Abstract
Calcium (Ca) is an important structural component of plant cell walls and an intracellular messenger in plants and animals. Therefore, plants tightly control the balance of Ca by regulating Ca uptake and its transfer from cell to cell and organ to organ. Here, we propose that Brassica juncea PCR1 (PCR1), a member of the plant cadmium resistance (PCR) protein family in Indian mustard, is a Ca2+ efflux transporter that is required for the efficient radial transfer of Ca2+ in the root and is implicated in the translocation of Ca to the shoot. Knock-down lines of BjPCR1 were greatly stunted and translocated less Ca to the shoot than did the corresponding WT. The localization of BjPCR1 to the plasma membrane and the preferential expression of BjPCR1 in the root epidermal cells of WT plants suggest that BjPCR1 antisense plants could not efficiently transfer Ca2+ from the root epidermis to the cells located inside the root. Protoplasts isolated from BjPCR1 antisense lines had lower Ca2+ efflux activity than did those of the WT, and membrane vesicles isolated from BjPCR1-expressing yeast exhibited increased Ca2+ transport activity. Inhibitor studies, together with theoretical considerations, indicate that BjPCR1 exports one Ca2+ in exchange for three protons. Root hair-specific expression of BjPCR1 in Arabidopsis results in plants that exhibit increased Ca2+ resistance and translocation. In conclusion, our data support the hypothesis that BjPCR1 is an exporter required for the translocation of Ca2+ from the root epidermis to the inner cells, and ultimately to the shoot.
Keywords: calcium translocation, plasma membrane H+/Ca2+ antiporter, nutrition, calcium homeostasis
Calcium (Ca) is an essential nutrient for plants. It is required for Ca2+-mediated signal transduction, the stabilization of the cell wall and plasma membrane, ion balance, and vacuolar osmoregulation (1–3). The diverse functions of Ca2+ in the plant require that the concentration of Ca2+ be maintained and regulated differently in different compartments, and in a timely manner, and this is achieved by the activity of numerous Ca transporters.
Ca2+-mediated signal transduction is necessary for the proper response of plants to touch, cold, and drought, as well as for the closure of stomata in response to abscisic acid (ABA), cold, and atmospheric CO2 (4, 5). Ca2+-mediated signal transduction is often initiated by rapid Ca2+ influx through selective or nonselective Ca2+ channels located in the plasma membrane and intracellular organelles, such as the Ca2+-permeable outward-rectifying K+ channel, depolarization-activated Ca2+ channel, hyperpolarization-activated Ca2+ channel, and voltage-dependent Ca2+ channel (4, 5). In order that changes in Ca2+ concentration are perceived as a signal, the cytosolic Ca2+ concentration has to be maintained at submicromolar concentrations. Such a low cytosolic Ca2+ concentration can be maintained by Ca2+ efflux transporters, such as Ca2+-ATPases (ECA1, ACA1, and ACA4) and H+/Ca2+ antiporters (CAXs) at the endoplasmic reticulum membrane and tonoplast, respectively (2, 5), and Ca2+-ATPases and H+/Ca2+ exchangers at the plasma membrane (6, 7).
In contrast to this need of individual cells to maintain cytosolic Ca2+ at a very low level, large quantities of Ca2+ are needed at the whole-plant level because of the structural role that Ca2+ plays in stabilizing cell walls and the plasma membrane, as well as its function as a counter ion for the massive amount of anions in the vacuole. In crops, the drop of Ca2+ levels to below a critical level in fast-growing tissues causes diseases, such as black heart in Apium graveolens (celery), blossom end rot in Solanum lycopersicum (tomatoes), and bitter-pit in Malus domestica (apples) (1, 2). These phenomena demonstrate the importance of regulating Ca2+ uptake and allocation. In the root, Ca2+ is taken up by epidermal cells, radially transferred to the inner parts of the root, and then finally loaded into the xylem for transport to the shoot. However, a detailed understanding of the mechanism underlying each step of Ca2+ transport is lacking. For example, it has been debated which part of the root is involved in Ca2+ uptake from the rhizosphere and whether the apoplastic or symplastic pathway is the predominant route for Ca2+ transport across the endodermal layer of the root (8–10). It is also not known which transporters are necessary for xylem loading of Ca2+.
In an effort to gain insight into the function of the plant cadmium resistance (PCR) family, we identified two members of this family from Brassica juncea. We demonstrate that, although B. juncea PCR1 (BjPCR1) exhibits strong sequence similarity to Arabidopsis thaliana PCR2 (AtPCR2), which plays a role in heavy metal transport (11, 12), BjPCR1 is not involved in heavy metal transport but contributes to Ca translocation from the root to the shoot via a Ca2+ efflux mechanism located in root epidermis.
Results
Identification of B. juncea PCRs.
