Abstract
Vibrio cholerae is an estuarine bacterium and the human pathogen responsible for the diarrheal disease cholera. In the environment, arthropods are proposed to be carriers and reservoirs of V. cholerae. However, the molecular basis of the association between V. cholerae and viable arthropods has not been elucidated previously. Here, we show that the V. cholerae Vibrio polysaccharide (VPS)-dependent biofilm is highly activated upon entry into the arthropod intestine and is specifically required for colonization of the arthropod rectum. Although the V. cholerae VPS-dependent biofilm has been studied in the laboratory for many years, the function of this biofilm in the natural habitats of V. cholerae has been elusive. Our results provide evidence that the VPS-dependent biofilm is required for intestinal colonization of an environmental host.
Keywords: Drosophila melanogaster, invertebrate model, exopolysaccharide, cholera, bacteria
Each year, thousands of people living without access to adequate sanitation facilities contract cholera by ingesting food or water that carries the pandemic Vibrio cholerae bacillus (1). After passing through the acidic barrier of the stomach, V. cholerae replicates rapidly in the human gastrointestinal tract and ultimately leaves the body at concentrations as high as 109 per mL in a fast-developing, voluminous diarrhea characteristic of the disease. Release of trillions of bacteria into the environment from a single host results in rapid epidemic spread. Once the disease reaches a particular locale, it can become endemic to the region for years, reflecting the ability of V. cholerae to persist in the environment.
Both pathogenic and nonpathogenic members of the diverse species of V. cholerae, which comprises more than 200 serogroups, are part of the normal aquatic microbial assemblage in the temperate coastal marine and estuarine waters of the world (2). In these environments, culture-dependent and culture-independent analyses have found V. cholerae in association with marine organisms such as fish (3) and copepods (4, 5). That V. cholerae can also be carried by insects such as chironomids (6) hints that V. cholerae interactions with arthropods are extensive. Indeed, V. cholerae has been isolated from houseflies in areas where cholera is endemic (7–12), signifying that both terrestrial and aquatic arthropods may act as disease reservoirs. Although the presence of arthropods has been correlated with cholera epidemics, a specific interaction between V. cholerae and arthropods has not been described.
Bacterial biofilm formation mediates colonization of both biotic and abiotic surfaces. The V. cholerae multilayer biofilm, which is dependent on elaboration of a matrix comprised of the Vibrio polysaccharide (VPS) and several matrix-associated proteins (13, 14), has long been hypothesized to play a role in environmental survival. However, because this biofilm has been studied only in laboratory media, concrete evidence for such a role is lacking.
Here, we demonstrate that formation of the V. cholerae VPS-dependent biofilm is highly activated upon entry into the fly intestine and absolutely required for colonization of a specific compartment within the arthropod intestine. This evidence supports the long-standing hypothesis that the V. cholerae VPS-dependent biofilm is a factor in environmental survival. However, while V. cholerae attachment to the arthropod exoskeleton has been the focus of previous studies, these findings suggest a different paradigm for the interaction of V. cholerae with arthropod hosts.
Results
The V. cholerae Biofilm Polysaccharide VPS Plays a Role in Oral Infection of Drosophila melanogaster.
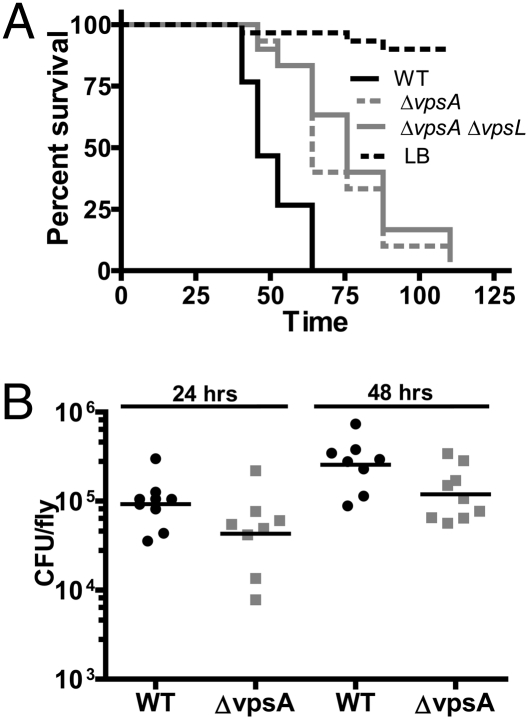
We previously described the use of Drosophila melanogaster as a model in which to study the host–pathogen interaction (15, 16). In a targeted screen for V. cholerae virulence factors in a D. melanogaster model of oral infection, we discovered that, when delivered in LB broth, V. cholerae mutants defective in synthesis of the biofilm exopolysaccharide VPS killed flies significantly more slowly than WT V. cholerae (Fig. 1A). We hypothesized that VPS might be required for colonization of the fly intestine. To investigate this, we quantified the numbers of V. cholerae in orally infected flies after 24 or 48 h of continuous V. cholerae ingestion. As shown in Fig. 1B, numbers of VPS mutant and WT cells were similar after 24 h and displayed a small but statistically significant colonization defect after 48 h.
