Abstract
The protein C (PC) pathway is a well-characterized coagulation system. Endothelial PC receptors and thrombomodulin mediate the conversion of PC to its activated form, a potent anticoagulant and anti-inflammatory molecule. Here we show that the PC pathway is expressed on intestinal epithelial cells. The epithelial expression of PC and endothelial PC receptor is down-regulated In patients with inflammatory bowel disease. PC−/−/PC(Tg) mice, expressing only 3% of WT PC, developed spontaneous intestinal inflammation and were prone to severe experimental colitis. These mice also demonstrated spontaneous elevated production of inflammatory cytokines and increased intestinal permeability. Structural analysis of epithelial tight junction molecules revealed that lack of PC leads to decreased JAM-A and claudin-3 expression and an altered pattern of ZO-1 expression. In vitro, treatment of epithelial cells with activated PC led to protection of tight junction disruption induced by TNF-α, and in vivo, topical treatment with activated PC led to mucosal healing and amelioration of colitis. Taken together, these findings demonstrate that the PC pathway is a unique system involved in controlling intestinal homeostasis and inflammation by regulating epithelial barrier function.
Keywords: intestinal barrier integrity, DSS-induced colitis, intestinal permeability and tight junction proteins
Inflammation and coagulation are closely linked, interdependent processes (1, 2). Under physiological conditions, the molecules within the microcirculation of tissues act in an anticoagulant and anti-inflammatory manner. However, when inflammation occurs, coagulation is also triggered and participates in the spreading of inflammation. Unexpected roles of hemostasis in the humoral and cellular mechanisms of innate immunity have been reported recently (2). One of the major systems that forms a bridge between inflammation and coagulation is the protein C (PC) pathway (3). This pathway is composed of thrombomodulin (TM), endothelial cell PC receptor (EPCR), PC, and protease-activated receptor-1 (PAR-1). TM and EPCR are expressed mainly by vascular endothelial cells and form a complex on the surface of these cells that converts circulating PC into its active form (3).
Although the function of the PC pathway is classically considered anticoagulative, mounting evidence indicates that this pathway also plays a dominant role in inflammation, with each component of the pathway displaying remarkably potent anti-inflammatory activity (2–4). Indeed, PC is now emerging as a participant in the pathogenesis of acute and chronic inflammatory diseases, such as sepsis, asthma, inflammatory bowel disease (IBD), atherosclerosis, and lung and heart inflammation, and might represent a previously unexpected therapeutic target for intervention (3, 5).
Crohn's disease (CD) and ulcerative colitis (UC) are the two major forms of IBD. Although these are immune-mediated diseases, we and others have shown that nonimmune cells [e.g., epithelial cells (ECs), vascular endothelium, platelets] and coagulation are deeply involved in the pathogenesis of IBD (6–10). In particular, we recently reported that the PC pathway controls microvascular inflammation by down-regulating endothelial EPCR and TM expression, thereby impairing activation of PC by the inflamed mucosal microvasculature (5, 10). Whether PC also could be involved in intestinal barrier function or in cytoprotection is unclear, however (5, 10).
In this paper we report the surprising observation that, along with its known expression in the microcirculation of the gut mucosa, the epithelial layer of the intestine expresses proteins of the PC pathway, which play a unique role in regulating intestinal permeability and controlling the integrity of tight junctions. Expression of epithelial PC is altered in patients with CD or UC, and its deletion in mice leads to spontaneous colitis. Restoring epithelial PC is therapeutically effective in mice and could represent a novel treatment approach in humans with IBD.
Results
Expression of EPCR, PAR-1, PC, and TM by ECs from Healthy Individuals and Patients with IBD.
To investigate whether intestinal epithelium expresses components of the PC system, we performed confocal microscopy using specific antibodies to PC, EPCR, PAR-1, and TM, and an antibody for pan-cytokeratin, a specific epithelial marker, on sections of colon obtained from 16 healthy individuals (Fig. 1A) (11). PC, EPCR, and PAR-1, but not TM, were expressed by ECs in the intestine, as indicated by colocalization with pan-cytokeratin (Fig. 1A). Staining for the same proteins in specimens from patients with active CD (n = 12) and active UC (n = 13) revealed a significant decrease in the epithelial expression of EPCR and PC compared with healthy individuals (Fig. 1A). No significant difference was found in PAR-1 expression on ECs between NL subjects and patients with CD, whereas PAR-1 was significantly down-regulated in patients with UC compared with NL subjects and patients with CD. We quantified protein expression in the tissue by a semiquantitative score (10, 12). We found a significant decrease in the expression of epithelial EPCR in tissue samples from patients with UC (0.8 ± 0.2) and those with CD (0.8 ± 0.2) compared with mucosa from normal individuals (2.7 ± 0.1) (P < 0.001). Similar results were obtained for PC expression, which was significantly decreased in the epithelium of patients with UC (0.5 ± 0.1) and those with CD (0.6 ± 0.2) compared with mucosa from control individuals (2.4 ± 0.1) (P < 0.001). Furthermore, TM was not expressed by the intestinal epithelium. No decrease in PC and EPCR staining was found in specimens from patients in an inactive disease state.
