Abstract
The mechanism of IFN-β therapy in relapsing-remitting multiple sclerosis (RRMS) is not well understood, but induction of apoptosis in specific leukocyte subsets is likely to be important. Enhanced expression of TNFSF10 or TNF-related apoptosis-inducing ligand (TRAIL) mRNA in unseparated leukocytes has been put forward as a therapeutic response marker, but it is unclear which leukocyte subsets express TRAIL. We investigated the basis of TRAIL expression in response to IFN-β by studying activation of STATs 1, 3, and 5, p38 MAPK, and NF-κB in different leukocyte subsets of patients with RRMS. Monocytes, B cells, and T cells showed substantial differences in the activation of p38 and the STATs in response to i.m. injection of IFN-β1a or stimulation in vitro. Induction of cell-surface TRAIL, analyzed in nine leukocyte subsets, was observed only on monocytes and granulocytes and correlated with the activation of p38 and/or NF-κB in these subsets only, in agreement with previous work in fibroblasts showing that the induction of TRAIL in response to IFN-β depends on the activation of p38 and NF-κB as well as STATs 1 and 2. We propose that, in myeloid cells, the differential activation of p38 and NF-κB and induction of TRAIL, which sensitizes cells to apoptosis, can help to explain differences in responsiveness to IFN-β therapy among patients with RRMS and, furthermore, that such differential patterns of activation and expression may also be important in understanding the therapeutic responses to IFN-α/β in hepatitis and cancer.
Keywords: humans, phosphoproteins, signal transduction, flow cytometry
IFN-β has been the main treatment for patients with relapsing-remitting multiple sclerosis (MS; RRMS) for many years and, despite the development of other treatments, still remains a widely used first-line disease-modifying drug in MS. IFN-β therapy reduces RRMS relapses by approximately 33% and reduces the development of new brain lesions by approximately 66%. Furthermore, IFN-β slows development of brain atrophy and neurological disability (1, 2). The molecular basis of the therapeutic response to IFN-β is still not well understood, although several immunomodulatory mechanisms have been proposed (3). Furthermore, therapeutic (2) and biological (4, 5) responses to IFN-β vary greatly among individual patients with RRMS, and again the basis is unknown. Inhibition of growth or induction of apoptosis are major therapeutic effects of IFN-α/β in cancer (6) and, likewise, one of the proposed immunoregulatory mechanisms of IFN-β therapy in RRMS is the induction of apoptosis in immune cells involved in pathogenesis (3, 7). IFN-α/β has opposing biological effects on different leukocyte subsets, namely, induction of apoptosis in monocytes and enhanced survival in T and B cells (8, 9). The molecular responses to IFN-β are initiated when it binds to its receptor, leading to activation of the receptor-associated kinases JAK1 and TYK2 and also to the activation of additional kinases (PI3K/Akt, p38, ERK, and JNK), and then to the activation of several transcription factors (TFs), including STATs 1–6, NF-κB, AP-1, IRF1, IRF4, IRF8, and PU1 (8). Activation of STAT3, STAT5, and NF-κΒ are linked to increased cell survival, whereas activation of STAT1 in general, NF-κΒ under certain conditions, and p38 especially in hematopoietic cells, lead to apoptosis (6, 9–11). We hypothesized that differential activation of these various signaling proteins could help to explain cell type-specific induction of apoptosis in response to IFN-α/β (9). In our previous study, by using freshly drawn whole blood from healthy subjects, we observed significant differences in the IFN-β–induced activation of STATs 1, 3, and 5 in primary human T cells, B cells, and monocytes in vitro, with STAT1-dependent gene induction linked to apoptosis in monocytes and STAT3-dependent gene-induction associated with survival in B cells (9). TNF-related apoptosis-inducing ligand (TRAIL), a member of the TNF family of type II membrane proteins, induces apoptosis upon binding to death receptors DR4 and DR5 (12). Both TRAIL on the cell surface and picomolar concentrations of soluble TRAIL rapidly induce apoptosis in a wide variety of transformed cell lines (12). Notably, melanoma cells that are resistant to induction of apoptosis in response to IFN-α/β failed to induce TNFSF10 (TRAIL) mRNA (13). Interestingly, TRAIL mRNA is induced in peripheral blood mononuclear cells (PBMCs) of only those patients with RRMS who show a clinical response to IFN-β treatment. However, it is not yet known whether all or only some leukocyte subsets from responders express TRAIL mRNA (7) and what the molecular mechanism might be. Of note, induction of TRAIL mRNA by IFN-β in fibrosarcoma cells (14, 15) depends on the activation of p38, PI3K/Akt, and NF-κB, as well as on the formation of ISGF3 (a trimer of tyrosine-phosphorylated STATs 1 and 2 and IRF9), a major TF mediating responses to type I IFNs. Here we have studied cell type-specific activation of STATs 1, 3, and 5, and p38 in leukocyte subsets of RRMS after injection with IFN-β1a, and how these cell type-specific responses relate to the induction of TRAIL on the cell surface. We go on to propose a model to explain differences in individual responsiveness to IFN-β therapy in patients with RRMS.
Results
Differential Activation of STATs and Cell Type-Specific Activation of p38 in Leukocytes of RRMS Patients After Injection with IFN-β1a or Stimulation in Vitro.