Fig. 1.
VPS mutants have virulence and colonization defects when ingested by Drosophila from LB broth. (A) Survival over time of flies fed LB alone or inoculated with WT V. cholerae, a ΔvpsA mutant or a ΔvpsAΔvpsL mutant. By log-rank analysis, survival of WT V. cholerae is significantly different from the ΔvpsA or ΔvpsAΔvpsL mutants (P < 0.0001). (B) Bacterial load of flies fed WT V. cholerae or a ΔvpsA mutant. The ΔvpsA mutant is impaired for colonization after 48 h compared with WT V. cholerae (P = 0.0360; Mann–Whitney U test).
A Colonization Assay Suggests That V. cholerae Pass Through an Intestinal Bottleneck and Proliferate Within the Fly Until a Colonization Threshold Is Reached.
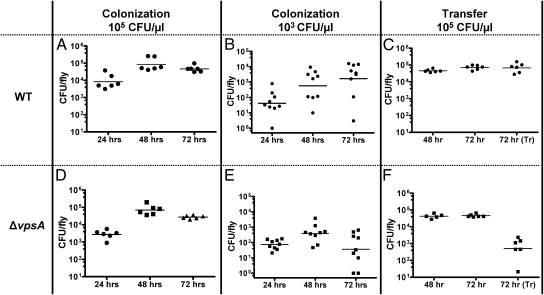
In the experimental design described above, V. cholerae were delivered to the fly in LB broth, a medium that allows the bacteria to proliferate outside as well as inside the fly. We hypothesized that this could mask a colonization defect. To limit bacterial growth in the delivery medium, we devised a colonization assay in which flies were given access to ∼105 cfu/μL of V. cholerae resuspended in PBS. In the PBS, numbers of viable, culturable bacteria remained constant, but no bacterial proliferation was observed. Under these conditions, bacterial loads in the fly were ∼10-fold lower than those observed in virulence assays, and fly death was not observed over the course of the 72-h experiment (Fig. 2A).
Fig. 2.
Low inocula and colonization transfer experiments highlight the ΔvpsA mutant colonization defect. (A and D) Bacterial burden of flies fed a PBS solution containing 105 cfu/μL of either WT V. cholerae (A) or a ΔvpsA mutant (D). (B and E) Bacterial burden of flies fed a PBS solution containing 103 cfu/μL of WT V. cholerae (B) or a ΔvpsA mutant (E). At 72 h, colonization of flies with WT V. cholerae and a ΔvpsA mutant were significantly different according to the Mann–Whitney U test (P = 0.0078). (C and F) Bacterial burden of flies measured after 48 h of access to 105 cfu/μL of WT V. cholerae (C) or a ΔvpsA mutant (F) in PBS. Bacterial burden was again measured after an additional 24 h of access to bacteria (72 h) or an additional 24 h after transfer to sterile PBS [72 h (Tr)]. After transfer, the burden per fly of the ΔvpsA mutant was significantly lower than that of the WT strain (P = 0.0022; Mann–Whitney U test).
To determine whether the numbers of V. cholerae in the fly were attributable to growth within the fly rather than accumulation of ingested bacteria, we assessed the burden over time of flies given access to a more dilute suspension of 103 bacteria per μL. Based on recent studies demonstrating that flies ingest at least 1 μL per day and higher volumes when the ingested solution is nutrient-poor (17), we predicted that we would find at least 1,000 bacteria per fly after 24 h. However, flies accumulated, on average, 50 cfu of WT V. cholerae per fly after 24 h of exposure (Fig. 2B). This suggests that there is a colonization bottleneck.
