Abstract
Acute myocardial infarction (AMI) initiates an intense inflammatory response that promotes cardiac dysfunction, cell death, and ventricular remodeling. The molecular events underlying this inflammatory response, however, are incompletely understood. In experimental models of sterile inflammation, ATP released from dying cells triggers, through activation of the purinergic P2X7 receptor, the formation of the inflammasome, a multiprotein complex necessary for caspase-1 activation and amplification of the inflammatory response. Here we describe the presence of the inflammasome in the heart in an experimental mouse model of AMI as evidenced by increased caspase-1 activity and cytoplasmic aggregates of the three components of the inflammasome—apoptosis speck-like protein containing a caspase-recruitment domain (ASC), cryopyrin, and caspase-1, localized to the granulation tissue and cardiomyocytes bordering the infarct. Cultured adult murine cardiomyocytes also showed the inducible formation of the inflammasome associated with increased cell death. P2X7 and cryopyrin inhibition (using silencing RNA or a pharmacologic inhibitor) prevented the formation of the inflammasome and limited infarct size and cardiac enlargement after AMI. The formation of the inflammasome in the mouse heart during AMI causes additional loss of functional myocardium, leading to heart failure. Modulation of the inflammasome may therefore represent a unique therapeutic strategy to limit cell death and prevent heart failure after AMI.
Keywords: interleukin-1, NALP3, NLRP3, pyroptosis
Despite the progress in the treatment of acute myocardial infarction (AMI), many patients die early during AMI and those who survive are at risk for developing heart failure (1–4), suggesting that the current treatment paradigm still misses one or more key pathophysiologic mechanisms. There is therefore a need to develop additional treatment strategies to prevent heart failure after AMI.
Ischemic injury initiates an intense inflammatory response that promotes further dysfunction and heart failure (5, 6). Cryopyrin (Nalp3/Nlrp3) is an intracellular Nod-like receptor that functions as a danger signal sensor that becomes activated in response to intracellular infections (i.e., bacterial and viral proteins), ATP, and other cellular debris released during tissue injury, or intracellular accumulation of uric acid, or cholesterol crystals (7, 8). Cryopyrin activation leads to recruitment of the apoptosis speck-like protein containing a caspase-recruitment domain (ASC) and formation of the inflammasome, a multiprotein complex necessary for caspase-1 activation and interleukin-1β (IL-1β) release (7, 8). Caspase-1 is a key modulator of the inflammatory response to tissue injury and participates both in the amplification of the inflammatory response and also in the promotion of cell death (7–9). Experimental studies in mice with genetic deletion of caspase-1 have identified caspase-1 inhibition as a potential target for pharmacologic intervention in the setting of AMI (10–12). A recent report described formation of the inflammasome in a mouse model of ischemia/reperfusion and reports that ASC knockout mice were protected (13). Herein we describe formation of the inflammasome in the myocardium leading to adverse cardiac remodeling and increased caspase-1–mediated cell death in a more severe model of ischemia without reperfusion. We also describe pharmacologic inhibition of cryopyrin and P2X7 to prevent inflammasome formation and ameliorate cardiac damage as a potential basis for translational investigation.
Results
Caspase-1 Is Activated in AMI.
Caspase-1 mRNA synthesis increased severalfold in the heart at 3 and 7 d after AMI (Fig. S1). Caspase-1 activation was also increased at 7 d as measured by increased procaspase-1, increased cleaved caspase-1, and increased cleaved/procaspase-1 ratio (Fig. S1). The marked increase in procaspase-1 mRNA and cleaved caspase-1 protein compared with a relatively small increase in procaspase-1 protein suggests rapid cleavage of procaspase-1. Increased caspase-1 enzymatic activity in the heart was detectable as early as 6 h after surgery, reached a peak between 3 and 7 d, and persisted for up to 14 d (Fig. S1). Parallel increases in the expression of ASC, a structural component of the inflammasome, were also noted over a similar time course after AMI (Fig. S2).
Expression of the Inflammasome Components in the Heart During AMI.
