Abstract
One of the main challenges in cancer research is the development of vaccines that induce effective and long-lived protective immunity against tumors. Significant progress has been made in identifying members of the cancer testis antigen family as potential vaccine candidates. However, an ideal form for antigen delivery that induces robust and sustainable antigen-specific T-cell responses, and in particular of CD8+ T lymphocytes, remains to be developed. Here we report the use of a recombinant nonpathogenic clone of Trypanosoma cruzi as a vaccine vector to induce vigorous and long-term T cell-mediated immunity. The rationale for using the highly attenuated T. cruzi clone was (i) the ability of the parasite to persist in host tissues and therefore to induce a long-term antigen-specific immune response; (ii) the existence of intrinsic parasite agonists for Toll-like receptors and consequent induction of highly polarized T helper cell type 1 responses; and (iii) the parasite replication in the host cell cytoplasm, leading to direct antigen presentation through the endogenous pathway and consequent induction of antigen-specific CD8+ T cells. Importantly, we found that parasites expressing a cancer testis antigen (NY-ESO-1) were able to elicit human antigen-specific T-cell responses in vitro and solid protection against melanoma in a mouse model. Furthermore, in a therapeutic protocol, the parasites expressing NY-ESO-1 delayed the rate of tumor development in mice. We conclude that the T. cruzi vector is highly efficient in inducing T cell-mediated immunity and protection against cancer cells. More broadly, this strategy could be used to elicit a long-term T cell-mediated immunity and used for prophylaxis or therapy of chronic infectious diseases.
Keywords: cytokine, prophylaxis, protozoa, innate immunity, Trypanosoma cruzi CL-14
The NY-ESO-1 antigen, a member of the cancer testis antigen (CTA) family, has been extensively characterized in terms of its distribution in tumor cells and immunological properties in humans (1). It induces integrated responses involving both the cellular and humoral arms of the immune system and is currently being tested in different clinical trials of antitumor vaccines (2). However, a major challenge for the development of an effective anticancer vaccine is the process and context of antigen delivery.
In addition to antigen retention and slow release, an inflammatory milieu is necessary for development of T cell-mediated immunity with the characteristics required to control tumor cells. The discovery of Toll-like receptors (TLRs), which recognize microbial molecular patterns, has led to significant progress in the field of immunological adjuvants, necessary to initiate adaptive immune responses and T cell-mediated immunity in vaccine protocols (3, 4). Although able to induce potent B- and T-cell responses, the frequency of antigen-specific T lymphocytes induced by TLR agonists rapidly decays. Furthermore, the conventional delivery of antigens associated to adjuvants favors antigen processing and presentation by MHC class II and results in relatively weak responses of CD8+ T cells, which are critical for immunological control of different types of tumors (5).
The development of nonreplicative viral vectors partially resolved this question and brought advancement to the field (6). However, clinical trials with existing vectors have yielded poor results. Here, we propose that the intracellular protozoan Trypanosoma cruzi would be a highly effective vaccine vector to induce antitumor protective immunity mediated by T lymphocytes. The rationale for using T. cruzi as a tumor vaccine vector is based on earlier immunological studies demonstrating the capacity of this parasite to elicit a strong and persistent T cell-mediated immunity (7, 8). The critical immunostimulatory characteristics of this parasite are (i) the existence of intrinsic agonists for TLRs (9–13), which favor induction of highly polarized T helper cell type 1 (Th1) response; (ii) the ability to persist in host tissues and keep boosting the immune response; and (iii) the ability to replicate in the host cell cytoplasm, resulting in direct antigen presentation to CD8+ T-cell response (14).
To test our hypothesis, we have chosen to use the highly attenuated CL-14 clone of T. cruzi (15). Through the use of the CL-14 clone we generated three different T. cruzi lineages expressing NY-ESO-1. The transgenic parasites were shown to induce strong NY-ESO-1–specific immune responses, both in human cells in vitro and in the mouse model in vivo. The development of NY-ESO-1–specific immunity, including CD8+ T-cell responses, and antitumor activity were dependent on both IL-12 and MyD88 pathway. Furthermore, immunization with CL-14 stably expressing NY-ESO-1 resulted in prevention and delay of tumor development in prophylactic and therapeutic protocols, respectively. Hence, we demonstrated that T. cruzi is an effective antigen delivery vector for induction of T cell-mediated immunity and should be further explored as a potential vaccine vector for anticancer vaccines.
Results
Stable Transgenic T. cruzi-NY-ESO-1 Are Able to Express the Exogenous Protein.
