Abstract
Multiwalled carbon nanotubes (MWCNTs) have the potential for widespread applications in engineering and materials science. However, because of their needle-like shape and high durability, concerns have been raised that MWCNTs may induce asbestos-like pathogenicity. Although recent studies have demonstrated that MWCNTs induce various types of reactivities, the physicochemical features of MWCNTs that determine their cytotoxicity and carcinogenicity in mesothelial cells remain unclear. Here, we showed that the deleterious effects of nonfunctionalized MWCNTs on human mesothelial cells were associated with their diameter-dependent piercing of the cell membrane. Thin MWCNTs (diameter ∼ 50 nm) with high crystallinity showed mesothelial cell membrane piercing and cytotoxicity in vitro and subsequent inflammogenicity and mesotheliomagenicity in vivo. In contrast, thick (diameter ∼ 150 nm) or tangled (diameter ∼ 2–20 nm) MWCNTs were less toxic, inflammogenic, and carcinogenic. Thin and thick MWCNTs similarly affected macrophages. Mesotheliomas induced by MWCNTs shared homozygous deletion of Cdkn2a/2b tumor suppressor genes, similar to mesotheliomas induced by asbestos. Thus, we propose that different degrees of direct mesothelial injury by thin and thick MWCNTs are responsible for the extent of inflammogenicity and carcinogenicity. This work suggests that control of the diameter of MWCNTs could reduce the potential hazard to human health.
Keywords: environmental health, inflammation, nanotoxicology
Multiwalled carbon nanotubes (MWCNTs) (1) have received much interest since their discovery because of their unique physical and chemical properties (2). However, serious concerns have been raised that nanomaterials may induce malignant mesothelioma (3, 4), a cancer derived from mesothelial cells, which line somatic cavities. The similarity of these MWCNTs to asbestos fibers that are well known carcinogens for the mesothelium has been discussed (5–7).
The mechanism of fiber-induced carcinogenesis remains elusive. Currently, the interaction of fibers with cells may be divided into two distinct processes (7), direct effects of fibers on mesothelial cells (8–10) and indirect effects of fibers by inducing frustrated phagocytosis by macrophages (11), in which fiber length and rigidity are thought to be critical (12, 13). Rigid, durable fibers longer than 15 to 20 μm with a high aspect ratio have been proposed to be carcinogenic based on their indirect effects, which promote the continuous activation of macrophages (14–16). Indeed, long MWCNTs and long asbestos fibers induce frustrated phagocytosis and granuloma formation (17). This “length-dependent theory” has been recently reviewed (14).
With regard to the direct effects on the mesothelial tissue, previous studies have demonstrated the potential cytotoxicity of MWCNTs (18). However, critical factors that determine mesothelial cytotoxicity are still poorly understood. The direct interaction between MWCNTs and mesothelial cells appears to be a key event for the initiation of mesothelial carcinogenesis and for inducing inflammation (7, 19).
The morphological resemblance between MWCNTs and asbestos fibers has been emphasized. However, based on the variation in density, chemical composition, and surface reactivity, there should be multiple differences between nanotube toxicity and asbestos toxicity that would give us a clue to assist in the establishment of appropriate safety regulations and prevention of environmental health problems. To find such differences, we studied the direct interaction of MWCNTs with mesothelial cells in comparison with that of asbestos in the present study.
Results
Fiber Characteristics and Cell Lines.
We used five different types of MWCNT grown in the vapor phase: NT50a was from Mitsui and NT50b, NT115, NT145, and NTtngl were from Showa Denko. NT50a, NT50b, NT115, and NT145 were named after their diameter in nanometers. NTtngl nanotubes were the thinnest MWCNTs used in this study and had a tangled conformation (Fig. 1A). Detailed information for all materials used is summarized in Table S1. We suspended MWCNTs in saline solution containing 0.5% BSA (henceforth A-saline solution). Three different asbestos fibers [chrysotile A (Chry), crocidolite (Cro), and amosite (Amo); Union for International Cancer Control] were used and were suspended in saline or A-saline solution. Two human mesothelial cell lines, MeT5A (SV40-transformed mesothelial cells) and E6/E7 and hTERT-immortalized human peritoneal mesothelial cells (HPMCs), were used. The HPMC culture was isolated and expanded according to an established protocol (20) with some modifications (21, 22) (SI Materials and Methods). Murine macrophages (RAW264.7) and canine kidney epithelial cells (MDCK II) were used as positive and negative controls, respectively.
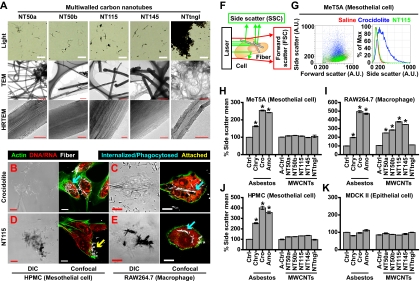
Fig. 1.
Mesothelial cells did not phagocytose MWCNTs. (A) Optical and transmission EM (TEM) images and high-resolution (HR) Transmission EM images for five types of MWCNTs. Each carbon nanotube is named after its diameter (Fig. 2 and Table S1) except NTtngl, which stands for tangled NTs. High-resolution transmission EM images show the presence of graphite layers in the MWCNTs. (Scale bars: light microscopy, 20 μm; all transmission EM images except NTtngl, 500 nm; NTtngl transmission EM image, 200 nm; and high-resolution transmission EM images, 10 nm.) (B and D) Differential interference contrast and confocal Images of HPMCs show Cro internalization (blue arrow) and NT115 attachment (yellow arrow) after a 24-h incubation. (C and E) Images of RAW264.7 cells show internalization of both fibers (blue arrows). (B–E) Differential interference contrast images (Left) and confocal microscopy images (Right) after staining of the cells with Alexa 488-phalloidin (green) and propidium iodide (PI, red), indicate cytoskeletal actin and DNA/RNA, respectively. (Scale bars: B–E, 14 μm.) (F) A schematic showing the SSC and forward scatter (FSC) values for uptake of fibers. (G) Left: Dot plot of forward scatter and SSC values of MeT5A cells show a marked increase in SSC only upon incubation with Cro, but not with NT115. Right: Histogram shows SSC value of the image on the left. (H–K) Mean of the SSC value of each cell after a 3-h incubation with each fiber type (*P < 0.001; n = 3, mean ± SEM). P values were calculated between control (saline) and asbestos, or A-control (A-saline solution) and MWCNTs.
Mesothelial Cells Did Not Internalize MWCNTs.
