Abstract
Thyroid hormone (TH) metabolism, mediated by deiodinase types 1, 2, and 3 (D1, D2, and D3) is profoundly affected by acute illness. We examined the role of TH metabolism during ventilator-induced lung injury (VILI) in mice. Mice exposed to VILI recapitulated the serum TH findings of acute illness, namely a decrease in 3,5,3′-triiodothyronine (T3) and thyroid-stimulating hormone and an increase in reverse T3. Both D2 immunoreactivity and D2 enzymatic activity were increased significantly. D1 and D3 activity did not change. Using D2 knockout (D2KO) mice, we determined whether the increase in D2 was an adaptive response. Although similar changes in serum TH levels were observed in D2KO and WT mice, D2KO mice exhibited greater susceptibility to VILI than WT mice, as evidenced by poorer alveoli integrity and quantified by lung chemokine and cytokine mRNA induction. These data suggest that an increase in lung D2 is protective against VILI. Similar findings of increased inflammatory markers were found in hypothyroid WT mice exposed to VILI compared with euthyroid mice, indicating that the lungs were functionally hypothyroid. Treatment of D2KO mice with T3 reversed many of the lung chemokine and cytokine profiles seen in response to VILI, demonstrating a role for T3 in the treatment of lung injury. We conclude that TH metabolism in the lung is linked to the response to inflammatory injury and speculate that D2 exerts its protective effect by making more TH available to the injured lung tissue.
Keywords: nonthyroidal illness, euthyroid sick, reverse triiodothyronine, bronchial alveolar lavage, sepsis
Thyroid hormone [TH, here denoting the active hormone 3,5,3′-triiodothyronine (T3) and its precursor thyroxine (T4)] is necessary for the normal development and function of virtually every vertebrate tissue. Although the thyroid gland is predominantly responsible for the production and release of T4, the cellular bioavailability of the active T3 depends on TH-specific transporters and tissue TH metabolism. Metabolism of TH is regulated by three iodothyronine deiodinases. Both type 1 (D1) and type 2 (D2) deiodinases serve to generate bioactive T3 by outer-ring deiodination of T4. D2 has a major role in the intracellular generation of the active TH in mouse; the role of the D1, with its broad substrate specificity and ability to carry out both outer- and inner-ring deiodination, is less well understood. In contrast, type 3 iodothyronine deiodinase (D3) inactivates TH by inner-ring deiodination of T4 to reverse T3 (3′,5,3-triiodothyronine; rT3) and T3 to 3,3′-diiodothyronine (T2), both biologically inactive compounds (1).
Studies have elucidated the dynamic effects of TH action on brain, bone, liver (2–5), and maturation of the fetal lung (6, 7), but little is known regarding the role of TH in adult lung physiology. Until now, evidence suggesting that TH influences adult lung physiology has been indirect and based on the presence of TH receptors (8), deiodinases (9–12), and measured TH concentrations (10, 13, 14) in the lung.
In severe illness associated with lung injury, there are systemic changes in circulating TH levels similar to those observed in other illnesses known as “nonthyroidal illness” (NTI). These changes are characterized by a decrease in serum T4, T3, and thyroid-stimulating hormone (TSH) with an increase in rT3 (15, 16). The mechanisms behind changes in serum TH concentrations that occur in NTI include induction of central hypothyroidism resulting from a diminution in hypothalamic thyrotrophin-releasing hormone. This decrease can be signaled by a localized increase in hypothalamic T3 catalyzed by altered expression of hypothalamic iodothyronine deiodinases D2 and D3 (17).