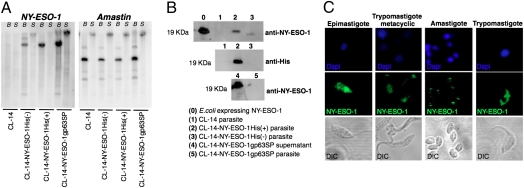
We engineered the CL-14 to express NY-ESO-1, a well-defined and highly immunogenic CTA, which is currently being tested as a vaccine candidate against cancer in different clinical trials (16–19). For homologous recombination, we used the pROCKNeo plasmid (20) that promotes insertion of the transgene in one of the many copies of the β-tubulin gene in the T. cruzi genome (Fig. S1A). Parasites were stably transfected with three different constructs of NY-ESO-1. Two plasmids encoded the NY-ESO-1 gene, with or without a 6x histidine (His) tag, were used to promote NY-ESO-1 expression in the parasite cytoplasm (Fig. S1 B and C). The third plasmid encoded the NY-ESO-1 gene along with a signal peptide (SP) from a T. cruzi metalloprotease, named glycoprotein 63 (gp63) (21), and used to favor NY-ESO-1 secretion by the parasite (Fig. S1D). Fig. S1E shows an agarose gel confirming the plasmid constructs. As shown in Fig. 1A, each of the constructs containing NY-ESO-1 gene were efficiently inserted into the genomic DNA from transgenic parasites. After digestion of parasite genomic DNA with BamHI and SacI, only one major band hybridized with the NY-ESO-1 probe. Thus, we assume that NY-ESO-1 gene was inserted in the β-tubulin locus. Fig. 1A, Right, shows control hybridization with the T. cruzi gene named amastin, which was present in the genome from all WT (22) and transgenic parasites. Recombinant NY-ESO-1 (rNY-ESO-1) was recovered in the pellet of parasites transfected with the coding region of the gene for NY-ESO-1 protein with 6x-His tag [CL-14-NY-ESO-1His(+)] or without the tag [CL-14-NY-ESO-1His(-)], being consistent with the localization in the cytoplasm. In contrast, the rNY-ESO-1 was found primarily in the supernatants of cultures with parasites transfected with NY-ESO-1 gene, which included the gp63 SP (CL-14-NY-ESO-1gp63SP) (Fig. 1B). Immunofluorescence analyses indicate that rNY-ESO-1 is expressed by all different stages of the parasite (Fig. 1C), including the trypomastigote metacyclic form, which was used to vaccinate mice and infect human cells in all experiments described below.
Fig. 1.
Expression of recombinant NY-ESO-1 by stably transfected T. cruzi parasites. (A) Genomic DNA from WT and transgenic parasites was digested with BamHI (B) or SacI (S), submitted to Southern blot, and hybridized with NY-ESO-1 or amastin probes. (B) Western blot analysis was used to detect NY-ESO-1 expression, in metacyclic lysates (lanes 1–3 and 5) or supernatants (lane 4) of parasite cultures, using anti-NY-ESO-1 or anti-His tag mAbs. (C) NY-ESO-1 expression by the four different stages of the CL-14-NY-ESO-1His(+) was analyzed by immunofluorescence. Parasites stained with anti-NY-ESO-1 mAb (green), or DAPI for DNA, were visualized by differential interference contrast (DIC).
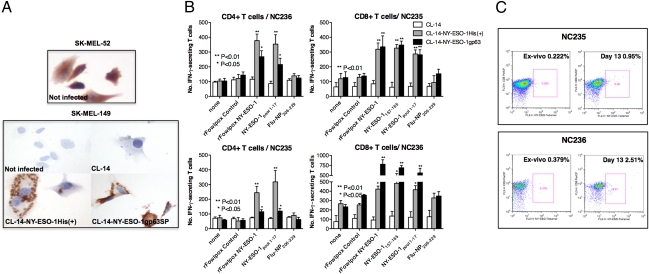
The amastigote forms are the replicative intracellular stage of T. cruzi and persist in the host tissues for life. This is thought to be critical for eliciting long-lasting CD8+ T lymphocytes during T. cruzi infection. We carefully examined the expression of rNY-ESO-1 by intracellular amastigotes in two human cell lines, SK-MEL-149 and SK-MEL-52, which were selected on the basis of their ability to constitutively express NY-ESO-1. SK-MEL-52 that expresses NY-ESO-1 was used as a positive control (Fig. 2A, Upper). As negative control, the SK-MEL-149 cell line, which does not express NY-ESO-1, was used uninfected or infected with WT parasite (Fig. 2A, Lower). The immunocytochemistry results clearly show the presence of NY-ESO-1 only in the SK-MEL-149 cell line infected with either CL-14-NY-ESO-1His(+) or CL-14-NY-ESO-1gp63SP. NY-ESO-1 was localized in the intracellular amastigotes expressing NY-ESO-1His(+) protein. Consistent with protein secretion, the recombinant CTA was homogeneously diffused in the cytoplasm of cells infected with CL-14-NY-ESO-1gp63SP (Fig. 2A, Lower).
Fig. 2.