We exposed two mesothelial cell lines, macrophages and epithelial cells, to 5 μg/cm2 MWCNTs or 5 μg/cm2 asbestos. After a 24-h incubation, we used confocal microscopy to detect the fibers (23) and found that HPMCs internalized Cro but not NT115, whereas macrophages phagocytosed both fibers (Fig. 1 B–E). NT115 fibers were found only attached to the surface of mesothelial cells (Fig. 1D). To quantitatively evaluate cell internalization, the side scatter (SSC) value in flow cytometry was used because it has been reported that fibers inside a cell increase the SSC value (24, 25) (Fig. 1F and Fig. S1A). The two mesothelial cell lines and macrophages showed a marked increase in SSC after a 3-h incubation with asbestos (Fig. 1 G, H, and J), consistent with previous reports (26, 27). However, MWCNTs differentially affected each cell line. Macrophages showed an increase in SSC after incubation with all types of MWCNTs except NTtngl (Fig. 1I), indicating that NTtngl was not phagocytosed by macrophages. Interestingly, the two mesothelial cell lines did not exhibit increased SSC after incubation with MWCNTs (Fig. 1 G, H, and J). These results were also confirmed by time-lapse microscopic analysis (Movies S1–S4). A slight increase in SSC was observed in HPMCs after incubation with MWCNTs because of the attachment of the fibers to the cells (Fig. 1 D and J). MDCK II epithelial cells showed no increase in SSC with any of the fibers used (Fig. 1K), indicating no internalization.
To further elucidate the internalization mechanism of Cro into mesothelial cells, we labeled rab5a (an early endosome marker) with GFP and actin with mCherry. Upon exposure to asbestos, HPMCs exhibited rab5a assembly at the periphery of Cro, whereas no rab5a concentration was observed after incubation with NT50a or NT145 (Fig. S1B), indicating that Cro fibers were actively internalized by mesothelial cells, but NT50a and NT145 were not. These data were also validated by using an inactive form of rab5a (28, 29) (Fig. S1C).
Inverse Correlation Between MWCNT Diameter and Mesothelial Cell Toxicity.
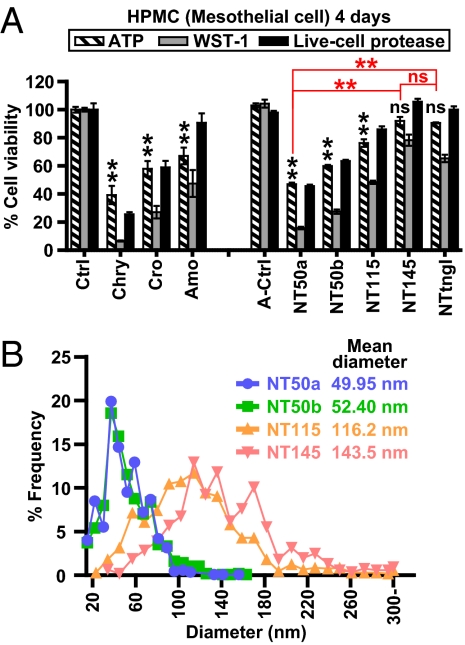
Because mesothelial cells did not internalize MWCNTs, we suspected that the toxicity of MWCNTs would be limited because internalization of asbestos fibers has been shown to be important in asbestos-induced cell death (10, 19). We used three different assays (luminescence, light absorbance, and fluorescence) to evaluate cell viability, and the results were consistent among all the assays. Unexpectedly, different types of MWCNTs induced different levels of cytotoxicity in mesothelial cells. NT145 and NTtngl induced cytotoxicity to a limited extent, whereas NT50a was highly cytotoxic (Fig. 2A). We then found that the degree of cytotoxicity was inversely correlated with the mean diameter of dispersed MWCNTs (Fig. 2B).
Fig. 2.
Diameter of MWCNTs inversely correlated with mesothelial toxicity. (A) Cell viability assays of HPMCs after a 4-d incubation with each fiber at 5 μg/cm2 show variable mesothelial cell cytotoxicity by MWCNTs. NT50a showed strong cytotoxicity against mesothelial cells, whereas NT145 and NTtngl did not. Ctrl, control. P values were calculated between control (saline) and asbestos or A-control (A-saline solution) and MWCNTs (n = 3, mean ± SEM; **P < 0.01; ns, not significant). (B) Distribution of the diameters of MWCNTs.
Thin and Rigid MWCNTs Penetrated Plasma and Nuclear Membranes of Mesothelial Cells.
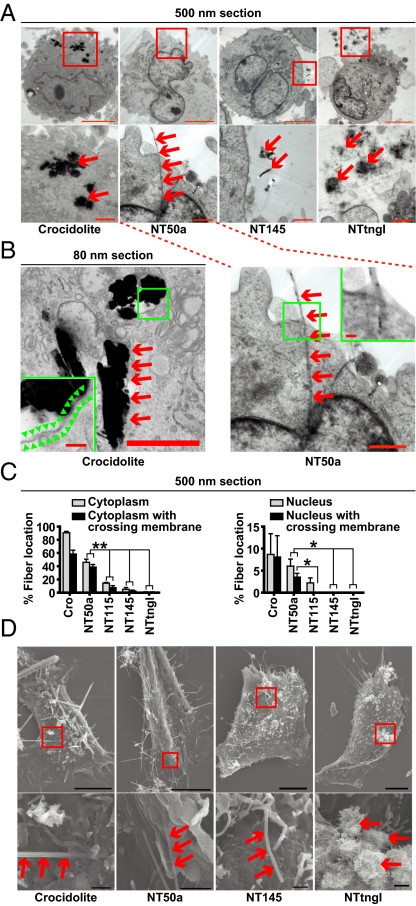
Given that the diameter of dispersed MWCNTs was important in influencing mesothelial injury, we hypothesized that thin MWCNTs were cytotoxic by piercing the cell membrane, as the force to penetrate cell membranes has been found to be proportionally dependent on fiber diameter (30). For these studies, we defined internalization, phagocytosis, and piercing as follows: internalization as an active behavior of nonphagocytic cells (e.g., mesothelial cells) to engulf foreign materials, phagocytosis as an active process by phagocytes to engulf foreign materials, and piercing as an energy-independent penetration of cell membranes by fibrous materials that may or may not lead to translocation inside cells (depending on the type of material). After a 3-h incubation with fibers, HPMCs were fixed, embedded in Epon resin, and sliced into 80-nm or 500-nm ultrathin sections. After visualization by transmission EM, we classified Cro and MWCNTs as inside a cell/nucleus, crossing a cellular/nuclear membrane, or both. We found that NT50a nanotubes penetrated the plasma and nuclear membranes, whereas NT115 and NT145 did not (Fig. 3 A–C). Remarkably, the MWCNTs that pierced the membranes were not covered by vesicular membrane structures (Fig. 3B), indicating that the penetration occurred without active cellular processes, such as endocytosis or phagocytosis. In contrast, Cro was surrounded by vesicular structures (Fig. 3B). We concluded from these results that MWCNTs and asbestos enter mesothelial cells in a different manner.
Fig. 3.
