Abstract
The prevailing paradigm in aquatic science is that microbial methanogenesis happens primarily in anoxic environments. Here, we used multiple complementary approaches to show that microbial methane production could and did occur in the well-oxygenated water column of an oligotrophic lake (Lake Stechlin, Germany). Oversaturation of methane was repeatedly recorded in the well-oxygenated upper 10 m of the water column, and the methane maxima coincided with oxygen oversaturation at 6 m. Laboratory incubations of unamended epilimnetic lake water and inoculations of photoautotrophs with a lake-enrichment culture both led to methane production even in the presence of oxygen, and the production was not affected by the addition of inorganic phosphate or methylated compounds. Methane production was also detected by in-lake incubations of lake water, and the highest production rate was 1.8–2.4 nM⋅h−1 at 6 m, which could explain 33–44% of the observed ambient methane accumulation in the same month. Temporal and spatial uncoupling between methanogenesis and methanotrophy was supported by field and laboratory measurements, which also helped explain the oversaturation of methane in the upper water column. Potentially methanogenic Archaea were detected in situ in the oxygenated, methane-rich epilimnion, and their attachment to photoautotrophs might allow for anaerobic growth and direct transfer of substrates for methane production. Specific PCR on mRNA of the methyl coenzyme M reductase A gene revealed active methanogenesis. Microbial methane production in oxygenated water represents a hitherto overlooked source of methane and can be important for carbon cycling in the aquatic environments and water to air methane flux.
Keywords: epilimnic methane peak, methanogens
Although methane makes up <2 parts per million by volume (ppmv) of the atmosphere, it accounts for 20% of the total radiative forcing among all long-lived greenhouse gases (1). In the aquatic environments, methane is also an important substrate for microbial production (2). The prevailing paradigm is that microbial methanogenesis occurs primarily in anoxic environments (3, 4). A commonly observed paradox is methane accumulation in well-oxygenated waters (2, 5), which is often assumed to be the result of physical transport from anoxic sediment and water (6–8) or in situ production within microanoxic zones (9–11). Two recent studies challenged this paradigm and suggested that microbes in oligotrophic ocean can metabolize methylated compounds and release methane even aerobically (12, 13). These claims are not without caveats, because the amounts of methylated compounds added [1–10 μM methylphosphonate (12) and 50 μM dimethyl sulfoniopropionate (13)] were far higher than their environmental concentrations, and therefore, the ecological relevance remains obscure. Moreover, dissolved oxygen (DO) was not monitored during the long incubation (5–6 d), and the possibility that the experimental setups had become anoxic before methane production could not be dismissed.* Despite the uncertainty, if microbial methane production can occur in oxygenated water, it will have profound implications for carbon cycling and climate.
Here, we test if microbial methane production occurs in the well-oxygenated water column of a temperate oligotrophic lake (Lake Stechlin, Germany). To obtain conclusive evidence, we established several criteria: (i) verification of methane production even in unamended lake water, (ii) confirmation that DO remains at high levels when methane production occurs, and (iii) demonstration of uncoupling between methane production and consumption causing accumulation of methane in well-oxygenated water.
Results
In Situ Methane Profiles and Microbial Composition.
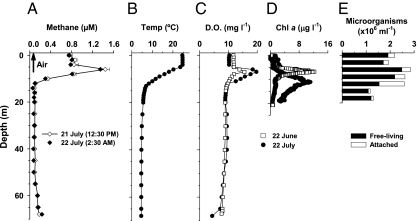
Methane oversaturation was observed within the upper 10 m on July 21 and 22, 2010, when the lake was calm and strongly stratified (Fig. 1A). A methane maxima of 1.35–1.44 μM coincided with the thermocline and DO oversaturation at 6 m (Fig. 1 B and C). DO oversaturation was also present 1 mo earlier (Fig. 1C), coinciding with the chlorophyll (Chl) a peak (Fig. 1D). However, the Chl a maximum was below 10 m on July 22, and the observed DO peak was likely the result of a past phytoplankton bloom. Microbial abundance was higher in the upper 12 m than below the thermocline, and of this abundance, 11–40% were particle-associated microbes (Fig. 1E). The dominant autotrophs were the cyanobacterium Aphanizomenon flos-aquae and diatoms (Asterionella formosa and Fragillaria crotonensis) in the upper 9 m. Free-living methanogenic Archaea belonging to uncultured Methanomicrobiales were present at 6 m (Fig. S1), and particle-associated methanogenic Archaea were found at 6 (closely related to uncultured Methanomicrobiales and Methanosaeta) and 9 m (closely related to Methanosaeta and the Candidatus Methanoregula sp.) (Fig. S1); however, they were undetectable at 18 m. Methane-oxidizing bacteria (MOB) were not detectable in the epilimnion, whereas at 18 m, a single species of MOB type I (99% similarity to Methylobacter tundripaludum) was detected. MOB type II were not detectable.