Previously, we identified and characterized two PCRs involved in heavy metal homeostasis in Arabidopsis (11, 12). B. juncea is a crop with a high intrinsic heavy metal tolerance and accumulation (13, 14), and it may therefore contain members of the PCR family with distinct characteristics. Because Brassica has coding sequences that are very similar to those of A. thaliana (15), we used primers specific for AtPCR1 to isolate PCR genes from B. juncea (11). Using a genomic PCR approach, we identified three different BjPCRs. All three BjPCR genes have four exons and three introns at the same positions as AtPCR1 and AtPCR2 (Fig. S1). The lengths of the exons of the three BjPCR genes were very similar to those of AtPCR1 and AtPCR2. In contrast, the introns exhibited some variation. Brassica PCR1 was more similar to AtPCR2 than to AtPCR1 and exhibited 76% identity at the amino acid level with AtPCR2 (Fig. 1A). Because of the high overall identity between AtPCR1, AtPCR2, and BjPCRs, particularly in the hydrophobic domain, which contains the CCXXXXCPC (CC-CPC) motif shown to be required for cadmium (Cd) resistance (11), we expected that BjPCRs would also be implicated in conferring Cd resistance. To test this hypothesis, we isolated the corresponding BjPCR1 and BjPCR2 cDNAs and expressed them in the Cd-sensitive yeast mutant DTY167. On normal half-strength synthetic galactose (SG)-agar medium, BjPCR1-, BjPCR2-, and AtPCR2-expressing yeast cells showed similar growth. Surprisingly, and in contrast to Arabidopsis PCR2, BjPCR1 conferred only weak Cd tolerance and BjPCR2 did not restore any tolerance at all to Cd (Fig. S2A). To determine why BjPCR1 does not confer Cd tolerance, we undertook domain-swapping experiments with BjPCR1 and AtPCR2. The results showed that mBP1, a hybrid construct consisting of the N-terminal part of AtPCR2 and the C-terminal part of BjPCR1, conferred Cd tolerance (Fig. S2B). Site-directed mutagenesis analysis within the N-terminal part of BjPCR1 revealed that the exchange of the naturally occurring Q11 with a His residue resulted in a BjPCR1 form that conferred Cd tolerance (Fig. S2C) and decreased Cd content (Fig. S2D) in ycf1 yeasts. Interestingly, the single amino acid change, Q11H, caused a shift in the protein band mobility, although it did not change the BjPCR1 protein level (Fig. S2E). This band mobility shift suggests a change in protein structure that might have contributed to the dramatic change in PCR function to confer Cd tolerance.
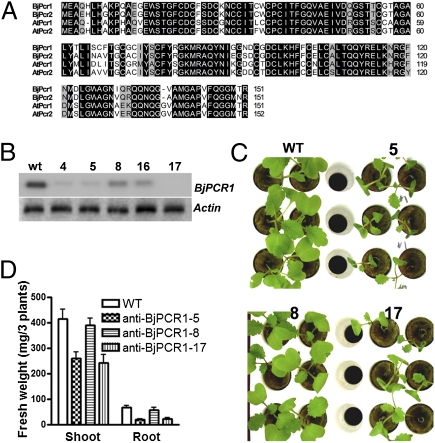
Fig. 1.
Characterization of BjPCR1. (A) Comparison of PCRs of A. thaliana and B. juncea by amino acid sequence alignment. Identical or similar amino acid residues are shown in black or gray boxes. ClustalW (http://align.genome.jp) was used to generate the alignment. (B–D) Phenotype analysis of BjPCR1 knock-down mutants. (B) Transcription levels of BjPCR1 in WT (wt) and anti-BjPCR1 B. juncea (lines 4, 5, 8, 16, and 17) plants. (C) Growth of 2-wk-old WT and anti-BjPCR1 (lines 5, 8, and 17) plants in hydroponic culture. (D) Fresh weight of the shoots and roots of WT and anti-BjPCR1 (lines 5, 8, and 17) plants grown as shown in C. The average ± SE is shown (n = 20, N = 3).
Phenotypic Analysis of BjPCR1 Antisense Lines.
To investigate the physiological function of BjPCRs in B. juncea, we produced a silencing construct for BjPCR1 and BjPCR2 that down-regulated the expression of both genes. We examined BjPCR1 and BjPCR2 transcript levels in the roots of 20 transformants exhibiting bar gene-mediated phosphinothricin resistance, using RNA blot analysis with a BjPCR1 probe that can cross-react with both BjPCR genes. Based on the expression levels of BjPCRs (Fig. 1B), we selected two lines with the lowest transcript levels (lines 5 and 17) and one where the transcript levels were only partially decreased and which could be used as a control (line 8). When these plants were grown under hydroponic conditions, lines 5 and 17 exhibited impaired growth, whereas line 8 grew at similar rates as the WT plants (Fig. 1C). Quantification of the shoot and root biomass confirmed our visual impression (Fig. 1D). In lines 5 and 17, the shoot biomass was decreased by nearly 40%. The reduction in biomass was even more pronounced at the root level, where the biomass of lines 5 and 17 decreased by more than 70% of WT values (Fig. 1D).
To determine the reason for this drastic phenotype, we first measured the levels of major cations in the mutant lines and WT plants grown under control conditions (Fig. 2 and Fig. S3). We did not detect any difference in cation content between our control anti–BjPCR1-8 plant and the WT, which corresponded well with the absence of a difference in growth phenotype in this line. In contrast, we observed a pronounced difference in Ca, iron (Fe), manganese (Mn), and sodium (Na) concentrations between anti–BjPCR1-5 and anti–BjPCR1-17 and the corresponding WT (Fig. 2A and Fig. S3 A–D). Only a slight effect was detected for Mg2+, whereas no differences were observed for zinc (Zn), copper (Cu), and potassium (K) (Fig. 2B and Fig. S3 E and F). The most drastic differences between the silenced lines and WT plants were observed for Ca (Fig. 2A). Ca concentrations in anti–BjPCR1-5 and anti–BjPCR1-17 were only 65–75% of those observed in the WT, whereas they were at least twofold higher than in the WT in the root. Consequently, the shoot-to-root ratio of Ca2+ was dramatically altered in the anti–BjPCR1-5 and anti–BjPCR1-17 lines, whereas that of other ions was less affected (Fig. 2C and Fig. S3G). A comparison of biomass (Fig. 1D) with Ca concentrations (Fig. 2A) revealed that the growth of roots of the antisense lines was strongly impaired despite the fact that they contained high levels of Ca2+. This may be because the high level of Ca2+ exerted a toxic effect or the shoot, which did not have sufficient levels of Ca2+, could not develop normally, and thus failed to provide sufficient energy for root growth. The remarkable difference in the shoot-to-root ratio of Ca between the WT and the antisense lines 5 and 17 indicated that BjPCR1 plays a major role in the transfer of Ca2+ from the root to the shoot, and thus differs from its Arabidopsis homolog, AtPCR2, which transports Zn (12). Therefore, we concentrated our further studies on the role of BjPCR1 in Ca2+ distribution and transport.