Thin, dispersed MWCNTs with high crystallinity pierced mesothelial cells. (A) Representative transmission EM images of HPMCs after a 3-h incubation with indicated fibers. Ultrathin sections were prepared at a thickness of 500 nm. Squares (Upper) indicate insets (Lower). Cro was fully internalized. NT50a penetrated the mesothelial cell membrane, but NT145 and NTtngl did not (red arrow). (Scale bars: Upper, 10 μm; Lower, 1 μm.) (B) Cro was internalized and surrounded by a vesicular membrane structure (Left), whereas NT50a pierced the plasma membrane of the mesothelial cell without a vesicular structure surrounding the nanotubes (Right). We prepared 80-nm sections to show vesicular structure. Green arrowhead indicates vesicular structure. (Scale bars: 1 μm; Insets, 100 nm.) (C) Localization of fibers in transmission EM images (n = 3, mean ± SEM; *P < 0.05 and **P < 0.01). P values were calculated by Student's t test. (D) Representative scanning EM images of HPMCs after a 3-h incubation with indicated fibers.
Scanning EM was also used to observe localization of fibers that penetrated the plasma membrane or attached onto a cell surface (Fig. 3D). The thin dispersed MWCNTs (e.g., NT50a) were confirmed to pierce mesothelial membranes, whereas the thick dispersed MWCNTs (e.g., NT145) were unable to injure mesothelial cells. This diameter-dependent membrane piercing by fibers was specific to MWCNTs. In the case of Cro, even thicker fibers than NT145 were internalized (Fig. S2). The thinnest fiber (NTtngl) did not penetrate the cells because it formed bundled aggregates.
Physicochemical Factors of MWCNTs Other Than Diameter Were Not the Primary Causes of Mesothelial Injury.
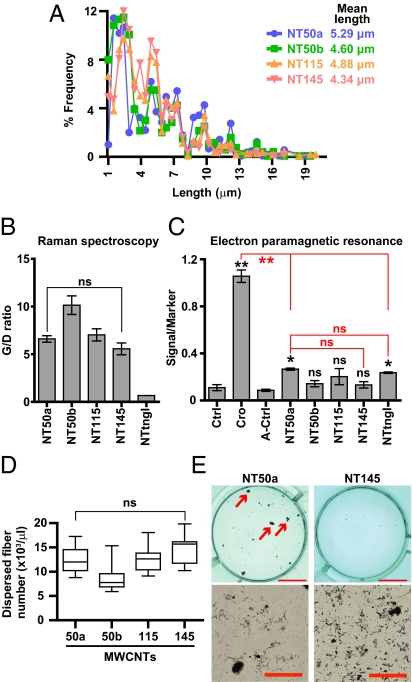
Besides diameter, there could be multiple factors involved in MWCNT-induced toxicity. The distribution of length in each MWCNT type was very similar, and the majority of the nanotubes were less than 10 μm in length (Fig. 4A). Lengths greater than 10 μm have been shown to promote increased toxicological reactivity (31). We performed Raman spectroscopy and found no significant difference between NT50a and NT145 in the relative amounts of structural defects (32, 33) (Fig. 4B). We measured free radical generation (34) by electron paramagnetic resonance and soluble metals (35) by inductively coupled plasma MS. The results showed no significant difference between NT50a and NTtngl (Fig. 4C and Fig. S3A), indicating that contaminating metals, at least when they are outside cells, do not play a central role in the current toxicity assays.
Fig. 4.
Other factors, including length, structural defects, free radical generation, and difference in number of fibers, were not the primary causes of mesothelial cell cytotoxicity by thin, dispersed MWCNTs with high crystallinity. (A) Distribution of the lengths of MWCNTs. (B) Graphite/defect (G/D) ratio shows no significant difference in defect abundance between NT50a and NT145. (C) EPR shows that NT50a and NTtngl produce hydroxyl radicals in the presence of hydrogen peroxide, whereas NT145 does not. Cro was most potent in free radical generation. P values were calculated between control (saline) and asbestos or A-control (A-saline) and MWCNTs. (D) Number of dispersed fibers in 0.5 mg/mL fiber suspensions. (E) Macroscopic and microscopic images of NT50a and NT145 suspensions under experimental conditions (5 μg/cm2). Arrows indicate nanotube aggregation, which greatly reduces the suspension of dispersed fibers. (Scale bars: Upper, 10 mm; Lower, 100 μm.) P values (*P < 0.05 and **P < 0.01; ns, not significant) were determined by one-way ANOVA with post-hoc Dunnett test (C) or Tukey test (B and D).
We tested the cytotoxicity of MWCNTs based on their weight. To exclude the possibility that the thin MWCNTs were toxic just because the number of fibers was greater than in the thick MWCNTs, we assessed the number of dispersed fibers in suspension. There was no significant difference in the number of dispersed fibers between NT50a and NT145 (Fig. 4D). This is because NT50a nanotubes were likely to form agglomerates because of their high aspect ratio, leading to a decrease in the number of dispersed fibers in suspension (Fig. 4E).
The large surface area of MWCNTs is also considered to be an important factor in toxicology because it depletes nutrients and growth factors in culture medium, resulting in cell death from starvation (36). We tested whether this phenomenon contributed to cellular toxicity by saturating the surface area with serum proteins. The serum-treated NT50a was still highly cytotoxic, whereas NT145 did not cause cell death (Fig. S3B). We also analyzed the profile of protein absorption on each fiber (37), but there was no significant difference between NT50a and NT145 (Fig. S3 C and D). Accordingly, surface area was not the primary cause of mesothelial injury in the present study.
Taken together, length (largely <10 μm), structural defects, free radical generation, difference in number of fibers, contaminating metals, and surface area were not considered to be the primary causes of the direct cytotoxicity of NT50a toward mesothelial cells. Instead, our studies demonstrated that the diameter of MWCNTs was one of the critical factors responsible for mesothelial cell injury.
Thin and Rigid MWCNTs Induced Severe Chronic Inflammation.
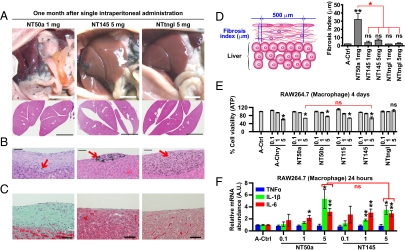
To study the effects of each MWCNT type in vivo, rats were killed 1 mo after an i.p. injection with 1 or 5 mg of NT50a, NT145, or NTtngl. Distinct macroscopic differences were observed among the three nanotube types. NT50a caused severe fibrous peritonitis and induced dull edge in the liver. In contrast, NT145 and NTtngl did not cause such effects, even at five times higher concentration (Fig. 5A and Fig. S4A). All MWCNTs administered to rats were phagocytosed by macrophages, and local granuloma formation was observed (Fig. S4D). Notably, rats treated with NT145 and NTtngl showed negligible fibrosis surrounding fiber deposition, in contrast to rats administered NT50a (Fig. 5 B and C and Fig. S4 B and C). Because fibrosis was observed around each i.p. organ regardless of MWCNT deposition, we calculated a fibrosis index and confirmed that NT50a was the most potent inducer of fibrotic inflammation (Fig. 5D). We observed iron deposition in NT50a-induced fibrotic tissue, which was derived from the rat organs because NT50a deposition itself did not react to Perls' iron staining (Fig. S4E). Furthermore, the proliferation of mesothelial cells was observed only in rats treated with NT50a (Fig. S4F).