Priming with transgenic parasites elicits IFN-γ–producing T cells specific for NY-ESO-1. (A) A melanoma cell line (SK-MEL-149) that does not express NY-ESO-1 was infected with either WT or transgenic parasites and expression of rNY-ESO-1 detected by immunohistochemistry. SK-MEL-52 cells were used as positive control. (B) CD8+ T as well as CD4+ T cells from healthy donors (NC235 and NC236) were cocultured with autologous APCs infected with WT or transgenic parasites expressing different forms of rNY-ESO-1. Induction of antigen-specific T cells was analyzed by quantifying the number of IFN-γ–producing cells by ELISPOT after restimulation with autologous EBV-B cells pulsed with peptides or infected with rFowlpox virus, as indicated. (C) The frequency of NY-ESO-1–specific CD8+ T cells was determined by flow cytometry. Percentages indicate the frequency of CD8+ T cells stained with NY-ESO-1 tetramer.
Human T-Cell Immunity Induced by Transgenic Parasites.
We also assessed the ability of CL-14-NY-ESO-1His(+) or CL-14-NY-ESO-1gp63SP to stimulate in vitro CD4+ T and CD8+ T cells purified from peripheral blood mononuclear cells (PBMCs) from unprimed healthy donors. Autologous antigen-presenting cells (APCs, CD4−/CD8−) were infected with WT (negative control) or transfected parasites and used for in vitro sensitization followed by evaluation of both CD4+ T and CD8+ T-cell responses specific for NY-ESO-1. To detect the T-cell response elicited by transgenic parasites, presensitized cells were washed and cocultured with target APCs (EBV-transformed B lymphocytes) from the same donor, infected with T. cruzi, fowlpox expressing or not NY-ESO-1, or APCs primed with a pool of immunostimulatory peptides derived from NY-ESO-1 amino acid sequence. Our results with two donors (NC235 and NC236) presented in Fig. 2 B and C demonstrate the ability of transgenic parasites to elicit NY-ESO-1–specific CD4+ T as well as CD8+ T lymphocytes. The latter results were confirmed by the increased frequency of CD8+ T lymphocytes, which reacted with a tetramer construct containing HLA*0201 compatible to the donors used in the study, and the NY-ESO-1157–165 peptide (Fig. 2C). Thus, we concluded that CL-14 clone expressing NY-ESO-1 was a potent inducer of both CD4+ T as well as CD8+ T-cell responses in humans.
T. cruzi-NY-ESO-1 Parasites Induce Antigen-Specific Immune Response and Tumor Inhibition in Mice.
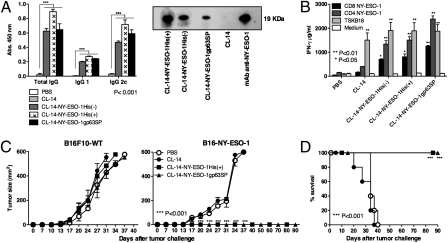
We next tested the ability of transgenic CL-14 (CL-14-NY-ESO-1) to elicit NY-ESO-1–specific humoral and cellular responses in mice. We first performed an experiment to define the protocol of immunization. One hour and 20 h after i.p. injection of 1 × 107 carboxyfluorescein succinimidyl ester-labeled CL-14-NY-ESO-1 metacyclics, a significant proportion of the dendritic cells in the lymph nodes (≈39%) and spleens (≈67%), respectively, contained intracellular parasites. Thus, we decided to use this dose and route of parasite inoculation. Furthermore, we tested a single vs. a homologous prime and boost protocol. As shown in Fig. S2 the prime-boost protocol, using CL-14-NY-ESO-1gp63, induced a stronger T-cell response to NY-ESO-1 peptides than a single-dose immunization. Thereafter, we used in all experiments described in this study two immunization doses 30 d apart. The results shown in Fig. 3 were obtained in vivo and demonstrated that all stably transfected parasites elicited high levels of antibodies specific for rNY-ESO-1 (Fig. 3A). In particular, we observed the induction of the isotype IgG2c, which is normally induced by IFN-γ produced by CD4+ Th1 lymphocytes. Consistently, we demonstrated a strong IFN-γ response by splenocytes from vaccinated mice, stimulated in vitro with NY-ESO-1–derived peptides that encode epitopes recognized by either CD4+ T or CD8+ T lymphocytes (Fig. 3B). As control, we used the CD8+ T cell-specific epitope derived from transialidase, a surface antigen from T. cruzi. Both WT and transgenic parasites primed CD8+ T cells to produce high levels of IFN-γ in response to this epitope. We also observed that the induction of NY-ESO-1–specific IgG2c, the levels of IFN-γ, and the frequency of IFN-γ–producing CD4+ T as well as CD8+ T lymphocytes correlated with protection, which was significantly higher in mice vaccinated with transgenic parasites than with recombinant NY-ESO-1 protein associated to TLR agonists (Fig. S3 A and B).
Fig. 3.