Fig. 1.
Decreased expression of components of the PC pathway in intestinal epithelial cells of patients with IBD. (A) Immunofluorescent staining shows the expression and colocalization of EPCR, PC, and PAR-1, but not of TM (in green) with the EC marker pan-cytokeratin (in red) in normal subjects (NL). Marked reductions in EPCR, PC, and PAR-1 were detected in the colon of patients with active Crohn's disease (CD) and patients with active ulcerative colitis (UC) with DAPI nuclear stain (blue). The images were acquired with an oil immersion objective (60×, 1.4 NA Plan-Apochromat; Olympus). (B) Relative changes in mRNA expression of EPCR, PC, PAR-1, and TM relative to GAPDH in NL subjects (n = 10), patients with CD (n = 6), and patients with UC (n = 6). *P < 0.05.
We next analyzed the expression of the PC pathway in primary ECs by FACS analysis. ECs in healthy subjects expressed PC, EPCR, and PAR-1. In patients with IBD, PC and EPCR expression was decreased by 47%, and PAR-1 expression was decreased by 30% (Fig. S1). These results suggest that inflammation down-regulates the expression of EPCR and PC on ECs within the intestine.
We also investigated the expression of EPCR, PAR-1, PC, and TM by RT-PCR. Results from primary ECs isolated from intestinal tissue of healthy subjects and patients with active IBD confirmed down-regulation of mRNA for EPCR, PC, and PAR-1 in patients with active CD or UC. The expression of mRNA for TM was undetectable (Fig. 1B). No decrease in PC or EPCR mRNA was found in ECs from patients with quiescent IBD. Taken together, these results demonstrate that the intestinal epithelium of healthy subjects expresses PC and EPCR, and that this expression is significantly reduced in the inflamed intestines of patients with active IBD.
Spontaneous Colitis in PC-Deficient Mice.
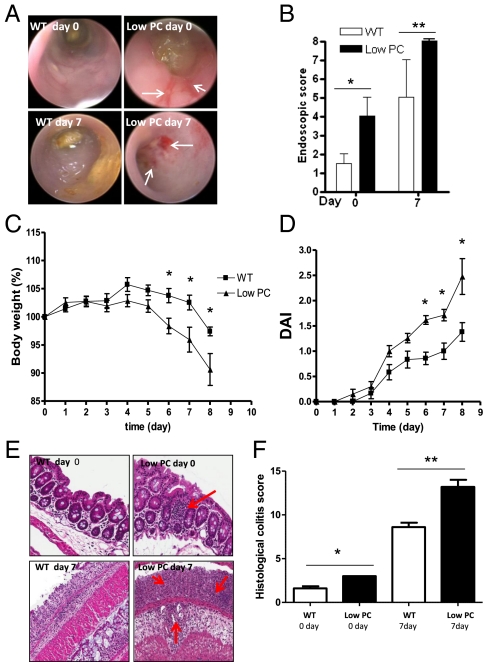
To study the consequences of the decreased expression of epithelial PC in the inflamed intestine, we evaluated the susceptibility to experimental colitis in PC-deficient mice. Given that the genetic deletion of PC leads to death soon after birth, we used PC−/−/PC(Tg) (“low-PC”) mice, which express only 3% of the amount of PC expressed by WT mice (13, 14). When mice (6–8 wk old) underwent colonoscopy, low-PC mice had an endoscopic colitis score indicating the presence of spontaneous colitis characterized by mucosal hyperemia and friability (P < 0.05) (Fig. 2 A and B). The degree of colitis increased after administration of dextran sodium sulfate (DSS), with more significant colon damage seen in low-PC mice (P < 0.05). Bleeding and the presence of mucosal erosions that were absent in WT mice characterized this damage.
Fig. 2.