It generally is accepted that extrathyroidal conversion of T4 to T3 is decreased in illness because of a reduction in both hepatic/renal D1 activity and skeletal muscle D2 activity (18). In addition to these changes, there may be a reactivation of hepatic and skeletal muscle D3, which leads to increased production of rT3 (19, 20). However, data from D1, D2, and D3 knockout mice suggest that these enzymes may have little contribution to the low serum T3 found in acute illness (9, 21). Because circulating T3 decreases before many T3-dependent tissues become T3 deficient, it is controversial whether tissues during severe illness are functionally hypothyroid, as indicated by the low serum or tissue levels of TH (21, 22). The purpose of the current study was to determine whether deiodinase activity as a marker of TH metabolism in the lung is altered in response to lung injury and whether the changes associated with NTI are adaptive or maladaptive. We examined the role of TH metabolism in a mouse model of ventilator-induced lung injury (VILI) (23–26). We demonstrate that TH concentrations in blood after VILI recapitulate those seen with NTI. Using mice deficient in deiodinases, we were able to demonstrate that D2 is protective against the effects of VILI. Furthermore, hypothyroid mice had more severe acute inflammatory lung injury than euthyroid mice in response to VILI, and administration of triiodo-l-thyronine (l-T3) to type 2 deiodinase knockout (D2KO) mice reduced the inflammatory lung injury. We conclude that TH metabolism in the lung may be linked to the response to inflammatory injury and speculate that D2 exerts its protective effect by making more TH available to the injured lung tissue.
Results
Serum Thyroid Function Tests in WT Mice After Exposure to VILI.
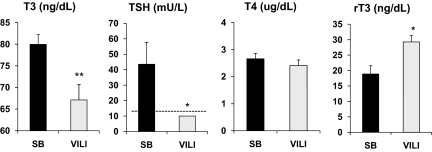
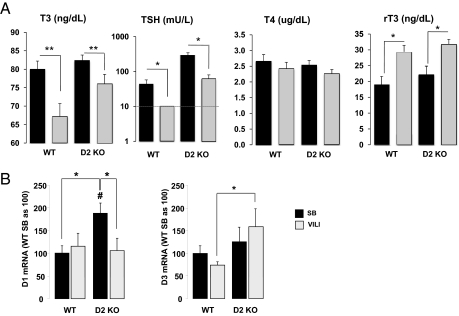
After 4 h of VILI, T3 serum levels were reduced by 16.1 ± 4.5% compared with spontaneously breathing (SB) animals (67.1 ± 3.6 vs. 80.0 ± 2.3 ng/dL, P < 0.01) (Fig. 1). Similarly, after VILI, the circulating TSH levels were reduced significantly, to undetectable levels, compared with SB controls (<10 vs. 44.5 ± 14.3 mU/L, P < 0.05) (Fig. 1). Although no significant differences were found in T4 serum levels between ventilated and SB animals (2.4 ± 0.2 vs. 2.6 ± 0.2 μg/dL) (Fig. 1), a significant increase in rT3 was observed after mechanical ventilation (18.9 ± 2.7 in SB animals vs. 29.3 ± 2.1 ng/dL after VILI, P < 0.05) (Fig. 1). The decreased T3 and TSH and the increased rT3 serum levels observed in this model of acute inflammatory lung injury are in agreement with those seen in the clinical setting of critical illness in patients with acute lung injury (ALI) and in animal models of acute illness (27–34).
Fig. 1.
Serum TH and TSH levels in WT mice after VILI. Serum T3, TSH, T4, and rT3 concentrations in WT mice exposed to VILI (gray bars) compared with SB animals (black bars). Note that TSH levels were undetectable after VILI and were assigned a value equal to the sensitivity of the assay (10 mU/L, indicated by a dashed line) for statistical analysis. Values shown are mean ± SEM. Differences between groups are indicated: *P < 0.05; **P < 0.01. n = 8 animals per group.
Genotyping of D2.
Because the D2 Thr92Ala polymorphism has been reported to have a protective effect against ALI in humans (35), we searched for the presence of a polymorphism in an homologous amino acid position in mice (ACA Thr in humans; CCT Pro in mice). Nineteen strains of mice (SJL/J, NZW/LacJ, NZB/BINJ, SPRET/EiJ, MA/MyJ, LP/J, JF1/Ms/ I/LnJ, FVB/NJ, DBA/2J, CZECHII/EiJ, C58/J, C57BL/6J, C3H/HeJ, BTBR_T+_tf/J, BALB/cByJ, AKR/J, A/J, 129S1/SvImJ) were examined; 18 were found to have the CCT genotype, the exception being CCC in the SPRET/EiJ strain.