Fig. 1.
Depth profiles for Lake Stechlin. (A) Mean methane concentrations during day and night (July 21 and 22, 2010, respectively). Error bars during the day are SDs of two to three measurements of the same bottles. Arrow indicates methane concentration in the air. (B) Average temperature on July 21 and 22. (C) Dissolved oxygen (DO) on June 22 and July 22. (D) Chlorophyll (Chl) a concentrations on June 22 and July 22. (E) Free-living and attached microorganism abundances on July 22.
Repeated measurements in September of 2010 showed temporal regression of the methane peak at 6 m, decreasing to only 0.10 μM by September 12. The reverse temporal development was observed in 2011, when the methane peak at 6 m increased from 0.18 μM in May to 1.25 μM in June (Fig. S2). As in the previous year, the methane maxima in 2011 coincided with the thermocline and DO oversaturation. We detected the active methyl coenzyme M reductase A (mcrA) gene for methanogenesis based on mRNA at 6 m in May and June of 2011. The mcrA gene was also present at 18–20 m, but it was undetectable at 0 m in May (Table 1).
Table 1.
Results of specific PCR on cDNA of the mcrA gene
| Date | Sample (m) | PCR products |
| May 14, 2011 | 0 | −/− |
| May 14, 2011 | 6 | +/+ |
| May 14, 2011 | 18 | +/+ |
| June 3, 2011 | 6 | +/+ |
| June 3, 2011 | 20 | +/− |
| June 13, 2011 | 6 | +/+ |
Equal amounts (5 μL) of the respective PCR products were added to the 2% agarose gel together with negative and positive controls plus the DNA size standard. Samples were tested in parallels; presence (+) and absence (−) of PCR products are given for each parallel.
Methane Production in Laboratory Incubation Experiments.
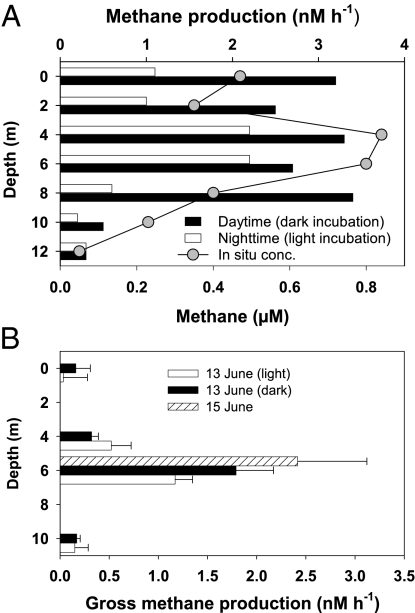
Laboratory incubation of unamended water samples from discrete depths in July showed positive methane production (Fig. 2A). Significantly higher production rates were observed when daytime water samples were incubated in the dark rather than when nighttime samples were incubated in the light. Water samples from the upper 8 m where cyanobacteria and green algae were abundant showed higher methane production rates (maximum = 3.40 nM⋅h−1), which corresponded with the high in situ methane concentrations (Fig. 2A). The water in the incubation bottles never became anoxic, and the DO was at 94–97% saturation at the end of the experiment. Despite the earlier reports (12, 13), addition of inorganic phosphate, methylphosphonate, or trimethylamine to lake surface water did not result in any significant increase in methane concentrations in our experiments (Table S1).
Fig. 2.
(A) Methane production by Lake Stechlin water collected from different depths in laboratory experiments in July of 2010. Excess methane was stripped by vigorous shaking before incubation. Daytime water samples were incubated in the dark, and nighttime water samples were incubated in the light (43 μmol photons⋅m−2 s−1) for 8 h at room temperature. Final DO was 7.4–7.7 mg⋅L−1 (94–97% saturation). Methane production in dark incubation was significantly higher than production in light incubation (paired t test; n = 7, P = 0.022). Ambient methane concentration profiles are included for comparison. (B) Methane production by Lake Stechlin water during in-lake incubation experiments. Water was collected from different depths on June 13, 2011 and stripped of excess methane. Afterward, the water was used to fill up three sets of gas-tight bottles in triplicate (n = 3). One set was used as the light treatment, one set was wrapped in aluminum foil as the dark treatment, and one set was spiked with >10−4 M 2-bromoethanesulfonic acid (BES) to inhibit methanogenesis. The three sets of bottles were then suspended at their original depths for 8.5 h. At the end of the incubation, dissolved methane concentrations in the bottles were measured by headspace analysis. Gross methane production rates were calculated as the difference between the light or dark treatment and the BES treatment divided by the incubation time. Two-way ANOVA test indicated that methane production rate varied significantly with depth (n = 8 P < 0.0001) but not between the light and dark treatments (n = 8, P = 0.188). The in-lake incubation experiment was repeated on June 15 at the 6-m depth with light and BES treatments, and methane production was calculated in the same manner.