Spontaneous inflammation and increased susceptibility to DSS-induced colitis in low-PC mice. (A) Endoscopic images of mucosal damage in the colon of WT and low-PC mice before (day 0) and after 7 d (day 7) of 2% DSS administration demonstrates the spontaneous mucosal hyperemia and friability in low-PC mice and the presence of mucosal erosions at day 7, as indicated by the white arrows. (B) Quantification of mucosal injury by endoscopic score in WT and low-PC mice before and after treatment. (C and D) Trends toward greater body weight loss (C) and disease activity index (DAI) (D) in low-PC mice compared with their littermates over the course of DSS treatment. (E) Red arrows indicate the presence of small lymphoid follicles at day 0 and increased leukocyte infiltrate at day 7 in the mucosa of low PC mice. (F) Histological damage scoring at day 0 and day 7 in low-PC and WT mice. Histological images were taken using a 20× objective.
In addition, minimal doses of DSS were sufficient to induce significant clinical colitis in low-PC mice, characterized by a significant loss in body weight and a progressive increase in the overall disease activity index (Fig. 2 C and D). Indeed, administration of 3% DSS led to 100% mortality in low-PC mice after 3–4 d of administration.
After mice were killed, the low-PC mice exhibited spontaneous intestinal inflammation characterized by the presence of small lymphoid follicles in the mucosa and submucosa mainly in the colon but not in the small bowel (Fig. 2 E and F). Moreover, after DSS challenge, the mucosa of low-PC mice displayed a significantly higher degree of histological inflammation (P < 0.05), characterized by total crypt loss and diffuse leukocyte infiltration that was not observed in WT mice (Fig. 2 E and F). The finding that deletion of PC leads to spontaneous colitis unveils a role for PC in intestinal inflammation.
Increased Mucosal Cytokine Production and Intestinal Permeability in PC-Deficient Mice.
We next measured the mucosal production of various inflammatory mediators, including IL-6, IL-8 (KC), and macrophage inflammatory protein 2. Consistent with our histological findings indicating the presence of spontaneous inflammation, low-PC mice displayed a significant increase in the spontaneous production of all inflammatory mediators compared with WT mice (all mediators; P < 0.05), even before DSS administration. These differences were maintained over 7 d of DSS administration (Fig. 3A).
Fig. 3.
Changes in mucosal cytokine production and the integrity of intestinal barrier function in low-PC mice. (A and B) Secretion of IL-6, KC, and macrophage inflammatory protein 2 by the mucosa of the colon (A) and TEER measured from the colon (B) of low-PC and WT mice on days 0, 3, and 7 of DSS administration. (C and D) Immunofluorescent staining and bar graph showing the expression of JAM-A, CL-3, CL-10, and ZO-1 tight junction proteins (green) in the colon of low-PC and WT mice before DSS administration (day 0); nuclei were stained with DAPI (blue). (Scale bar: 30 μm.) Values represent mean ± SEM; n = 13 for WT mice and n = 20 for low-PC mice. These data are representative of three independent experiments. *P < 0.05; **P < 0.01.
Given that PC is expressed by ECs, which function to maintain an intact intestinal barrier, we hypothesized that PC could play a key role in this process. Thus, we investigated paracellular epithelial permeability in low-PC and WT mice before and after DSS administration by measuring transepithelial electrical resistance (TEER) using a Ussing chamber and the Evans blue dye test. TEER was monitored as an indicator of epithelial barrier integrity. Low-PC mice demonstrated a spontaneous increase in intestinal permeability, with significantly lower values of TEER (28.66 ± 2 Ω/cm2) compared with WT mice (88 ± 2 Ω/cm2) before DSS challenge (P < 0.0001) (Fig. 3B). After 3 d of DSS administration, epithelial permeability was still significantly higher in WT mice (45.9 ± 3.74 Ω/cm2) compared with low-PC mice (29.85 ± 3.66 Ω/cm2) (P = 0.0005). After 7 d of DSS administration, TEER values were markedly diminished in both groups of mice, with no significant differences (Fig. 3B). Taken together, these data indicate a leaky intestinal barrier in low-PC mice.
Epithelial permeability is governed by tight junctions, and alterations in the expression of the proteins forming these junctions might lead to a leaky intestinal barrier (15). Thus, we investigated the expression levels of the tight junction proteins claudin-3, ZO-1, JAM-A, and claudin-10 in low-PC and WT mice. Consistent with the observed intestinal permeability, low-PC mice showed a significant decrease in the expression of claudin-3 and JAM-A compared with WT mice (P < 0.05) (Fig. 3C), a finding that could explain the leaky intestinal barrier in low-PC mice. In addition, the expression pattern of ZO-1 was significantly altered and irregular in low-PC mice compared with WT mice (Fig. 3C). There was no between-group difference in claudin-10 expression (Fig. 3C). In addition, there was no between-group difference in the overall expression of tight junctions after 7 d of DSS-induced colitis.
PC Controls Epithelial Tight Junction Integrity, Wound Healing, and EC Proliferation.
