Abstract
Terrestrial wildlife is the primary source of meat for hundreds of millions of people throughout the developing world. Despite widespread human reliance on wildlife for food, the impact of wildlife depletion on human health remains poorly understood. Here we studied a prospective longitudinal cohort of 77 preadolescent children (under 12 y of age) in rural northeastern Madagascar and show that consuming more wildlife was associated with significantly higher hemoglobin concentrations. Our empirical models demonstrate that removing access to wildlife would induce a 29% increase in the numbers of children suffering from anemia and a tripling of anemia cases among children in the poorest households. The well-known progression from anemia to future disease demonstrates the powerful and far-reaching effects of lost wildlife access on a variety of human health outcomes, including cognitive, motor, and physical deficits. Loss of access to wildlife could arise either from the diligent enforcement of existing conservation policy or from unbridled unsustainable harvest, leading to depletion. Conservation enforcement would enact a more rapid restriction of resources, but self-depletion would potentially lead, albeit more slowly, both to irrevocable local wildlife extinctions and loss of the harvested resource. Our research quantifies costs of reduced access to wildlife for a rural community in Madagascar and illuminates pathways that may broadly link reduced natural resource access to declines in childhood health.
Keywords: ecosystem services, epidemiology, protected areas, hunting
Biodiversity loss and large-scale wildlife declines are now globally pervasive and well-documented (1–3). These losses have had severe consequences for ecosystems, such as trophic meltdown (4), loss of critical ecological interactions (5, 6), and extinctions of fish and game species (2, 7). Surprisingly few studies have attempted to quantify the effects of wildlife declines on human economies and health (8, 9), despite the fact that wildlife consumption is central in the diet of hundreds of millions of rural people across the globe (8, 10, 11). The widespread harvest of wildlife for human consumption is a multibillion dollar provisioning service (see ref. 12) worth tens of millions of dollars to the rural poor (8, 13), but research to date has largely overlooked potential links between wildlife declines and human health.
Wildlife declines are likely to have direct and powerful effects on human health and nutrition, particularly via lost access to critical micronutrients (14). Animal source foods, such as wildlife, are rich in energy, protein, and micronutrients that have greater bioavailability than vegetable sources (14). Micronutrient deficiencies, described as “hidden hunger” because of their often asymptomatic nature, are the most prevalent form of malnutrition globally and have a range of health sequelae (15). Iron deficiency is the most prevalent nutrient deficiency worldwide and results in negative consequences for brain metabolism, myelination, neurotransmitter function, motor development, physical activity, and emotional regulation (16, 17). Its most severe form is iron-deficiency anemia (IDA), which is characterized by a deficiency of red blood cells, and affects more than 2 billion people worldwide, including 46–66% of children under age 4 y in developing countries (18). IDA is caused by the inadequate intake of iron-rich foods or excessive blood loss because of bleeding or infectious diseases, such as malaria or parasitic infections.
Our research examined how access to wildlife as a food source affected the risk of anemia for a longitudinal cohort of 77 children (Table S1) living in a remote area of the eastern rainforest in Madagascar (Fig. S1), who were measured monthly from March 2008 to February 2009. The rural community where the study was conducted relies heavily on local wildlife resources (Fig. S2), as do more than 300 million people globally who are supported nutritionally by forest products (13). Unlike protein, which can be acquired at adequate levels from multiple dietary sources, bioavailable iron is almost exclusively derived from animal-source foods (14, 19). We hypothesized that increased wildlife consumption would be associated with a reduced incidence of anemia based on clinical evidence linking animal source foods with improved human nutritional status (14).
Results
We found strong support for our primary hypothesis in our year-long monitoring of hemoglobin levels and wildlife consumption in children. Children who consumed a greater quantity of wildlife had higher hemoglobin concentrations [β (95% confidence interval, CI) = 0.20 (0.0078, 0.39), P = 0.041], when controlling for domesticated meat consumption, household income, sex, age, and nutritional and disease status (Table S2). Of the confounding variables, only household income (β = 0.55, P < 0.0005) and age (β = 0.17, P < 0.0005) were significantly, positively associated with hemoglobin concentration.