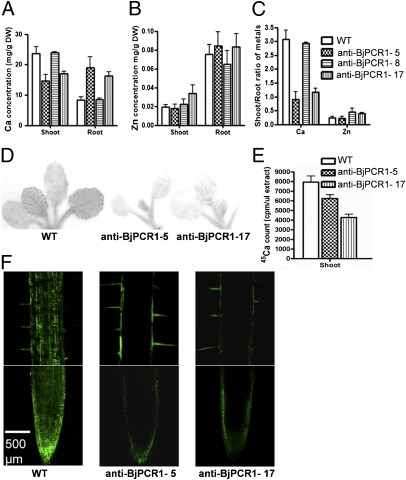
Fig. 2.
BjPCR1 antisense lines exhibited reduced translocation of Ca2+ to the shoot and reduced radial translocation of Ca2+ to the inner part of the root. Ca (A) and Zn (B) concentrations in the shoots and roots of 4-wk-old WT and anti-BjPCR1 B. juncea plants. DW, dry weight. (C) Shoot-to-root ratios of Ca and Zn concentrations as shown in A and B. (D) Autoradiography of 3-wk-old B. juncea plants incubated in hydroponic medium supplemented with 1.5 mM CaCl2 containing 0.4 MBq of 45CaCl2 supplied through the root for 15 h. (E) Counts of 45Ca normalized by the volume of cell sap extracted from the shoot of plants treated with 45CaCl2 as in D. All data represent average ± SE (n = 5, N = 2). (F) Distribution of free Ca2+ in the roots of the WT and anti-BjPCR1 lines visualized using fluo-3 fluorescence. Roots of 5-d-old B. juncea plants were stained with fluo-3 for 4 h, washed with PBS solution, and observed by confocal microscopy. (Scale bar = 500 μm.)
Ca2+ Translocation in Anti-BjPCR1 Lines.
To confirm the decreased root-to-shoot Ca translocation observed in the BjPCR1-5 and BjPCR1-17 lines, we performed short-term uptake experiments using 45Ca2+ (Fig. 2 D and E). When grown in hydroponic medium and exposed for 15 h to 0.4 MBq of 45Ca2+, the leaves of the anti-BjPCR1 mutant lines 5 and 17 contained less 45Ca radioactivity than did those of the corresponding WT (Fig. 2 D and E). To test if BjPCR1 is involved in the lateral transport of Ca2+, we analyzed Ca2+ distribution in the root hair zone and root tip, where the Casparian band has not yet formed, using a cell-permeable Ca2+ dye, the acetoxymethyl ester derivative of fluo-3 (Fluo-3-AM; Molecular Probes). This dye permeates into cells and is hydrolyzed by nonspecific esterases, and the cleavage product, fluo-3, emits a green fluorescence when bound to Ca2+, allowing the visualization of intracellular Ca2+ (13). Roots of WT plants exhibited a strong Ca2+-dependent fluorescence signal in the tip and epidermal layer of the tip (Fig. 2F). A Ca2+ signal was also observed in the stele. In the root hair zone of the root, the strongest Ca2+ signal was observed in the tissue inside the epidermis adjacent to the root tip and in the stele (Fig. 2F). In contrast, the Ca2+-dependent fluorescence signal of the two anti-BjPCR1 mutant lines was less pronounced in the root tip. In the root hair zone, Ca2+-dependent fluorescence could only be observed in the epidermal cells and root hairs but not in the stele (Fig. 2F and Fig. S4). No difference in the fluorescence pattern of zinpyr-1 (Sigma–Aldrich), an indicator dye for Zn, was observed between the roots of the WT and the antisense lines (Fig. S5), which corresponded to the absence of differences in Zn concentration in WT and mutant plants (Fig. 2B). Furthermore, this result indicates that the difference in the fluo-3 pattern between the antisense and WT lines did not originate from any difference in dye penetration. Taken together, these results suggest that anti-BjPCR1 lines do not efficiently translocate Ca2+ from the root epidermal cells to the inner cells of the root.
Tissue-Specific Expression of BjPCRs.
To understand the function of BjPCR1 and BjPCR2 in Brassica further, we analyzed the tissue-specific expression. BjPCR1 was expressed mainly in roots but was also present in leaves (Fig. 3A). Expression in stems and flowers was low. The expression pattern of BjPCR2 was similar to that of BjPCR1, but the overall expression level was lower than that of BjPCR1 (Fig. 3A). To obtain a clue as to where in the root BjPCR1 and BjPCR2 are expressed, we first used a stepwise grinding method. BjPCR1 was highly expressed in root hair cells, which fell off at the first step of grinding, and its expression pattern was similar to that of EXP7, a root hair marker (14), but was opposite to that of HMA4, which is mainly expressed in vascular tissues (15) (Fig. 3B). Whole-mount in situ RNA hybridization confirmed that BjPCR1 is indeed strongly expressed in the epidermal layer (antisense probe of Fig. 3C). We cannot completely exclude the possibility that BjPCR1 is also expressed in other parts of the root; however, in this case, the expression level would be very low compared with that in epidermal cells. The grinding method indicates that BjPCR2 exhibits a similar expression pattern as BjPCR1 (Fig. 3B), but the expression level of BjPCR2 was 1/10th that of BjPCR1 (Fig. 3 A, B, and D). BjPCR1 was strongly induced under Ca2+ starvation conditions but not under Ca2+ excess conditions (Fig. 3D). BjPCR2 exhibited a similar response, but its expression level remained lower than that of BjPCR1.