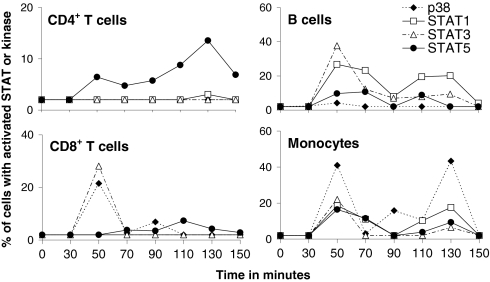
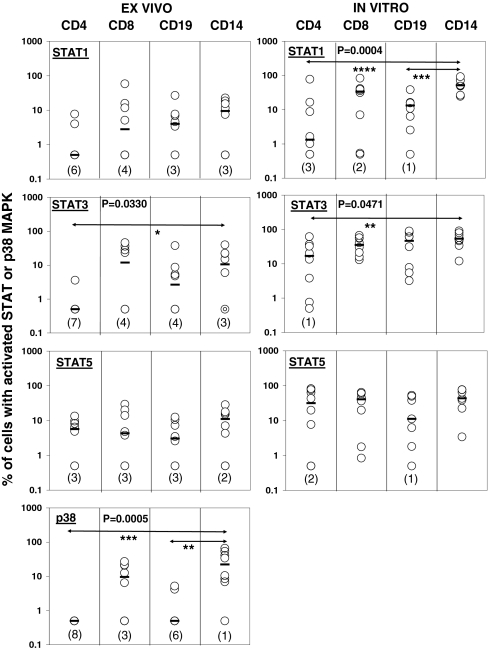
We previously found significant differences in the activation of STATs 1, 3, and 5 among leukocyte subsets after stimulation of whole blood of healthy donors in vitro with IFN-β1a, and linked these differences to differential induction of apoptosis (9). In a control experiment (Fig. S1), the activation of STATs 1, 3, and 5 was similar on two different occasions in monocytes and CD4+ and CD8+ T cells of one healthy subject after stimulation with IFN-β1a in vitro. To investigate whether similar differential activation of STATs 1, 3, and 5 also occurs after injection of IFN-β1a, eight patients with RRMS were initially studied who had previously been treated with IFN-β1a for various amounts of time. Because the activation of p38 MAPK by IFN-α/β appears to be an important means of generating proapoptotic signals (6), we also studied the phosphorylation of Thr180 and Tyr182 of p38, which are involved in its activation (6, 11). Fig. 1 shows the activation of the three STATs and p38 in T cells, B cells, and monocytes present in whole blood from RRMS patient no. 1 before injection and at 20-min intervals, starting 30 min after injection with IFN-β1a. Even though all the leukocyte subsets had an initial maximal response 50 min after injection, the data clearly reveal different patterns of STAT and p38 MAPK activation among the various subsets. Fig. S2 shows the activation of the three STATs and p38 in CD4+ T cells, CD8+ T cells, B cells, and monocytes for all eight patients with RRMS. The blood of four patients was sampled before injection and at 20-min intervals between 30 and 150 min (Fig. S2, Left) after i.m. injection with IFN-β1a, and the blood of the other four patients was collected before and at 10-min intervals between 30 and 80 min after injection (Fig. S2, Right). B cells, monocytes, and CD4+ and CD8+ T cells all showed an initial peak in the activation of signaling proteins between 30 and 70 min after injection when blood was sampled every 20 min (Fig. S2, Left). Moreover, when blood was collected every 10 min (Fig. S2, Right), it became clear that, in general, all leukocyte subsets showed the highest activation of signaling proteins at 40 and 50 min after injection. In contrast, in patient no. 7, previously shown to have developed neutralizing antibodies against IFN-β1a, we could not detect any activation of STAT1, 3, or 5 in monocytes, B cells, or CD4+ and CD8+ T cells in response to injection with IFN-β1a (Fig. S3). Table S1 shows a summary of the data from the eight patients with RRMS (patients 1, 3, 5, 6, and 8–11) in whom at least 3% of the monocytes, B cells, and CD4+ and CD8+ T cells were positive for activated STAT1, STAT3, STAT5, or p38 within 30 to 70 min after injection with IFN-β1a. P38 was activated in a strikingly cell type-specific manner after injection with IFN-β1a; CD4+ T cells never showed activation, whereas monocytes in 88% of the patients did. To study these differences in more depth, the highest amount of activated STATs 1, 3, 5, or p38 within each leukocyte subset observed within the 30- to 70-min timeframe after IFN-β1a injection was plotted for each individual patient (Fig. 2, Left). The Friedman test indicated significant differences among the leukocyte subsets for the activation of STAT3 (P = 0.0330) and p38 (P = 0.0005). Further analysis showed a trend toward lower numbers of CD4+ T cells with activated STAT3 than monocytes (P = 0.0625), and significantly lower numbers of CD4+ T cells (P < 0.01) and B cells (P < 0.05) demonstrated activation of p38 in comparison with monocytes (Fig. 2, Left) after injection of IFN-β1a.
Fig. 1.
Analysis of STAT and p38 activation after injection of IFN-β1a into a single patient with MS. Patient 1 was injected i.m. with IFN-β1a and blood was sampled before injection and at 20-min intervals between 30 and 150 min after injection. Flow cytometric analysis of CD4+ T cells, CD8+ T cells, B cells, and monocytes revealed cell type-specific activation of STAT1, STAT3, STAT5, and p38 after injection.
Fig. 2.