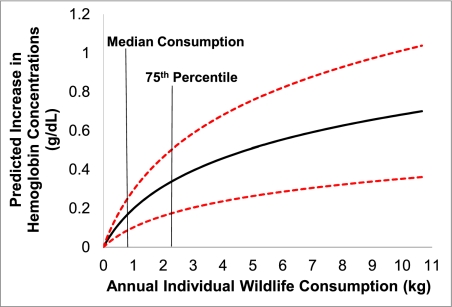
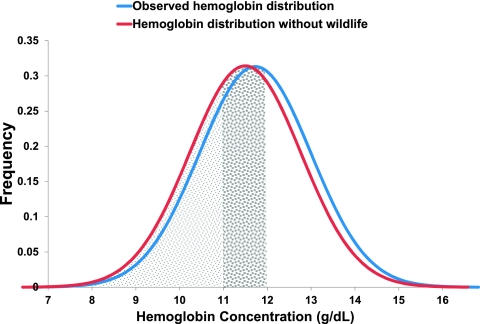
To examine wildlife's relative nutritional importance, we used an empirical model of hemoglobin levels in response to wildlife consumption (SI Text). The maximum benefit of wildlife consumption for a child, when controlling for age and household income, is a mean increase in hemoglobin concentration of 0.69 g/dL (95% CI: 0.36, 1.02 g/dL) (Fig. 1). Using our empirically derived estimates of the contribution of wildlife to hemoglobin levels, we modeled the health impact of removing household access to wildlife, such as would occur if current laws in Madagascar against wildlife hunting were strictly enforced or if wildlife populations collapsed from overharvest or other factors (e.g., habitat conversion, climate change, and drought) (SI Text). Under this scenario, individual's hemoglobin concentrations would decrease up to 0.7 g/dL and the prevalence of anemia would increase from 42% to 54% in our study group, a nearly 30% increased risk of anemia at the population level (Fig. 2).
Fig. 1.
Individual wildlife consumption predicts children's hemoglobin concentrations (n = 77). Bootstrapped estimates of the impact of wildlife consumption on hemoglobin concentrations in Malagasy children show a log-linear positive association (mean shown in black and 95% CI shown in red). Household wildlife consumption was measured with daily diet calendars and individual-level consumption was calculated from direct observation of intrahousehold allocation. As a point of reference, increases in hemoglobin concentration between 0.85 and 1.13 g/dL are expected from iron-supplementation efficacy trials (18).
Fig. 2.
Removing access to wildlife causes a downward shift in population-level hemoglobin concentrations. A lack of wildlife for consumption would cause a downward shift in the observed population hemoglobin distribution (blue) to a predicted future population (red). Anemia was defined as below 11.0 g/dL (small dots) for children less than 5 y of age and below 12.0 g/dL (large dots) for children 5–12 y old (n = 77). Although this downward shift in hemoglobin may appear small in magnitude (<0.7 g/dL), it reflects a 12% absolute increase in the number of children suffering from anemia (from 42 to 54%) and a 29% relative increase over the currently observed prevalence.
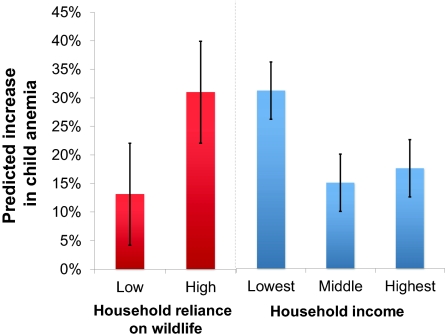
By comparing the modeled population with no access to wildlife to the observed population that frequently consumed wildlife, we estimated that the odds of becoming anemic following wildlife loss would be four-times higher (odds ratio = 4.00, 95% CI: 1.90, 8.40) in households with greater dependency on wildlife than in households with less dependency (dependency determined through a median split of total mass of wildlife consumed over total mass of meat consumed) (Fig. 3). Because households at the highest income levels showed the lowest dependency on wildlife consumption (correlation: −0.254, P < 0.001) and were most able to afford domesticated meat as an alternative to wildlife, children in the economically poorest households would be three times (odds ratio = 3.05, 95% CI: 1.29, 7.45) (Fig. 3) more likely to become anemic than either middle-income or high-income households if access to wildlife were restricted. Based on our calculation of the association between wildlife consumption and hemoglobin levels, ∼1,280 kg⋅km2⋅y of wildlife would need to be harvested from local hunting grounds before anemia would be eliminated in our study community (SI Text). This biomass would be equivalent to an increased annual harvest of 142 animals per household in total (range, 1–51 animals per species per household) (SI Text).