Antigen-specific humoral and cellular responses and complete protection induced by immunization with transgenic parasites expressing NY-ESO-1. (A) ELISA plates coated with rNY-ESO-1 were used to quantify the levels of NY-ESO-1–specific total IgG, IgG1, and IgG2c isotypes present in sera of control and immunized mice; Western blot membranes containing rNY-ESO-1 were individually incubated with sera of mice immunized with each of the three transgenic or WT parasites, or the anti-NY-ESO-1 mAb. (B) Splenocytes from control or immunized mice were cultured in the presence or absence of NY-ESO-1 peptides encoding epitopes specific for CD4+ T or CD8+ T cells. As positive control we used a T. cruzi–derived immunodominant epitope named TSKB18. IFN-γ production was measured at 72 h after stimulation by a sandwich ELISA. Control and immunized mice were challenged with 5 × 104 B16F10 melanoma cells expressing or not NY-ESO-1 (n = 4). Tumor growth (C) and survival (D) were measured for 90 d (n = 6).
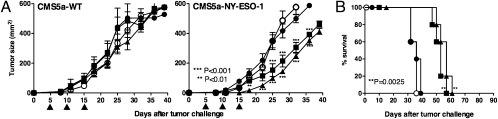
Importantly, vaccination with transgenic parasites expressing NY-ESO-1gp63SP as well as NY-ESO-1His(+) induced complete protection and prevented mortality in mice challenged with a syngeneic transgenic melanoma, the B16F10 cell line expressing NY-ESO-1 (23) (Fig. 3 C and D). In contrast, the formulations using rNY-ESO-1 protein and TLRs agonists were only able to delay tumor development and mortality (Fig. S3 C and D). We also tested the ability of transgenic parasites in a therapeutic protocol to reverse/delay B16-NY-ESO-1 growth in C57BL/6 mice (Fig. S4 A and B) or in BALB/c mice challenged with fibrosarcoma (CMS5a) (24) or colon adenoma (CT26) (25) cell lines. The results shown in Fig. 4 also demonstrate that repeated injections with trypomastigote metacyclics expressing NY-ESO-1 were able to delay tumor growth and prolong survival for both tumor cell lines CMS5a-NY-ESO-1 (Fig. 4 A and B) and CT26-NY-ESO-1 (Fig. S4 C and D). It is noteworthy that no parasitemia/parasitism or any sign of T. cruzi infection was observed, even after 90 d of immunization with CL-14-NY-ESO-1 (Fig. 3D and Fig. S3D).
Fig. 4.
Therapeutic protocols using transgenic parasites delay the growth of tumor cells expressing NY-ESO-1. 1 × 106 cells of the fibrosarcoma CMS5a (A and B) expressing or not rNY-ESO-1 were used to challenge BALB/c mice (n = 6). Mice were treated with three doses of 107 metacyclic forms of WT (CL-14) or transgenic parasites given 5 d apart (arrows), starting at day 5 after challenge. Tumor growth (A) and survival (B) were monitored for 40 and 90 d, respectively.
T. cruzi-NY-ESO-1 Leads to IL-12 and MyD88 Dependent Pathway.
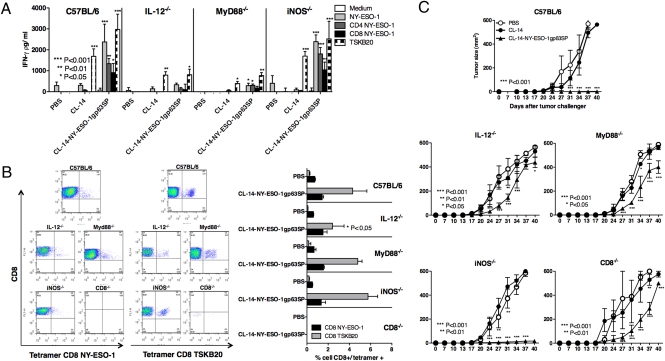
To investigate the mechanism by which CL-14-NY-ESO-1gp63SP induces protective immunity against the B16-NY-ESO-1 cell line, we used the MyD88−/−, IL-12p40−/−, iNOS−/−, and β2-microglobulin−/− (CD8 T cell-deficient) mice. None of these knockout mice that are otherwise highly susceptible to T. cruzi (11, 14, 26, 27) showed any sign of disease when vaccinated with the live transgenic parasites or the WT CL-14 (Fig. S5). Importantly, our results show that upon in vitro stimulation with either rNY-ESO-1, NY-ESO-1, or T. cruzi–derived peptide (TSKB20), the IFN-γ production by CD4+ T and CD8+ T lymphocytes was severely impaired in both MyD88−/− and IL-12p40−/− mice vaccinated with CL-14-NY-ESO-1gp63SP (Fig. 5A). Similar results were obtained when we immunized knockout mice with either Alum/CpG/NY-ESO-1 or Alum/MPL/NY-ESO-1 (Fig. S6). On the other hand, we found no significant decrease in the frequency of CD8+ T lymphocytes specific for NY-ESO-1 epitope in the various knockout (KO) mice vaccinated with the transgenic parasites (Fig. 5B).
Fig. 5.