We calculated that, if the bottleneck remained constant over the course of the experiment and no growth occurred within the fly, then we should have found ∼150 cfu per fly after 72 h of bacterial ingestion. However, over 72 h, bacterial counts increased to ∼1,600 cfu per fly. We hypothesized that this increase was attributable to growth within the fly. Finally, regardless of the density of V. cholerae fed to flies, a colonization threshold of ∼104–105 cfu per fly was always reached. This suggests that there is an upper limit to the number of bacteria that can colonize the fly.
V. cholerae Colonization of the Fly Remains Constant in the Absence of Continued Ingestion.
As a more stringent test of a stable relationship between V. cholerae and the fly intestine, we performed a variation of the colonization assay in which flies were transferred to sterile PBS after 48 h (Fig. 2C). We found that colonization of the fly by V. cholerae was maintained even 24 h after access to the bacteria was terminated.
In summary, we suggest that V. cholerae encounters a colonization bottleneck within the fly intestine. Bacteria that survive this bottleneck are able to grow inside the fly until a particular bacterial density is reached. Finally, the V. cholerae population within the fly can be maintained in the absence of continued ingestion.
VPS Is a Colonization Factor in Oral Infection of the Fly.
We then tested the ability of a ΔvpsA mutant to colonize the fly. As shown in Fig. 2D, when flies were continuously fed a PBS solution containing high concentrations of the ΔvpsA mutant, the bacterial densities reached were comparable to those reached by WT V. cholerae. In contrast, if the flies were fed very dilute suspensions of a ΔvpsA mutant, then similar numbers of bacteria were seen at early time points, but much lower numbers of colonizing bacteria were observed at later time points compared with ingestion of WT V. cholerae (Fig. 2E). This suggests that WT V. cholerae and the ΔvpsA mutant survive the intestinal bottleneck equally well, but the ΔvpsA mutant is unable to proliferate in and/or colonize the fly intestine. In colonization transfer experiments, ΔvpsA mutant cells were rapidly lost from the fly (Fig. 2F). Taken together, these results suggest that VPS is a colonization factor in oral V. cholerae infection of the fly.
Biofilm Genes Are Specifically Activated in the Drosophila Intestine.
The V. cholerae biofilm is tightly regulated by a complex network of signal-transduction cascades. We predicted that if the V. cholerae biofilm were important for colonization of the arthropod intestine, then it should be specifically and significantly activated upon entry of the bacterium into this environment. To investigate this, we compared vpsL transcript levels in bacteria that had entered the fly from a PBS solution with those of bacteria incubated in PBS. In multiple experiments, the transcript levels of vpsL were found to be between 80- and 180-fold higher in the fly than in PBS (Fig. 3).
Fig. 3.
V. cholerae biofilm genes are strongly induced inside the fly. Results from three independent experiments measuring the transcription of the vpsL gene in flies after ingestion of V. cholerae in PBS for 48 h relative to V. cholerae resuspended in PBS for 2 h are shown. Each experiment was performed in triplicate. Normalization was performed with the clpXP gene, and fold change was calculated using the 2−ΔΔCt method.
The Colonization Defect of a VPS Mutant Is Not Affected by the Presence of WT V. cholerae in the Fly Intestine.
We reasoned that VPS might enhance colonization of the Drosophila intestine in a variety of ways. First of all, VPS might change the intestinal environment or dampen the innate immune response. If this were the case, then co-colonization with WT V. cholerae would be expected to improve colonization by the ΔvpsA mutant. Secondly, VPS might be coregulated with a gene or genes that improve access to or utilization of key resources in the Drosophila intestine. In this case, we would expect co-colonization with WT V. cholerae to further decrease the ability of a ΔvpsA mutant to colonize the fly intestine. Lastly, as part of the biofilm matrix, VPS might simply be an adhesion factor. Because VPS is not a shared resource in the V. cholerae biofilm (13), we predicted that, if this were the case, then co-colonization with WT V. cholerae would have no effect on the ability of the ΔvpsA mutant to colonize the intestine. To evaluate these three possibilities, we infected Drosophila with equal numbers of WT V. cholerae and a ΔvpsA mutant and evaluated colonization at 48 and 72 h using a colonization-transfer assay. In these experiments, WT V. cholerae was maintained in the intestine in numbers similar to those observed for single-strain infections (Fig. 4A). Similarly, the ΔvpsA mutant was lost in numbers comparable to those observed for a single-strain infection (Fig. 4B). Based on these observations, we hypothesized that VPS does not alter the intestinal environment of the fly or improve competition for a limited resource but rather acts as an adhesion factor.