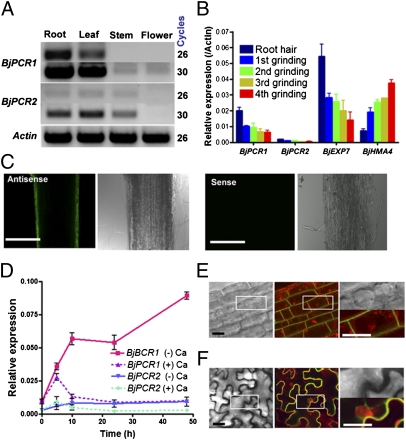
Fig. 3.
Expression pattern and subcellular localization of BjPCR1. (A) RT-polymerase chain reaction analysis of BjPCR1 and BjPCR2 in B. juncea plants. (B) Localization of BjPCR1 and BjPCR2 transcripts in the roots of B. juncea grown on agar medium for 5 d. BjEXP7 and BjHMA4 were used as marker genes that are expressed in root hairs and vascular tissue, respectively. Root cell layers were collected in liquid nitrogen, sequentially ground four times, and collected again, and their mRNA was extracted as described in SI Materials and Methods. The average ± SE is shown (n = 3, N =2). (C) Localization of BjPCR1 in the roots of B. juncea, as detected by the whole-mount in situ RNA hybridization technique, using a fluorescein-12-UTP–labeled antisense (Left) or sense (Right; background control) probe. Optically sectioned images of the median planes of the samples were obtained by confocal microscopy. (Scale bars = 500 μm.) (D) Expression pattern of BjPCR1 and BjPCR2 under excess (10 mM CaCl2) and deficient (0 mM CaCl2) Ca conditions. The average ± SE is shown (n = 3, N = 2). (E and F) Plasma membrane localization of BjPCR1-GFP. (E) Fluorescence at the root epidermis of a BjPCR1-GFP transgenic Arabidopsis plant. The red fluorescence indicates the vacuoles and endosomes stained with FM4-64. (Scale bar = 5 μm.) (F) Fluorescence of BjPCR1-GFP at the leaf epidermis of a BjPCR1-GFP–expressing tobacco plant. Red fluorescence indicates the cell walls and nuclei of epidermal cells stained with propidium iodide. Bright-field images (Left), merged images of red fluorescence and green fluorescence (Center), and images enlarged from the boxed areas in the first two columns (Right). (Scale bar = 5 μm.)
Plasma Membrane Localization of BjPCR1-GFP.
To investigate the subcellular localization of BjPCR1 in planta, transgenic Arabidopsis lines expressing the 35S::BjPCR1-GFP construct were generated. Green fluorescence in the root epidermal cells of these plants was localized to the plasma membrane (Fig. 3E). Transient expression of the construct in tobacco epidermal cells by infiltration confirmed that BjPCR1-GFP was targeted to the cell surface in close proximity to the cell wall, which was stained with propidium iodide (Fig. 3F). These results indicate that BjPCR1-GFP is located at the plasma membrane of plant cells.
Ca2+ Transfer by BjPCR1 in the Root Epidermis.
Ca2+ transport analysis and epidermal plasma membrane localization of BjPCR1 indicated that BjPCR1 acts as a Ca2+ efflux transporter at the epidermis for shoot Ca2+ translocation in B. juncea. If BjPCR1 indeed facilitates Ca2+ efflux from the epidermis to the apoplast, the pathway for Ca2+ translocation to the shoot involves both the symplast of the epidermal cells and the adjacent apoplast. To estimate the portions of the apoplast/symplast combinatorial pathway and the entirely apoplastic pathway in the total transfer of Ca2+ to the shoot, we compared the short-term root uptake of 45Ca at 0 °C and 25 °C. The results demonstrated that, although Ca2+ transport was significant at 0 °C (70% for WT), which is probably through the apoplast alone, 30% of the transport is mediated by energy-dependent mechanism(s) that might include uptake into the epidermis and subsequent release into the cortical apoplast (Fig. S6, WT). Furthermore, the same temperature-dependent transport assays with antisense BjPCR1 lines 5 and 17 revealed that they contained higher levels of Ca2+ than the WT, especially at 25 °C (Fig. S6), which suggests that BjPCR1 is important for the energy-dependent removal of Ca2+ from the epidermis to the apoplast.
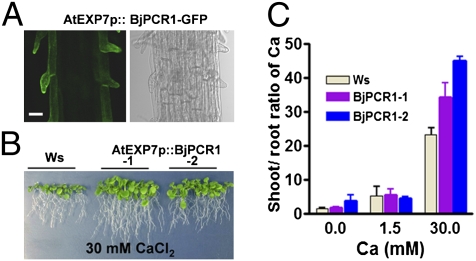
To test further whether Ca2+ is indeed a physiological substrate of BjPCR1 important for Ca2+ transfer at the epidermis, we expressed BjPCR1 in Arabidopsis root hair cells using the AtEXP7 promoter, and thereby generated EXP2p::BjPCR1-V5 and EXP2p::BjPCR1-GFP transgenic plants. As shown in Fig. 4A, BjPCR1-GFP was indeed specifically expressed in the root hair cells of these Arabidopsis plants. When EXP2p::BjPCR1-V5 or EXP2p::BjPCR1-GFP transgenic Arabidopsis plants were grown on media containing different concentrations of Ca, Mn, or Fe, Arabidopsis plants expressing BjPCR1 grew better than WT under the Ca2+-deficient, Ca2+-sufficient, Ca2+-excessive, and Mn2+-excessive conditions but grew similar to WT in medium containing excess Fe (Fig. 4B and Fig. S7 A, B, and E). The most dramatic effect was observed when Ca2+ was present at high concentrations, which impaired plant growth (Fig. 4B and Fig. S7 A and B). Ca2+ was present at higher concentrations in the shoots but at similar concentrations in the roots of transgenic Arabidopsis lines relative to the WT (Fig. S7C), resulting in an increase in shoot-to-root Ca ratio in the transgenic plants (Fig. 4C) and indicating that Ca2+ is translocated more efficiently in the transgenic plants. Together, these results indicate that Ca2+ is a physiological substrate of BjPCR1, and they further suggest that, in the epidermis, BjPCR1 contributes to Ca2+ translocation to the shoot.