MyD88- and IL-12–dependent induction of IFN-γ–producing T cells and protective immunity elicited by immunization with CL-14 expressing NY-ESO-1. (A) Splenocytes from vaccinated WT (C57BL/6) and KO mice (MyD88−/−, IL-12−/−, iNOs−/−, and CD8−/−) were restimulated with T CD4+- or T CD8+-specific peptides or with rNY-ESO-1 protein (n = 4). IFN-γ production was measured by ELISA at 72 h after stimulation. (B) Before restimulation, the splenocytes were stained with anti-CD3, anti-CD8, and NY-ESO-1 or TSKB20 tetramers and analyzed by flow cytometry. Representative dot blots and a graph summarizing the percentage of double-positive cells are shown at Left and Right, respectively. (C) Immunized C57BL/6 and KO mice were challenged with 5 × 104 of the B16F10 melanoma cells expressing the NY-ESO-1 and monitored 40 d for tumor growth (n = 6).
We also challenged the different KO mice with B16-NY-ESO-1 (Fig. 5C). We observed that protective immunity elicited by vaccination was not effective in either MyD88−/− or IL-12p40−/− mice, suggesting the critical role of TLRs in inducing IL-12 and consequently IFN-γ production and protection elicited by the CL-14-NY-ESO-1gp63SP. We also observed that vaccinated β2-microglobulin−/− mice (CD8−/−), which lack CD8+ T lymphocytes, were unable to control tumor growth, suggesting a critical role of this T-cell subset in host resistance to B16-NY-ESO-1. In contrast, mice deficient in inducible nitric oxide synthase controlled tumor growth when vaccinated with CL-14-NY-ESO-1 (Fig. 5C). Together, these results indicate that the adjuvant activity of CL-14 clone is highly dependent on MyD88 and IL-12, whereas CD4+ T as well as CD8+ T lymphocytes are the main cellular sources of IFN-γ.
Discussion
Both attenuated viruses and bacteria have been largely explored as vaccine vectors to elicit T cell mediated immunity and in particular CD8+ T-cell responses (6, 24). Although effective, some of these vectors induce strong but not persistent immunity. A possible explanation for the decay of the antigen-specific T-cell response is related to the fact that these attenuated viruses or bacteria are often not replicative, and infection is rapidly eliminated by the immune system. One possible alternative is to vaccinate with vectors that persist in the host, ensuring long-term immunity. In this regard, like other parasites, T. cruzi coevolved with their hosts, to maintain a long-lived infection (8). As a consequence, the parasite induces a strong immunity that persists throughout the infection, which prevents reemergence of intense parasitism and secondary infection with T. cruzi.
Different studies associate the presence of local tissue parasitism with the development of chronic cardiac and digestive pathology, both in humans and mice (28–30). Hence, we used CL-14, a highly attenuated clone of T. cruzi that was derived from the CL strain (15, 31) in the early 1980s and kept attenuated, even after continuous passages in liquid cultures. The CL-14 clone is consistently avirulent, and both parasitemia and tissue parasitism are absent or very scarce, even when infecting newborn mice that are highly susceptible to T. cruzi infection (31). In addition, our results show that various KO mice, which are otherwise very susceptible to T. cruzi infection (11, 14, 26, 27), did not show signs of parasitemia or lethality upon vaccination with the live CL-14 clone or the CL-14 clone expressing NY-ESO-1. As a consequence, the systemic aberrant activation of the immune system and pathogenesis are not observed even in highly susceptible strains of mice, as one would predict from studies with virulent strains of T. cruzi (32). Importantly, vaccination with live CL-14 was repeatedly shown to induce a potent and long-lasting parasite-specific antibody and T cell-mediated immunity, characterized by high levels of IgG2, IFN-γ, and CD8+ T-cell responses, as well as solid protection against highly virulent strains of T. cruzi (33). Thus, we assume that the immunological adjuvant properties of CL-14 are still effective. T. cruzi parasites have intrinsic TLR agonists, such as glycosylphosphatidylinositol anchors (9, 10), unmethylated CpG motifs found on its nuclear DNA (11, 12) and RNA (13) that ensure the continuous stimulation of polarized antigen-specific Th1 lymphocytes. In addition, long-lived intracellular parasitism is likely to be another important characteristic of CL-14-NY-ESO-1 for the prolonged stimulation of CD8+ T cells by MHC class I from parasitized host cells (34) and persistent immunity.
Inspired by the “malariotherapy,” which used infection with Plasmodium to treat neurosyphilis (35), Roskin and colleagues thought that parasitic infections that cause serious diseases produced a “toxin” that could act on and kill tumor cells (36–38). Almost 10 y after he started his studies, Roskin published an article demonstrating tumor regression after infection with T. cruzi in mice (37). Later, he published an article demonstrating tumor regression in human patients infected with T. cruzi (38). A growing interest in using T. cruzi to treat cancer was pursued by different research groups (39, 40), and T. cruzi preparations for cancer therapy were launched by French and Russian pharmaceutical companies (36). Although similar results were found, the ambiguity of the findings obtained by various investigators regarding efficacy against experimental tumors (41) led to the discontinuation of this line of investigation and commercial distribution of this anticancer compound. We assume that, at least in part, the antitumor activity of T. cruzi infection was due to the strong activation of the innate immune system. The immunostimulatory effect of T. cruzi infection results in the production of high levels of proinflammatory cytokines, such as TNF-α (42), which leads to activation of effector mechanisms that efficiently destroy tumor cells (43).