Differential activation of STAT1, STAT3, and p38 in leukocyte subsets after injection of IFN-β1a into patients with MS or after stimulation with IFN-β1a in vitro. The highest percentages of leukocytes with activated STAT1/3/5 or p38 that were observed within 30 to 70 min after i.m. injection with IFN-β1a of eight patients with RRMS (patients 1, 3, 5, 6, and 8–11) are depicted (Left). From seven of the same patients with RRMS and one additional patient with RRMS (patients 3–6 and 8–11), blood was drawn before IFN-β1a injection and stimulated with 500 IU/mL IFN-β1a for 25 min in vitro to determine the activation of STAT1/3/5 (Right). The median percentages of activated STAT or p38 in leukocytes of eight patients with RRMS are indicated with horizontal bars. The Friedman test indicated significant differences among leukocytes in the activation of STAT1 in vitro, STAT3 ex vivo and in vitro, and p38 ex vivo (P values shown). CD4, CD4+ T cells; CD8, CD8+ T cells; CD19, B cells; CD14, monocytes (*P < 0.065, **P < 0.05, ***P < 0.01, and ****P < 0.001). The numbers between brackets indicate the numbers of patients in which the activation of STAT1/3/5 or p38 was not detected within a certain leukocyte subset.
Similarly, our previous study of healthy subjects showed significantly fewer CD4+ T cells than monocytes with activated STAT3 in response to 500 IU/mL IFN-β1a in vitro (9). However, we also observed previously, in healthy subjects, by using the same in vitro conditions, that significantly fewer CD4+ T cells and B cells activated STAT1 in comparison with monocytes (9). The reason for the observed differences between patients with RRMS and healthy donors might be because the i.m. injection of IFN-β1a in patients results in a concentration of approximately 100 IU/mL IFN-β1a in serum (16), which is considerably lower than the 500 IU/mL IFN-β1a used for healthy subjects in vitro. We therefore studied whether differential activation of STATs 1, 3, and 5 occurred in leukocytes of patients with RRMS in response to a higher concentration of IFN-β in vitro. Blood obtained before IFN-β1a injection from seven of the eight patients with RRMS initially studied (Fig. 2, Left), and an additional patient with RRMS (patient 4), was stimulated in vitro with 500 IU/mL IFN-β1a for 25 min. Initial statistical analysis revealed significant differences in the activation of STAT1 (P = 0.0004) and STAT3 (P = 0.0471) among leukocyte subsets of patients in response to stimulation with 500 IU/mL IFN-β1a in vitro (Fig. 2, Right). Further statistical analysis showed that significantly fewer CD4+ T cells (P < 0.001) and B cells (P < 0.01) than monocytes activated STAT1, and fewer CD4+ T cells activated STAT3 compared with monocytes (P < 0.05), in agreement with previous results in healthy donors (9). Indeed, when the IFN-β–induced activation of STATs 1, 3, and 5 in monocytes and B and T cells of the eight patients with RRMS (Fig. 2, Right) was compared with activation of STATs in leukocytes of nine healthy donors in response to 500 IU/mL IFN-β in vitro, no significant differences were observed between patients with MS and healthy subjects (Fig. S4). In summary, our data show that, among leukocyte subsets of patients with RRMS, monocytes had the most STAT3-positive and p38-positive cells after IFN-β1a injection and monocytes had the most STAT1-positive and STAT3-positive cells after stimulation with IFN-β1a in vitro.
IFN-β1a–Induced Surface TRAIL Expression ex Vivo and in Vitro Is Seen on Monocytes and Granulocytes only.
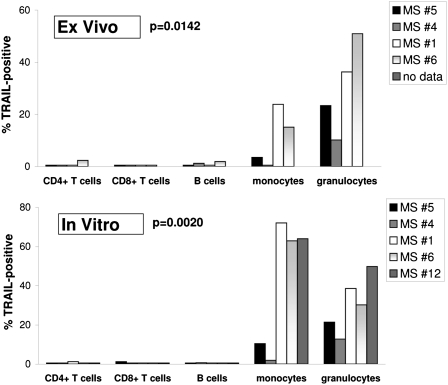
The activation of p38 by IFN-β is an unusual but important requirement for the induction of a small minority of IFN-stimulated genes, with CXCL11 (β-R1) and TRAIL as major examples (14). TRAIL is proposed to be a marker for responsiveness to IFN-β treatment in MS (7) and is a potent inducer of apoptosis (12). Because we found that none of the CD4+ T cells from patients with MS, but monocytes in 88% of the patients, showed p38 activation after injection with IFN-β1a (Table S1), we examined whether such cell type-specific activation of p38 by IFN-β would also lead to cell type-specific induction of TRAIL on monocytes. To this end, four patients with RRMS were injected with IFN-β1a and, 18 h later, the induction of TRAIL on the surfaces of CD4+ T cells, CD8+ T cells, CD25+CD4+ regulatory T cells, natural killer (NK) cells, NK T cells, B cells, monocytes, plasmacytoid dendritic cells (pDCs), and granulocytes was studied by flow cytometry. Fig. 3 (Upper) shows significant differences (P = 0.0142) in the expression of TRAIL on B cells, CD4+ and CD8+ T cells, granulocytes, and monocytes, with significant induction for only the latter two leukocyte subsets. In addition, NK cells, NK T cells, CD25+CD4+ regulatory T cells, and pDCs also did not show TRAIL expression after IFN-β1a injection (only ≤1.6% of these subsets were positive; Fig. S5A).
Fig. 3.
Differential induction of TRAIL on leukocytes of patients with RRMS after stimulation with IFN-β1a in vitro and in vivo. The percentage of TRAIL-positive leukocytes was determined for four patients with RRMS (patients 1 and 4–6) 18 h after injection with IFN-β1a (Upper) or after stimulation of whole blood samples of five patients with RRMS with 1,500 IU/mL IFN-β1a in vitro for 18 h (Lower). For the latter, blood drawn before injection with IFN-β1a was treated in vitro. The Friedman test revealed significant differences in TRAIL induction among monocytes, granulocytes, T cells, and B cells ex vivo and in vitro (P values shown).