Effect of VILI on D1, D2, and D3 Expression in Lung.
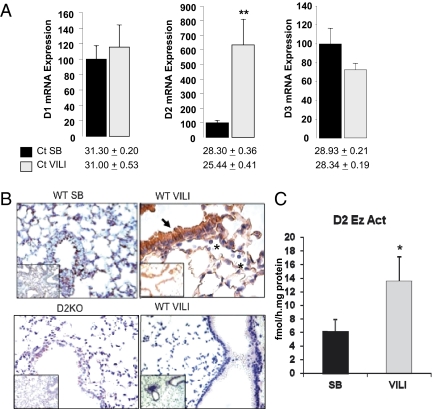
Although we detected D1, D2, and D3 mRNA in the lungs of SB animals, in agreement with previous reports (11, 12), expression of D1 was lower than expression of D2 [cycle threshold (Ct) for D1, 31.3 ± 0.2; Ct for D2, 28.3 ± 0.4]. No differences in D1 and D3 mRNA levels were observed between VILI and SB WT animals (Fig. 2A). Curiously, D2 mRNA expression was increased by 6.4 ± 1.8-fold (P < 0.01) over SB WT mice after VILI (Fig. 2A). Retrospective review of our prior expression profiling results in murine models of VILI indicated that D2 mRNA was induced in VILI-challenged WT mice (9.5-fold increase at 30 mL/kg tidal volume and 26.2-fold increase at 40 mL/kg tidal volume) (36, 37).
Fig. 2.
D1, D2, and D3 mRNA levels and D2 protein levels and enzymatic activity in the lungs of WT animals exposed to VILI. (A) Lung D1, D2, and D3 mRNA levels in WT mice after VILI (gray bars) compared with SB animals (black bars). Values shown are mean ± SEM. Significant differences between groups are indicated: *P < 0.05; **P < 0.01. n = 8 animals per group. The number of cycles in the quantitative PCR (Ct) is given below the bars to indicate the relative abundance of each enzyme. (B) (Upper Left) Immunohistochemical staining of D2 (brown) is shown for an SB WT mouse in which D2 protein was graded as 1+ in the epithelium and 1+ in pulmonary endothelium. Minimal inflammatory cells were observed. (Upper Right) Sections from a WT mouse exposed to VILI demonstrate 3+ D2 in the epithelium and 3+ in pulmonary endothelium. More inflammatory cells were observed, but no immunoreactivity was observed in these cells (indicated by asterisk). (Lower Left) Staining of D2 in a section from a D2KO mouse. The absence of D2 staining shows the specificity of the antibody. (Lower Right) Negligible D2 staining of a WT mice after VILI by using the preimmune serum (n = 2 animals per group). Inset shows a lower magnification a representative area of lung. (C) D2 enzymatic activity after VILI (gray bar) compared with SB animals (black bar). Values shown are mean ± SEM. Significant differences between groups are indicated: *P < 0.05. n = 8 animals per group.
Immunohistochemistry of the SB WT lungs revealed D2 present in airway epithelium and in the pulmonary endothelium (Fig. 2B; inflammatory cells were not present in control lungs). VILI-challenged WT mice demonstrated markedly increased immunoreactive D2 protein in pulmonary endothelium and epithelium compared with SB WT mice (Fig. 2B). D2 immunoreactivity was not detected in the inflammatory cells (indicated by asterisks in Fig. 2B). Specificity of the anti-D2 antibody was demonstrated by negligible staining of lungs of D2KO mice and lungs of WT mice stained with the preimmune serum (Fig. 2B, Lower). In agreement with the strong induction of D2 immunoreactivity, D2 enzymatic activity was 2.2 ± 0.6-fold higher (P < 0.05) after VILI challenge than in SB WT animals (Fig. 2C).