Fig. 4.
Evidence that the biofilm genes play a role in attachment in the fly. (A and B) Drosophila burden of WT V. cholerae (A) and the ΔvpsA mutant (B) in a coinfection colonization transfer experiment in which one strain was labeled by mutation of the lacZ gene. Gray and black circles reflect results of experiments in which the bacterial label was swapped. Asterisk (*) indicates measurements below the level of detection. (C) Colonization transfer experiment with the ΔrbmC Δbap1 mutant alone. The ΔrbmC Δbap1 mutant was significantly lost after transfer to sterile PBS compared with bacterial burden of the ΔrbmC Δbap1 mutant at 48 h (P = 0.0022) and 72 h (P = 0.0022) in the absence of transfer (Mann–Whitney U test).
The V. cholerae biofilm matrix contains two highly similar proteins, Bap1 and RbmC (13). These proteins have a redundant and essential function in biofilm formation. However, deletion of these proteins does not affect transcription of VPS genes (13, 18, 19). We discovered recently that these proteins are located at the base of the biofilm and function specifically to stabilize the attachment of the V. cholerae biofilm to surfaces (13). We hypothesized that if the V. cholerae biofilm were simply required for attachment, then a Δbap1ΔrbmC mutant should also have a defect in colonization of the Drosophila intestine. As shown in Fig. 4C, this was, in fact, the case.
The V. cholerae Biofilm Is Required for Colonization of a Specific Compartment Within the Fly Intestine.
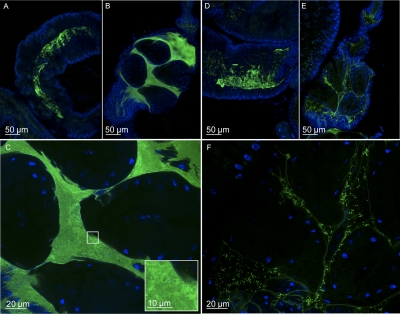
To support our hypothesis that VPS was an attachment factor, we subsequently used confocal microscopy to visualize the distribution of V. cholerae within the Drosophila intestine. We fed flies either LB broth or PBS containing GFP-labeled WT V. cholerae or a ΔvpsA mutant and examined the distribution of these two strains in the Drosophila intestine. In LB broth, large numbers of both WT V. cholerae and the ΔvpsA mutant were observed throughout the proximal intestine and midgut (Fig. 5 A and D). However, strain-specific differences in colonization were observed more distally in the rectum. WT V. cholerae coated the rectal surface (Fig. 5 B and C), whereas the ΔvpsA mutant attached to the rectal surface in much smaller numbers and was also observed floating throughout the rectum (Fig. 5 E and F). For comparison, the midgut and rectal pouch of Drosophila fed LB alone are included in Fig. S1.
Fig. 5.
After 48 h of ingestion in LB broth, the ΔvpsA mutant has a specific defect in colonization of the Drosophila rectal pouch. (A) WT V. cholerae in the midgut. (B) WT V. cholerae in the rectal pouch. (D) ΔvpsA mutant in the midgut. (E) ΔvpsA mutant in the rectal pouch. (C and F) Magnified view of WT V. cholerae (C) and a ΔvpsA mutant (F) in the rectum. The oval structures in the rectum are the rectal papillae. V. cholerae carry a constitutively expressed chromosomal copy of GFP. Nuclei were stained with DAPI.
In PBS colonization experiments, intestinal bacteria were sparser, and occasionally intestines were not colonized at all. Very few WT V. cholerae were visible in the midgut (Fig. 6A). Large numbers of fluorescent bacteria were observed within both the crop, a proximal intestinal food storage appendage, and the rectal pouch (Figs. 6 B and C and Fig. S2A). In most intestines, a sheet or thick coating of V. cholerae covered the inner surface of the rectal pouch. By comparison, whereas densities of the ΔvpsA mutant were similar to those of WT V. cholerae in midgut and crop (Fig. 6D and Fig. S2B), in the rectum, only individual bacteria were observed, and these were mostly not attached to the rectal surface (Fig. 6 E and F).
Fig. 6.