Fig. 3.
Wildlife loss induces major increases in childhood anemia that is modified by household-level characteristics. Predictive models of the association between wildlife consumption and children's hemoglobin concentrations (n = 77) demonstrate that removing wildlife from the diet engenders a disproportionate risk of developing anemia in households with a high reliance on wildlife (odds ratio = 4.00; 95% CI: 1.90, 8.40) and in low-income households (odds ratio = 3.05; 95% CI: 1.29, 7.45). Thresholds for anemia were defined as hemoglobin concentrations below 11.0 g/dL for children under age 5 y and below 12.0 g/dL for children 5–12 y old. Income is a composite of products sold, wages earned, and items bartered and was split into tertiles according to natural breaks in the income variable.
Discussion
Anemia has significant and life-long impacts on human health outcomes (17). Thus, the increased prevalence of childhood anemia that would occur with decreased access to wildlife may have significant and lasting effects at the individual and population level. In our population, we showed that access to wildlife could increase hemoglobin levels by almost 0.7 g/dL, providing ∼61–81% of the expected effect of iron supplementation on hemoglobin as determined in efficacy trials (18). Decreasing hemoglobin concentrations by as little as 1.0 g/dL has been associated with a decline of 1.73 IQ points (18) at a population level and a 1.28-fold increased risk of mild-to-moderate cognitive delay (20). Many cognitive deficits driven by IDA in infancy have been shown to persist until adulthood (21) and are likely to negatively affect the functioning of a healthy work force in developing countries where iron-deficiency anemia is pervasive (22). We assumed that anemia in this system was mainly IDA, as hemoglobin was tightly linked to meat consumption and we controlled for malaria incidence and deworming of intestinal parasites. Studies relating anemia to numerous domains of human health and well-being suggest the tremendous importance of wildlife to local people in Madagascar and highlight the potential for health reductions as a result of wildlife loss in the absence of meat alternatives.
Wildlife hunting is illegal for the majority of mammalian species in Madagascar, but local human populations continue to exploit them for local consumption because of lack of enforcement of national conservation policies (23). Of the five harvested species in our study area for which there are life history and consumption data, analyses suggest four of them are hunted unsustainably (23). Using even the most conservative estimates of current wildlife harvest, wildlife populations in this region of Madagascar will not be sufficient to sustain local human health into the future (23). The approximate 1,280 kg⋅km2⋅y of wildlife needed to be harvested from local hunting grounds before anemia would be eliminated in our study community would exceed current rates of exploitation by 6- to 11-times and maximum sustainable levels of estimated wildlife production by 8-times (see ref. 10 and SI Text).
Although many studies have suggested that wildlife can provide a food security safety net (11), our study illuminates quantitative links between micronutrients derived from wildlife and critical human health outcomes. These results suggest a pathway of how rapid global declines of access to wildlife for consumption, either because of conservation measures (24) or wildlife depletion, could significantly affect the health of local human populations. The enforcement of national wildlife conservation policies that heavily restrict hunting could have a negative effect on the nutritional status of local people, which would be similar to the effects of unsustainable harvesting. However, the absence of conservation enforcement could result in either unsustainable hunting or habitat loss that leads indirectly to wildlife depletion. Seasonal coping strategies, such as hunting, foraging, and off-farm income are necessary to compensate for seasonal health stresses and reductions in crop productivity (25).
In 2003, Madagascar's former President Ravalomanana pledged to triple the system of nature reserves to ∼10% of the nation's surface area (26). This bold commitment was justified with ecological and environmental considerations and it was universally applauded by the conservation community (27). Our results suggest that there may be unanticipated health costs and consequences of this far-reaching change in environmental policy. In fact, both unsustainable hunting and conservation enforcement can lead to the same livelihood outcome on different scales. Conservation enforcement would enact a more rapid restriction of resources, but self-depletion would potentially lead, albeit more slowly, both to irrevocable local population extinctions and loss of the harvested resource. Thus, conservation policy makers and health practitioners must implement integrated conservation and development solutions to mitigate both the effects of wildlife loss on human health and livelihoods, and the potentially severe consequences to biodiversity.