Increased D2 mRNA expression as reported as a ratio: SB as 100, was not observed in liver [SB 100 ± 29.7 (n = 5) vs. VILI 147.5 ± 29.3 (n = 7] or in kidney [SB 100 ± 16.5 (n = 4) vs. VILI 107.0 ± 15.4 (n = 3)].
D2KO Mice Have Increased Susceptibility to Inflammatory Lung Injury.
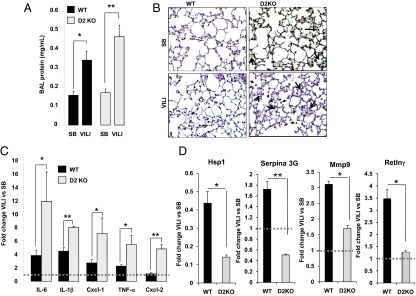
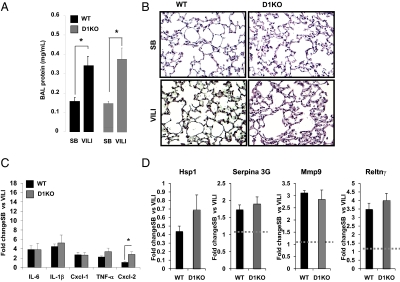
To define whether D2 induction by mechanical ventilation in WT mice had a protective or detrimental role in inflammatory lung injury, we exposed mice with targeted D2 deletion to VILI. Both VILI-challenged WT and D2KO mice exhibited an increase in lung vascular permeability compared with SB animals, as shown by the increased protein content in bronchoalveolar lavage (BAL) fluid (1.9 ± 0.3-fold change for WT mice, P < 0.05, and 2.9 ± 0.4-fold change for D2KO mice, P < 0.01) (Fig. 3A). D2KO mice had a greater increase in BAL protein content following VILI than WT mice, although this difference did not reach significance (P = 0.058) (Fig. 3A). There were no differences in inflammatory content as determined by histopathologic analysis in SB WT and D2KO mice (Fig. 3B). In contrast, after exposure to VILI, both groups of animals showed increased inflammatory cell infiltration in the alveolar wall and hyaline membrane formation. However, D2KO mice exposed to VILI demonstrated greater polymorphonuclear leukocyte infiltration and hyaline membrane formation than WT animals (Fig. 3B, arrows). After VILI exposure, D2KO mice displayed markedly higher elevations in lung chemokine and cytokine levels over SB compared with WT animals: 3.1 ± 1.1-fold for IL-6 (D2KO vs. WT, P < 0.05); 1.8 ± 0.2-fold for IL-1β (P < 0.001); 2.6 ± 0.9-fold for chemokine (C-X-C motif) ligand 1 (Cxcl-1) (P < 0.05); 2.4 ± 0.7-fold for TNF-α (P < 0.05); and 4.4 ± 0.6-fold for chemokine (C-X-C motif) ligand 2 (Cxcl-2) (P < 0.001) (Fig. 3C).
Fig. 3.
D2KO mice have increased susceptibility to VILI. (A) BAL protein content in D2KO mice compared with WT mice exposed to VILI. VILI evoked significant elevations in BAL protein levels in WT (*P < 0.05) and D2KO mice (**P < 0.01). (B) H&E staining of representative lung sections of SB and VILI-exposed WT and D2KO mice. No pathological differences were seen between SB WT and D2KO mice. Cell infiltration in the alveolar wall and hyaline membrane formation were increased in WT mice exposed to VILI (arrows). Histology of a representative D2KO mouse exposed to VILI that shows higher polymorphonuclear cells infiltration and hyaline membrane formation compared with their correspondent WT mice (three animals per group). (C) Fold change of cytokines and chemokines (IL-6, IL-1β, Cxcl1, TNF-α and Cxcl2) by qPCR in WT mice (n = 6) (black bars) or D2KO mice (n = 6) (gray bars) exposed to VILI relative to SB animals as control. Differences between groups are indicated: *P < 0.05; **P < 0.01. (D) Fold change of Hsp1, Serpina 3G, Mmp9, and Retlnγ by qPCR in WT mice (n = 8) (black bars) or D2KO mice (n = 6) (gray bars) exposed to VILI relative to SB animals as control. Differences between groups are indicated: *P < 0.05; **P < 0.01. Dashed line is drawn at a fold change of “1” for purposes of comparison.