Fig. 5.
Thin, dispersed MWCNTs with high crystallinity caused severe fibrotic peritonitis in rats, although affected macrophages in vitro similarly compared with the other MWCNTs. (A–C) Macroscopic and microscopic images of rat organs 1 mo after a single i.p. injection of 1 mg NT50a (Left), 5 mg NT145 (Middle), or 5 mg NTtngl (Right). (A) Macroscopic images of i.p. organs immediately after dissection (Top). Low-power view of H&E-stained livers show an altered dull edge (Lower Left). (Scale bar: 1 cm.) (B) Microscopic images of the liver surface show the deposition of fibers (arrow) and various fibrotic responses around fibers. (Scale bars: 50 μm.) (C) Masson trichrome stain showing abundant light green collagen (Left). (Scale bars: 50 μm.) (D) Fibrosis index was calculated by measuring the distance from the entire outer surface of the liver to the mesothelium at intervals of 500 μm (Left). Fibrosis index shows that NT50a induced the strongest fibrotic inflammation [Right; n = 3–6, mean ± SEM; *P < 0.05; ns, not significant between A-control (A-saline) and MWCNT]. (E) Cell viability assays of macrophages (RAW264.7) after a 4-d incubation with each fiber at indicated concentrations (0.1, 1, or 5 μg/cm2) show that there was no significant difference in cytotoxic effects between NT50a and NT145. (F) Macrophages showed increased mRNA levels of IL-1β and IL-6 after a 24-h incubation with NT50a and NT145 at 5 μg/cm2, and there was no significant difference between NT50a and NT145 for mRNA of inflammatory cytokines (n = 3–6, mean ± SEM; *P < 0.05 and **P < 0.01; ns, not significant). P values were determined by one-way ANOVA with post-hoc Tukey test (D and E) or by Student's t test (F).
Inflammation is a systemic response to foreign bodies or wounds and is driven by various stimuli, such as cytokines and necrotic cell debris. In the abdominal cavity, there are resident macrophages as well as mesothelial cells surrounding the cavity. Here, we hypothesized that the difference in the extent of inflammation induced by NT50a and NT145 was caused by differences in the ability to induce direct mesothelial injury because all types of MWCNT affected macrophages similarly in terms of cytotoxic effects and inflammatory responses (Fig. 5 E and F). Indeed, mesothelial injury caused by asbestos can induce inflammation as previously shown (19).
Thin and Rigid MWCNTs Induced Frequent and Early Mesothelial Carcinogenesis in Rats.
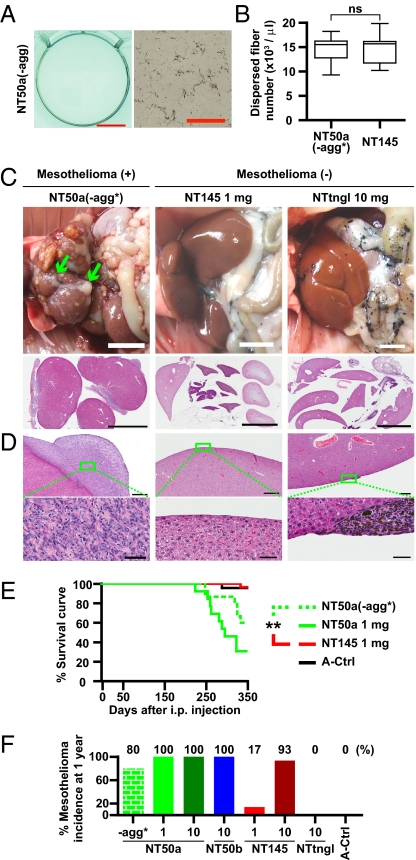
Finally, we tested the carcinogenicity of each MWCNT type by i.p. injection into rats to allow MWCNTs to directly interact with mesothelial cells immediately after administration. We injected 1 mL of MWCNT suspension into 6-wk-old rats twice with a 1-wk interval and observed them for 1 y. We used total doses of 1 mg or 10 mg per animal in addition to a special group of rats injected with NT50a, referred to as NT50a(-agg*), meaning no aggregation. We prepared this special group to eliminate the agglomeration activity of NT50a and equalize the number of fibers to 1 mg NT145. We centrifuged 0.5 mg/mL NT50a and collected the supernatant, which we named NT50a(-agg), to eliminate agglomerate (Fig. 6A). We doubled the concentration of NT50a (1 mg/mL) for centrifugation to equalize the number of dispersed fibers in the NT50a(-agg*) suspension and the 0.5 mg/mL NT145 suspension (Fig. 6B). For the other dosing, the 10-mg injection was a relatively high dose, and nearly half the rats died within several days (Table S2). Consequently, we used the remaining animals as the 10-mg injection group. None of the rats administered 1 mg of MWCNTs died immediately after injection. Rats were killed when bloody ascites or weight loss was prominent or after 1 y from the initial administration.
Fig. 6.
Thin, dispersed MWCNTs with high crystallinity had higher mesotheliomagenicity than thick MWCNTs when injected intraperitoneally into rats. (A) Macroscopic and microscopic images of NT50a(-agg). NT50a(-agg) consisted of the supernatant of the original NT50a suspension after centrifugation. No agglomeration can be seen (compare vs. Fig. 4E). (Scale bars: Left, 10 mm; Right, 100 μm.) (B) NT50a(-agg*) had twice as many fibers in suspension as NT50a(-agg). The number of dispersed fibers in NT50a(-agg*) and NT145 was almost the same (ns, not significant). The text provides further details. (C) Macroscopic images of i.p. organs immediately after dissection (Upper). Mesothelioma covers liver surface (Upper Left). Low-power view of H&E-stained livers shows malignant mesothelioma (Lower Left) or absence of mesothelioma (Lower Middle, Lower Right). (Scale bars: 1 cm.) (D) Microscopic images of the liver surface show mesothelioma (Left). Granuloma is seen (Right), and squares (Upper) indicate insets (Lower). (Scale bars: Upper, 500 μm; Lower, 50 μm.) (E) Survival curve of rats injected with MWCNTs and vehicle control shows that there was significant difference in mesotheliomagenicity between NT50a(-agg*) and NT145 (**P < 0.01). (F) Overall tumor probability induced by each fiber during the 1-y observation period. The number below the x axis indicates the amount of injected fibers [in mg; n = 15 in NT50a(-agg*), n = 13 in 1 mg NT50a, n = 43 in 10 mg NT50a, n = 6 in 10 mg NT50b, n = 29 in 1 mg NT145, n = 30 in 10 mg NT145, n = 15 in 10 mg NTtngl, and n = 23 in A-saline solution (A-Ctrl)]. Table S2 includes the numbers of rats used and histology of mesothelioma. P values were determined by Student’s t test (B) or by log-rank test (E).