Balancing the needs of economic development, biodiversity conservation, and human health and rights is a tall order, especially when the goals of these interests are incongruent with each other. Historically, there have been few successes in managing wildlife for harvest in tropical developing countries (28, 29), and a few glimmers of hope in successful management of marine fisheries (e.g., ref. 30) that could perhaps inform terrestrial wildlife management. However, no solution will be generally applicable across all geographical and cultural divisions. Site-specific solutions to the tension between environmental conservation and human health and livelihoods are required. Cost-effective public health solutions would not likely include iron supplementation or fortification on the population level (SI Text). In the Makira Protected Area, specifically, improving poultry husbandry conditions and the dissemination of poultry vaccines might provide a preferred nutritional alternative to wildlife that could ease hunting pressure on endangered biodiversity. Wildlife harvest is a critical component of nutritional security in the developing world (11, 31, 32), and it is the responsibility of policymakers to garner broad support from all constituencies to resolve the tension between biodiversity conservation and human health.
Materials and Methods
Site Description.
The Makira Protected Area in northeastern Madagascar covers 3,712 km2 of lowland and midaltitude rainforest. It is one of the most biologically diverse ecosystems in Madagascar and represents one of the nation's largest remaining blocks of contiguous forest. Two ethnic groups dominate the area, with Betsimisaraka predominating in the east and south, Tsimihety predominating in the north and west, and a mixing where these regions overlap. This forest supports 18 species of lemurs, all of which are endemic to Madagascar. There are also several unique species of carnivores, bats, and micromammals, all of which are hunted in this ecosystem. Although the majority of hunting was already illegal before the recent inclusion of the Makira Forest as a protected area in 2005, the dawning of conservation attention through its new protection status and its comanagement by the Wildlife Conservation Society is engendering increased monitoring and enforcement over a geographical area that was previously habituated to customary access rights. In this region, we have focused this research in one village adjacent to the border of the protected area (Fig. S1).
Randomization and Adherence.
The village selected for this research had a total of 105 households. We had previously used systematic random sampling from a census list of households to select 48 households for an environmental resource-use study examining rates of wildlife and other nontimber forest product extraction. Of the 48 households already enrolled in the environmental cohort, 29 had children 12 y of age or younger and were asked to join the current study. We restricted the study base to children 12 y of age and younger to exclude girls who had reached menarche (a factor known to impact hemoglobin concentrations). Informed consent or assent was obtained for all study participants (Protocol # 2007–2-3, issued by the Office for the Protection of Human Subjects at the University of California, Berkeley, CA). After screening, we individually spoke to the females in the study group to assure that they had not yet reached menarche. One household was not included in the analysis because a detailed diet calendar was not maintained. All 28 households joined the study in March 2008, enrolling 77 children. Before the end of the study in February 2009, four children withdrew from the study (one after 6 mo of follow-up and three after 9 mo of follow-up).
Health and Diet Measurements and Protocols.
Every household was visited each month for anthropometric measurements (33), illness recalls, and hemoglobin sampling using a HemoCue Hb 201+ Analyzer supplied by HemoCue (34). For children who were too young to answer their own health questions (typically under age 5 y), surrogate responses from mothers were accepted. Outcome assessors were blinded to the exposure status of children and children were not aware of the purpose of the study. Information regarding the baseline characteristics and health of individuals included in the study can be found in Table S1. The female head of household maintained a diet calendar throughout the duration of the study, on a daily basis, recording the type (i.e., chicken, duck, fish, beef, pork, or species of wildlife) and weight of every meat consumed by the household. Meat weights were measured through the use of scales and recorded daily in diet calendars over the course of 1 y. Dressed meat weight (after hair removal, feather plucking, etc.) was used.
Intrahousehold Food Allocation.
