci U S A. 2001;98:8762–8767. doi: 10.1073/pnas.151033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes Dev. 2001;15:1383–1392. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Troya S, Perez-Perez ME, Florencio FJ, Crespo JL. The role of TOR in autophagy regulation from yeast to plants and mammals. Autophagy. 2008;4:851–865. doi: 10.4161/auto.6555. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 27.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 28.Jacquel A, Herrant M, Legros L, Belhacene N, Luciano F, Pages G, Hofman P, Auberger P. Imatinib induces mitochondria-dependent apoptosis of the Bcr-Abl-positive K562 cell line and its differentiation toward the erythroid lineage. FASEB J. 2003;17:2160–2162. doi: 10.1096/fj.03-0322. [DOI] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 31.Hresko RC, Mueckler M. mTOR. RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 32.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology. 2009;50:216–229. doi: 10.1002/hep.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djouder N, Metzler SC, Schmidt A, Wirbelauer C, Gstaiger M, Aebersold R, Hess D, Krek W. S6K1-mediated disassembly of mitochondrial URI/PP1gamma complexes activates a negative feedback program that counters S6K1 survival signaling. Mol Cell. 2007;28:28–40. doi: 10.1016/j.molcel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 39.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A Critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]