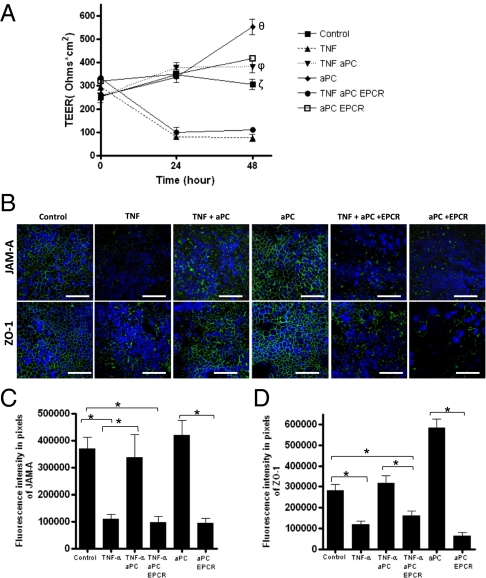
Increased leakiness of the intestinal barrier is considered a crucial event in the pathogenesis of IBD, and TNF-α actively contributes to the disruption of barrier function by down-regulating epithelial tight junctions. To investigate the role of PC in epithelial barrier function, we first analyzed the expression of the PC pathway in a monolayer of the colonic EC line (Caco-2) by immunofluorescence. Similar to primary ECs, Caco-2 cells expressed PC, PAR-1 and EPCR, but not TM (Fig. S2). We then cultured Caco-2 cells with and without stimulation by TNF-α in the absence and presence of exogenous activated PC (aPC). Changes in TEER were measured over 48 h. As expected, a decrease in TEER was observed after 48 h of stimulation with TNF-α (t = 0: 304 ± 23.31 Ω/cm2; + TNF, t = 48: 78.75 ± 6.57 Ω*cm2) (Fig. 4A). However, the addition of exogenous aPC totally abrogated the effects of TNF-α on TEER (TNF + aPC, t = 48: 381.8 ± 26.20 Ω*cm2; P < 0.05). Notably, when the cells were treated only with aPC an increase of TEER was observed compared with nontreated cells (+ aPC, t = 48: 552 ± 34.5 Ω*cm2; control, t = 48: 304.8 ± 23.31 Ω*cm2) (Fig. 4A). To test whether the protective effect observed with aPC was dependent on EPCR signaling, we performed the same experiments in the presence and absence of anti-EPCR antibodies. The protective effects of aPC disappeared in the presence of anti-EPCR antibodies, suggesting that aPC exerts a direct effect on paracellular epithelial permeability in an EPCR-dependent manner. No effects on TEER values were seen in the presence of anti-EPCR alone. Because the aPC–EPCR complex cross-activates sphingosine 1 phosphate (S1P), a signaling sphingolipid that promotes increased endothelial barrier protection, we investigated whether aPC induces the potent epithelial barrier protective effects via S1P activation. To assess this hypothesis, we evaluated the changes in TEER values in Caco-2 cells after 0, 6, and 12 h of stimulation with S1P (1 μM). Interestingly, S1P not only enhanced TEER values similarly to APC (1 μg/mL), but also abrogated TNF-dependent TEER reduction (Fig. S3A). These data support the evidence suggesting the involvement of S1P in the epithelial barrier protection. It has been reported that once formed after the phosphorylation of sphingosine by enzyme sphingosine kinase (SphK1 and SphK 2), S1P regulates the endothelial barrier function mediating the S1P1 receptor. Because the Caco-2 cell line expresses S1P1, SphK1, and SphK2 mRNA (Fig. S3 B and C), we verified whether the effects of aPC on TEER were S1P1-dependent. After 5 h of pretreatment with FTY720 (1 μM), which is known to inhibit the S1P1 receptor, the Caco-2 cells were stimulated with and without TNF-α (25 ng/mL) in the presence or absence of aPC (1 μg/mL) for 0, 6, and 12 h. FTY720 treatment displayed no inhibitory effects on aPC activity. Indeed, aPC in the presence of FTY720 significantly abrogated (P < 0.01) TNF-dependent TEER values (Fig. S3D). No differences were observed between control and FTY720 alone. These results suggest that aPC-enhanced epithelial barrier protection is S1P1-independent.
Fig. 4.