Immunofluorescence staining for ASC appeared as perinuclear cytoplasmic aggregates in leukocytes, endothelial cells, and fibroblasts in the granulation tissue as well as in cardiomyocytes in the infarct border zones, with minimal expression in the remote myocardium, and virtually no staining in the hearts of sham-operated mice (Fig. 1 and Figs. S3 and S4). ASC was highly expressed in the granulation tissue 3 d after AMI, in CD45+ leukocytes as well as in S100A4+ fibroblasts and caveolin-1+ endothelial cells, and also in the cytoplasm of cardiac actin+ cardiomyocytes bordering the infarct (Fig. 1 and Fig. S3). Seven days after AMI, ASC staining was more prominently detected in the cardiomyocytes (cardiac actin+) in the infarct border zone (Fig. 1). Similar to ASC, cryopyrin was mainly expressed in the granulation tissue and colocalized with CD45+ leukocytes, S100A4+ fibroblasts, and caveolin-1+ endothelial cells 3 and 7 d after AMI (Fig. 1 and Figs. S3 and S4). In sham-operated animals (control group) and in the remote zone 3 and 7 d after AMI, very little expression of ASC, cryopyrin, or caspase-1 was detected in cardiomyocytes, leukocytes, fibroblasts, or endothelial cells (Fig. 1 and Fig. S3). A semiquantitative assessment of the formation of the inflammasome in the different cell types is presented in Fig. 2.
Fig. 1.
Formation of the inflammasome in the granulation tissue and cardiomyocytes. Immunofluorescence shows overlap of cardiac actin (green) and the different components of the inflammasome [ASC (red, A–D), cryopyrin (red, E–H), and caspase-1 (red, I–L)] in the granulation tissue and border zone, 3 and 7 d after AMI (C, G, and K and D, H, and L, respectively), with minimal staining and overlap in the sham-operated mouse or the remote zone (A, E, and I and B, F, and J, respectively). Counterstaining with DAPI (blue). Original magnification 40×. (Scale bar, 20 μm.)
Fig. 2.
Cell type distribution of the inflammasome in acute myocardial infarction. The graph shows results of a semiquantitative assessment of ASC expression by immunofluorescence (examples in Fig. 1). Expression was quantified as intense (3+), moderate (2+), weak (1+), or no expression (0) (Materials and Methods) by three investigators separately and then averaged. Mean ± SEM values are reported (n = 3–4 per group).
Cryopyrin mainly colocalized with ASC and caspase-1 in the cytoplasm of noncardiomyocytes in the granulation tissue and in some cardiomyocytes in the border zone, 3 d after AMI, whereas costaining for ASC, cryopyrin, and caspase-1 was found to be stronger in the cardiomyocytes of the border zone 7 d after AMI (Fig. S4).
The Inflammasome Is Induced in Cardiomyocytes.
We further characterized the formation of the inflammasome in cardiomyocytes using a stable adult murine cardiomyocyte cell line (HL-1). In vitro “simulated ischemia” induced formation of the inflammasome, activation of caspase-1, and cell death (Fig. 3), whereas addition of “ischemic medium” did not activate the inflammasome, showing that in vitro ischemia activates the inflammasome by a mechanism other than true soluble mediator (Fig. S5).
Fig. 3.
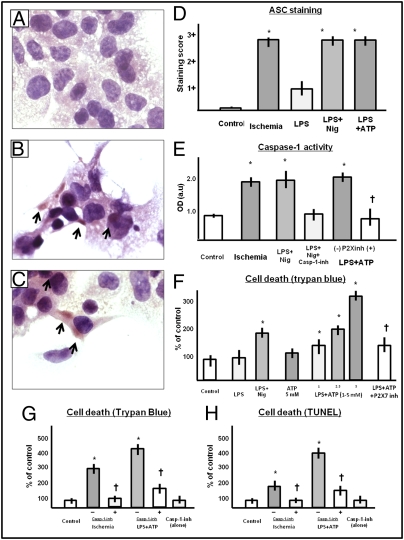
Formation of the ASC inflammasome in isolated cardiomyocyte in culture. (A–C) Effects of LPS pretreatment (priming) and nigericin or ATP triggering on adult cardiomyocytes (HL-1) in culture. (A) Untreated cell culture. (B) Culture treated with LPS and nigericin. (C) Culture treated with LPS and ATP. ASC aggregates in the cytoplasm are evident in B and C (arrows). (D–F) Mean ± SEM of ASC staining expressed in a semiquantitative scale in which 1+ is mild expression and 3+ is intense expression (D), caspase-1 activity assessed using an enzymatic assay (N-Ac-Tyr-Val-Ala-Asp-CHO was used as caspase-1 inhibitor) (E), cell death assessed by either Trypan blue or TUNEL (F–H) in cultured adult HL-1 cardiomyocytes treated with LPS and triggered with nigericin, or ATP, or simulated ischemia. P2X7 inh refers to the use of a pharmacologic P2X7 inhibitor, PPADS (5 mM, equimolar to ATP), used as an ATP antagonist. Simulated ischemia refers to simulated ischemia in vitro obtained by exposing cells to hypoxia and an “ischemic” buffer. A caspase-1 inhibitor (casp-1 inh, benzyl-oxycarbonyl-Trp-Glu(OMe)-His-Asp(OMe)-fluoromethylketone) was added to determine whether cell death was caspase-1 dependent or not. *P < 0.05 vs. control conditions, †P < 0.05 for P2X7 inh vs. no treatment. Details of D–H are found in Materials and Methods.