Importantly, either CL-14-NY-ESO-1-His(+) or CL-14-NY-ESO-1gp63 induced in vitro, both human CD4+ T as well as CD8+ T lymphocytes to produce IFN-γ after restimulation with either fowlpox expressing NY-ESO-1 or NY-ESO-1 peptides. Our results also show that in a prophylactic protocol, CL-14-NY-ESO-1 was highly effective in inducing immunity to tumor development, compared with vaccination with recombinant NY-ESO-1 associated with classic TLR agonists. Furthermore, we show that the levels of IFN-γ responses and frequency of IFN-γ–producing T cells nicely correlate with protective immunity and were dependent of MyD88, IL-12, and CD8+ T lymphocytes. In contrast, the effect of therapeutic vaccination with CL-14-NY-ESO-1 was only partial. We believe that this might be explained in part by the ability of tumors to elicit immunoregulatory mechanisms, such as regulatory T cells, expression of CTLA-4, IL-10, and TGF-β (44–47), that prevent establishment of an optimal immune response and complete reversion of tumor growth. This is an important question, which is currently being investigated in our laboratory.
It is noteworthy that the technology we developed for stable transfection targets the β-tubulin gene, which has multiple copies (≈40 copies) in the T. cruzi genome (48). Although CL-14 is highly susceptible to the existing drug (benznidazole) used to treat Chagas disease (49), we also have engineered T. cruzi to simultaneously express both NY-ESO-1 and the herpes virus thymidine kinase. Thus, an additional safety mechanism for eliminating persistent parasites could be the use of acyclovir therapy. Another attractive idea that has already been tested in the laboratory is the generation of CL-14 expressing different tumor antigens, which could be used as polyvalent antitumor vaccine. One important question that is currently being addressed in our laboratory concerns the molecular basis of the attenuated phenotype of the CL-14 clone. CL-14 has been kept stable for 3 decades, and this strategy could lead us to develop genetically engineered parasites with lower risk of reversion of the attenuated phenotype.
In conclusion, on the basis of the ability of T. cruzi parasites to strongly activate both innate and acquired immunity, we explored genetically engineered attenuated parasites to express tumor antigens as a strategy to develop an anticancer vaccine. Our initial hypothesis was that by triggering innate immunity and production of proinflammatory cytokines, in particular IL-12, T. cruzi parasites stimulate strong and long-lasting antigen-specific CD4+ Th1 as well as CD8+ T-cell responses. As a consequence, vaccination with T. cruzi infection would lead to activation of immunological effector mechanisms, including the production of TNF-α, that are highly effective in killing or controlling the growth of tumor cells. We envisage ethical barriers of using T. cruzi parasites to prevent or treat cancer. Nevertheless, this study highlights a new strategy to induce a highly and long-lasting T cell-mediated immune response, which is often poorly achieved in vaccine protocols against different types of tumors. Hence, the experiments presented here indicate that use of T. cruzi parasites as a live vector for an anticancer vaccine should be further explored.
Materials and Methods
Parasite Construction and Characterization.
Transgenic parasites were obtained by cloning the NY-ESO-1 Open reading frame (ORF), fused to His tag or to the gp63 SP sequences into the pROCKNeo plasmid. T. cruzi epimastigotes were transfected and maintained as previously described (20). The integration of the exogenous gene into the parasite genome was confirmed by Southern blot analysis of genomic DNA digested with BamHI and SacI and hybridized with NY-ESO-1 and amastin probes labeled with 32P. Expression of rNY-ESO-1 was detected by Western blot and fluorescence microscopy using the anti-NY-ESO-1 or anti-His tag mAbs.
Immunohistochemistry and Human T-Cell Immune Response.
For immunohistochemistry, the SK-MEL-149 melanoma cell line infected or not with WT or transgenic parasites and the SK-MEL-52 cell line that constitutively expresses NY-ESO-1 (positive control) were stained with anti-NY-ESO-1 mAb (PowerVison Kit; Leica Microsystems). For human T-cell responses we used PBMC-derived irradiated APCs (CD4−CD8−) infected with 30 parasites per cell and cocultured with purified autologous CD8+ T or CD4+ T cells (50). The cellular immune response was evaluated by an IFN-γ enzyme-linked immunosorbent spot assay (ELISPOT), 72 h after restimulation with autologous EBV-B cells pulsed with peptides or infected with recombinant Fowlpox (rFowlpox) virus. For flow cytometry analysis, CD8+ T cells were stained with anti-CD8 mAb and NY-ESO-1 tetramer using the HLA-A*0201 and NY-ESO157–165 peptide.
Mouse Immune Response and Tumor Challenge.