The i.m. injection of IFN-β1a is reported to result in a concentration of approximately 100 IU/mL in serum (16). We therefore investigated whether in vitro stimulation with a much higher concentration of IFN-β1a would result in expression of TRAIL on other leukocyte subsets in addition to monocytes and granulocytes. To this end, blood, collected from the same four patients with RRMS and one additional patient with RRMS before injection with IFN-β1a, was stimulated in vitro with 1,500 IU/mL IFN-β1a for 18 h, to mimic the duration of stimulation in the in vivo situation. Fig. 3 illustrates that the same significant differences (P = 0.0020) occurred in vitro (Fig. 3, Lower) and in vivo (Fig. 3, Upper), even when a much higher dose of IFN-β1a was used to stimulate in vitro. Notably, the four patients with RRMS studied for TRAIL induction after injection with IFN-β1a showed the same expression patterns for TRAIL on monocytes and granulocytes at the individual patient level after in vitro stimulation with IFN-β1a (Fig. 3). Moreover, B cells, CD4+ and CD8+ T cells (Fig. 3, Lower), NK cells, NK T cells, CD25+CD4+ regulatory T cells, and pDCs did not show TRAIL expression after stimulation by IFN-β1a in vitro (only ≤4% of these subsets were positive; Fig. S5B). In summary, of nine leukocyte subsets, we found that only monocytes and granulocytes express TRAIL after IFN-β1a injection or stimulation in vitro.
Differential Induction of TRAIL on Leukocytes of Patients with RRMS in Response to IFN-β1a Correlates with Differential Activation of p38 and NF-κB.
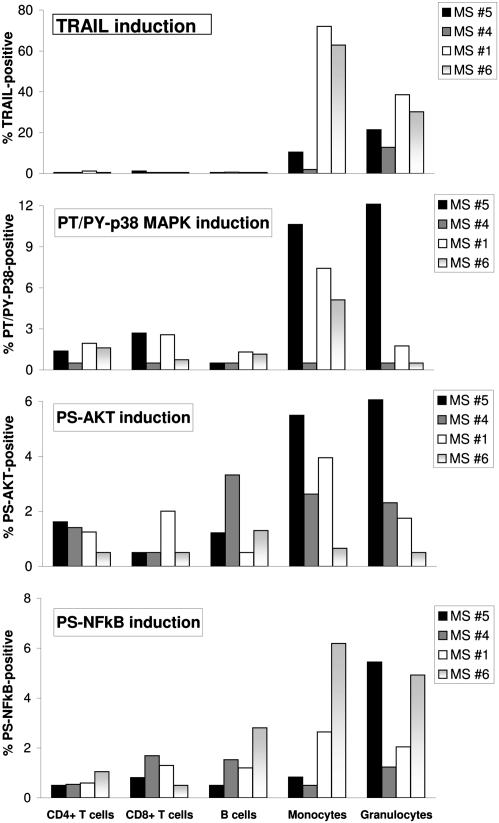
The expression of TRAIL mRNA in fibrosarcoma cells in response to IFN-β depends on the formation of ISGF3 and the activation of p38, PI3K/Akt, and NF-κB (14, 15). To determine whether early activation of specific kinases and TFs could be linked to cell type-specific induction of TRAIL, whole blood samples from four patients with RRMS (nos. 1, 4, 5, and 6) who had been studied for TRAIL protein expression on leukocytes in response to IFN-β in vitro after 18 h (Fig. 3, Lower) were simultaneously studied after 15, 30, and 45 min of stimulation with 1,500 IU/mL of IFN-β1a in vitro (Fig. 4). Stimulation resulted in STAT1 and STAT2 activation in granulocytes, monocytes, B cells, and CD4+ and CD8+ T cells in all four samples (Fig. S6) and, therefore, differential formation of ISGF3 cannot be the reason for cell type-specific induction of TRAIL on monocytes and granulocytes. Interestingly, in addition to these cell type-specific effects, we also found differences in individual patients; one of four patients did not show expression of surface TRAIL on monocytes in the ex vivo experiments, and one of five patients did not show TRAIL expression on monocytes in the in vitro experiments (patient 4 in both experiments; Fig. 3). In contrast, TRAIL was expressed on granulocytes in 100% of the patients with RRMS after IFN-β1a injection and in response to IFN-β1a in vitro (Fig. 3). Notably, activation of p38 could be linked qualitatively to induction of TRAIL on monocytes (Fig. 4). Patient 4, who showed no p38 activation, also did not shown induction of TRAIL, whereas patients 1, 5, and 6, who showed differing amounts of activation of p38, all showed expression of TRAIL on monocytes (Fig. 4). However, activation of p38 was not linked at all to the induction of TRAIL on granulocytes (Fig. 4), suggesting that activation of another signaling protein might be responsible.
Fig. 4.
Differential induction of TRAIL on leukocytes of patients with RRMS in response to IFN-β1a in vitro correlated with differential activation of p38 and NF-κB in monocytes and activation of NF-κB in granulocytes. In whole blood of four patients with RRMS (patients 1 and 4–6), activation of p38, Akt, and NF-κB and induction of TRAIL protein was determined after short (15, 30, and 45 min) and long (18 h) stimulation with 1,500 IU/mL IFN-β1a in vitro. Shown for each individual patient are the highest percentages of kinase- and NF-κB–positive leukocytes observed within the 15- to 45-min timeframe.