To study a more classical way to trigger the formation of the inflammasome, we primed HL-1 cells with lipopolysaccharide (LPS) and then triggered with nigericin or adenosine-triphosphate (ATP) inducing a reproducible formation of the inflammasome (Fig. 3). Addition of LPS or ATP alone were not sufficient to stimulate the formation of the inflammasome (Fig. 3). Induction of the inflammasome in HL-1 cells led to a significant increase in cell death (measured by Trypan blue exclusion or TUNEL for DNA fragmentation, Fig. 3) that paralleled caspase-1 activity, and was inhibited by the addition of a caspase-1 inhibitor (Fig. 3).
Whereas cryopyrin is the intracellular danger sensor (Nod-like receptor) leading to the formation of the inflammasome, P2X7 is the purinergic receptor channel that is activated by extracellular ATP (a byproduct of cell death) and leads to K+ efflux and cryopyrin activation (7–9). Addition of a pharmacologic P2X7 inhibitor, pyridoxalphosphate-6′-azopheny-2′,4′-disulphonate (PPADS), inhibited the effects of ATP on both caspase-1 activity and cell death. Taken together, these data demonstrate the formation of the inflammasome in the myocardium leading to caspase-1 activation and cell death and support the targeting of inflammasome formation as a potential strategy to protect the myocardium following AMI.
Cryopyrin and P2X7 Gene Silencing Inhibit Caspase-1 Activation During AMI.
Gene silencing was achieved by systemic administration of a high dose of commercially available small interfering (si)RNAs directed toward cryopyrin or P2X7 using a previously validated protocol (see Materials and Methods). Silencing efficacy by Western blot (WB) showed a marked decrease (>80%, P < 0.05) in the targeted protein expression (Fig. 4). Cryopyrin- and P2X7 directed siRNA prevented the increase in caspase-1 activity 72 h after coronary artery ligation surgery, inducing AMI and ameliorated cardiac remodeling at 7 d as reflected in less enlargement and dysfunction, whereas scrambled (nontargeted) siRNA had no effect (Fig. 4). These data support the concept that inhibition of one of the components of the inflammasome may be sufficient to blunt caspase-1 activation during AMI.
Fig. 4.
Cryopyrin- and P2X7 targeted interventions in acute myocardial infarction. (A) Cryopyrin- and P2X7 targeted small interfering (si)RNAs administered intraperitoneally 24 h before surgery leading to a >80% reduction in target protein expression (Western blot) and a significant reduction in caspase-1 activity. Treatment with a pharmacological P2X7 inhibitor (PPADS 25 mg/kg intraperitoneally daily) provided a similar effect on caspase-1 activity. Values are expressed as mean ± SEM % of control values; n = 4–6 per group, *P < 0.05 vs. sham-operated mice. (B and C) Histology (Masson's trichrome) of cardiac left ventricle midsections of mice 7 d after AMI and treatment with vehicle (NaCl 0.9%) or a P2X7 inhibitor (PPADS), respectively. (D–F) Reductions in ASC expression (Western blot, day 3) and in infarct size (Masson's trichrome, day 7) and apoptosis (in situ end labeling of DNA fragmentation, day 7), and in mice treated with a P2X7 inhibitor (PPADS) vs. vehicle (NaCl 0.9%) after AMI. Values are expressed as mean ± SEM; n = 4–6 per group, *P < 0.05 vs. sham-operated mice, †P < 0.05 vs. control AMI. (G and H) Monodimensional echocardiography recordings obtained 7 d after AMI in mice treated with NaCl 0.9% (control) or a P2X7 inhibitor (PPADS), respectively. (I–N) Reductions in left ventricular enlargement (end-diastolic and end-systolic diameters) in mice 7 d after AMI treated with a P2X7 inhibitor (PPADS) vs. vehicle, without any difference in left ventricular hypertrophy. (O–R) Similar reductions in left ventricular enlargement (end-diastolic and end-systolic diameters) in mice 7 d after AMI treated with silencing RNA targeted to cryopyrin and P2X7 but not with scrambled siRNA sequences. Values are expressed as mean ± SEM; n = 6–8 per group, *P < 0.05 vs. sham-operated mice, †P < 0.05 vs. control AMI.