Methane Production in In-Lake Incubation Experiments.
Methane production was observed in the first in-lake incubation experiment (June 13) (Fig. 2B). The gross methane production rate varied significantly among water samples suspended at different depths but not between the light and dark treatments. The highest average production rates were at 1.2–1.8 nM⋅h−1 at 6 m. Methane production was observed again in the second experiment (June 15) at an even higher average rate of 2.4 nM⋅h−1 at 6 m (Fig. 2B) when in situ methane concentrations had further increased.
Uncoupled Methane Oxidation in Laboratory Experiments.
When unamended Lake Stechlin surface water samples were incubated over a longer time, methane concentrations in all three replicates increased at an average rate of 52 nM·h−1 (Fig. S3). Two of the replicates were killed for DO measurement, which was at ≥80% saturation. Methane concentration in the remaining replicate subsequently decreased at a rate of 52 nM·h−1 (Fig. S3). Threshold-dependent methane oxidation was observed in another experiment. In unamended lake water and water with added methane and oxidation inhibitor, methane concentrations did not change significantly over time; in the sample augmented with >50 μM methane, methane oxidation proceeded at a rate of 0.19 μM·h−1 (Fig. S4).
Enrichment Culture Experiments.
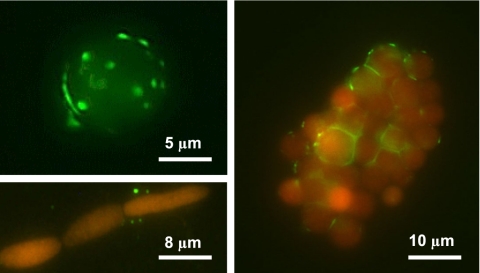
Microbial enrichment culture established from lake water was used to inoculate cultures of three photoautotrophs: (i) A. flos-aquae (SAG 31.87), (ii) axenic Microcystis aeruginosa (HUB W333), and (iii) a xenic Chlorella-like green alga from Lake Stechlin. In all cases, inoculation led to significantly higher methane production under well-oxygenated conditions compared with those conditions without the inoculum or the inoculum alone (Table S2). FISH revealed direct attachment of potentially methanogenic Archaea to the photoautotrophic cells (Fig. 3).
Fig. 3.
Direct attachment of methanogenic Archaea (green, FITC-labeled oligonucleotide probe) to autotrophs (red, autofluorescence) observed by FISH. (Upper Left) A single Chlorella-like algal cell. (Right) A colony of Chlorella-like green alga. (Lower Left) A filament of the cyanobacterium A. flos-aquae.
Discussion
Our study showed that epilimnetic methane oversaturation is a recurring phenomenon in Lake Stechlin during the stratified season. Similar temporal development of epilimnetic methane oversaturation has also been observed in Lake Constance, which was assumed to be caused by anoxic aggregates within the water column (11). Here, we showed positive methane production in both laboratory and in-lake incubation experiments with unamended lake water excluding any external methane sources. Although internal sources caused by microanoxic zones could not be completely dismissed, metazoans, fecal pellets, or large phytoaggregates were not present in our incubations, and the short incubation time (<1 d) should have prevented oxygen depletion even for large (>1 mm) aggregates (16). The overlapping of the methane peak, mcrA gene transcription, and strong DO oversaturation at 6 m also implies that the methane production was positively, not negatively, related to oxygen availability. Our results, therefore, provide direct empirical evidence of microbial methane production under well-oxygenated conditions.†
The depth-specific differences in methane production rates in the incubation experiments corresponded well with the vertical variations in ambient methane concentrations (Fig. 2), indicating that the ambient profile was strongly influenced by depth-specific methane production and consumption processes. The methane production rate was highest at 6 m in both in-lake incubation experiments, consistent with the strong expression of the mcrA gene for methanogenesis (Table 1). Based on the ambient profiles, the methane concentration at 6 m increased from 0.59 to 1.25 μM between June 10 and 15, 2011 (Fig. S2), which is equivalent to an average accumulation rate of 5.5 nM⋅h−1. The estimated average production rates (1.8–2.4 nM·h−1 at 6 m) (Fig. 2B), therefore, suggest that in situ methane production could account for 33–44% of the observed methane accumulation at that depth in mid-June when assuming the total absence of methane oxidation.