Fourteen of the 28 households in the study base, also randomly selected at the beginning of the study, were visited without forewarning once a month and observed during dinner to determine patterns of intrahousehold food allocation. Because the daily diet calendars measure the amount of food consumed at the level of the household, it is also necessary to develop estimates of how this food is then partitioned to individuals within households. Methods of observation and estimation have been shown to be rigorous (35–37) and were adapted from these studies to apply to the particular sociocultural context in this region of Madagascar. The 14 households were visited each month during the study and 40 of the 77 children in the study sample were observed. A research assistant counted the number of spoonfuls of stew consumed by each household member from a communal stew bowl. These stews were occasionally comprised of meat, vegetables, or a mixture of both. These observations permitted the calculation of a mean proportion of stew typically consumed by individuals by summing all spoonfuls and then calculating an individual's allotment. Determining the proportion of stew consumed by individuals based on age and sex permitted modeling of individual-level wildlife consumption.
Statistical Analysis.
All statistical analyses were conducted using STATA Version 10.0. To test the hypothesis that wildlife consumption was associated with hemoglobin concentrations, we used a generalized linear mixed-model (GLMM) regression. The use of a GLMM permitted multilevel modeling and we clustered observations at the repeated measures for individuals and by the households in the study population. Thus, the model treated individuals and households as latent random effects. The model was bootstrapped to produce robust SEs. We generated two models: Model 1 included the full set of covariates; Model 2 included the statistically significant (P < 0.05) variables from the Model 1 (Table S2). In Model 1, we included household-level variables, including domesticated meat consumption and annual income. We also included temporally varying individual-level variables, such as body mass index for age z-score, malaria incidence in the previous month, and the number of months since the consumption of deworming medication. The best-fit equation for predicting the hemoglobin concentration of an individual from Model 2 was (Eq. 1):
where Yijk is the concentration of hemoglobin (g/dL) for the ith individual in the jth household at the kth visit, β0 (10.48) is a constant, β0i is the random effect for the individual, β0ij is the random effect for the household, β1 (0.20) is the annual amount of individual wildlife consumption, β2 (0.55) is the log-transformed annual household income centered around the mean, β3 (0.17) is the age in years of the individual, and eijk is the error term (Table S2).
Modeling the Effect of Wildlife Loss on Hemoglobin.
To understand the potential health impact of the loss of access to wildlife, we modeled the response of hemoglobin concentrations of individual children to changes in wildlife consumption. Using the best-fit equation from the Model 2 GLMM (Eq. 1), we estimated the change in hemoglobin concentrations from wildlife consumption by subtracting the expected hemoglobin concentrations with zero wildlife consumption from the hemoglobin concentrations under observed levels of wildlife consumption. We do not present new data on trends in wildlife loss or deforestation. The difference in hemoglobin concentrations for an individual is the effect of the simulated loss of access to wildlife and thus is the maximum effect. By comparing the prevalence of anemia under observed conditions to the prevalence following a scenario where wildlife access is lost, we estimated the predicted increase in childhood anemia. Anemia was determined based on a threshold of 11.0 g/dL for children less than 5 y of age and 12.0 g/dL for children 5 to 12 y of age.
Calculating Harvest Required to Eliminate Anemia.
In our study village, we determined the amount of additional hunting required to eliminate anemia from the population of children. We calculated the additional amount of harvest required by solving the GLMM (Eq. 1) for the amount of wildlife consumption required to raise all individuals above the thresholds for anemia (38), finding that harvest would need to be increased 11-fold. For the purposes of this analysis, we assumed that the study village was representative of other villages in the Makira region in hemoglobin levels and wildlife consumption, and then used a larger dataset on wildlife consumption (481 households distributed among 26 villages, median village size is 57.5 households) (SI Text) to assess the effects of increasing hunting 11-fold on wildlife. From this larger dataset, we calculated the average number of individuals per animal species consumed by each household over the course of a year. We then multiplied the number of individual animals harvested per household per year (determined from oral recall) by the lower and upper range in species body mass from the literature (39) to determine the average biomass consumed by each household per year. Following this determination, we extrapolated the biomass consumed by households to the level of each village and multiplied this biomass by 11. We took the difference between the harvest needed for anemia elimination and the current rates of harvest to determine the number of additional animals required. We divided the difference between required and current harvest by the maximum body weight of each species, assuming that the relative proportion of each species in the diet remained unchanged. To determine the potential sustainability of this required harvest, we used the range in observed household wildlife biomass consumption produced from the lower and upper body masses of each species and compared it to the required amount of biomass needed for harvest per square kilometer. The wildlife biomass harvest required (1,280.8 kg/km2) would be 6- to 11-times more than the observed rates of current wildlife harvest and 8-times more than the expected sustainable rates of maximum sustainable yield (ref. 10 and SI Text). This finding means that current rates of biomass harvest are right at the fringe of sustainability, although this is not indicative of species-specific sustainability.