Inhibition of P2X7 Limits Cell Death and Ameliorates Cardiac Remodeling Following AMI.
PPADS is a noncompetitive antagonist of the P2X7 receptor with a receptor affinity (pIC50) for P2X7 of 4.3 μM widely used in preclinical mouse models (14, 15). In vitro, PPADS (100 μM) added to HL-1 cells exposed to simulated ischemia for 2.5 h significantly reduced cell death (control conditions 4.8 ± 0.4%, simulated ischemia 8.2 ± 0.9%, simulated ischemia + PPADS 5.4 ± 1.0%, P < 0.05 vs. simulated ischemia alone, P = NS vs. control). In vivo, PPADS was administered intraperitoneally using a previously validated dose of 25 mg/kg given daily for 7 d starting 5 min after surgery (14, 15). P2X7 inhibition by PPADS treatment blunted the increase in ASC expression and caspase-1 activity 3 d after AMI in a fashion similar to that of P2X7 targeted siRNA (Fig. 4). Cell death, measured as the number of TUNEL+ cardiomyocytes in the infarct border zone (reflecting DNA fragmentation in programmed cell death) or assessed by the extent of the replacement fibrosis using Masson's trichrome stain (reflecting necrosis), was also significantly reduced by P2X7 inhibition with PPADS (Fig. 4). P2X7 inhibition by PPADS led to a reduction in cardiac enlargement without inhibition of compensatory hypertrophy (Fig. 4).
Discussion
Myocardial ischemia leads to initial damage that is characterized by an intense inflammatory response. Caspase-1 activity is increased in the myocardium minutes after the onset of ischemia, remains elevated for several days, and leads to cardiomyocyte death (10–12). Herein we confirm the presence of cryopyrin inflammasomes in leukocytes, endothelial cells, and fibroblasts in the granulation tissue early during the infarct period and describe formation of active cryopyrin inflammasomes in cardiomyocytes bordering the infarct zone later during the infarct process. Using an isolated culture of adult cardiac myocytes, we also observed that the induction of the inflammasome led to a significant increase in caspase-1 activity accompanied by a dose-dependent increase in cell death. Taken together, these findings suggest a significant role for the inflammasome in the cardiomyocyte and identify disruption of the inflammasome as a unique strategy to prevent further loss-of-functional myocardium following AMI and to prevent adverse cardiac remodeling. Unfavorable cardiac remodeling is indeed the substrate for heart failure and related mortality after AMI (16). The favorable changes in cardiac remodeling seen with inflammasome-targeted therapy (30–50% less enlargement of the left ventricle (LV) compared with the control group) suggest that such treatment may potentially translate into the clinical arena in which small improvements in LV dimensions have led to a significant survival benefit after AMI (16).