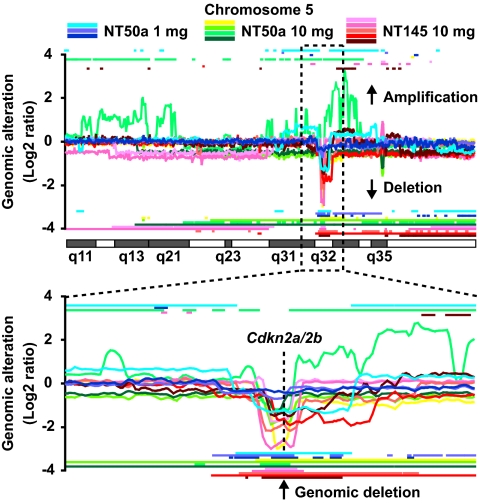
Injections with NT50a(-agg*) or 1 mg of NT50a induced malignant mesothelioma with a higher frequency and earlier progression than injections with 1 mg of NT145 and 10 mg of NTtngl. Representative images of mesothelioma are shown in Fig. 6 C and D. The survival curve and overall tumor incidence showed that NT50a(-agg*) and 1 mg of NT50a were more carcinogenic than 1 mg of NT145 (Fig. 6 E and F). Even in the same group, tumor progression and histologic findings were variable (Fig. S5 and Table S2). Notably, most of the tumors induced by MWCNTs exhibited sarcomatoid histology (epithelioid, 0.9%; biphasic, 12.1%; sarcomatoid, 86.0%; not determined, 0.9%), indicating that the mesotheliomas induced by MWCNTs were more aggressive in nature. To study the genomic alteration of the tumors, we performed an array-based comparative genomic hybridization (CGH) analysis and found that there were homozygous deletions of Cdkn2a/2b in almost all the tumors tested (Fig. 7 and Fig. S6), which is one of the most common genetic aberrations found in mesotheliomas in both humans (38, 39) and rodents (40, 41). There were numerous other genomic amplifications and deletions, but they were not consistent among tumors.
Fig. 7.
Cdkn2a/2b was homozygously deleted in MWCNT-induced mesothelioma of rats. Array-based CGH analysis of mesotheliomas shows that the genomic locus, Cdkn2a/2b, was homozygously or heterozygously deleted in all mesothelioma cases tested. Horizontal lines above and below the CGH ratio curve were delineated based on z-score (Materials and Methods), indicating amplification and deletion of the indicated loci, respectively. At the Cdkn2a/2b locus, all tumors have horizontal lines below the CGH curve and thus have genomic deletion. Fig. S6 shows signal intensity and extent of genomic deletion at the Cdkn2a/2b locus.
Discussion
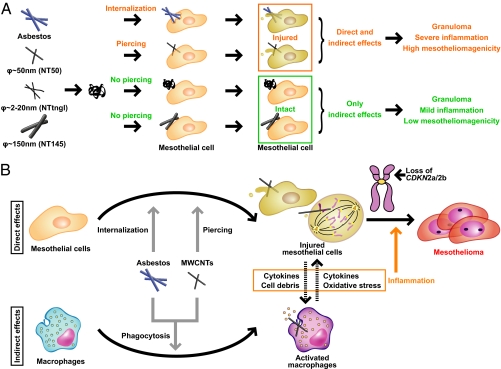
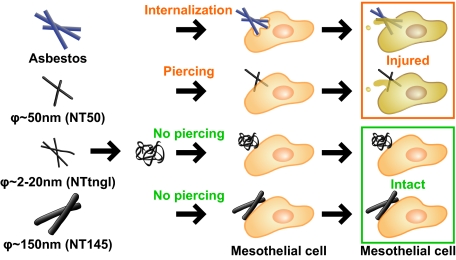
We investigated five different types of MWCNTs and their direct effect on mesothelial cells in comparison with three types of asbestos fibers by studying cellular internalization, injury, inflammogenicity, and carcinogenicity (as summarized in Fig. 8 and Table S1). We focused on how MWCNTs differed from asbestos, not on how MWCNTs behaved like asbestos. We demonstrated that MWCNTs directly pierced mesothelial plasma and nuclear membranes, whereas asbestos fibers were internalized by mesothelial cells via encapsulation by vesicular membranous structures. Because of this difference, thick MWCNTs (e.g., NT145) were less likely to pierce mesothelial cells than thin MWCNTs (e.g., NT50a), even though Cro, which is even thicker than NT145, was easily internalized by the cells. Therefore, although MWCNTs and asbestos both share needle-like structures and have high durability, they do not necessarily enter mesothelial cells in the same way. Thus, nanotube toxicology should be evaluated differently than asbestos toxicology under certain conditions.
Fig. 8.
Schematic of the effect of different MWCNTs on mesothelial cells. (A) The present study shows that asbestos and thin, dispersed MWCNTs with high crystallinity (diameter∼ 50 nm; .e.g., NT50a) penetrate mesothelial cells and further induce cell injury. However, aggregative MWCNTs (diameter ∼ 2–20 nm; e.g., NTtngl) and thick MWCNTs (diameter ∼ 150 nm; e.g., NT145) do not penetrate mesothelial cells, and thus there is no cell injury, which may explain the difference in the carcinogenicity between thin and thick MWCNTs. (B) Current schematic for mechanisms of mesotheliomagenesis focused on mesothelial injury and macrophage activation. Interplay of these two factors may lead to persistent inflammation and subsequent mesotheliomagenesis.
Surface characteristics of MWCNTs, such as functionalization, degree of hydrophobicity, and protein modification, are thought to be important in internalization events (31, 42–44). The present study used pristine MWCNTs without any chemical functionalization and suspended MWCNTs in saline solution containing albumin as previously described (17). To determine possible environmental or toxicological risks of MWCNTs, the use of pristine MWCNTs was considered more appropriate because chemical functionalization of bulk MWCNTs is unrealistic and has been shown to significantly improve the toxicological profile of nanotubes (45). Albumin was used as a dispersing medium for MWCNTs because it is abundant in cell culture media supplemented with FBS as well as in serum and pleural fluid in vivo (46). We also evaluated the profile of protein adsorption on the MWCNTs (Fig. S3 C and D), but there were no significant differences found between the MWCNTs.