On the basis of chemokine and cytokine induction and histological analysis of VILI-mediated alterations in lung morphology, D2KO mice appear to be more susceptible than WT mice to VILI, suggesting that the presence of D2 is protective against VILI. As an unbiased approach to elucidate additional transcriptional pathways that underlie the D2KO lung phenotype, we used microarray analysis of lungs from WT and D2KO mice, both SB and after VILI. This approach led to the identification of additional genes with reported roles in regulation of inflammation and damage, including Heat shock protein 1 (Hsp1), Serine (cysteine) peptidase inhibitor, clade A, member 3G (Serpina 3G), Matrix metallopeptidase 9 (Mmp9) and Resistin-like γ (Retnlγ) (Fig. 3D).
Expression of Hsp1 is well described in lung injury and in response to a variety of stressors. In vitro data, as well as data from various animal models of ALI, demonstrate that Hsp1 has an important cytoprotective role during lung inflammation and injury (38, 39). We found much lower expression of Hsp1 in D2KO mice than in WT mice after VILI challenge (Fig. 3D), suggesting the possibility of further decreased cytoprotection during lung inflammation in D2KO mice. Also, in WT animals, exposure to VILI induced Serpina 3G (Fig. 3D), a serine protease inhibitor that is down-regulated by an hypoxia-inducible factor mechanism (40). However, Serpina 3G was down-regulated after VILI in D2KO mice. Most dramatically, Mmp9 was significantly less induced (55% of levels in WT animals) in VILI-exposed D2KO mice (Fig. 3D). Mmp9 is a gelatinase that has a protective role during inflammatory lung injury (41, 42). Finally, Retnlγ is a member of a family of cysteine-rich secreted proteins, referred to as “resistin-like molecules” or “found in inflammatory zone,” that increase in expression during inflammation and cytokine immune responses (43), although its function still is not clear. After VILI, the induction of Retnlγ was completely absent in D2KO mice, whereas a 3.5-fold induction was observed in WT animals (Fig. 3D). In summary, these data indicate that the absence of D2 modifies the lung transcriptome after VILI challenge, increasing the inflammatory response and decreasing cytoprotective defenses against VILI.
Treatment of D2KO with Triiodo-l-Thyronine Decreases Susceptibility to Inflammatory Lung Injury.
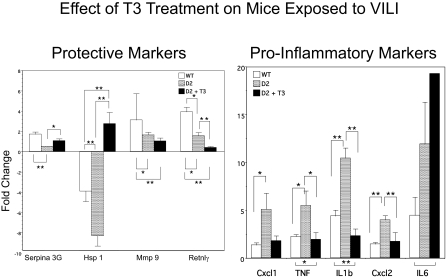
We determined whether a potential lung local hypothyroidism accounts for the increased susceptibility of D2KO mice to inflammatory lung injury by pretreating these mice with 0.2 μg/100 g l-T3 before challenging them to VILI (Fig. 4). In general, the protective markers of inflammation (e.g., Serpina 3G) in untreated D2KO mice were only 0.5-fold increased relative to SB compared with an increase of 1.1-fold in D2KO mice treated with l-T3. More dramatic was the 8.3-fold reduction in Hsp1 expression after VILI; the same gene was stimulated 2.8-fold after l-T3 treatment in D2KO mice after VILI. Mmp9 and Retnlγ did not change significantly with treatment. In addition, l-T3 treatment significantly reduced the proinflammatory markers Cxcl1, TNF, IL-1β, and Cxcl2, but not IL-6, in D2KO mice.
Fig. 4.