Fig. 4.
Arabidopsis lines expressing BjPCR1 in root hairs exhibited enhanced Ca resistance and translocation to the shoot. (A) Root hair-specific localization of BjPCR1 in EXP7promoter::BjPCR1-GFP transgenic Arabidopsis. (Scale bar = 5 μm.) (B) Ca tolerance phenotype of EXP7promoter::BjPCR1 transgenic Arabidopsis lines (EXP7p::BjPCR1-1 and BjPCR1-2). Plants were grown on 30 mM CaCl2 containing half-strength Murashige and Skoog medium for 3 wk. Ws, WT plants. (C) Shoot-to-root Ca ratio in EXP7promoter::BjPCR1-expressing Arabidopsis lines (BjPCR1-1 and BjPCR1-2). Ca content was measured, and the shoot-to-root Ca ratio was analyzed using data from Fig. S7C. Average values ± SE are shown (n = 3, N = 2).
Ca2+ Efflux Activity by BjPCR1.
To test whether BjPCR1 acts directly as a Ca2+ efflux transporter, we performed transport experiments using mesophyll protoplasts isolated from the anti-BjPCR1 lines and WT plants. The 45Ca2+ uptake activity of protoplasts of the antisense lines was about twice that of control protoplasts (Fig. 5A). This result could indicate that the Ca2+ taken up by control plants is readily exported, whereas that taken up by antisense lines is not. To test this hypothesis, we preloaded protoplasts isolated from control and mutant plants for 30 min with 45Ca2+ and then investigated the release of 45Ca2+. Indeed, Ca2+ efflux rates were slower in protoplasts isolated from antisense lines than in those from WT plants (Fig. 5B), indicating that BjPCR1 acts as a Ca2+ efflux transporter and supporting the conclusions drawn from the experiments on whole plants.
Fig. 5.
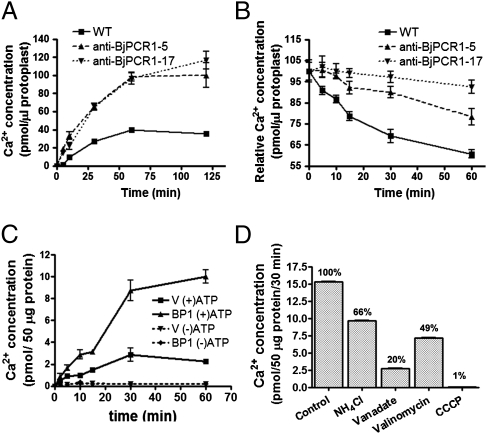
Ca2+ transport mediated by BjPCR1. (A) Time-dependent Ca2+ uptake by protoplasts of WT and anti–BjPCR1-5 and anti–BjPCR1-17. The protoplasts were suspended in loading buffer containing 100 μM CaCl2, 18.5 kBq of 45CaCl2, and 18.5 kBq of 3H2O, and they were then incubated for the indicated periods of time. Only the intact protoplasts were collected by centrifugation. (B) Time-dependent release of Ca2+ from protoplasts of WT and anti-BjPCR1 plants. The protoplasts were preloaded in medium containing 100 μM CaCl2 and 18.5 kBq of 45CaCl2 for 30 min, washed briefly with ice-cold bathing solution, and incubated in the bathing medium. Only intact cells were collected, and radioactive disintegrations from the samples were counted. The Ca2+ content was normalized against the 3H2O content of the protoplasts. The average ± SE is shown (n = 4, N = 3). (C and D) Ca2+ uptake experiment in yeast microsomes isolated from Saccharomyces cerevisiae transformed with the empty vector (V) or BjPCR1 (BP1). (C) Time course of Ca2+ uptake by vesicles from cells transformed with V or BP1. Ca2+ uptake was performed in the absence (−ATP) or presence (+ATP) of 4 mM Mg-ATP in Ca transport medium containing a standard transport buffer at 25 °C for the indicated period. The microsomes were collected by filtration on a nitrocellulose filter. (D) Effects of inhibitors of ion transport on Ca2+ uptake by vesicles derived from BP1-expressing cells. Uptake assay was performed using yeast microsomes expressing V or BP1 in the Ca2+ transport medium containing 4 mM Mg-ATP (Control) plus the compounds indicated [i.e., NH4Cl, 5 mM; vanadate, 1 mM; valinomycin, 2 μM; carbonyl cyanide m-chlorophenylhydrate (CCCP), 10 μM]. The bars represent the Ca2+ concentrations in vesicles expressing BjPCR1 minus those in vesicles transformed with empty vector (n = 4, N = 2). The values (%) in the graph are the rates of uptake expressed as a percentage of the control. Average values ± SE are shown (n = 3, N = 2).