Our findings are consistent with the recent report by Kawaguchi and colleagues that reported the formation of the inflammasome in the mouse heart, mainly in cardiac fibroblasts and infiltrating cells 48 h after ischemia/reperfusion injury (13). The presence of the inflammasome in cardiomyocytes at 48 h was also noted but considered marginal, likely due to the fact that the cardiomyocyte failed to release active IL-1β (13). Our study expands upon these findings to show that the inflammasome is present in cardiomyocytes, that the cardiomyocyte is the most prevalent location of the inflammasome by day 7, and, most importantly, that induction of the inflammasome in the cardiomyocyte leads to cell death. The formation of the inflammasome appears to be independent of whether or not cardiomyocytes release active IL-1β, because the release of active IL-1β is dependent upon transcription of pro–IL-1β and regulated by cell-specific transcription factors. We indeed show that, independent of IL-1β release, the cardiomyocyte contains the structural components of the inflammasome (ASC, cryopyrin, and caspase-1) and active caspase-1 which ultimately induce caspase-1–dependent cell death. Although overlap of different modalities of cell death may occur, the observation of caspase-1–dependent cell death, DNA fragmentation, and loss-of-membrane integrity indicate the presence of pyroptosis or inflammatory cell death (9, 17). Accordingly, we show that strategies targeted to disrupt inflammasome activation led to significantly less cardiomyocyte loss and more favorable cardiac remodeling.
P2X7 is activated by extracellular ATP and leads to K+ efflux, cryopyrin activation, and cell death (7–9). ATP is a byproduct of cell death and is also actively released by monocytes at the site of acute inflammation (18, 19). Our data show that inhibition of cryopyrin or P2X7 using silencing RNA is sufficient to blunt caspase-1 activation during AMI. Moreover we show that prevention of the formation of the inflammasome using a P2X7 inhibitor reduced cell death and adverse cardiac remodeling. These data confirm the central role of caspase-1 in the myocardial response to ischemia and begin to explain the mechanisms leading to enhanced caspase-1 activity. Ischemia may lead caspase-1 activation through several mechanisms (direct and indirect) and the exact mechanism involved in the formation of the inflammasome in the heart during AMI cannot be defined. Overexpression of caspase-1 caused cell death in cultured cardiomyocytes, whereas deletion of endogenous caspase-1 was previously shown to protect against ischemia/reperfusion-induced death (10–12). Accordingly, mice overexpressing caspase-1 had larger areas of ischemic damage, more severe cardiac enlargement, and a reduced survival after AMI; whereas caspase-1–deficient mice were protected after AMI (10–12). In the model of cardiomyocyte-specific overexpression of caspase-1 mice developed heart failure after AMI, independent of detectable formation of active IL-1β and IL-18 (10–12), which is consistent with the notion that activation of the inflammasome and caspase 1 in the cardiomyocyte may be disconnected from IL-1β processing and release. Caspase-1 activation and the formation of the inflammasome occur also in the brain in response to cerebral ischemia and traumatic injury (20–24). The concept of P2X7 activation by cellular debris (including ATP, other purines, and other cellular contents) leading to inflammasome formation during sterile inflammation likely represents a general and ubiquitous modality of amplification and regulation of the inflammatory cascade (20–26). P2X7 targeted strategies have so far been effective in limiting neuronal damage, lung, liver, and kidney injury in several preclinical disease models (14, 15, 27, 28). The results of our experiments demonstrate that ischemia and necrosis of cardiomyocytes during AMI trigger the formation of the ASC/cryopyrin inflammasome via activation of the P2X7 receptor channel and that pharmacological inhibition of P2X7 is sufficient to prevent cell death and adverse cardiac remodeling in a model of severe ischemic damage in the setting of a nonreperfused AMI. We chose the model of nonreperfused AMI because although most of the patients with AMI receive some form of intervention aimed at obtaining reperfusion, incomplete tissue level reperfusion (no reflow) occurs in a large number of patients, which negates the benefit of reperfusion and is associated with a greater risk of subsequent heart failure (29). Fig. 5 shows a simplified scheme proposing the central roles of the ASC/cryopyrin inflammasome and the P2X7 receptor in the promotion of heart failure after AMI. Release of active IL-1β from activated leukocytes or endothelial cells following formation of the inflammasome triggers a sterile inflammation response that amplifies the initial injury (25, 26). Furthermore “IL-1 induces more IL-1” and therefore release of active IL-1β likely induces more caspase-1 activation in an autocrine and paracrine fashion (30). This is in agreement with the beneficial effects seen in this model with IL-1 blockade with anakinra (31). caspase-1 however, may also be involved in the release of other proinflammatory molecules other than IL-1β (32).