After 72 h of ingestion in PBS, WT V. cholerae densely colonize the rectal pouch, whereas very few ΔvpsA mutants are visible. WT colonization of the rectal pouch is maintained after transfer to sterile PBS. (A) WT V. cholerae in the midgut after 72 h of continuous feeding. (B) WT V. cholerae in the rectal pouch after 72 h of continuous feeding. (C) Magnified view of WT V. cholerae in the rectal pouch and individual bacteria (inset). (D) ΔvpsA mutant in the midgut after 72 h of continuous feeding. (E) ΔvpsA mutant in the rectal pouch after 72 h of continuous feeding. (F) Magnified view of ΔvpsA mutant in the rectal pouch. (G) WT V. cholerae in midgut after colonization transfer experiment. (H) WT V. cholerae in rectal pouch after colonization transfer experiment. (I) Magnified view of WT V. cholerae in the rectal pouch and individual bacteria (inset). Nuclei were stained with DAPI.
To determine whether the WT V. cholerae biofilm could persist on the rectal surface in the absence of continued ingestion, we performed a colonization-transfer experiment with GFP-labeled WT bacteria. In this experiment, V. cholerae were largely absent from the midgut and crop (Fig. 6G and Fig. S2C). However, the rectal surface remained densely colonized with bacteria (Fig. 6 H and I). We conclude that the V. cholerae biofilm is maintained in the Drosophila rectal pouch in the absence of continued ingestion.
Discussion
Biofilm formation and attachment to arthropods such as zooplankton and terrestrial insects have been proposed as mechanisms for environmental survival and spread of V. cholerae (6, 7, 9, 10, 13, 20). Here, we demonstrate that V. cholerae is able to multiply and form a biofilm within the model terrestrial insect D. melanogaster. This biofilm forms within a specific compartment of the fly intestine, the rectum, and requires genes encoding the synthesis of the VPS biofilm matrix, as well as matrix-associated proteins that are essential for stability and surface attachment. Here, we provide evidence that the V. cholerae VPS-dependent biofilm is required for colonization of an epithelial surface and, in particular, that of an insect.
There is evidence that VPS genes are expressed in the mammalian intestine; however, the role of the VPS-dependent biofilm in pathogenesis is less clear. In vivo-expression technology-based screens for genes that are selectively transcribed in the human intestine have identified VPS genes (21). Furthermore, V. cholerae aggregates have been observed in the stool of cholera patients (22). V. cholerae introduced into rabbit ileal loops form visible clumps of cells that are dependent upon the VPS-synthesis genes (23). However, most studies suggest that the VPS genes not only are unnecessary for intestinal colonization but even interfere with it (24–26). Therefore, it seems unlikely that VPS-dependent biofilm formation contributes significantly to attachment to the human intestine.
Although a role for the V. cholerae VPS-dependent biofilm in the ecology and pathogenesis of this diarrheal pathogen has long been hypothesized, definitive proof has been elusive. Environmental studies suggest that the majority of V. cholerae are associated with large biotic or abiotic particulates and, in particular, zooplankton and larger crustaceans (4, 27). Furthermore, V. cholerae has been linked to terrestrial insects such as the nonbiting midge and the common house fly (7, 28). Attachment to the exoskeleton of the nonbiting midge has been proposed as a mechanism for environmental spread (6). Whereas most studies have focused on attachment of V. cholerae to exposed environmental surfaces, here, we show that the VPS-dependent biofilm is absolutely required for persistent colonization of an internal surface, the insect intestinal epithelium. These data suggest a different paradigm for the role of the VPS-dependent biofilm in environmental survival as a colonization factor in the intestine of an insect host.
Exopolysaccharide-dependent biofilm formation may be a common theme in colonization of invertebrate hosts by Gram-negative bacteria. Yersinia pestis, the plague bacterium, is transmitted to humans by fleas. In the process of taking a blood meal, the bacterium is ingested and makes a biofilm, blocking the proventriculus of the flea. Because this increases the attempts of the flea to feed, biofilm formation increases disease transmission (29). A V. fischeri exopolysaccharide-dependent biofilm, which is formed during attachment of the bacterium to the opening of the squid light organ, is required for efficient colonization of the light organ itself (30). The role of bacterial exopolysaccharide-dependent biofilms in attachment to invertebrate but not mammalian epithelia likely reflects the very different compositions of these two surfaces.