To confirm further that BjPCR1 is indeed a Ca2+ transporter, we expressed BjPCR1 in the yeast strain SM17, which is deficient in CNB1 and the Ca2+ transporters PMR1, PMC1, and VCX1. The 45Ca uptake experiment in the BjPCR1-expressing yeast cells showed that BjPCR1 decreased the Ca2+ content of the cell (Fig. S8A), which indicated that BjPCR1 had a role in Ca2+ efflux. This result is also consistent with the increased Ca2+ level in protoplasts isolated from anti-BjPCR1 lines (Fig. 5A). For the in-depth analysis of BjPCR1-mediated transport, vesicles were isolated from yeast cells and used in the Ca2+ transport assay. The vesicles prepared from yeast cells expressing BjPCR1 exhibited significantly increased Ca2+ transport activity relative to those isolated from the empty vector control. During the first 30 min of incubation, yeast vesicles expressing BjPCR1 took up Ca2+ about fourfold faster than the empty vector control (Fig. 5C). This activity demonstrates Ca2+ efflux in vivo, because only the inside-out vesicles can use the magnesium (Mg)-ATP required to drive the transport. To exclude the possibility that the difference observed was attributable either to variation in the stability of vesicles or to the amount of vesicles used, we performed a control experiment using leukotriene, which is glutathionated and taken up by ABCC-type transporters in yeast. We did not see any difference in leukotriene uptake activity between the two preparations (Fig. S8B). Concentration-dependent Ca2+ transport assays revealed that BjPCR1 is a high-capacity and low-affinity Ca2+ transporter exhibiting an apparent Km of 50 μM (Fig. S8C). To determine substrate specificity, competition of 45Ca2+ transport assay was performed using cold Ca2+, Fe2+, and Mn2+. The 45Ca2+ transport activity was inhibited by 93% and 38% by addition of 500 μM Ca2+ and Mn2+ but not by 500 μM Fe2+ (Fig. S8D). The result suggests that Ca2+ is a preferred substrate for BjPCR1 compared with other ions.
To determine how the BjPCR1-mediated Ca2+ transport was energized, we performed inhibitor studies (Fig. 5D). Vanadate, an inhibitor of P-type ATPases, such as the plasma membrane proton pump, inhibited the transport by 80% compared with the Mg-ATP control, suggesting that the plasma membrane-localized H+-ATPase generates the driving force for Ca2+ uptake. To test this hypothesis, we first examined the effect of ammonium chloride, which abolishes the ΔpH but not the membrane potential (Δψm). In the presence of 5 mM ammonium chloride, Ca2+ transport was inhibited by 34%. This result indicated that BjPCR1-mediated Ca2+ uptake into yeast vesicles is partially ΔpH-dependent and that BjPCR1 does not act as a simple Ca2+ channel. To identify the additional driving force that supports the BjPCR1-mediated Ca2+ fluxes, we performed Ca2+ uptake experiments in the presence of valinomycin, which dissipates the Δψm. In this case, Ca2+ transport was inhibited by 51%, indicating that Ca2+ transport is electrogenic. Finally, the addition of carbonyl cyanide m-chlorophenylhydrate, which disrupts both the ΔpH and Δψm, had a drastic effect and inhibited Ca2+ transport activity by 99%. Two additional experiments provided further confirmation that the proton motive force drives BjPCR1-mediated Ca2+ transport: (i) Preincubation with Mg-ATP resulted in faster Ca2+ uptake into yeast vesicles (Fig. S8E), and (ii) yeast vesicles expressing BjPCR1 exhibited a more pronounced recovery of pH when challenged with Ca2+, as indicated by the larger increase in 9-amino-6-chloro-2-methoxyacridine (ACMA) fluorescence in BjPCR1-expressing yeast microsomes than in empty vector (EV)-expressing ones (Fig. S8F). In this experiment, low pH-induced quenching of ACMA fluorescence was transiently reversed by the addition of Ca2+ to the medium, which is most likely attributable to H+ release by Ca2+/H+ antiport activity. Together, these results indicate that Ca2+ transport by BjPCR1 is driven by a proton-coupled antiport mechanism. Because proton transport into vesicles by H+-ATPase generates an inside-positive membrane potential and a collapse of the membrane potential by valinomycin-inhibited Ca2+ uptake into the vesicles, it is likely that more positive charges are exported than imported by BjPCR1 in the vesicle membrane (i.e., more charges carried by H+ efflux than by Ca2+ influx; see below). This hypothesis is confirmed by a theoretical consideration, which, based on the following equation (details are provided in SI Materials and Methods),
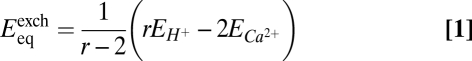 |
where r is the stoichiometric coefficient of the exchange mechanism (rH+:Ca2+), and EH+ and ECa2+ are the Nernst potential of H+ and Ca2+, respectively, shows that in the physiological ranges (Fig. S9B, shadowed area), the exchanger can always mediate Ca2+ efflux when one Ca2+ is exchanged with three protons.
Discussion
In this study, we demonstrated that BjPCR1, a homolog of AtPCR2, exports Ca2+ from plant cells and acts as a Ca2+ transporter in plant protoplasts and membrane vesicles isolated from yeast cells. AtPCR1 and AtPCR2 are small proteins that contain two predicted membrane-spanning α-helices and contribute to Cd resistance and Zn homeostasis, respectively (11, 12). The PCR family of genes, characterized by the common cysteine-rich PLAC8 domain, belongs to a large gene family that consists of many members in eukaryotes, including fungi, green algae, plants, and animals (16, 17). Two completely different functions have been associated with this gene family. On the one hand, the encoded proteins have been shown to act as transporters of Zn and Cd (11, 12), and on the other, they have been associated with the control of the number of cells in fruits (16, 18).