In the presence of oxygen, methane production needs to be uncoupled from methane oxidation to produce the observed methane accumulation. Our longer-term incubation experiment with surface water showed that average methane increase rate during the first 51 h was the same as the average methane decrease rate in the subsequent 42 h. This finding suggests that, although methane oxidation could potentially balance methane production, there was a temporal uncoupling between the two processes, which could result from a time lag in growth of methanotrophs, inhibitory effects of initially high DO concentration (17), or a threshold methane concentration required by methanotrophs (18). Threshold-dependent methane oxidation was also supported by the additional experiment in which methane oxidation was detectable only in the water spiked with >50 μM methane, suggesting that methane oxidation only proceeds at an appreciable rate when the ambient methane concentration has reached a high level. In situ methane oxidation may also be inhibited by nitrate, nitrite, and ammonium released by grazing and microbial degradation of organic matter in water layers with high algal and cyanobacterial biomass (19).
Spatial uncoupling between methanogenesis and methanotrophy within the water column was supported by our molecular analysis. Methanogenic Archaea were present, but MOB were not detectable in the epilimnion in July of 2010 when methane oversaturation was observed. Active methanogens, especially at 6-m depth, were also confirmed by our mRNA data. MOB were detectable only below the thermocline (e.g., at 18- and 20-m depth), where ambient methane concentrations were low but mcrA gene expression was still detectable. This spatial separation between methanogenic Archaea and MOB could also contribute to the accumulation of methane in the epilimnion. Our field measurements showed that the methane peak at 6 m tended to develop in May to July and then decline in September (Fig. S2). This wax and wane pattern may be related to temporal changes in the abundance and activities of methanogens (production) and methanotrophs (consumption). Understanding what factors determine these temporal variations will be an important topic for future studies.
Several mechanisms have been proposed for methanogenesis in oxygenated waters (20): cleavage of methyl-esters by UV radiation, hypoxia-induced methane production in mitochondria, methane release by organisms under oxidative stress, and microbial decomposition of methylated compounds. In oligotrophic lakes such as Lake Stechlin, UV penetration is limited to <2.0 m (21), much shallower than the observed methane peak. UV was also absent in our laboratory experiments where methane production was observed. The overlapping between the methane peak and the oxygen peak in situ suggests that hypoxia is not a factor. It is also unlikely that epilimnetic organisms would suffer from strong oxidative stress, because they frequently experience high oxygen concentration and have multiple mechanisms to cope with it (22). Although conversion of methylated compounds such as methanethiol to methane can be bioenergetically favorable for methanogenic Archaea (13), neither methanethiol nor other methylated compounds are commonly found in high concentrations in oxygenated epilimnion in lakes (23–25). In our experiments, the addition of neither inorganic phosphate nor methylated compounds affected methane production; hence, pelagic methanogenesis in Lake Stechlin did not seem to depend on phosphate or methylated substrates like other systems (12, 13).
The alternatives are hydrogenotrophic and acetoclastic methanogenesis, both of which are common among methanogenic Archaea (26). The Archaea in Lake Stechlin belong to groups that have the ability to perform hydrogenotrophic or acetoclastic methanogenesis (27–29). Many photoautotrophs can produce H2 through both direct and indirect photolysis (30). Cyanobacteria can also increase H2 yield through nitrogen-fixing activities at night (31). Indeed, the slightly higher methane production rates in our dark vs. light incubations (Fig. 2A) suggest that H2 production through intracellular fermentation using storage photosynthates (32) or nitrogen fixation in the dark may be particularly important for pelagic methanogenesis in Lake Stechlin. Contrary to common belief, many methanogens can tolerate and survive oxygen exposure (33), which helps explain their methanogenic activity within the water column. It has been shown that Methanosarcina and Methanocella in desert soils can transcribe the mcrA gene even under oxic/oxygenic conditions and at the same time, actively transcribe the gene for oxygen-detoxifying catalase (34). It is also noteworthy that particle-associated Methanosaeta were present in the epilimnion in Lake Stechlin (Fig. S1). This group of methanogens is known to tolerate oxygen exposure (35) and is particularly well-adapted to low acetate environments (28). The co-occurrence of high numbers of cyanobacteria, algae, and attached Archaea within the epilimnion in our study may enable a direct transfer of H2 or acetate from the autotrophs to the methanogenic Archaea to support methane production. Although our field observations suggest that, in Lake Stechlin, this methane production was closely associated with cyanobacteria, our laboratory experiments confirmed that methane production could also be supported by other photoautotrophs in the presence of methanogenic Archaea (Table S2). Given the ubiquitous distribution of Archaea and photoauthotrophs in the aquatic environments, this methane production process may be more widespread than previously recognized.