Protective effects of aPC on epithelial barrier function are mediated by EPCR. Confluent Caco-2 cells were treated without (control) and with TNF-α (25 ng/mL) in the presence (TNF aPC) or absence of aPC (1 μg/mL), and with (TNF aPC EPCR) or without (aPC EPCR) a 1-h pretreatment with the antibody inhibiting EPCR activity (5 μg/mL). (A) Values of TEER were measured over 48 h of stimulation. (B–D) After 48 h of stimulation, immunofluorescence analysis of JAM-A and ZO-1 (in green) was performed on Caco-2 cells grown on Transwell filters under different conditions: control, TNF, TNF + aPC, aPC, TNF + aPC + EPCR, and aPC + EPCR; DAPI nuclear stain (blue). (Scale bar: 60 μm.) Values are mean ± SEM; the data are representative of three independent experiments. θP < 0.05 (aPC vs. control); ζP < 0.05 (control vs. TNF and TNF aPC EPCR); ϕP < 0.01(aPC vs. TNF and TNF aPC EPCR); *P < 0.01.
To further explore the mechanism by which aPC reinforces epithelial barrier function, we investigated the expression levels of the major tight junction proteins in EC lines in the presence and absence of aPC and before and after stimulation with TNF-α. Consistent with the in vivo findings in low-PC mice, we found that aPC inhibits the altered expression of JAM-A and ZO-1 induced by TNF-α (Fig. 4 B and C). Furthermore, the use of anti-EPCR antibodies totally inhibited such modification of tight junctions, suggesting a functional role for EPCR in aPC-mediated regulation of tight junctions (Fig. 4 B and C), whereas the stimulation with α-EPCR alone did not alter the expression of JAM-A or ZO-1. Along with their crucial role in formation of tight junctions to maintain the epithelial barrier, ECs are able to actively respond to injury by migrating when wounds occur, and to heal the disrupted barrier. Thus, we tested whether aPC can induce intestinal wound closure and cell proliferation. To investigate wound closure, we seeded Caco-2 cells onto plates in designated areas in the absence or presence of exogenous aPC (1 μg/mL) and monitored cell migration for 48 h after creation of a scratch in the monolayers. Significant wound closure was seen in the presence of a PC compared with the control, at a level comparable to that induced by epithelial growth factor (EGF; 50 ng/mL) (Fig. S4 A and B). Pretreatment with α-EPCR antibody (5 μg/mL) 1 h before the stimulation decreased epithelial wound closure, supporting the suggestion of a functional role for EPCR in aPC-mediated epithelial migration (Fig. S4 A and B). Finally, a [3H]-thymidine incorporation assay revealed that a low concentration of aPC (1 μg/mL) induced a high rate of Caco-2 cell proliferation, comparable to that induced by EGF (50 ng/mL). The cells pretreated with α-EPCR and stimulated with aPC displayed significantly reduced cellular proliferation. Importantly, pretreatment with α-EPCR alone did not induce any effects on ECs, reinforcing the hypothesis that aPC signals through EPCR (Fig. S4C).
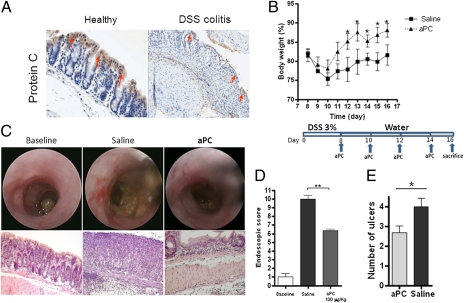
Topical aPC Treatment Leads to Improvement of Colitis.
Mucosal healing is believed to be an important target for the treatment of IBD (16). To explore the therapeutic potential of our in vitro findings, we investigated whether the intrarectal administration of aPC would exert a therapeutic effect in mice with experimental colitis. We began by investigating the expression of epithelial PC. Similar to human samples, the ECs of healthy WT mice expressed high but physiological levels of PC. In contrast, in mice developing colitis induced by DSS administration, a significant decrease in PC expression was detected by day 3 and persisted to the end of the experiment (Fig. 5A). Because intestinal inflammation leads to down-regulation of epithelial PC expression, we investigated whether topical intrarectal administration of aPC could improve colitis by favoring epithelial healing. Mice receiving aPC recovered more rapidly from colitis, as indicated by a significant increase in body weight compared with saline-treated mice (P < 0.05) (Fig. 5 A and B). Endoscopically, administration of aPC significantly ameliorated mucosal damage (Fig. 5 C and D), and the mice that received intrarectal aPC demonstrated significantly less colon shortening compared with saline-treated mice (P < 0.05) (Fig. S5). Finally, quantification of mucosal inflammation revealed that aPC-treated mice had a significantly less severe histological score, characterized by a decreased number of ulcers (Fig. 5E). This finding suggests that aPC has a beneficial and therapeutic effect. Taken together, these data support the potential therapeutic efficacy of topical aPC in intestinal inflammation.
Fig. 5.