We hypothesized that the diameter of MWCNTs played a major role in determining whether the nanotubes would be able to directly pierce cell membranes. A similar idea was suggested by Lacerda et al., who determined that MWCNTs pierced and directly translocated through cell membranes in an energy-independent manner (47); Vakarelski et al. showed that thin MWCNTs penetrated living cell membranes with minimal required force (i.e., energy) (30). We found that nanotube diameter critically determined whether MWCNTs led to increased risk of cytotoxicity after direct interaction, penetration, or injury of mesothelial cells. The exact mechanism of penetration of mesothelial cells by MWCNTs and the way in which membrane piercing by MWCNTs causes toxicity still remains unknown. Regarding the longitudinal dimension (i.e., length) of MWCNTs, significantly shorter MWCNTs have been previously reported to be nontoxic (42), whereas significantly longer MWCNTs compared with the ones used in the present study have been shown to be inflammogenic (14). The MWCNTs used in our study affected macrophages to the same extent (Fig. 5 E and F). Because length is an important factor in inducing macrophage activation (14, 16), the lack of difference in macrophage activation among each MWCNT type confirmed the evenness of fiber length and the similarity in mean length (Fig. 4A). Although length, contaminating metals, structural defects, and protein adsorption may be involved in cellular toxicity induced by penetrating MWCNTs, we confirmed here that these factors were not the primary causes of the difference in observed cytotoxicity. Notably, we showed that crystallinity, which determines MWCNT sharpness and rigidity, was an important structural feature because tangled MWCNTs, even though they were the thinnest (i.e., have the smallest diameter) of the nanotubes used, did not pierce mesothelial membranes or induce mesothelioma in rats, which is in agreement with previously reported studies that used similar material (18, 48).
In the mesotheliomagenesis experiment, we used several doses of MWCNTs, including 1 mg and 10 mg. However, we would like to focus on the carcinogenicity of NT50a(-agg*) compared with that of 1 mg of NT145 because these two fiber suspensions contained nearly the same numbers of dispersed fibers (Fig. 6B) and both lacked agglomerates (Figs. 4E and 6A). We used the 10-mg injection group to avoid false-negative results in the NT145-injected group. Fortunately, we could perform array CGH analysis of mesothelioma samples from the 10-mg NT145 group, but not from the 1-mg NT145 group, because of the low incidence of mesothelioma. Thus, the present study demonstrated a profiling of genetic alterations in the mesothelium following long-term exposure to carbon nanotubes, revealing a common mechanism of mesotheliomagenesis (homozygous deletion of Cdkn2a/2b) in MWCNTs (NT50a and NT145) and asbestos (40, 41).
The shorter latency period of mesothelioma in MWCNT-injected rats than in asbestos-injected rats is reported in the literature (3, 4). The temporal disparity in mesotheliomagenesis may come from differences between the two fiber types, including how they injure mesothelial cells. Here, we demonstrated that MWCNTs behaved differently than asbestos fibers against mesothelial cells. Both thin MWCNTs with high crystallinity and asbestos fibers should induce the main two events in mesotheliomagenesis, mesothelial injury and macrophage activation. However, we found that the mechanism of mesothelial injury was different. This distinctness may partially explain the question above. For example, it is possible that MWCNTs that are located in the cytoplasm and are uncovered by membranes would be more likely to injure chromosomes during mitosis than asbestos fibers surrounded by membranous structures, resulting in a difference in the progression of mesothelioma. It is unknown how genetic aberrations are introduced into mesothelial cells by fibers, although we have several hypotheses, including free radical generation, mitotic disturbance, molecule adsorption, and inflammation (7, 49). Notably, the entry of fibers into mesothelial cells was associated with all these mechanisms. Indeed, there was a large difference in the extent of fibrotic inflammation between groups injected with thin or thick MWCNTs (Fig. 5 A–D) but no difference in the activation of and toxicity to macrophages (Fig. 5 E and F), which indicates the involvement of mesothelial injury in inflammation. The interplay of mesothelial injury and macrophage activation was previously reviewed elsewhere (7). In brief, mesothelial cell injury per se can activate macrophages and neutrophils and thus induce inflammation (50), which would explain why thin MWCNTs were more inflammogenic and carcinogenic than thick MWCNTs.
Our previous findings have shown that the loss of Cdkn2a/2b occurs in sarcomatoid mesothelioma induced by iron overload without fibers (51), but the tumors developed 2 y after injection, and the nature of the tumors was less aggressive. This rat model suggests that free radical generation inside the rat peritoneal cavity is sufficient to induce mesothelioma with the same genetic features as asbestos-induced mesothelioma. However, when comparing the progression of fiber-induced mesothelioma with iron overload-induced mesothelioma, there should be factors specific to fibers involved in mesotheliomagenesis that exacerbate the tumor initiation and growth.
There are some limitations to the present study. As in various other studies, we used the i.p. model of mesothelial exposure. Thus, a careful consideration of the multiple factors involved in fiber translocation from the airways to the pleura is important before conclusive assessments of the health risks posed by inhaled MWCNTs as carcinogens can be reached.
Most previously reported studies focus mainly on the length of MWCNTs in inflammogenicity and carcinogenicity (14, 31), based on the observation that long (>15–20 μm), biopersistent fibers induce frustrated phagocytosis. Even though such hypotheses are certainly useful, they do not offer a complete explanation of the complex mechanisms leading to mesotheliomagenesis. Our results suggested that direct mesothelial injury was crucial in inducing carcinogenesis with the same genetic signature as that of asbestos in a rat i.p. exposure model. Furthermore, we found that the thin diameter of nonfunctionalized MWCNTs in combination with high crystallinity resulted in rigid, needle-shaped nanomaterials that caused direct injury to mesothelial cells and presented a risk for induction of mesothelioma. In general, this study and others have helped instruct the ways in which MWCNTs should be designed (i.e., as short, flexible, thick, and chemically functionalized as possible) to be less biologically reactive and more prone to biodegradation. Further investigation is warranted to elucidate the effect of different types of nanotubes on long-term carcinogenesis and the detailed genetic cascades involved.
Materials and Methods
Materials.
Five types of vapor-grown MWCNTs were donated. NT50a (MWNT-7, lot 061220–02) was obtained from Mitsui, and NT50b, NT115, NT145, and NTtngl were obtained from Showa Denko (Table S1). Three types of asbestos (Chry, Cro, and Amo) were obtained from the Union for International Cancer Control. The plasmids, mCherry-actin and EGFP-actin, were provided by Kozo Kaibuchi (Nagoya University, Nagoya, Japan) and Roger Y. Tsien (University of California, San Diego, La Jolla, CA). Plasmids with the WT and inactive form (S34N) of rab5a were kind gifts from Mitsunori Fukuda (Tohoku University, Tohoku, Japan). All rats were Fischer 344/Brown Norway F1 hybrids. Fischer 344 female rats and Brown Norway male rats were purchased from Charles River Japan. The animal experiment committee of Nagoya University Graduate School of Medicine approved these animal experiments. The MeT5A and RAW264.7 cell lines were obtained from the American Type Culture Collection. The MDCK II cell line was provided by Kozo Kaibuchi (Nagoya University, Nagoya, Japan). The HPMC line was established as described in SI Materials and Methods.