There are three NF-κB binding elements in the promoter of the TRAIL gene, and two of those are close to the ISRE element that binds to ISGF3 (14, 15). In addition, in vitro stimulation with IFN-β induces the phosphorylation of the p65 subunit of NF-κB on an unknown residue in human fibrosarcoma cells (14) and increases NF-κB (p65/p50) DNA binding in primary human microglia (17). Because optimal activation of NF-κB requires the phosphorylation of Ser529 and Ser536 in the transactivation domain of p65 (10), we studied the phosphorylation of Ser529 in response to IFN-β. Remarkably, IFN-β–induced activation of p65 correlated quantitatively with the induction of surface TRAIL expression, on both granulocytes and monocytes (Fig. 4). The IFN-β–induced phosphorylation of Ser473 of Akt was also studied, because activated Akt has been shown to mediate Ser529 phosphorylation of p65 through the activation of p38 (18). Furthermore, activation of PI3K/Akt, but not ERK, was linked to induction of TRAIL mRNA in fibrosarcoma cells by IFN-β (14, 15). However, the activation of Akt (Fig. 4) or ERK (Fig. S6) did not correlate with surface TRAIL induction on monocytes or granulocytes in a qualitative or quantitative fashion in individual patients. Whole blood samples from the four patients with RRMS were also pretreated with chemical inhibitors of PI3K/Akt and p38 for 30 min and subsequently stimulated with 1,500 IU/mL IFN-β1a for 18 h (Fig. S7). Inhibition of the IFN-β1a–induced activation of p38 decreased TRAIL expression only on monocytes (by 60% on average), whereas inhibition of Akt activation had no effect on TRAIL induction on monocytes or granulocytes. In conclusion, the cell type-specific induction of TRAIL on monocytes and granulocytes by IFN-β was correlated with the IFN-β–induced activation of p38 and NF-κB p65 in monocytes, and to the activation of p65 in granulocytes of patients with RRMS.
Discussion
IFN-β–Induced Cell Type-Specific Signaling in Leukocytes of Patients with RRMS Explains Selective TRAIL Induction.
IFN-β is one of the main first-line disease-modifying drugs for patients with RRMS, but the molecular basis of its activity is not understood, and clinical responsiveness varies among patients. Understanding the molecular mechanisms of therapeutic action may allow those who show a poor response to be identified in advance of treatment and may provide insight into MS pathogenesis. It is becoming clear that a complex set of signaling pathways are activated by IFN-β, and we previously proposed that genes stimulated by type I IFNs are dependent on the activation of STATs alone (i.e., “STAT-only genes”), of STATs plus other TFs (i.e., “STAT + TF-genes”), or of other TFs only (i.e., “TF-only genes” (8). Understanding the complexity of responses to type I IFNs is important for explaining cell type specificity, type I IFN subtype specificity, and individual responsiveness to treatment (8). With respect to cell type specificity, it was unclear why treatment with IFN-β caused apoptosis in certain cancer cells and monocytes but increased the survival of B and T cells (9). We recently linked increased survival of normal human B cells in response to IFN-β to low numbers of B cells with PY-STAT1 and higher numbers with PY-STAT3 that drive expression of prosurvival genes, whereas IFN-β–induced apoptosis in monocytes is caused by PY-STAT1–dependent proapoptotic genes (9). Here we describe that injection of IFN-β1a into patients with RRMS led to significant differences in the numbers of monocytes and T and B cells in which STAT3 and p38 become activated. We did not observe any activation of p38 in CD4+ T cells, and only in very few B cells, but much higher numbers of monocytes did activate p38 (in 88% of the patients with MS investigated). Because TRAIL mRNA induction in fibrosarcoma cells by IFN-β depends on the formation of ISGF3 and the activation of p38, PI3K/Akt, and NF-κB p65 (14, 15), we hypothesized that cell-surface expression of TRAIL would be induced primarily on monocytes. Induction of surface TRAIL by IFN-β1a injection has not been previously tested on CD4+ T cells, CD8+ T cells, CD25+CD4+ regulatory T cells, NK cells, NK T cells, B cells, monocytes, pDCs, and granulocytes of patients with RRMS. Strikingly, we found significantly increased levels of TRAIL after IFN-β1a injection only on the surfaces of monocytes and granulocytes, consistent with published data on the induction of TRAIL mRNA and release of soluble TRAIL only in normal human neutrophils and monocytes, but not in lymphocytes by IFN-α/β in vitro (19, 20). The selective IFN-β–induced expression of TRAIL observed here on monocytes and granulocytes of patients with MS cannot be explained by a failure to induce the formation of ISGF3 in some subsets, because activation of STAT1 and STAT2 occurred in all leukocyte subsets. Moreover, despite activation of STAT3 in monocytes of patients with MS (Fig. 2), IFN-β did not prevent induction of PY-STAT1–dependent TRAIL. Interestingly, by using chemical inhibitors of p38, we were able to link the selective IFN-β1a–induced expression of surface TRAIL on monocytes and granulocytes to activation of p38 only in monocytes. Furthermore, in accordance with previous data (14, 15, 18), we found that Ser529 phosphorylation of NF-κB p65 in granulocytes, and particularly in monocytes, could be connected to cell type-specific induction of TRAIL by IFN-β in patients with MS. However, by using a chemical inhibitor of PI3K, we could not demonstrate in monocytes or granulocytes that TRAIL induction depended on activation of PI3K/Akt by IFN-β, indicating that the observed serine phosphorylation of p65 is not dependent on upstream activation of PI3K/Akt by IFN-β (18) as it is in fibrosarcoma cells (14, 15). In monocytes, it is likely that the activation of p38 by IFN-β caused Ser529 phosphorylation of p65 (18). Perhaps casein kinase 2 is responsible for the activation of NF-κB in granulocytes in response to IFN-β, because this kinase can also phosphorylate p65 on Ser529 (21). In summary, monocytes and granulocytes are the only two leukocyte subsets in patients with RRMS that express TRAIL, but because only monocytes showed dependency on p38 MAPK activation, IFN-β seems to use different, cell type-specific signaling pathways to induce TRAIL in these two cell types. The reason that TRAIL, which is a STAT + TF gene (8), is induced only in granulocytes and monocytes and not in other leukocyte subsets seems to be that the necessary set of TFs is activated by IFN-β in only these two subsets.