Exogenous administration of aPC ameliorates recovery of intestinal mucosa in DSS-induced colitis. (A) Immunohistochemical staining of PC on sections of colon from WT mice before and after administration of 3% DSS. Arrows indicate the strong expression of PC on ECs in healthy colon and its dramatic reduction after DSS treatment. (B) After 8 d of DSS administration, WT mice were divided into two groups: One group received saline, and the other group received recombinant murine aPC (100 μg/kg) intrarectally in a volume of 80 μL using a straight gavage needle every other day after colitis was established. Loss of body weight was monitored daily during treatment. (C and D) Endoscopic images and evaluation of mucosal damage in the colons of healthy and saline- and aPC-treated mice on day 16. (E) Evaluation of the number of ulcers on day 16 after topical aPC or saline treatment. Values are mean ± SEM; n = 6 mice for all groups. These data are representative of two independent experiments. *P < 0.05; ** P < 0.01. Images were taken using a 20× objective.
Discussion
Inflammation is the result of an imbalance between proinflammatory and anti-inflammatory pathways. In the last several years, it has become clear that inflammation involves unique pathways that are not classically acknowledged as inflammatory systems, for example, pathways involved in hemostasis (1, 2). Evidence supporting this hypothesis has pointed to the involvement of the coagulation cascade in inflammatory processes, and it has been firmly established that coagulation and inflammation are interrelated processes, which is particularly true in IBD (8).
The specific hemostasis-related molecules involved in inflammation are only now beginning to be elucidated. The PC pathway is a major pathway bridging inflammation and coagulation (3). Mounting evidence also indicates that the PC pathway plays a dominant role in inflammation, with each component of the pathway displaying remarkably potent anti-inflammatory activity (3, 17, 18). Indeed, TM, EPCR, PAR-1, and aPC, individually and together, have emerged as crucial controllers of inflammation in multiple organs, suggesting the potential for development of novel therapeutic approaches for a wide range of inflammatory disorders (3, 17, 18). Recently, the PC pathway was suggested to be involved in endothelial barrier integrity and in cytoprotection (18). However, up to now, expression of the proteins of the PC pathway and their roles in controlling intestinal barrier integrity and cytoprotection, let alone the role of PC in the pathogenesis of the two major forms of IBD, have not been investigated. In patients with CD and UC, alterations of the coagulative system and of the cells involved in coagulation play important roles in the intestinal immune response (7, 8). In particular, the PC pathway has been implicated in the control of microvascular inflammation of the intestine (5, 10).
In this paper we report the surprising finding that beyond its classical role in vascular biology, PC also acts as an unexpected mediator of intestinal epithelial barrier function. We demonstrate that the intestinal epithelial layer expresses the PC pathway, and that the expression of PC system is lost in patients with active IBD, whereas no changes in the PC pathway were found in ECs from patients in an inactive disease state. These findings suggest that inflammation leads to dysregulation of PC system components in intestinal ECs, similar to what is seen in intestinal endothelial cells (10). However, consistent with these human results, we also found a down-regulation of epithelial PC in mice during DSS-induced colitis. The altered expression of EPCR and TM impairs the conversion of PC into its active form, aPC, which has been widely demonstrated to exert anti-inflammatory and cytoprotective properties (3, 10). Interestingly, we found that intestinal ECs are positive for all PC pathway components except TM, a protein crucial for aPC conversion. Therefore, these data suggest that although the epithelium expresses EPCR, PC, and PAR-1, it does not provide an appropriate microenvironment for aPC production. It is plausible to hypothesize that ECs expressing EPCR and PAR-1, even if they synthesize PC, must obtain aPC from other types of cells. Previous reports have described the interactions between immune mucosal cells and ECs that contribute to intestinal homeostasis and intestinal epithelial permeability (19), indicating the likely presence of cross-talk between ECs and other cells expressing TM. However, a TM-soluble form, derived from the endothelial membrane, is released in plasma, but whether this form is functional is unknown (20).