Detection of Cellular Uptake of Fibers.
We grew cells at a density of 1 × 104 cells/cm2 at 1 d before the addition of each fiber. In each experiment, 0.5 mg/mL fibers were added to the culture medium to a final concentration of 5 μg/cm2. We used an LSM5PASCAL microscope (Zeiss) to obtain confocal images. Cells were fixed with 4% paraformaldehyde 24 h after the addition of the fibers and stained with Alexa 488-phalloidin (Invitrogen) and propidium iodide without RNase to stain the cytosol and nucleus at the same time. Fibers were detected with reflected or scattered laser light. For flow cytometry analysis, cells were collected 3 h after the addition of fibers (FACSCalibur; BD Biosciences).
Cell Viability Assays.
We grew cells at a density of 3 × 103 cells/cm2 for MeT5A cells and HPMCs and at 3 × 104 cells/cm2 for RAW264.7 macrophages 24 h before the addition of the fibers (final concentration, 5 μg/cm2). Four days after fiber addition, the following three types of live cell assays were performed: the live cell luminescence ATP detection assay (Cell Titer-Glo; Promega), the light absorbance mitochondrial activity assay (WST-1, Roche), and the fluorescence live-cell caspase activity assay (MultiTox-Fluor; Promega).
In Vivo Chronic Inflammation Assays.
To observe the chronic phase, 6-wk-old male rats were injected with 1 mL of 1 mg/mL NT50a or 1 mL of 1 or 5 mg/mL NT145 or NTtngl. Tissues were harvested after 1 mo. We used three to six rats in each group. Paraffin-embedded tissues were subjected to H&E, Masson trichrome, or Perls' iron staining. Podoplanin (T-20; Santa Cruz Biotechnology) immunohistochemistry was performed as previously reported (52) to identify mesothelial cells. Fibrosis index was calculated by measuring the distance from the outer surface of the liver to the mesothelium at intervals of 500 μm. The measurement was made on a digital slide taken by Scanscope (Aperio Technologies).
Mesotheliomagenesis in Rats.
Six-week-old male and female rats were injected with 1 mL of 0.5 or 5 mg/mL of the MWCNT suspension or 1 mL of NT50a(-agg*) twice with a 1-wk interval. NT50a(-agg*) consisted of the supernatant of 1 mg/mL NT50a after centrifugation at 2,200 × g for 10 s. We used 1 mg/mL of the NT50a suspension because the supernatant of the centrifuged 0.5 mg/mL NT50a suspension, NT50a(-agg), contained nearly half the number of fibers as 0.5 mg/mL NT145. NT50a(-agg*) contained no agglomerate and had approximately the same number of dispersed fibers as 0.5 mg/mL NT145. Thus, in the NT50a(-agg*) and 1 mg NT145 groups, rats were injected with almost the same number of fibers. Rats were killed when bloody ascites or weight loss was prominent. Rats that did not exhibit this phenotype were killed after approximately 1 y (350 d) had passed from the initial injection of MWCNTs. The cumulative probability of death was calculated for each group according to the Kaplan–Meier method and compared by using the log-rank test.
Array-Based CGH Analysis.
We performed array-based CGH with a Rat Genome CGH Microarray 4x180K (G4841A; Agilent Technologies), as described in the Agilent Oligonucleotide Array-based CGH for Genomic DNA Analysis Protocol, version 6.2. For each array, a normal kidney was used as a reference and labeled with Cy-3. Samples of interest were labeled with Cy-5. Three, four, and five cases of mesothelioma that were induced by 1 mg NT50a, 10 mg NT50a, and 10 mg NT145, respectively, were assessed, and the results were analyzed with the Agilent Genomic Workbench Standard Edition (version 5.0). We identified regions of copy number alteration based on the z-score (threshold, 3.0). Centralization was performed with a threshold of 6.0 and bin size of 10. Genomic positions were based on the University of California, Santa Cruz, November 2004 rat reference sequence (rn4). We calculated moving averages of the signal log ratio with a window size of 0.5 Mb. We determined that all of the tumors have genomic deletion at Cdkn2a/2b because of the results of the chromosomal aberration analysis based on the z-score. Then, the extent of genomic deletion, namely whether it was homozygous or heterozygous, was determined by the value of y at the locus. We determined that the mesothelioma samples, whose signal intensity at the Cdkn2a/2b locus was largely lower than −1, had homozygous deletion of the gene (Fig. S6).
Statistics.
All experiments were performed in triplicate. Data are expressed as mean values ± SEM. All statistics except the survival curve were calculated by using a one-way ANOVA with a post-hoc Tukey test, a one-way ANOVA with a post-hoc Dunnett test, or a Student's t test. The cumulative probability of death was compared by using a log-rank test. All statistics were calculated with Prism 5 software (GraphPad).
Supplementary Material
Acknowledgments
We thank Midori Tanaka (Kyoto University) for schematics; Atsushi Enomoto, Takuya Kato (Nagoya University), and Shigekazu Nagata (Kyoto University) for comments on the manuscript; Takashi Watanabe (Nagoya University) for technical advice; and Yoshikazu Fujita and Ikuyo Mizuguchi (Division for Medical Research Engineering, Nagoya University Graduate School of Medicine) for technical support. This study was supported by a Long-range Research Initiative Grant from the Japan Chemical Industry Association; Princess Takamatsu Cancer Research Fund Grant 10-24213; grant-in-aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan; grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; a MEXT Special Coordination Funds for Promoting Science and Technology Grant; a grant from the Takeda Science Foundation; and grant-in-aid from the Japan Society for the Promotion of Science Fellows. K.K. is the recipient of a professorial fellowship from the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 19467.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110013108/-/DCSupplemental.