Inverse modeling has been used to explain the observed atmospheric methane concentrations by constraining the uncertainties in known sources and sinks of methane, but this approach does not consider methane production in oxygenated water bodies (36). It is estimated that freshwater environments contribute >70% of the natural source of methane to the atmosphere (37). Because of the traditional emphasis on anaerobic methanogenesis, this methane is assumed to originate from anoxic sediments and bottom waters (38). Our study provides compelling evidence that microbial methane production can also take place within the oxygenated water and is high enough to contribute substantially to epilimnetic methane accumulation. Quantifying the fate of this methane source—whether through water to air flux or carbon cycling within the water column—may improve our understanding of the global methane budget and climate.
Materials and Methods
Study Site.
Lake Stechlin is a dimictic oligotrophic lake in northeastern Germany (53° 10′ N, 13° 02′ E) with low anthropogenic impact (39). The lake has been continuously studied for almost 50 y, and it serves as a reference lake for the European Water Framework Directive. The lake has a maximum depth of 69.5 m and an area of 4.3 km2, with a hypolimnetic oxygen saturation level of up to 60%.
In Situ Methane and Hydrographical Profiles.
Water samples for dissolved methane were taken in 2-m depth intervals filled into 120-mL preweighed gas-tight crimp bottles. Within 1–2 h after sampling, dissolved methane was determined by headspace analysis (40) on a gas chromatograph with flame ionization detector (Shimadzu). Concurrent DO and water temperature were measured with an in situ probe (Wissenschaftlich Technische Werkstätten). Chl a profiles were measured by a fluorescence probe (Hardt). Attached and free-living microbes were operationally separated by a 5.0-μm polycarbonate membrane and measured by DAPI direct count (41) at 1,000× magnification with an epifluorescence microscope (Zeiss).
Laboratory Incubation Experiments.
Laboratory incubation experiments were conducted to test for methane production in July of 2010 (Fig. 2). To test for the effects of inorganic phosphate and methylated compounds, surface water samples were incubated with or without the addition of 3 μM inorganic phosphate, 1 μM methylphosphonate, or 1 μM trimethylamine, and the methane concentrations were monitored over 55–69 h. To test for temporal uncoupling between methanogenesis and methanotrophy, methane concentrations in triplicate bottles of unamended surface lake water samples were monitored over a longer time, and the average rate of methane increase in the first 51 h was compared with the average rate of methane decrease in the next 42 h (Fig. S3). To test for threshold-dependent methane oxidation, surface lake water samples were incubated for 58 h with added methane or methane plus the inhibitor difluoromethane to prevent methane oxidation (42). Unamended lake water sample was used as the control (Fig. S4).
In-Lake Incubation Experiments.
The first in-lake incubation experiment was conducted on June 13, 2011 to test for methane production under near in situ condition. Water samples collected from different depths were stripped of excess methane by shaking and incubated at the original depth with light, without light, or with added 2-bromoethane sulfonic acid (BES; final concentration >10−4 M) to inhibit methanogenesis (43). Gross methane production rates were calculated as the difference between the light or dark treatment and the BES treatment divided by the incubation time. The experiment was repeated on June 15 at 6-m depth with light and BES treatments (Fig. 2).
Enrichment Culture Experiments.
A microbial enrichment culture was established by incubating Lake Stechlin water with Z-Medium at 26 °C and 72 μmol photons·m−2 s−1 with a 16:8 light:dark cycle. The enrichment culture was then used to inoculate A. flos-aquae (31.87; SAG), axenic M. aeruginosa HUB W333 (Humboldt University), and a green alga isolate from Lake Stechlin (Chlorella-like unicellular, round green alga). Inoculated cultures were kept in gas-tight crimp bottles, and methane production rates were calculated based on methane concentrations measured after 2 and 6 d. Untreated photoautotrophic cultures and inoculum were used as controls, where no methane production was detectable.
Molecular Analysis.