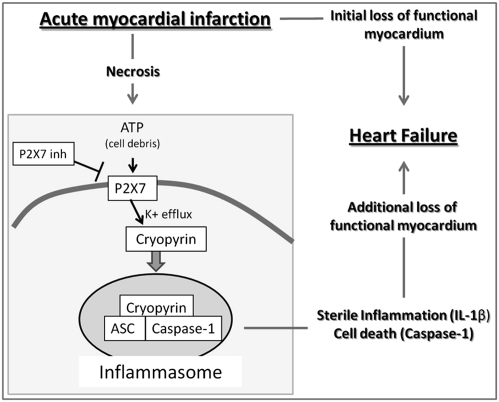
Fig. 5.
Central role of the inflammasome in acute myocardial infarction. Simplified scheme proposing the central roles of the P2X7 receptor and the ASC/cryopyrin inflammasome in the promotion of heart failure after AMI. ATP and other cellular debris released during ischemic myocardial necrosis activates the membrane P2X7 receptor channel and leads to cryopyrin activation by facilitating K+ efflux from the cell. Activated cryopyrin recruits the ASC scaffolding protein and procaspase-1 allowing for homodimerization and autocatalytic activation of caspase-1. Active caspase-1 cleaves pro–IL-1β and other proinflammatory cytokines into the active isoforms thus amplifying the inflammatory response (sterile inflammation). Active caspase-1 also triggers cell death, further contributing to the additional loss of functional myocardium and subsequent heart failure following AMI.
Our experimental studies are limited in that (i) other forms of the inflammasome not containing the ASC/cryopyrin complex have been described and were not investigated in this study; (ii) we find evidence of activation of the inflammasome in several different cell types, and by the means of systemic silencing RNA or pharmacologic inhibition we cannot distinguish whether inhibition of the inflammasome in cardiomyocytes or noncardiomyocytes drives the benefits on cardiac remodeling; (iii) the P2X7 receptor is involved in other processes (i.e., IL-1β release), which may also affect the inflammatory response; and (iv) PPADS can also inhibit other members of the purinergic P2 receptor family. Moreover, given the close relationship of inflammation and tissue injury, we suspect that inflammation is the result of injury and the cause of further injury. However, the exact causal link between these two processes could not be determined in our studies. Despite such limitations, however, the results of our study point toward a key pathologic role of the ASC/cryopyrin inflammasome in postinfarction remodeling.
Conclusions
The results of our study provide evidence for the formation of the ASC/cryopyrin inflammasome in the myocardium during AMI and suggest that cryopyrin and P2X7 are potential targets for intervention for the prevention of heart failure following AMI.
Materials and Methods
Experimental AMI Model.
Adult male out-bred Institute of Cancer Research (CD1) mice were supplied by Harlan Labratories. Experimental AMI was induced by permanent left coronary artery ligation as described previously (31).
Caspase-1
Hearts were collected 3 and 7 d after surgery (n = 4–6 per group) for evaluation of caspase-1 mRNA (real-time PCR) and protein (Western blot) synthesis, and caspase-1 enzymatic activity. Detailed procedures are included in SI Materials and Methods.
Expression of the Component of the Inflammasome in the Heart During AMI.
The whole hearts were collected at 3 and 7 d after surgery. Procedures for immunofluorescence staining of ASC, cryopyrin, and cleaved caspase-1 in the heart during AMI are included in SI Materials and Methods. Expression of the components of the inflammasome was quantified by three different investigators using a semiquantitative scale and expressed as mean and SEM (SI Materials and Methods). Analysis was performed considering the granulation tissue in the infarct area composed of tissue debris, leukocytes, endothelial cells and fibroblasts, the periinfarct border zone, and the remote myocardium in an area of the heart opposite to the infarct.
Formation of the Inflammasome in Isolated Cardiomyocytes in Vitro.
HL-1 cells represent an immortalized adult murine cardiomyocyte cell line kindly donated by Dr. Claycomb (Louisiana State University, New Orleans, LA) and cultured in Claycomb medium (Sigma-Aldrich) as suggested by the manufacturer (33). We exposed the HL-1 cells to simulated ischemia. HL-1 cells were plated at a density of 400,000 cells per 35-mm dish 24 h before simulated ischemia for 6 h as described in SI Materials and Methods. As an additional experiment, we transferred the medium of HL-1 cells exposed to 2.5 h, or 12 h of ischemia to healthy HL-1 and then measured caspase-1 activation as a marker of the formation of the inflammasome.