Why the ECs express the inactive form of PC but are not able to covert it remains unclear. Once activated, aPC plays an important role in governing intestinal homeostasis by inhibiting the release of proinflammatory cytokines and limiting leukocyte adhesion (10). When PC activation is impaired, as in low-PC mice, the intestinal homeostasis is altered, and the transgenic mice develop spontaneous intestinal inflammation and a more pronounced experimental colitis phenotype after challenge with DSS, reinforcing the evidence that the low PC levels in these mice induce a proinflammatory state, as also indicated by the high levels of proinflammatory cytokines at baseline. Low-PC mice, besides being associated with high mortality and prothrombotic and proinflammatory phenotypes, display altered intestinal barrier function. Tight junction proteins strictly control intestinal permeability, and their alteration can lead to spontaneous intestinal inflammation. We found that low-PC mice had altered or reduced expression of ZO-1, JAM-A, and claudin-3, three tight junction proteins required for intestinal barrier function. Most notable was the down-regulation of JAM-A, a molecule that we recently identified as a crucial mediator of intestinal barrier permeability (11, 21). In addition, consistent with the in vivo findings, aPC had a strong effect on tight junctions in vitro; its presence was able to inhibit the altered expression of ZO-1 and JAM-A induced by TNF-α. As expected, TNF-α stimulation induced an increase in epithelial permeability, but notably, this effect was abrogated by recombinant aPC. This indicates that aPC controls the intestinal barrier function enhancing the expression of tight junction proteins. It has been reported that most of the cytoprotective effects of aPC are mediated by EPCR. In the present study, we have demonstrated that the aPC's beneficial protective effects in controlling intestinal barrier function are EPCR-dependent, suggesting that EPCR expressed by the epithelium is functionally active and is essential for the regulation of cellular permeability.
The protective effects of aPC on endothelial barrier integrity are mediated through its interaction with EPCR and the subsequent cleavage and activation of protease-activated receptor-1 (PAR-1) (3). The aPC/EPCR/PAR-1 complex cross-activates S1P, a signaling sphingolipid. It has been reported that S1P is involved in regulating the proliferation, survival, differentiation, and migration of many types of cells, including endothelial cells (22, 23). Furthermore, a recent study indicated that S1P regulates the function of the intestinal epithelial barrier by altering the expression of E-cadherin, which is an adherens junction (24). That study demonstrated that ECs express S1P and that the signaling pathway of S1P is involved in maintaining the integrity of the epithelial barrier, but it did not address the mechanism of these effects. The cell-signaling properties of aPC are known to enhance vascular endothelial barrier function via the S1P1 receptor. Although the level of S1P1 mRNA expression was very low in Caco-2 cells, we tested whether the aPC-dependent intestinal barrier protective effects were mediated by S1P1. Both S1P and aPC stimulation exerted similar effects, completely abrogating the TNF-dependent decrease in TEER, suggesting that the cytoprotective effects of aPC that we observed in ECs can be mediated by S1P signaling; however, the inhibition of S1P1 using FTY720 did not abolish the effect of aPC. FTY720 is an immunomodulator drug that has been proven to induce internalization of S1P1 in endothelial cells after 60 min of treatment (25). Here we pretreated the cells with FTY720 for 5 h before aPC stimulation, but found no difference in TEER values in the presence or absence of FTY720. It is plausible to suppose that aPC in ECs activates S1P signaling, but not via S1P1. S1P1 receptor is not a unique receptor for S1P ligand; both S1P2 and S1P3 are expressed by ECs at higher levels than S1P1 (22). Thus, further studies are needed to provide evidence of the involvement of S1P receptors in aPC effects.
To maintain intestinal homeostasis, the epithelium must be able to proliferate and migrate after injury, especially when inflammation occurs. Evidence points to the capacity of aPC to stimulate proliferation, migration, and wound closure in human keratinocytes (26). In particular, aPC was found to increase keratinocyte proliferation in a dose-dependent manner. Thus, we investigated whether aPC promoted this function in intestinal ECs as well. We found that at low concentrations, aPC accelerated wound closure and induced proliferation of ECs similarly to EGF, in an EPCR-dependent manner.
Because aPC promotes wound closure and intestinal epithelial proliferation, it is plausible that the prolonged healing process seen in patients with UC and CD could be at least partially dependent on the reduced expression of PC and EPCR by the intestinal epithelium. Thus, topical administration of aPC could promote mucosal healing. A clinical study of four patients found that treatment of chronic leg ulcers with topical aPC stimulated wound healing by activating skin reepithelialization (27). Furthermore, the local aPC treatment was well tolerated, with no adverse effects or complications (27). Consistent with these findings, our in vivo studies demonstrated the capacity of aPC treatment to favor mucosal healing. Indeed, mice treated with intrarectal injection of aPC every other day after colitis was established displayed faster recovery of body weight loss and endoscopic and histological changes, with a reduced number of ulcers, compared with mice receiving saline. Importantly, the intrarectal administration of aPC did not increase hemorrhage. Given the increase in bleeding complications associated with the administration of i.v. aPC, the intrarectal route could provide a safer route of administration.
In conclusion, our study provides evidence of an unexpected function of the anticoagulative PC pathway in the intestine. The PC pathway controls the process of mucosal homeostasis that is mediated by ECs through the maintenance of barrier function and cytoprotection. Altered PC expression in the epithelium of the gut leads to spontaneous intestinal inflammation, a previously unrecognized component of the pathogenesis of IBD. Restoring the function of the PC pathway in intestinal epithelium by acting on a molecule classically recognized as an anticoagulant may help guide the development of new treatments for IBD and mucosal inflammation.