We hypothesize that V. cholerae forms a specialized, nonpathogenic relationship with one or several arthropod hosts in the environment. Because arthropods are abundant in both aquatic and terrestrial habitats, such relationships would significantly improve the odds of V. cholerae survival and proliferation. Furthermore, the greater mobility afforded by association with arthropods would increase environmental dissemination. Lastly, the high density of V. cholerae associated with the chitinous surface of the arthropod rectum could greatly accelerate sharing of genetic material between different V. cholerae species, as well as between V. cholerae and the commensal bacterial community. In summary, by conferring on V. cholerae the ability to colonize the insect rectum, the VPS-dependent biofilm may have a dramatic impact on the ecological fitness of this bacterium.
Methods
Colonization Assays.
To measure the ability of V. cholerae to colonize and grow in the fly intestine in the absence of bacterial growth in the ingested medium, we performed a colonization assay as follows. Overnight cultures of V. cholerae in LB broth were pelleted by centrifugation for 6 min at 2,300 × g. Spent medium was removed, the pellet was washed with PBS, a volume of PBS supplemented with streptomycin equal to that of the original culture volume was added, and the cells were resuspended. Two milliliters of a dilution of this bacterial suspension were added to a standard fly vial containing a cellulose acetate plug. In this experimental format, the majority of flies remained viable throughout the course of each experiment, which generally lasted ≤72 h. At the indicated time, flies were collected; the entire vial of flies was homogenized together, and the resulting bacterial suspension was plated as described for LB broth assays. Three replicates were included for each test condition, and each experiment was repeated at least twice.
Confocal Microscopy.
Overnight cultures of V. cholerae strains constitutively expressing GFP from a chromosomal location were fed to male yw flies using either the virulence or colonization assay protocols. After 48 h (LB broth) or 72 h (PBS), the gastrointestinal tracts of flies were dissected in Grace's medium, immediately fixed in 4% paraformaldehyde in 0.5× PBS for 30 min, washed once in PBS, and washed again in PBS supplemented with DAPI (1 μg/mL) for at least 30 min at 4 °C. Gastrointestinal tracts were mounted on slides with Vectashield (Vector Labs) and stored at 4 °C until use. V. cholerae in the Drosophila gastrointestinal tract were visualized on a LSM700 confocal microscope (Zeiss) with 25× or 63× oil immersion objectives (Intellectual and Developmental Disabilities Research Imaging Core, Children's Hospital Boston). Additional experimental details are provided in SI Methods.
Supplementary Material
Acknowledgments
Microscopy imaging was performed at the Intellectual and Developmental Disabilities Research Imaging Core, Children's Hospital Boston (National Institutes of Health Grant P30-HD-18655). This work was supported by National Institutes of Health Grants R01 AI AI071147 (to P.I.W.) and T32 HD007466-12 and F32 AI082940 (to A.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111530108/-/DCSupplemental.
References
- 1.Zuckerman JN, Rombo L, Fisch A. The true burden and risk of cholera: Implications for prevention and control. Lancet Infect Dis. 2007;7:521–530. doi: 10.1016/S1473-3099(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 2.Faruque SM, Nair GB. Molecular ecology of toxigenic Vibrio cholerae. Microbiol Immunol. 2002;46:59–66. doi: 10.1111/j.1348-0421.2002.tb02659.x. [DOI] [PubMed] [Google Scholar]
- 3.Senderovich Y, Izhaki I, Halpern M. Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE. 2010;5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lizárraga-Partida ML, et al. Association of Vibrio cholerae with plankton in coastal areas of Mexico. Environ Microbiol. 2009;11:201–208. doi: 10.1111/j.1462-2920.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 5.Baffone W, et al. Detection of free-living and plankton-bound vibrios in coastal waters of the Adriatic Sea (Italy) and study of their pathogenicity-associated properties. Environ Microbiol. 2006;8:1299–1305. doi: 10.1111/j.1462-2920.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 6.Broza M, Gancz H, Halpern M, Kashi Y. Adult non-biting midges: Possible windborne carriers of Vibrio cholerae non-O1 non-O139. Environ Microbiol. 2005;7:576–585. doi: 10.1111/j.1462-2920.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 7.Fotedar R. Vector potential of houseflies (Musca domestica) in the transmission of Vibrio cholerae in India. Acta Trop. 2001;78:31–34. doi: 10.1016/s0001-706x(00)00162-5. [DOI] [PubMed] [Google Scholar]