Although BjPCRs are highly similar in amino acid sequence to their Arabidopsis counterparts AtPCR1 and AtPCR2, antisense lines for BjPCR1 were not compromised in Zn translocation; however, surprisingly, they exhibited reduced translocation of Ca2+ to the shoot, which resulted in impaired growth. The impaired Ca2+ translocation into the shoot from the root of BjPCR1 antisense lines 5 and 17 is likely attributable to the impaired transfer of Ca2+ from the epidermal cells, where BjPCR1 is highly expressed, to the inner cells of the root, as evidenced by the accumulation of fluo-3 signal at the root epidermis of the antisense lines (Fig. 2F). Interestingly, in Arabidopsis, the Ca concentration in roots seemed to be tightly controlled through Ca2+ translocation to the shoot, because Arabidopsis lines grown on low and high Ca2+ concentrations exhibited similar Ca2+ concentration in the roots, whereas the shoots of plants grown on higher Ca2+ concentrations contained higher Ca2+ concentrations than those grown on lower Ca2+ concentrations (Fig. S7D). Thus, under high Ca2+ concentration conditions, Arabidopsis plants expressing BjPCR1 in the epidermis translocated more Ca from the root to the shoot, which contributed to their improved Ca tolerance (Fig. 4), most likely attributable to dilution effect. Together, these results indicate that the extrusion of Ca2+ by BjPCR1 from the epidermal cells to the apoplast of the cortical layer of the root is required for the efficient movement of Ca2+ from the root to the shoot. In addition, our temperature-dependent Ca2+ transport assay (Fig. S6) revealed that the antisense BjPCR1 plants retained more Ca2+ in the root than the WT, indicating that the energy-dependent activity of BjPCR1 is responsible for the removal of Ca2+ from the root. Because there is no extensive symplastic connection via plasmodesmata between the epidermal and cortical layers of cells in the root (19, 20), BjPCR1 is expected to remove Ca2+ to the apoplast of the root, and thereby contributes to the translocation of Ca2+ from the root to the shoot. There is some debate on whether Ca2+ is delivered to the xylem by the apoplastic or symplastic pathway across the endodermal layer of the root (1, 8, 21, 22). So far, the available data indicate that Ca2+ uptake and transfer to the xylem are achieved by a complex mechanism, which is highly regulated and may differ from one plant to another. However, at least in B. juncea, it is clear from our results that apoplastic transfer of Ca2+ at the interface of epidermal/cortical cells is an important step in the radial transfer of Ca2+ across the root. A similar function in radial translocation of metal ions at the root epidermis has been described for AtPCR2 (12). AtPCR2 is a Zn efflux transporter located at the plasma membrane of root xylem cells and epidermal cells, and an AtPCR2 knockout mutant exhibited reduced Zn translocation to shoots. Thus, efflux transport systems may be required for the radial transfer of mineral ions from epidermal to inner layers through the apoplastic pathway.
No transporter has yet been shown to be responsible for the radial transport of Ca2+ in the root. Plant roots need to transport high levels of Ca2+ in a radial direction from the epidermis to the vascular tissue, because shoots require a large amount of Ca2+ (4). To translocate high levels of Ca2+ through the epidermal cells to the inner part of a root, a high-capacity Ca2+ transporter, such as a plasma membrane-localized CAX or Na+/Ca2+ exchanger (NCX), has been postulated to exist, because epidermal cells do not contain enough plasmodesmata for an efficient symplasmic transfer of Ca2+. In mammals, an Na+/Ca2+ exchanger (NCX) prevents significant increases in intracellular Ca2+ by exhibiting low-affinity and high-capacity efflux activity (23). In plants, the presence of plasma membrane-localized CAXs was suggested based on a biochemical assay that used plasma membrane-derived vesicles from Zea mays (corn) leaves and roots; however, no plasma membrane-localized CAX gene has yet been reported in plants (6, 7). Ca2+ transport assays using BjPCR1-expressing yeast vesicles imply that BjPCR1 can function as a high-capacity and low-affinity H+/Ca2+ exporter. Experiments with agents abolishing the ΔpH, ΔΨ, or both, together with theoretical considerations, revealed that using a stoichiometry of at least three protons per exported Ca2+, BjPCR1 can efficiently export Ca2+ from the cell. A mammalian NCX (24, 25) has a stoichiometry of 3Na+/Ca2+ or 4Na+/Ca2+, whereas for a vacuolar Ca2+ proton antiporter, a stoichiometry of 3H+/Ca2+ has been postulated (26). Furthermore, studies of a CAX from Escherichia coli also pointed to a stoichiometry higher than 2H+/Ca2+ (27).
Although Ca2+ is available in sufficient amounts in the soil, Ca-related disorders, such as bitter pit in apple fruit, blossom-end rot in tomato fruit, and tip burn in the leaves of vegetables, can occur, especially in vigorously growing plants and in parts of the plant that demand a high level of Ca2+. It is therefore likely that these plants are limited in their ability to transfer Ca2+ to the above-ground parts and that genetically engineering crops with BjPCR1 might improve the quality and yield of these plants.
In addition to acting as cation transporters, members of the PLAC8 motif-containing family have been associated with the control of cell number (16, 18). Therefore, it remains an open question as to whether other genes that contain the common PLAC8 motif regulate cell number through the transport of divalent cations in a manner similar to other members of the PCR family. The fact that PCRs act as transporters of the classic signaling compound Ca2+ (in the case of BjPCR1) and the important enzyme cofactor Zn2+ (in the case of AtPCR2) may indicate that cell number is also adjusted by the transport of such cations.
Materials and Methods
The B. juncea 182921 line (28) was grown on rock-wool block containing hydroponic nutrient solution (SI Materials and Methods). For the 45CaCl2 uptake experiment, anti-BjPCR1 lines and WT B. juncea plants were grown in half-strength hydroponic medium for 3 wk. The plants were then incubated in hydroponic nutrient solution supplemented with 0.4 MBq of 45CaCl2 for 5 and 12 h, and shoots were separated from the roots. The radioactivity was measured using a liquid scintillation counter (Perkin–Elmer). Autoradiography of 45CaCl2 was performed on plants incubated in medium supplemented with 0.4 MBq of 45CaCl2 for 12 h. Other methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Armando Carpaneto for performing the oocyte experiments and Prof. Ueli Grossniklaus for help in initial in situ hybridization experiments. This work was supported by Grant K20607000006 from the Global Research Laboratory program of the Ministry of Education, Science, and Technology of Korea (to Y. L. and E. M.); Grant R31-10105 from the World Class University program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology; Grant PJ0074482011 from the Cooperative Research Program of Rural Development Administration (to Y.L.); Grant FOOD-CT-2006-0016253 from the European Union project PHIME (Public health aspects of long-term, low-level mixed element exposure in susceptible population strata) (to E.M.); and European Molecular Biology Organization Fellowship ALTF 872009 (to D.A.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.G.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104905108/-/DCSupplemental.