Supplementary Material
Acknowledgments
We thank M. Florine, N. Lilien, C. D. Zafidahy, and E. J. G. Anjaranirina for research assistance; A. Ayson, Jr. and C. Zulueta for data management; K. Smith for comments on the manuscript; and A. Weber and A. Hubbard for comments on the statistical model. This study was supported in part by National Geographic Society Conservation Trust C135-08 (to C.D.G.); the Margot Marsh Biodiversity Fund 023815 (to C.D.G.); the Mohamed bin Zayed Species Conservation Fund 1025935 (to C.D.G.); and a National Science Foundation (NSF) predoctoral graduate research Fellowship and NSF DDIG 1011714 (to C.D.G.) and Grant NSF-IOS 0818185 (to J.S.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112586108/-/DCSupplemental.
References
- 1.Pauly D, et al. The future for fisheries. Science. 2003;302:1359–1361. doi: 10.1126/science.1088667. [DOI] [PubMed] [Google Scholar]
- 2.Brashares JS, et al. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science. 2004;306:1180–1183. doi: 10.1126/science.1102425. [DOI] [PubMed] [Google Scholar]
- 3.Butchart SHM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 4.Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- 5.Sekercioğlu CH, Daily GC, Ehrlich PR. Ecosystem consequences of bird declines. Proc Natl Acad Sci USA. 2004;101:18042–18047. doi: 10.1073/pnas.0408049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peres CA, Palacios A. Basin-wide effects of game harvest on vertebrate population densities in Neotropical forests: Implications for animal-mediated seed dispersal. Biotropica. 2007;39:304–315. [Google Scholar]
- 7.Roberts CM, Hawkins JP. Extinction risk in the sea. Trends Ecol Evol. 1999;14:241–246. doi: 10.1016/s0169-5347(98)01584-5. [DOI] [PubMed] [Google Scholar]
- 8.Milner-Gulland EJ, et al. Wild meat: The bigger picture. Trends Ecol Evol. 2003;18:351–357. [Google Scholar]
- 9.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 10.Robinson J, Bennett E. In: Hunting for Sustainability in Tropical Forests. Robinson J, Bennett E, editors. New York: Columbia Univ Press; 2000. pp. 13–30. 499–521. [Google Scholar]
- 11.Brashares JS, Golden CD, Weinbaum KZ, Barrett CB, Okello GV. Economic and geographic drivers of wildlife consumption in rural Africa. Proc Natl Acad Sci USA. 2011;108:13931–13936. doi: 10.1073/pnas.1011526108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millennium Ecosystem Assessment . Ecosystems and Human Well-Being: Biodiversity Synthesis. Washington, DC: World Resources Institute; 2005. [Google Scholar]
- 13.Pimentel D, McNair M, Duck L, Pimentel M, Kamil J. The value of forests to world food security. Hum Ecol. 1997;25:91–120. [Google Scholar]
- 14.Neumann CG, et al. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: Background, study design and baseline findings. J Nutr. 2003;133(11, Suppl 2):3941S–3949S. doi: 10.1093/jn/133.11.3941S. [DOI] [PubMed] [Google Scholar]
- 15.Black RE, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 16.Pollitt E. The developmental and probabilistic nature of the functional consequences of iron-deficiency anemia in children. J Nutr. 2001;131(2S-2):669S–675S. doi: 10.1093/jn/131.2.669S. [DOI] [PubMed] [Google Scholar]
- 17.Lozoff B, et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43, discussion S72–S91. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoltzfus RJ, Mullany L, Black RE. In: Global and Regional Burden of Diseases Attributable to Selected Major Risk Factors. Ezzati M, Lopez A, Rogers A, Murray C, editors. Geneva: World Health Organization; 2004. pp. 163–209. [Google Scholar]
- 19.Beaton GH, Calloway DH, Murphy SP. Estimated protein intakes of toddlers: Predicted prevalence of inadequate intakes in village populations in Egypt, Kenya, and Mexico. Am J Clin Nutr. 1992;55:902–911. doi: 10.1093/ajcn/55.4.902. [DOI] [PubMed] [Google Scholar]