For molecular analysis, water samples were taken between July 21 and 26, 2010 at 0-, 3-, 6-, 9-, 12-, 16-, and 18-m depth; 500 mL were prefiltered through a 5.0-μm Nuclepore polycarbonate membrane, and genomic DNA of free-living microbes was subsequently collected by filtering 500 mL prefiltered sample onto a 0.2-μm Nuclepore polycarbonate filter. DNA was extracted with chloroform-phenol-isoamylalcohol and zirconium beads (44). The reaction mixtures for PCR amplification contained 2 μL template DNA, 200 nM each of the appropriate primers (Table S3), 250 μM each deoxyribonucleoside triphosphate, 3 mM MgCl2, 5 μL 10× PCR buffer, and 0.5 U BIOTAQ Red DNA polymerase (Bioline) in a total volume of 50 μL. Cloning of partial 16S rRNA genes was done using primers specific for Archaea including methanogenic Archaea (Table S3) and the pGEM-T-Easy Vector System II (Promega) according to the manufacturer's protocol. Insert length was checked by primers T7 and SP6. Of each clone library, 20–30 clones were picked and sequenced. PCR products of clones were purified using an established protocol (45). Sequences are deposited in GenBank under the accession numbers JF510050–JF510160. To test for active methanogenesis as indicated by mRNA expression, samples for RNA extraction were taken in May and June of 2011; 1 L water from selected depths (0, 6, 18, or 20 m) was filtered over a PES filter (Millipore). The samples were stored at −80 °C. Extraction of DNA and RNA used an established protocol (46). DNA was then removed by digestion with a Turbo DNA-Free Kit (Ambion) using the manufacturer's instructions. The digestion was proofed by a PCR with universal primers. cDNA synthesis from extracted RNA was performed with random oligohexamers and the Array Script (Ambion) using the manufacturer's instructions. Specific primers (Table S3) were then used in a nested PCR approach to target cDNA from mRNA for mcrA. PCR conditions were the same as above. For FISH, photoautotrophic cells were embedded on slides (47) and fixed with 70% ethanol for 1.5 min. Hybridization was done for 2 h using the probe MG3 for predominantly methanogens (48) and used here to target potentially methanogenic Archaea. The probe was labeled with fluorescein-isothiocyanate (FITC). Afterward, hybridized cells were analyzed with an epifluorescence microscope using the FITC filter set (Leica).
Supplementary Material
Acknowledgments
We thank Martin Allgaier and Ivette Salka for support in phylogenetic tree construction and Solvig Pinnow and Peter Casper for technical support. Kirsten Pohlmann, the editor, and two reviewers provided helpful comments to improve the manuscript. The research was supported by the German Research Foundation (DFG) Grants GR1540/11-1,2 and GR1540/15-1. K.W.T. was also supported by a Humboldt Research Fellowship for Experienced Researchers and a visiting scientist fellowship from the Leibniz-Institute for Freshwater Ecology and Inland Fisheries (IGB)-Berlin, Germany.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JF510050–JF510160).
This article is a PNAS Direct Submission. M.R.T. is a guest editor invited by the Editorial Board.
*We estimated oxygen consumption in the experiments in the work by Karl et al. (12) as follows. According to table 2 in ref. 12, particulate carbon increased by 104 μM within 48 h. Assuming a bacterial growth efficiency of 0.33 and a respiratory quotient of one (14), the estimated O2 consumption because of bacterial biomass production would have been 311 μM, which was more than the ambient O2 concentration (205–215 μM) (ref. 12, table 1). Alternatively, based on table 2 in ref. 12, we calculated a dissolved organic carbon (DOC) consumption of 217 μM within 48 h. Assuming a 1:1.2 molar ratio of DOC oxygen to O2 consumption by microbes (15), the incubation bottles would have become anoxic in 38–40 h. In the experiments, glucose was added (100–200 μM) to stimulate bacterial growth, which would also stimulate oxygen consumption. Assuming a molar ratio of 1 glucose to 6 O2, the addition of glucose would have led to an O2 consumption of 600–1,200 μM, which is three to six times the oxygen that was present in the water. Hence, using their data and estimating oxygen consumption in various ways, we came to the same conclusion. It is very likely that their incubation bottles had become anoxic before substantial methane production was observed.
†The term aerobic methane production strictly means that the production process requires oxygen, which has not been shown in this study or earlier studies (12, 13); hence, we describe our results as methane production under oxygenated conditions.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110716108/-/DCSupplemental.