Cell Type-Specific Activation of STATs Plus Other TFs as a Model to Explain Individual Responsiveness to IFN-β Treatment.
TRAIL mRNA in unseparated PBMCs has been proposed as a marker of responsiveness to IFN-β therapy in MS (7), but it is not clear whether the higher TRAIL mRNA expression in responders was induced in all or only some of the leukocyte subsets present in PBMCs. Remarkably, a recent study shows exclusive TRAIL mRNA induction in monocytes and not in T cells or B cells of clinical responders to IFN-β therapy (22). In our study, TRAIL expression was found on granulocytes in all patients with RRMS after IFN-β1a stimulation, both ex vivo and in vitro. In contrast, not all patients with RRMS showed surface TRAIL expression on monocytes in response to IFN-β1a; we found such expression in only three of four patients after injection with IFN-β1a, and in four of five patients after in vitro stimulation (Fig. 3). Therefore, 20% to 25% of patients with RRMS did not show expression of TRAIL protein on monocytes, which approximates the expected percentage of nonresponders to IFN-β1a treatment (2). Based on availability of brain MRI scans, we could only verify that RRMS patient 12 (Fig. 3, Lower) is a responder to IFN-β1a therapy, and this patient is one of four who did show expression of TRAIL on monocytes in vitro. Although our study was not designed to draw definite conclusions about differences in cell type-specific expression of TRAIL by responders and nonresponders, the data are in line with a recent study showing selective expression of TRAIL on monocytes of responders only (22). Therefore, the previously described increase in TRAIL mRNA expression in unseparated PBMCs of responders to IFN-β treatment (7) is likely to be caused by selective TRAIL mRNA expression in monocytes only (22). Interestingly, the reason that the latter occurred simultaneously with an increase in secretion of soluble TRAIL in responders and nonresponders (22) might be that all patients with MS show increased TRAIL production on granulocytes, as our preliminary data here suggest. In melanoma cells, sensitivity to induction of apoptosis in response to IFN-α/β depends on the induction of TRAIL mRNA (13). In responders, selective induction of TRAIL (22) and other proapoptotic genes in monocytes in response to IFN-β may contribute to the immediate induction of apoptosis in monocytes (9) or may prime monocytes to undergo programmed cell death later, when they differentiate into macrophages during migration across the blood–brain barrier (23). Likewise, the induction of TRAIL, observed on the granulocytes of all the patients with RRMS we have studied, may contribute to the observed apoptosis of granulocytes of patients with MS after IFN-β treatment (24). Remarkably, the protective effect of IFN-α/β in experimental autoimmune encephalomyelitis, the animal model for MS, depends entirely on the response of myeloid cells, because the deletion of IFNAR on myeloid cells, but not on other cells, increases disease burden during the effector phase of CNS autoimmunity, through enhanced macrophage invasion, cytokine/chemokine production, and demyelination (25). Granulocytes and monocytes can both cause breakdown of the blood–brain barrier, and infiltrating macrophages are believed to cause demyelination and axonal loss in MS as well (26–28). Together, our data indicate that myeloid cells constitute a very important target of IFN-β therapy in MS. Therefore, triggering the apoptosis of monocytes and macrophages might be an important mechanism through which IFN-β treatment reduces active lesions in the brains of patients with MS. Notably, monocytes and macrophages play a common pathogenic role in several neuroinflammatory and neurodegenerative diseases, such as neural injury following stroke, Alzheimer's and Parkinson diseases, and HIV-associated dementia (29, 30). Alternative therapeutic strategies that diminish macrophage recruitment into the CNS might be of benefit in all these neurological diseases (26, 31).
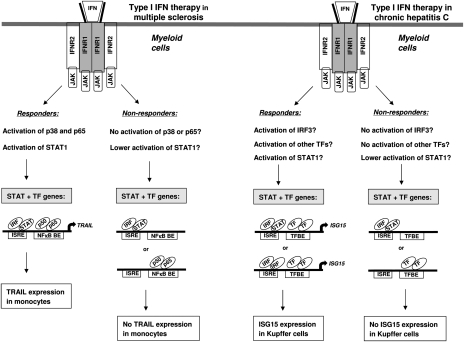
The results of our exploratory study of whether cell type-specific signaling occurs after IFN-β injection suggest that the differential activation of p38 and NF-κB in monocytes and the activation of NF-κB in granulocytes can help explain the selective expression of TRAIL on these leukocyte subsets in individual patients with RRMS. Based on these data, we hypothesize that individual responsiveness to IFN-β treatment in MS and individual responsiveness to type I IFN therapy in other diseases as well might be explained by cell type-specific induction of certain STAT + TF genes by IFN-α/β. This notion is supported by recent work showing that responsiveness to pegylated IFN-α2 in chronic hepatitis C infection of the liver can be linked to a cell type-specific increase of ISG15 in Kupffer cells of responders, in contrast to cell type-specific increase of ISG15 in hepatocytes of nonresponders (32). Because ISG15, like TRAIL, is a STAT + TF gene (8, 33), it remains to be established which STATs or TFs are differentially activated by pegylated IFN-α2 in Kupffer cells and hepatocytes of responders and nonresponders, respectively, leading to differential induction of ISG15. Kupffer cells are the resident macrophages of the liver, and it is striking that IFN-α/β therapy, both in patients with RRMS and with chronic hepatitis C infection, induces the expression of a STAT + TF gene in myeloid cells of responders only. We therefore speculate that successful therapy with type I IFNs in cancer may rely not only on direct toxic effects on tumor cells, but might also depend on altering the myeloid-derived suppressor cells that otherwise would blunt the antitumor immune response (34). Fig. 5 shows a model to explain individual responsiveness to IFN-α/β therapy in patients with MS and chronic hepatitis C.