- 20.Hurtado EK, Claussen AH, Scott KG. Early childhood anemia and mild or moderate mental retardation. Am J Clin Nutr. 1999;69:115–119. doi: 10.1093/ajcn/69.1.115. [DOI] [PubMed] [Google Scholar]
- 21.Lukowski AF, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: Evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13:54–70. doi: 10.1179/147683010X12611460763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton S, Ross J. The economics of iron deficiency. Food Policy. 2003;28:51–75. [Google Scholar]
- 23.Golden CD. Bushmeat hunting and use in the Makira Forest north-eastern Madagascar: A conservation and livelihoods issue. Oryx. 2009;43:386–392. [Google Scholar]
- 24.Agrawal A, Chhatre A, Hardin R. Changing governance of the world's forests. Science. 2008;320:1460–1462. doi: 10.1126/science.1155369. [DOI] [PubMed] [Google Scholar]
- 25.Barrett C, Reardon T, Webb P. Nonfarm income diversification and household livelihood strategies in rural Africa––Concepts, dynamics and policy implications. Food Policy. 2001;26:315–331. [Google Scholar]
- 26.Kremen C, et al. Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science. 2008;320:222–226. doi: 10.1126/science.1155193. [DOI] [PubMed] [Google Scholar]
- 27.Duffy R. Non-governmental organizations and governance states: The impact of transnational environmental management networks in Madagascar. Env Polit. 2006;15:731–749. [Google Scholar]
- 28.Alexander J, McGregor J. Wildlife and politics: CAMPFIRE in Zimbabwe. Dev Change. 2000;31:605–627. [Google Scholar]
- 29.Fa JE, Currie D, Meeuwig J. Bushmeat and food security in the Congo Basin: Linkages between wildlife and people's future. Environ Conserv. 2003;30:71–78. [Google Scholar]
- 30.Russ GR, Alcala AC. Enhanced biodiversity beyond marine reserve boundaries: The cup spillith over. Ecol Appl. 2011;21:241–250. doi: 10.1890/09-1197.1. [DOI] [PubMed] [Google Scholar]
- 31.Brown D, Williams A. The case for bushmeat as a component of development policy: Issues and challenges. Int Forest Rev. 2003;5:148–155. [Google Scholar]
- 32.Bennett EL, et al. Hunting for consensus: Reconciling bushmeat harvest, conservation, and development policy in West and Central Africa. Conserv Biol. 2007;21:884–887. doi: 10.1111/j.1523-1739.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 33.de Onis M, Onyango AW, Van den Broeck J, Chumlea WC, Martorell R. for the WHO Multicentre Growth Reference Study Group Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25(1, Suppl):S27–S36. doi: 10.1177/15648265040251S104. [DOI] [PubMed] [Google Scholar]
- 34.Bäck S-E, et al. Multi-site analytical evaluation of a new portable analyzer, HemoCue Hb 201+, for point of care testing. Point of Care. 2004;3:60–65. [Google Scholar]
- 35.Gittelsohn J. Opening the box: Intrahousehold food allocation in rural Nepal. Soc Sci Med. 1991;33:1141–1154. doi: 10.1016/0277-9536(91)90230-a. [DOI] [PubMed] [Google Scholar]
- 36.Graham MA. Food allocation in rural Peruvian households: Concepts and behavior regarding children. Soc Sci Med. 1997;44:1697–1709. doi: 10.1016/s0277-9536(96)00372-3. [DOI] [PubMed] [Google Scholar]
- 37.Gittelsohn J, Vastine AE. Sociocultural and household factors impacting on the selection, allocation and consumption of animal source foods: Current knowledge and application. J Nutr. 2003;133(11, Suppl 2):4036S–4041S. doi: 10.1093/jn/133.11.4036S. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization . Indicators and Strategies for Iron Deficiency and Anemia Programmes. Geneva: WHO/UNICEF/UNU Consultation; 1994. [Google Scholar]
- 39.Garbutt N. Mammals of Madagascar: A Complete Guide. New Haven, CT: Yale Univ Press; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.