References
- 1.IPCC . In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Marquis M, Averyt KB, Tignor M, Miller HL, editors. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 2.Conrad R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ Microbiol Rep. 2009;1:285–292. doi: 10.1111/j.1758-2229.2009.00038.x. [DOI] [PubMed] [Google Scholar]
- 3.Mah RA, Ward DM, Baresi L, Glass TL. Biogenesis of methane. Annu Rev Microbiol. 1977;31:309–341. doi: 10.1146/annurev.mi.31.100177.001521. [DOI] [PubMed] [Google Scholar]
- 4.Wahlen M. The global methane cycle. Annu Rev Earth Planet Sci. 1993;21:407–426. [Google Scholar]
- 5.Reeburgh WS. Oceanic methane biogeochemistry. Chem Rev. 2007;107:486–513. doi: 10.1021/cr050362v. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann H, Federwisch L, Peeters F. Wave-induced release of methane: Littoral zones as a source of methane in lakes. Limnol Oceanogr. 2010;55:1990–2000. [Google Scholar]
- 7.Murase J, Sakai Y, Kametani A, Sugimoto A. Dynamics of methane in mesotrophic Lake Biwa, Japan. Ecol Res. 2005;20:377–385. [Google Scholar]
- 8.Scandella BP. Cambridge, MA: Massachusetts Institute of Technology; 2010. Numerical modeling of methane venting from lake sediments. PhD thesis. [Google Scholar]
- 9.De Angelis MA, Lee C. Methane production during zooplankton grazing on marine phytoplankton. Limnol Oceanogr. 1994;39:1298–1308. [Google Scholar]
- 10.Oremland RS. Methanogenic activity in plankton samples and fish intestines: A mechanism for in situ methanogenesis in oceanic surface waters. Limnol Oceanogr. 1979;24:1136–1141. [Google Scholar]
- 11.Schulz M, Faber E, Hollerbach A, Schröder HG, Güde H. The methane cycle in the epilimnion of Lake Constance. Arch Hydrobiol. 2001;151:157–176. [Google Scholar]
- 12.Karl DM, et al. Aerobic production of methane in the sea. Nat Geosci. 2008;1:473–478. [Google Scholar]
- 13.Damm E, et al. Methane production in aerobic oligotrophic surface water in the central Arctic Ocean. Biogeosciences. 2010;7:1099–1108. [Google Scholar]
- 14.Del Giorgio PA, Cole JJ. Bacterial growth efficiency in natural aquatic systems. Ann Rev Ecol Syst. 1998;29:503–541. [Google Scholar]
- 15.Grossart H-P, Ploug H. Bacterial production and growth efficiencies: Direct measurements on riverine aggregates. Limnol Oceanogr. 2000;45:436–445. [Google Scholar]
- 16.Ploug H, Kühl M, Buchholz-Cleven B, Jørgensen BB. Anoxic aggregates—an ephemeral phenomenon in the pelagic environment? Aquat Microb Ecol. 1997;13:285–294. [Google Scholar]
- 17.Rudd JWM, Furutani A, Flett RJ, Hamilton RD. Factors controlling methane oxidation in Shield Lakes: The role of nitrogen fixation and oxygen concentration. Limnol Oceanogr. 1976;21:357–364. [Google Scholar]
- 18.Whiticar MJ, Faber E. Methane oxidation in sediment and water column environments—isotope evidence. Org Geochem. 1986;10:759–768. [Google Scholar]
- 19.Dunfield P, Knowles R. Kinetics of inhibition of methane oxidation by nitrate, nitrite, and ammonium in a humisol. Appl Environ Microbiol. 1995;61:3129–3135. doi: 10.1128/aem.61.8.3129-3135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keppler F, et al. Methane formation in aerobic environments. Environ Chem. 2009;6:459–465. [Google Scholar]
- 21.Morris DP, et al. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol Oceanogr. 1995;40:1381–1391. [Google Scholar]
- 22.Pisciotta JM, Zou Y, Baskakov IV. Light-dependent electrogenic activity of cyanobacteria. PLoS One. 2010;5:e10821. doi: 10.1371/journal.pone.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz M, Bachofen R. Volatile organic sulfur compounds in a meromictic alpine lake. Acta Hydrochim Hydrobiol. 2000;28:185–192. [Google Scholar]
- 24.Gun J, et al. Formation of polysulfides in an oxygen rich freshwater lake and their role in the production of volatile sulfur compounds in aquatic systems. Environ Sci Technol. 2000;34:4741–4746. [Google Scholar]
- 25.Henatsch JJ, Juettner F. Occurrence and distribution of methane thiol and other volatile organic sulfur compounds in a stratified lake with anoxic hypolimnion. Arch Hydrobiol. 1990;119:315–323. [Google Scholar]