To study a more classical way to trigger the formation of the inflammasome, HL-1cells were primed with Escherichia coli 0111:B4 LPS (25 ng/mL; Sigma-Aldrich) for 2 h and then aggregation of the inflammasome was induced by nigericin (20 μM; Sigma-Aldrich) or ATP (1–5 mM; Sigma-Aldrich) for 1 h (34). To test the inhibitory effects of PPADS on ATP-induced formation of the inflammasome and caspase-1 activation, equimolar PPADS was added to ATP (5 mM). The formation of the inflammasome in HL-1 cells was determined and quantified by immunohistochemistry as described above. The cells were plated on 24 × 24 mm glass covers pretreated with gelatin/plasma human-fibronectin (0.02–0.5%) at 2.5 × 105 in 35-mm dishes 24 h before the experiment. ASC is not or minimally expressed in HL-1 cells. Upon triggering of the inflammasome, ASC expression was detected as circumscribed cytoplasmic perinuclear aggregates and expressed semiquantitatively on a 1–3 scale. In a parallel experiment 1 × 106 cells were plated in 60-mm dishes and 24 h later, primed and induced as described above. After the treatment the cells were washed, harvested, and frozen for caspase-1 activity assessment as described above. Each experiment was performed in triplicate.
Assessment of Cell Death in Isolated Cardiomyocytes.
Cell death in HL-1 cardiomyocytes in vitro was assessed determining loss of membrane integrity using Trypan blue as well as determining nuclear DNA fragmentation using in situ end labeling (TUNEL) (SI Materials and Methods). Trypan-blue positivity reflected loss of membrane integrity as seen in necrotic/oncotic cell death or in pyroptosis (17). TUNEL was performed according to the supplier's instructions (31). Nuclear DNA fragmentation is a hallmark of programmed cell death, namely apoptosis or pyroptosis (17). Pyroptosis is caspase-1–dependent cell death; to determine whether HL-1 cardiomyocytes death was caspase-1 dependent, a caspase-1 inhibitor was added in vitro.
Silencing RNA.
Targeting and scrambled siRNA were purchased from Santa Cruz. We used a previously validated approach of high-dose (0.45 mg/kg) systemic (intraperitoneal) injection mixed with an equal volume of siPORT amine (Ambion) in the live animal (35, 36). For both cryopyrin and P2X7 a dose of 0.45 mg/kg induced a significant >80% reduction in target protein at 24 h. We then used this approach in the AMI model by administering the siRNA 24 h before surgery and then again every 72 h. Silencing efficacy was evaluated by WB using an anti-cryopyrin polyclonal antibody (Santa Cruz) and an anti-P2X7 polyclonal antibody (Sigma-Aldrich).
Pharmacologic Treatment.
To evaluate the effects of pharmacologic P2X7 inhibition, after surgery mice were randomly assigned to treatment with PPADS or a matching volume of vehicle (NaCl 0.9%, n = 12 per group). PPADS was purchased from Tocris Bioscience and prepared according to the supplier's instructions, PPADS was administered intraperitoneally at a dose of 25 mg/kg given immediately after surgery and then daily for a period of 7 d.
Echocardiography.
All animals underwent transthoracic echocardiography before surgery and 7 d later. Echocardiography was performed with the Vevo770 imaging system (VisualSonics) and a 30-MHz probe as previously described (31).
Infarct Size and Programmed Cell Death.
Mice were killed on day 7 after echocardiogram and the hearts were explanted and fixed in formalin 10% for at least 48 h. A transverse section of the median third of the heart was dissected, included in paraffin, cut into 5-μm slides, and stained with Masson's trichrome (Sigma-Aldrich). The areas of fibrosis and the whole left ventricle were determined by computer morphometry using the Image Pro Plus 6.0 software (31). Programmed cell death was defined as fragmentation of nuclear DNA reflecting either apoptosis or pyroptosis. The number of cardiomyocytes nuclei showing DNA fragmentation at in situ end labeling (TUNEL) were counted and expressed as percentage of total nuclei. The TUNEL technique was performed according to the supplier's instruction as previously described (31).
Statistics.