Materials and Methods
Patients.
Intestinal tissues were obtained from surgical specimens taken from patients with active CD or UC. Healthy areas of intestine taken from patients admitted for bowel resection because of polyps or diverticulosis were used as controls. Specimens were formalin-fixed and paraffin-embedded or frozen in Cryoblock Compound (DiaPath) on dry ice and stored at −80 °C. Human studies were approved by the Ethical Committee of Istituto Clinico Humanitas.
Detailed information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sarah A. De La Rue of Readable Science for her assistance with the manuscript. This study was supported by grants from Innovative Medicines Initiative (IMI) (115142), Ministero della Salute (GR-2007 convenzione 23), Fondazione Cariplo (Grant 2010-0733), and Associazione Italiana Ricerca Cancro (IG-2010 10205) to S.D.; IBD Research Foundation (mini Grant 2010), European Crohn's and Colitis Organization (ECCO) (Grant 2009), and Ministero della Salute (GR -2009 convenzione 76) to S.V.; and National Institutes of Health Grant HL019982 (to F.J.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107140108/-/DCSupplemental.
References
- 1.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 2.Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25:536–542. doi: 10.1016/j.it.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ. The protein C pathway in tissue inflammation and injury: Pathogenic role and therapeutic implications. Blood. 2010;115:1121–1130. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esmon CT, et al. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254–259. [PubMed] [Google Scholar]
- 5.Lust M, Vulcano M, Danese S. The protein C pathway in inflammatory bowel disease: The missing link between inflammation and coagulation. Trends Mol Med. 2008;14:237–244. doi: 10.1016/j.molmed.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Danese S. Nonimmune cells in IBD pathogenesis: From victim to villain. Trends Immunol. 2008;29:555–564. doi: 10.1016/j.it.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Danese S, et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003;124:1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, et al. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 9.Danese S, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060–2073. doi: 10.1053/j.gastro.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Scaldaferri F, et al. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J Clin Invest. 2007;117:1951–1960. doi: 10.1172/JCI31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vetrano S, et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–184. doi: 10.1053/j.gastro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Scaldaferri F, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–595. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 13.Lay AJ, Liang Z, Rosen ED, Castellino FJ. Mice with a severe deficiency in protein C display prothrombotic and proinflammatory phenotypes and compromised maternal reproductive capabilities. J Clin Invest. 2005;115:1552–1561. doi: 10.1172/JCI24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lay AJ, Donahue D, Tsai MJ, Castellino FJ. Acute inflammation is exacerbated in mice genetically predisposed to a severe protein C deficiency. Blood. 2007;109:1984–1991. doi: 10.1182/blood-2006-07-037945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 16.Rutgeerts P, Vermeire S, Van Assche G. Mucosal healing in inflammatory bowel disease: Impossible ideal or therapeutic target? Gut. 2007;56:453–455. doi: 10.1136/gut.2005.088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esmon CT. Protein C anticoagulant pathway and its role in controlling microvascular thrombosis and inflammation. Crit Care Med. 2001;29:S48–S51. doi: 10.1097/00003246-200107001-00018. [DOI] [PubMed] [Google Scholar]
- 18.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2006;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 19.Groschwitz KR, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci USA. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii H, Majerus PW. Thrombomodulin is present in human plasma and urine. J Clin Invest. 1985;76:2178–2181. doi: 10.1172/JCI112225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetrano S, Danese S. The role of JAM-A in inflammatory bowel disease: Unrevealing the ties that bind. Ann N Y Acad Sci. 2009;1165:308–313. doi: 10.1111/j.1749-6632.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- 22.Young N, Van Brocklyn JR. Signal transduction of sphingosine-1-phosphate G protein-coupled receptors. ScientificWorldJournal. 2006;6:946–966. doi: 10.1100/tsw.2006.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Greenspon J, et al. Sphingosine-1-phosphate regulates the expression of adherens junction protein E-cadherin and enhances intestinal epithelial cell barrier function. Dig Dis Sci. 2011;56:1342–1353. doi: 10.1007/s10620-010-1421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullershausen F, et al. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 26.Xue M, Thompson P, Kelso I, Jackson C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp Cell Res. 2004;299:119–127. doi: 10.1016/j.yexcr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Whitmont K, et al. Treatment of chronic leg ulcers with topical activated protein C. Arch Dermatol. 2008;144:1479–1483. doi: 10.1001/archderm.144.11.1479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.