References
- 1.Marschner H. Mineral Nutrition of Higher Plants. San Diego: Academic; 1995. [Google Scholar]
- 2.White PJ, Broadley MR. Calcium in plants. Ann Bot (Lond) 2003;92:487–511. doi: 10.1093/aob/mcg164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maathuis FJM. Physiological functions of mineral macronutrients. Curr Opin Plant Biol. 2009;12:250–258. doi: 10.1016/j.pbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta. 2002;1564:299–309. doi: 10.1016/s0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 5.Kudla J, Batistič O, Hashimoto K. Calcium signals: The lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasai M, Muto S. Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from corn leaves. J Membr Biol. 1990;114(2):133–142. doi: 10.1007/BF01869094. [DOI] [PubMed] [Google Scholar]
- 7.Vicente JAF, Vale MGP. Activities of Ca2+ pump and low affinity Ca2+/H+ antiport in plasma membrane vesicles of corn roots. J Exp Bot. 1995;46:1551–1559. [Google Scholar]
- 8.White PJ. The pathways of calcium movement to the xylem. J Exp Bot. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- 9.Cholewa E, Peterson CA. Evidence for symplastic involvement in the radial movement of calcium in onion roots. Plant Physiol. 2004;134:1793–1802. doi: 10.1104/pp.103.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayter ML, Peterson CA. Can Ca2+ fluxes to the root xylem be sustained by Ca2+-ATPases in exodermal and endodermal plasma membranes? Plant Physiol. 2004;136:4318–4325. doi: 10.1104/pp.104.041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song WY, et al. A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. 2004;135:1027–1039. doi: 10.1104/pp.103.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song WY, et al. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell. 2010;22:2237–2252. doi: 10.1105/tpc.109.070185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang WH, Rengel Z, Kuo J. Determination of intracellular Ca2+ in cells of intact wheat roots: Loading of acetoxymethyl ester of fluo-3 under low temperature. Plant J. 1998;15:147–151. [Google Scholar]
- 14.Cho HT, Cosgrove DJ. Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell. 2002;14:3237–3253. doi: 10.1105/tpc.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain D, et al. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell. 2004;16:1327–1339. doi: 10.1105/tpc.020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M, et al. Cell Number Regulator1 affects plant and organ size in maize: Implications for crop yield enhancement and heterosis. Plant Cell. 2010;22:1057–1073. doi: 10.1105/tpc.109.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song WY, Hörtensteiner S, Tomioka R, Lee Y, Martinoia E. Common functions or only phylogenetically related? The large family of PLAC8 motif-containing/PCR genes. Mol Cells. 2011;31(1):1–7. doi: 10.1007/s10059-011-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frary A, et al. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289(5476):85–88. doi: 10.1126/science.289.5476.85. [DOI] [PubMed] [Google Scholar]
- 19.Zhu T, Lucas WJ, Rost TL. Directional cell-to-cell communication in the Arabidopsis root apical meristem. I. An ultrastructural and functional analysis. Protoplasma. 1998;203(1-2):35–47. [Google Scholar]
- 20.Ma F, Peterson CA. Frequencies of plasmodesmata in Allium cepa L. roots: Implications for solute transport pathways. J Exp Bot. 2001;52:1051–1061. doi: 10.1093/jexbot/52.358.1051. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin SB, Wimmer R. Calcium physiology and terrestrial ecosystem processes. New Phytol. 1999;142:373–417. [Google Scholar]
- 22.Yang HQ, Jie YL, Zhang LZ, Cui MG. The effect of IBA on the Ca2+ absorption and Ca2+-ATPase activity and their ultracytochemical localization in apple roots. Acta Hortic. 2004;636:211–219. [Google Scholar]
- 23.Noble D, Herchuelz A. Role of Na/Ca exchange and the plasma membrane Ca2+-ATPase in cell function. Conference on Na/Ca exchange. EMBO Rep. 2007;8:228–232. doi: 10.1038/sj.embor.7400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka Y, Hiroe K, Matsuoka S. Regulation kinetics of Na+-Ca2+ exchange current in guinea-pig ventricular myocytes. J Physiol. 2000;529:611–623. [PubMed] [Google Scholar]
- 25.Dong H, Dunn J, Lytton J. Stoichiometry of the Cardiac Na+/Ca2+ exchanger NCX1.1 measured in transfected HEK cells. Biophys J. 2002;82:1943–1952. doi: 10.1016/S0006-3495(02)75543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackford S, Rea PA, Sanders D. Voltage sensitivity of H+/Ca2+ antiport in higher plant tonoplast suggests a role in vacuolar calcium accumulation. J Biol Chem. 1990;265:9617–9620. [PubMed] [Google Scholar]
- 27.Brey RN, Rosen BP, Sorensen EN. Cation/proton antiport systems in Escherichia coli. Properties of the potassium/proton antiporter. J Biol Chem. 1980;255:39–44. [PubMed] [Google Scholar]
- 28.Ebbs SD, Kochian LV. Phytoextraction of zinc by oat (Avena sativa), barley (Hordeum vulgare), and Indian mustard (Brassica juncea) Environ Sci Technol. 1998;32:802–806. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.