Differences between the groups were analyzed using the one-way ANOVA and changes in repeated measures in echocardiographic data were analyzed using the random effects ANOVA for repeated measures to determine the main effect of time, group, and time-by-group interaction using the SPSS 15.0 package for Windows.
Supplementary Material
Acknowledgments
This work was supported in part by an American Heart Association Beginning Grant-in-Aid (Mid-Atlantic affiliate) (to A.A.). B.W.V.T. is supported by National Institutes of Health Grant KL2RR031989-01. This work was also supported by funds from the Victoria Johnson Research Laboratories (to N.F.V.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108586108/-/DCSupplemental.
References
- 1.Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg H, McMaster P, Dwyer EM., Jr Left ventricular dysfunction after acute myocardial infarction: Results of a prospective multicenter study. J Am Coll Cardiol. 1984;4:867–874. doi: 10.1016/s0735-1097(84)80045-5. [DOI] [PubMed] [Google Scholar]
- 3.Velagaleti RS, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg RJ, et al. A 25-year perspective into the changing landscape of patients hospitalized with acute myocardial infarction (the Worcester Heart Attack Study) Am J Cardiol. 2004;94:1373–1378. doi: 10.1016/j.amjcard.2004.07.142. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz) 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stutz A, Golenbock DT, Latz E. Inflammasomes: Too big to miss. J Clin Invest. 2009;119:3502–3511. doi: 10.1172/JCI40599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, et al. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed FM, et al. Proapoptotic effects of caspase-1/interleukin-converting enzyme dominate in myocardial ischemia. Circ Res. 2005;96:1103–1109. doi: 10.1161/01.RES.0000166925.45995.ed. [DOI] [PubMed] [Google Scholar]
- 11.Merkle S, et al. A role for caspase-1 in heart failure. Circ Res. 2007;100:645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- 12.Frantz S, et al. Targeted deletion of caspase-1 reduces early mortality and left ventricular dilatation following myocardial infarction. J Mol Cell Cardiol. 2003;35:685–694. doi: 10.1016/s0022-2828(03)00113-5. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi M, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 14.Csölle C, Sperlágh B. Peripheral origin of IL-1β production in the rodent hippocampus under in vivo systemic bacterial lipopolysaccharide (LPS) challenge and its regulation by P2X(7) receptors. J Neuroimmunol. 2010;219:38–46. doi: 10.1016/j.jneuroim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Gourine AV, et al. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005;146:139–145. doi: 10.1038/sj.bjp.0706287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer DG, et al. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: A meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroemer G, et al. Nomenclature Committee on Cell Death 2009 Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ninomiya H, et al. Complementary role of extracellular ATP and adenosine in ischemic preconditioning in the rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H1810–H1820. doi: 10.1152/ajpheart.00760.2001. [DOI] [PubMed] [Google Scholar]
- 19.Piccini A, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trendelenburg G. Acute neurodegeneration and the inflammasome: Central processor for danger signals and the inflammatory response? J Cereb Blood Flow Metab. 2008;28:867–881. doi: 10.1038/sj.jcbfm.9600609. [DOI] [PubMed] [Google Scholar]
- 21.Rawat R, et al. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol. 2010;176:2891–2900. doi: 10.2353/ajpath.2010.090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Y, et al. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abulafia DP, et al. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 24.de Rivero Vaccari JP, et al. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MCDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 27.Riteau N, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama T, et al. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci. 2010;51:3236–3243. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- 29.Abbate A, Kontos MC, Biondi-Zoccai GGL. No reflow: The next challenge in the treatment of ST-segment elevation acute myocardial infarction. Eur Heart J. 2008;19:1785–1787. doi: 10.1093/eurheartj/ehn281. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139:1902–1910. [PubMed] [Google Scholar]
- 31.Abbate A, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 32.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 33.Claycomb WC, et al. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 35.Van Tassell BW, et al. Pharmacologic inhibition of myeloid differentiation factor 88 (MyD88) prevents left ventricular dilation and hypertrophy after experimental acute myocardial infarction in the mouse. J Cardiovasc Pharmacol. 2010;55:385–390. doi: 10.1097/FJC.0b013e3181d3da24. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan R, et al. Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am J Physiol Heart Circ Physiol. 2007;293:H1571–H1580. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.