- 26.Bapteste E, Brochier C, Boucher Y. Higher-level classification of the Archaea: Evolution of methanogenesis and methanogens. Archaea. 2005;1:353–363. doi: 10.1155/2005/859728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bräuer SL, Cadillo-Quiroz H, Ward RJ, Yavitt JB, Zinder SH. Methanoregula boonei gen. nov., sp. nov., an acidiphilic methanogen isolated from an acidic peat bog. Int J Syst Evol Microbiol. 2011;61:45–52. doi: 10.1099/ijs.0.021782-0. [DOI] [PubMed] [Google Scholar]
- 28.Smith KS, Ingram-Smith C. Methanosaeta, the forgotten methanogen? Trends Microbiol. 2007;15:150–155. doi: 10.1016/j.tim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Yashiro Y, et al. Methanoregula formicica sp. nov., a methane-producing archaeon isolated from methanogenic sludge. Int J Syst Evol Microbiol. 2011;61:53–59. doi: 10.1099/ijs.0.014811-0. [DOI] [PubMed] [Google Scholar]
- 30.Redwood MD, Paterson-Beedle M, Marcaskie LE. Integrating dark and light bio-hydrogen production strategies: Towards the hydrogen economy. Rev Environ Sci Biotechnol. 2009;8:149–185. [Google Scholar]
- 31.Conrad R. Contribution of hydrogen production by biological nitrogen fixation to the global hydrogen budget. J Geophys Res. 1980;85:5493–5498. [Google Scholar]
- 32.Bandyopadhyay A, Stöckel J, Min H, Sherman LA, Pakrasi HB. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat Commun. 2010;1:139. doi: 10.1038/ncomms1139. [DOI] [PubMed] [Google Scholar]
- 33.Jarrell KF. Extreme oxygen sensitivity in methanogenic Archaebacteria. Bioscience. 1985;35:298–302. [Google Scholar]
- 34.Angel R, Matthies D, Conrad R. Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen. PLoS One. 2011;6:e20453. doi: 10.1371/journal.pone.0020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huser BA, Wuhrmann K, Zehnder AJB. Methanothrix soehngenii gen. nov. sp. nov, a new acetotrophic non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1982;132:1–9. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- 36.Hein R, Crutzen PJ, Heimann M. An inverse modeling approach to investigate the global atmospheric methane cycle. Global Biogeochem Cycles. 1997;11:43–76. [Google Scholar]
- 37.Michmerhuizen CM, Striegl RG, McDonald ME. Potential methane emission from north-temperate lakes following ice melt. Limnol Oceanogr. 1996;41:985–991. [Google Scholar]
- 38.Bastviken D, Tranvik LJ, Downing JA, Crill PM, Enrich-Prast A. Freshwater methane emissions offset the continental carbon sink. Science. 2011;331:50. doi: 10.1126/science.1196808. [DOI] [PubMed] [Google Scholar]
- 39.Casper SJ. Lake Stechlin—a Temperate Oligotrophic Lake. Vol 58. Dordrecht, The Netherlands: Monogr Biol; 1985. [Google Scholar]
- 40.Schmidt U, Conrad R. Hydrogen, carbon monoxide, and methane dynamics in Lake Constance. Limnol Oceanogr. 1993;38:1214–1226. [Google Scholar]
- 41.Porter KG, Fieg YS. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 42.Miller LG, Sasson C, Oremland RS. Difluoromethane, a new and improved inhibitor of methanotrophy. Appl Environ Microbiol. 1998;64:4357–4362. doi: 10.1128/aem.64.11.4357-4362.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oremland RS. Biogeochemistry of methanogenic bacteria. In: Zehnder AJ, editor. Biology of Anaerobic Microorganisms. New York: Wiley; 1988. pp. 641–770. [Google Scholar]
- 44.Zhou J, Bruns MA, Tiedje JM. DNA recovery from soils of diverse composition. Appl Environ Microbiol. 1996;62:316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenthal A, Coutelle O, Craxton M. Large-scale production of DNA sequencing templates by microtitre format PCR. Nucleic Acids Res. 1993;21:173–174. doi: 10.1093/nar/21.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nercessian O, Noyes E, Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L. Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Appl Environ Microbiol. 2005;71:6885–6899. doi: 10.1128/AEM.71.11.6885-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daims H, Ramsing NB, Schleifer K-H, Wagner M. Cultivation-independent, semiautomatic determination of absolute bacterial cell numbers in environmental samples by fluorescence in situ hybridization. Appl Environ Microbiol. 2001;67:5810–5818. doi: 10.1128/AEM.67.12.5810-5818.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocheleau S, et al. Differentiation of Methanosaeta concilii and Methanosarcina barkeri in anaerobic mesophilic granular sludge by fluorescent In situ hybridization and confocal scanning laser microscopy. Appl Environ Microbiol. 1999;65:2222–2229. doi: 10.1128/aem.65.5.2222-2229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.