WT and KO mice received two i.p. doses, 30 d apart, of 107 metacyclic forms of T. cruzi. At 21 d after the boost, sera was collected to measure the levels of anti-NY-ESO-1 IgG isotypes by ELISA or Western blot; and spleen cells were cultured with or without 10 μM of either CD4-NY-ESO-1, CD8-NY-ESO-1, or TSKB18 peptides. IFN-γ was quantified in tissue culture supernatants by ELISA (R&D Systems). For flow cytometry analysis, splenocytes were stained with anti-CD3, anti-CD8 (BD Pharmingen), and NY-ESO-1 or TSKB18 tetramers. Alternatively, mice were challenged s.c. with 5 × 104 B16 melanoma cell lines expressing or not NY-ESO-1. For immunotherapy, mice were challenged s.c. with either 5 × 104 B16 melanoma, 1 × 106 CT26 colon adenoma, or CMS5a fibrosarcoma cell lines expressing or not NY-ESO-1 and treated with three doses of live parasites, 5 d apart, starting at day 5 after challenge.
Supplementary Material
Acknowledgments
We thank Andrew Simpson, Jonathan Skipper from the Ludwig Institute for Cancer Research (LICR), and Denise Golgher for incentive, scientific discussions, and suggestions during the development of this work; the New York branch of the LICR for providing the anti-NY-ESO-1 mAb and a plasmid containing the NY-ESO-1 coding region; the LICR Tetramer Facility for tetramers synthesis; the LICR–Cornell University for the recombinant NY-ESO-1 protein; Dr. Jonathan Cebon from LICR–Melbourne for the B16F10 and B16-NY-ESO-1 cell lines; and Dr. Hiroyoshi Nishikawa from Mie University Medical School for the CT26, CT26-NY-ESO-1, CMS5a, and CMS5a-NY-ESO-1 cell lines. This study was funded by the Atlantic Philanthropies/Program of Clinical Discoveries from the LICR, Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), Fundação Oswaldo Cruz, and the National Institute of Science and Technology for Vaccines/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). R.T.G. and C.J. are recipients of CNPq fellowships; B.G.-F. and L.I.S. are fellows from FAPEMIG; and C.J. is a fellow from Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.D.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110030108/-/DCSupplemental.
References
- 1.Gnjatic S, et al. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 2.Caballero OL, Chen YT. Cancer/testis (CT) antigens: Potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu MA. Immunologic basis of vaccine vectors. Immunity. 2010;33:504–515. doi: 10.1016/j.immuni.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: Implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 8.Junqueira C, et al. The endless race between Trypanosoma cruzi and host immunity: Lessons for and beyond Chagas disease. Expert Rev Mol Med. 2010;12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- 9.Campos MA, et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira AC, et al. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J Immunol. 2004;173:5688–5696. doi: 10.4049/jimmunol.173.9.5688. [DOI] [PubMed] [Google Scholar]
- 11.Bafica A, et al. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol. 2006;177:3515–3519. doi: 10.4049/jimmunol.177.6.3515. [DOI] [PubMed] [Google Scholar]
- 12.Bartholomeu DC, et al. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. J Immunol. 2008;181:1333–1344. doi: 10.4049/jimmunol.181.2.1333. [DOI] [PubMed] [Google Scholar]
- 13.Caetano BC, et al. Requirement of UNC93B1 reveals a critical role for TLR7 in host resistance to primary infection with Trypanosoma cruzi. J Immunol. 2011;187:1903–1911. doi: 10.4049/jimmunol.1003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarleton RL, Koller BH, Latour A, Postan M. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature. 1992;356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 15.Lima MT, Jansen AM, Rondinelli E, Gattass CR. Trypanosoma cruzi: Properties of a clone isolated from CL strain. Parasitol Res. 1991;77:77–81. doi: 10.1007/BF00934390. [DOI] [PubMed] [Google Scholar]
- 16.Davis ID, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–10702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aoki M, et al. Antibody responses against NY-ESO-1 and HER2 antigens in patients vaccinated with combinations of cholesteryl pullulan (CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine. 2009;27:6854–6861. doi: 10.1016/j.vaccine.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Bioley G, et al. Vaccination with recombinant NY-ESO-1 protein elicits immunodominant HLA-DR52b-restricted CD4+ T cell responses with a conserved T cell receptor repertoire. Clin Cancer Res. 2009;15:4467–4474. doi: 10.1158/1078-0432.CCR-09-0582. [DOI] [PubMed] [Google Scholar]
- 19.Bioley G, et al. Vaccination with a recombinant protein encoding the tumor-specific antigen NY-ESO-1 elicits an A2/157-165-specific CTL repertoire structurally distinct and of reduced tumor reactivity than that elicited by spontaneous immune responses to NY-ESO-1-expressing Tumors. J Immunother. 2009;32:161–168. doi: 10.1097/CJI.0b013e31819302f6. [DOI] [PubMed] [Google Scholar]