References
- 1.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 2.Thayer AM. Carbon nanotubes by the metric ton. Chem Eng News. 2007;85:29. [Google Scholar]
- 3.Sakamoto Y, et al. Induction of mesothelioma by a single intrascrotal administration of multi-wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci. 2009;34:65–76. doi: 10.2131/jts.34.65. [DOI] [PubMed] [Google Scholar]
- 4.Takagi A, et al. Induction of mesothelioma in p53+/- mouse by intraperitoneal application of multi-wall carbon nanotube. J Toxicol Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson K, Poland CA. Nanotoxicology: New insights into nanotubes. Nat Nanotechnol. 2009;4:708–710. doi: 10.1038/nnano.2009.327. [DOI] [PubMed] [Google Scholar]
- 6.Jaurand MC, Renier A, Daubriac J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part Fibre Toxicol. 2009;6:16. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai H, Toyokuni S. Biopersistent fiber-induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys. 2010;502:1–7. doi: 10.1016/j.abb.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Hei TK, Piao CQ, He ZY, Vannais D, Waldren CA. Chrysotile fiber is a strong mutagen in mammalian cells. Cancer Res. 1992;52:6305–6309. [PubMed] [Google Scholar]
- 9.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res. 2002;62:6065–6069. [PubMed] [Google Scholar]
- 10.Yang H, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci USA. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson K, Brown GM, Brown DM, Bolton RE, Davis JM. Inflammation generating potential of long and short fibre amosite asbestos samples. Br J Ind Med. 1989;46:271–276. doi: 10.1136/oem.46.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberdörster G. Toxicokinetics and effects of fibrous and nonfibrous particles. Inhal Toxicol. 2002;14:29–56. doi: 10.1080/089583701753338622. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanton MF, et al. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981;67:965–975. [PubMed] [Google Scholar]
- 16.Ye J, et al. Critical role of glass fiber length in TNF-alpha production and transcription factor activation in macrophages. Am J Physiol. 1999;276:L426–L434. doi: 10.1152/ajplung.1999.276.3.L426. [DOI] [PubMed] [Google Scholar]
- 17.Poland CA, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 18.Tabet L, et al. Adverse effects of industrial multiwalled carbon nanotubes on human pulmonary cells. J Toxicol Environ Health A. 2009;72:60–73. doi: 10.1080/15287390802476991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci USA. 2010;107:12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajiyama H, et al. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, et al. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun. 2002;295:354–361. doi: 10.1016/s0006-291x(02)00661-7. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita Y, Tsurumi T, Mori N, Kiyono T. Immortalization of Epstein-Barr virus-negative human B lymphocytes with minimal chromosomal instability. Pathol Int. 2006;56:659–667. doi: 10.1111/j.1440-1827.2006.02026.x. [DOI] [PubMed] [Google Scholar]
- 23.Shukla A, Stern M, Lounsbury KM, Flanders T, Mossman BT. Asbestos-induced apoptosis is protein kinase C delta-dependent. Am J Respir Cell Mol Biol. 2003;29:198–205. doi: 10.1165/rcmb.2002-0248OC. [DOI] [PubMed] [Google Scholar]
- 24.Al-Jamal KT, Kostarelos K. Assessment of cellular uptake and cytotoxicity of carbon nanotubes using flow cytometry. Methods Mol Biol. 2010;625:123–134. doi: 10.1007/978-1-60761-579-8_11. [DOI] [PubMed] [Google Scholar]
- 25.Stringer B, Imrich A, Kobzik L. Flow cytometric assay of lung macrophage uptake of environmental particulates. Cytometry. 1995;20:23–32. doi: 10.1002/cyto.990200106. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, et al. Characteristics and modifying factors of asbestos-induced oxidative DNA damage. Cancer Sci. 2008;99:2142–2151. doi: 10.1111/j.1349-7006.2008.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu W, Ernst JD, Broaddus VC. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am J Respir Cell Mol Biol. 2000;23:371–378. doi: 10.1165/ajrcmb.23.3.4094. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- 29.Tamura K, et al. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell. 2009;20:2900–2908. doi: 10.1091/mbc.E08-12-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vakarelski IU, Brown SC, Higashitani K, Moudgil BM. Penetration of living cell membranes with fortified carbon nanotube tips. Langmuir. 2007;23:10893–10896. doi: 10.1021/la701878n. [DOI] [PubMed] [Google Scholar]
- 31.Kostarelos K. The long and short of carbon nanotube toxicity. Nat Biotechnol. 2008;26:774–776. doi: 10.1038/nbt0708-774. [DOI] [PubMed] [Google Scholar]
- 32.Fenoglio I, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Physicochemical aspects. Chem Res Toxicol. 2008;21:1690–1697. doi: 10.1021/tx800100s. [DOI] [PubMed] [Google Scholar]
- 33.Muller J, et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem Res Toxicol. 2008;21:1698–1705. doi: 10.1021/tx800101p. [DOI] [PubMed] [Google Scholar]
- 34.Pulskamp K, Diabaté S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Toyokuni S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9–16. doi: 10.1111/j.1349-7006.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L, et al. Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing. Small. 2008;4:721–727. doi: 10.1002/smll.200700754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai H, et al. Asbestos surface provides a niche for oxidative modification. Cancer Sci. 2011 doi: 10.1111/j.1349-7006.2011.02087.x. 10.1111/j.1349-7006.2011.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JQ, et al. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res. 1994;54:5547–5551. [PubMed] [Google Scholar]
- 39.Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–2113. [PubMed] [Google Scholar]
- 40.Altomare DA, et al. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 2005;65:8090–8095. doi: 10.1158/0008-5472.CAN-05-2312. [DOI] [PubMed] [Google Scholar]
- 41.Jean D, et al. Syntenic relationships between genomic profiles of fiber-induced murine and human malignant mesothelioma. Am J Pathol. 2011;178:881–894. doi: 10.1016/j.ajpath.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostarelos K, et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nanotechnol. 2007;2:108–113. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 43.Nimmagadda A, Thurston K, Nollert MU, McFetridge PS. Chemical modification of SWNT alters in vitro cell-SWNT interactions. J Biomed Mater Res A. 2006;76:614–625. doi: 10.1002/jbm.a.30577. [DOI] [PubMed] [Google Scholar]
- 44.Raffa V, et al. Can the properties of carbon nanotubes influence their internalization by living cells? Carbon. 2008;46:1600–1610. [Google Scholar]
- 45.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4:627–633. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 46.Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219–225. doi: 10.1183/09031936.97.10010219. [DOI] [PubMed] [Google Scholar]
- 47.Lacerda L, Raffa S, Prato M, Bianco A, Kostarelos K. Cell-penetrating CNTs for delivery of therapeutics. Nano Today. 2007;2:38–43. [Google Scholar]
- 48.Muller J, et al. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicol Sci. 2009;110:442–448. doi: 10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- 49.Toyokuni S. Mechanisms of asbestos-induced carcinogenesis. Nagoya J Med Sci. 2009;71:1–10. [PMC free article] [PubMed] [Google Scholar]
- 50.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Q, et al. Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab Invest. 2010;90:360–373. doi: 10.1038/labinvest.2009.140. [DOI] [PubMed] [Google Scholar]
- 52.Okazaki Y, et al. A beverage containing fermented black soybean ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J Clin Biochem Nutr. 2010;47:198–207. doi: 10.3164/jcbn.10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]