Fig. 5.
Model to explain individual responsiveness to IFN-α/β therapy in MS and chronic hepatitis C. We propose that differential activation of TFs only in myeloid cells leads to selective induction of STAT + TF-dependent genes (like TRAIL and ISG15) in responders to type I IFN therapy, but not in nonresponders. TRAIL expression was selectively found in monocytes of responders to IFN-β therapy in MS (22), and here we show that selective activation of p38 and p65 leads to cell type-specific induction of TRAIL on monocytes of patients with MS. The reason that nonresponders do not express TRAIL on monocytes might be a result of failure to activate p38 or p65 (as shown here in MS patient 4; Fig. 4), or much lower activation of STAT1 (35). ISG15 is expressed only in Kupffer cells of responders to pegylated IFN-α2 therapy in patients with chronic hepatitis C (32). It has to be established whether Kupffer cells of nonresponders have lower activation of STAT1 (8), IRF3 (33), or other TFs, causing no expression of ISG15 in these myeloid cells. NFκB BE, NF-κB binding element; TFBE, TF binding element.
In conclusion, we have demonstrated cell type-specific signaling linked to selective expression of TRAIL in leukocyte subsets of patients with RRMS after IFN-β1a injection, a previously proposed determinant of clinical responsiveness to IFN-β therapy in MS (7). Therefore, the observed variability in the activation of STATs, a variety of kinases, and NF-κB by IFN-β1a in the various patients wit RRMS we describe here is likely to underlie the significant patient-to-patient variation in gene expression that has been observed in unseparated PBMCs and whole blood samples of patients after IFN-β injection (4, 5). We are currently determining in a larger study whether differential STAT, kinase, and NF-κB activation in myeloid cells and other leukocyte subsets in response to IFN-β injection predicts responsiveness to this therapy in MS.
Materials and Methods
Patients, Healthy Controls, and IFN-β Stimulation.
Ten healthy controls and 11 patients with RRMS consented to give whole blood. All patients donated blood before injection with IFN-β1a (Avonex; Biogen Idec), and nine patients with RRMS also donated blood at different times after injection. To enable determination of TRAIL induction by IFN-β, four of the 11 patients with RRMS returned after approximately 3 y. After stimulation with IFN-β, the undiluted whole blood was fixed immediately and erythrocytes were lysed for intracellular detection of PY-STAT1/3/5, PT/PY-p38, PS-Akt, PT/PY-ERK, or PS-p65 in leukocyte subsets by using flow cytometry (9). Further detailed information is provided in SI Materials and Methods.
Detection of Surface TRAIL Protein on Leukocytes by Flow Cytometry.
Blood was drawn before and 18 h after IFN-β1a injection, and surface markers to distinguish leukocyte subsets in whole blood were stained first. Samples were fixed and essentially further processed as described previously (9). To study the dependency of TRAIL induction on activation of PI3K/Akt and p38, whole blood was pretreated with 25 mM Ly294,002 or 20 mM SB203580, respectively (Sigma-Aldrich), for 30 min and subsequently stimulated with 1,500 IU/mL IFN-β1a. Further detailed information is provided in SI Materials and Methods.
Statistical Analysis.
InStat 3 software (GraphPad Software) was used for statistical analyses. The nonparametric Friedman test was used first, and subsequent post-hoc analysis was performed by using a Dunn test. Further detailed information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Sharon Parker and Vinette Zinkand for their support and skilful performance of venipuncture through an i.v. catheter. In addition, we are thankful that Claire Hara-Cleaver, Parianne Fatica, and Diane Ivancic coordinated this research study at the Mellen Center for Multiple Sclerosis Treatment and Research (Cleveland Clinic). Finally, we thank Drs. Elizabeth Fisher and Natasha Frost for facilitating the access to the patient samples. This work was supported by National Multiple Sclerosis Society Pilot Grant PP1086 and Career Transition Fellowship Award TA3032A1/1 (to A.H.H.v.B.-D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117347108/-/DCSupplemental.