- 20.DaRocha WD, et al. Expression of exogenous genes in Trypanosoma cruzi: improving vectors and electroporation protocols. Parasitol Res. 2004;92:113–120. doi: 10.1007/s00436-003-1004-5. [DOI] [PubMed] [Google Scholar]
- 21.Grandgenett PM, Coughlin BC, Kirchhoff LV, Donelson JE. Differential expression of GP63 genes in Trypanosoma cruzi. Mol Biochem Parasitol. 2000;110:409–415. doi: 10.1016/s0166-6851(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira SM, Russell DG, Kirchhoff LV, Donelson JE. A differentially expressed gene family encoding “amastin,” a surface protein of Trypanosoma cruzi amastigotes. J Biol Chem. 1994;269:20509–20516. [PubMed] [Google Scholar]
- 23.Maraskovsky E, et al. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ t-cell-mediated immunity and protection against NY-ESO-1+ tumors. Clin Cancer Res. 2004;10:2879–2890. doi: 10.1158/1078-0432.ccr-03-0245. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa H, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 26.Campos MA, et al. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004;172:1711–1718. doi: 10.4049/jimmunol.172.3.1711. [DOI] [PubMed] [Google Scholar]
- 27.Michailowsky V, et al. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am J Pathol. 2001;159:1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vago AR, Macedo AM, Adad SJ, Reis DD, Corrêa-Oliveira R. PCR detection of Trypanosoma cruzi DNA in oesophageal tissues of patients with chronic digestive Chagas’ disease. Lancet. 1996;348:891–892. doi: 10.1016/S0140-6736(05)64761-7. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi MLBT, et al. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: Light microscopy and immunohistochemical findings. Cardiovasc Pathol. 1993;2:101–106. doi: 10.1016/1054-8807(93)90021-S. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J Infect Dis. 1999;180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 31.Lima MT, Lenzi HL, Gattass CR. Negative tissue parasitism in mice injected with a noninfective clone of Trypanosoma cruzi. Parasitol Res. 1995;81:6–12. doi: 10.1007/BF00932410. [DOI] [PubMed] [Google Scholar]
- 32.Paiva CN, Castelo-Branco MT, Lannes-Vieira J, Gattass CR. Trypanosoma cruzi: Protective response of vaccinated mice is mediated by CD8+ cells, prevents signs of polyclonal T lymphocyte activation, and allows restoration of a resting immune state after challenge. Exp Parasitol. 1999;91:7–19. doi: 10.1006/expr.1999.4356. [DOI] [PubMed] [Google Scholar]
- 33.Paiva CN, Castelo-Branco MT, Rocha JA, Lannes-Vieira J, Gattass CR. Trypanosoma cruzi: Lack of T cell abnormalities in mice vaccinated with live trypomastigotes. Parasitol Res. 1999;85:1012–1017. doi: 10.1007/s004360050674. [DOI] [PubMed] [Google Scholar]
- 34.Tzelepis F, et al. Infection with Trypanosoma cruzi restricts the repertoire of parasite-specific CD8+ T cells leading to immunodominance. J Immunol. 2008;180:1737–1748. doi: 10.4049/jimmunol.180.3.1737. [DOI] [PubMed] [Google Scholar]
- 35.Whitrow M. Wagner-Jauregg and fever therapy. Med Hist. 1990;34:294–310. doi: 10.1017/s0025727300052431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krementsov N. Trypanosoma cruzi, cancer and the Cold War. Hist Cienc Saude Manguinhos. 2009;16(Suppl 1):75–94. doi: 10.1590/s0104-59702009000500005. [DOI] [PubMed] [Google Scholar]
- 37.Roskin G. Toxin therapy of experimental cancer; the influence of protozoan infections upon transplanted cancer. Cancer Res. 1946;6:363–365. [PubMed] [Google Scholar]
- 38.Klyueva NG, Roskin G. Cancerolytic substance of Schizotrypanum cruzi. Am Rev Sov Med. 1946;4:127–129. [PubMed] [Google Scholar]
- 39.Malisoff WM. The action of the endotoxin of Trypanosoma cruzi (KR) on malignant mouse tumors. Science. 1947;106:591–594. doi: 10.1126/science.106.2763.591-a. [DOI] [PubMed] [Google Scholar]
- 40.Hauschka TS, Goodwin MB. Trypanosoma cruzi endotoxin (KR) in the treatment of malignant mouse tumors. Science. 1948;107:600–602. doi: 10.1126/science.107.2788.600. [DOI] [PubMed] [Google Scholar]
- 41.Belkin M, Hardy WG. Effect of reserpine and chlorpromazine on sarcoma 37. Science. 1957;125:233–234. doi: 10.1126/science.125.3241.233. [DOI] [PubMed] [Google Scholar]
- 42.Silva JS, Vespa GN, Cardoso MA, Aliberti JC, Cunha FQ. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin BY, et al. Tumor necrosis factor and IFN induce a common set of proteins. J Immunol. 1988;141:1180–1184. [PubMed] [Google Scholar]
- 44.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 46.Ikushima H, Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 47.Steinbrink K, et al. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 48.El-Sayed NM, et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 49.Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 50.Dutoit V, et al. Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest. 2002;110:1813–1822. doi: 10.1172/JCI200216428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.