- 8.Echeverria P, Harrison BA, Tirapat C, McFarland A. Flies as a source of enteric pathogens in a rural village in Thailand. Appl Environ Microbiol. 1983;46:32–36. doi: 10.1128/aem.46.1.32-36.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotenok IaF, Chicherin IuV. [Houseflies (M. domestica L.) as transmitters of the agent of cholera] Zh Mikrobiol Epidemiol Immunobiol. 1977;(12):23–27. [PubMed] [Google Scholar]
- 10.Sukontason K, et al. Mechanical carrier of bacterial enteric pathogens by Chrysomya megacephala (Diptera: Calliphoridae) in Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health. 2000;31(Suppl 1):157–161. [PubMed] [Google Scholar]
- 11.Khin Nwe O, Sebastian AA, Aye T. Carriage of enteric bacterial pathogens by house flies in Yangon, Myanmar. J Diarrhoeal Dis Res. 1989;7:81–84. [PubMed] [Google Scholar]
- 12.Khan AR, Huq F. Disease agents carried by flies in Dacca city. Bangladesh Med Res Counc Bull. 1978;4:86–93. [PubMed] [Google Scholar]
- 13.Absalon C, Van Dellen K, Watnick PI. A communal bacterial adhesin anchors biofilm and bystander cells to surfaces. PLoS Pathog. 2011;7:e1002210. doi: 10.1371/journal.ppat.1002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yildiz FH, Schoolnik GK. Vibrio cholerae O1 El Tor: Identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkey CD, Blow N, Watnick PI. Genetic analysis of Drosophila melanogaster susceptibility to intestinal Vibrio cholerae infection. Cell Microbiol. 2008;11:461–474. doi: 10.1111/j.1462-5822.2008.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blow NS, et al. Vibrio cholerae infection of Drosophila melanogaster mimics the human disease cholera. PLoS Pathog. 2005;1:e8. doi: 10.1371/journal.ppat.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moorthy S, Watnick PI. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol Microbiol. 2005;57:1623–1635. doi: 10.1111/j.1365-2958.2005.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong JC, Yildiz FH. The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J Bacteriol. 2007;189:2319–2330. doi: 10.1128/JB.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein PR. Algal blooms in the spread and persistence of cholera. Biosystems. 1993;31:209–221. doi: 10.1016/0303-2647(93)90050-m. [DOI] [PubMed] [Google Scholar]
- 21.Lombardo MJ, et al. An in vivo expression technology screen for Vibrio cholerae genes expressed in human volunteers. Proc Natl Acad Sci USA. 2007;104:18229–18234. doi: 10.1073/pnas.0705636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen MA, Faruque SM, Mekalanos JJ, Levin BR. Modeling the role of bacteriophage in the control of cholera outbreaks. Proc Natl Acad Sci USA. 2006;103:4652–4657. doi: 10.1073/pnas.0600166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamruzzaman M, et al. Quorum-regulated biofilms enhance the development of conditionally viable, environmental Vibrio cholerae. Proc Natl Acad Sci USA. 2010;107:1588–1593. doi: 10.1073/pnas.0913404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watnick PI, Lauriano CM, Klose KE, Croal L, Kolter R. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol Microbiol. 2001;39:223–235. doi: 10.1046/j.1365-2958.2001.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong JC, Syed KA, Klose KE, Yildiz FH. Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology. 2010;156:2757–2769. doi: 10.1099/mic.0.040196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houot L, Chang S, Absalon C, Watnick PI. Vibrio cholerae PTS control of carbohydrate transport, biofilm formation, and colonization of the germ-free mouse intestine. Infect Immun. 2010;78:1482–1494. doi: 10.1128/IAI.01356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huq A, et al. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl Environ Microbiol. 1996;62:2508–2512. doi: 10.1128/aem.62.7.2508-2512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern M, Broza YB, Mittler S, Arakawa E, Broza M. Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microb Ecol. 2004;47:341–349. doi: 10.1007/s00248-003-2007-6. [DOI] [PubMed] [Google Scholar]
- 29.Darby C. Uniquely insidious: Yersinia pestis biofilms. Trends Microbiol. 2008;16:158–164. doi: 10.1016/j.tim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Visick KL. An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri. Mol Microbiol. 2009;74:782–789. doi: 10.1111/j.1365-2958.2009.06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.