References
- 1.Filippi M, et al. Interferon β-1b and glatiramer acetate effects on permanent black hole evolution. Neurology. 2011;76:1222–1228. doi: 10.1212/WNL.0b013e3182143577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudick RA. Interferon beta. In: Cohen JA, Rudick RA, editors. Multiple Sclerosis Therapeutics. 4th Ed. Cambridge, UK: Cambridge Univ Press; 2011. pp. 302–316. [Google Scholar]
- 3.Dhib-Jalbut S, Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. 2010;74(suppl 1):S17–S24. doi: 10.1212/WNL.0b013e3181c97d99. [DOI] [PubMed] [Google Scholar]
- 4.Reder AT, et al. IFN-beta1b induces transient and variable gene expression in relapsing-remitting multiple sclerosis patients independent of neutralizing antibodies or changes in IFN receptor RNA expression. J Interferon Cytokine Res. 2008;28:317–331. doi: 10.1089/jir.2007.0131. [DOI] [PubMed] [Google Scholar]
- 5.Rani MR, et al. Heterogeneous, longitudinally stable molecular signatures in response to interferon-beta. Ann N Y Acad Sci. 2009;1182:58–68. doi: 10.1111/j.1749-6632.2009.05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platanias LC. The p38 mitogen-activated protein kinase pathway and its role in interferon signaling. Pharmacol Ther. 2003;98:129–142. doi: 10.1016/s0163-7258(03)00016-0. [DOI] [PubMed] [Google Scholar]
- 7.Wandinger KP, et al. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet. 2003;361:2036–2043. doi: 10.1016/S0140-6736(03)13641-0. [DOI] [PubMed] [Google Scholar]
- 8.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 9.van Boxel-Dezaire AH, et al. Major differences in the responses of primary human leukocyte subsets to IFN-beta. J Immunol. 2010;185:5888–5899. doi: 10.4049/jimmunol.0902314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 12.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 13.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: Correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7:1821–1831. [PubMed] [Google Scholar]
- 14.Rani MR, Ransohoff RM. Alternative and accessory pathways in the regulation of IFN-beta-mediated gene expression. J Interferon Cytokine Res. 2005;25:788–798. doi: 10.1089/jir.2005.25.788. [DOI] [PubMed] [Google Scholar]
- 15.Rani MR, Pandalai S, Shrock J, Almasan A, Ransohoff RM. Requirement of catalytically active Tyk2 and accessory signals for the induction of TRAIL mRNA by IFN-beta. J Interferon Cytokine Res. 2007;27:767–779. doi: 10.1089/jir.2007.0005. [DOI] [PubMed] [Google Scholar]
- 16.Khan OA, Dhib-Jalbut SS. Serum interferon beta-1a (Avonex) levels following intramuscular injection in relapsing-remitting MS patients. Neurology. 1998;51:738–742. doi: 10.1212/wnl.51.3.738. [DOI] [PubMed] [Google Scholar]
- 17.Kim MO, et al. Interferon-beta activates multiple signaling cascades in primary human microglia. J Neurochem. 2002;81:1361–1371. doi: 10.1046/j.1471-4159.2002.00949.x. [DOI] [PubMed] [Google Scholar]
- 18.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 19.Tecchio C, et al. IFNalpha-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood. 2004;103:3837–3844. doi: 10.1182/blood-2003-08-2806. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich S, Infante-Duarte C, Seeger B, Zipp F. Regulation of soluble and surface-bound TRAIL in human T cells, B cells, and monocytes. Cytokine. 2003;24:244–253. doi: 10.1016/s1043-4666(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 22.Hebb AL, Moore CS, Bhan V, Robertson GS. Effects of IFN-B on TRAIL and decoy receptor expression in different immune cell populations from MS patients with distinct disease subtypes. Autoimmune Dis. 2010;2011:485752. doi: 10.4061/2011/485752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Weyenbergh J, Wietzerbin J, Rouillard D, Barral-Netto M, Liblau R. Treatment of multiple sclerosis patients with interferon-beta primes monocyte-derived macrophages for apoptotic cell death. J Leukoc Biol. 2001;70:745–748. [PubMed] [Google Scholar]
- 24.Mix E, et al. Lymphocyte subpopulations, oxidative burst and apoptosis in peripheral blood cells of patients with multiple sclerosis-effect of interferon-beta. Autoimmunity. 2003;36:291–305. doi: 10.1080/0891693031000152697. [DOI] [PubMed] [Google Scholar]
- 25.Prinz M, et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Izikson L, Klein RS, Luster AD, Weiner HL. Targeting monocyte recruitment in CNS autoimmune disease. Clin Immunol. 2002;103:125–131. doi: 10.1006/clim.2001.5167. [DOI] [PubMed] [Google Scholar]
- 27.Veldhuis WB, et al. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. J Cereb Blood Flow Metab. 2003;23:1060–1069. doi: 10.1097/01.WCB.0000080701.47016.24. [DOI] [PubMed] [Google Scholar]
- 28.Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Brain Res Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Downes CE, Crack PJ. Neural injury following stroke: Are Toll-like receptors the link between the immune system and the CNS? Br J Pharmacol. 2010;160:1872–1888. doi: 10.1111/j.1476-5381.2010.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadiu I, Glanzer JG, Kipnis J, Gendelman HE, Thomas MP. Mononuclear phagocytes in the pathogenesis of neurodegenerative diseases. Neurotox Res. 2005;8:25–50. doi: 10.1007/BF03033818. [DOI] [PubMed] [Google Scholar]
- 31.Eder C. Ion channels in monocytes and microglia/brain macrophages: Promising therapeutic targets for neurological diseases. J Neuroimmunol. 2010;224:51–55. doi: 10.1016/j.jneuroim.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, et al. Cell-type specific gene expression signature in liver underlies response to interferon therapy in chronic hepatitis C infection. Gastroenterology. 2010;138:1123–1133. doi: 10.1053/j.gastro.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Au WC, Moore PA, Lowther W, Juang YT, Pitha PM. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci USA. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apetoh L, Végran F, Ladoire S, Ghiringhelli F. Restoration of antitumor immunity through selective inhibition of myeloid derived suppressor cells by anticancer therapies. Curr Mol Med. 2011;11:365–372. doi: 10.2174/156652411795976574. [DOI] [PubMed] [Google Scholar]
- 35.Comabella M, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132:3353–3365. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.