Effect of l-T3 treatment on cytokine and chemokine expression in lungs of D2KO mice after VILI. Mice treated with 0.2 μg/100 g l-T3 for 5 d were subjected to VILI or SB 2 h after the last injection. Shown is the fold change in markers of inflammation and injury by qPCR in WT (n = 6), D2KO (n = 6), and l-T3–treated D2KO (n = 6) mice exposed to VILI relative to SB animals as control. In general, protective markers of inflammation (Serpina 3G, Hsp1) increased with l-T3 treatment (although Mmp9 and Retnlγ did not), and proinflammatory markers (Cxcl1, TNF, IL-1β, and Cxcl2) were suppressed with l-T3 (although IL-6 was not). Differences between groups are indicated: *P < 0.05; **P < 0.01.
Serum Thyroid Function and D1 and D3 mRNA Expression in D2KO Mice Exposed to VILI.
At baseline D2KO mice differed from WT mice only in a markedly elevated TSH with similar serum T4, T3, and rT3 concentrations (44, 45). After exposure to VILI, D2KO mice had a significant decrease in T3 and TSH concentrations (82.3 ± 1.5 vs. 76.1 ± 2.5 ng/dL and 262.3 ± 56.2 vs. 63.4 ± 17.0 mU/L, respectively) and a significant increase in serum rT3 levels (22.1 ± 2.8 vs. 31.7 ± 1.5 ng/dL) compared with SB animals (Fig. 5A). No significant changes were observed in serum T4 concentrations (2.5 ± 0.2 vs. 2.3 ± 0.1 μg/dL) (Fig. 5A). The serum TSH concentration remained significantly higher in D2KO mice than in WT animals when both received VILI (Fig. 4A).
Fig. 5.
Serum TH and TSH concentrations and D1 and D3 mRNA expression in D2KO mice after VILI. (A) Serum T3, TSH, T4, and rT3 concentrations in ventilated WT and D2KO mice (gray bars) compared with SB animals (black bars). Note that after VILI the TSH levels in WT mice were undetectable and were assigned a value equal to the sensitivity of the assay (10 mU/L, indicated by a dashed line) for statistical analysis. Values shown are mean ± SEM. *P < 0.05; **P < 0.01 VILI vs. SB. n = 6 animals per group. (B) Lung D1 and D3 mRNA levels in WT and D2KO mice after VILI compared with SB animals as control. n = 8 animals per group. Values shown are ± SEM. Significant differences between groups are indicated: *P < 0.05; #P < 0.05 vs. WT SB.
Lung D1 and D3 mRNA expression in SB D2KO mice and those exposed to VILI were analyzed by quantitative PCR (qPCR). In SB mice, D1 mRNA was 1.9 ± 0.2-fold higher in lung tissue of D2KO mice than in WT mice (P < 0.05) (Fig. 5B). After D2KO mice were exposed to VILI, D1 levels were reduced by 0.4 ± 0.1-fold, similar in absolute value to levels in WT mice after 4 h of mechanical ventilation (Fig. 5B). Although an increase of D3 levels was detected in D2KO mice after VILI compared with SB animals, the difference did not reach significance. However, a significant (2.4 ± 0.5-fold) increase in D3 mRNA was observed after VILI in D2KO mice compared with WT mice (Fig. 5B).
Response of Type 1 Deiodinase Knockout Mice to VILI.
To investigate further the role of D1 in inflammatory lung injury, we exposed type 1 deiodinase knockout (D1KO) mice to VILI. As shown in Fig. 6 A and B, WT and D1KO mice had the same degree of inflammatory inflation after VILI, as observed by BAL protein content and by histological examination, respectively. There were no differences in the expression of lung chemokines and cytokine (IL-6, IL-1β, Cxcl1, and TNF-α) levels between WT and D1KO mice exposed to VILI, but Cxcl2 expression was increased by 197% in D1KO mice relative to WT animals exposed to VILI (Fig. 6C). In addition, no significant differences in Hsp1, Serpina 3G, Mmp9, or Retnlγ were observed between D1KO and WT animals exposed to VILI (Fig. 6D). These results indicated that the absence of D2, but not D1, increases the susceptibility to VILI. The observation that D1 plays a lesser role than D2 in response to lung injury is in agreement with the low expression of D1 mRNA and undetectable D1 enzymatic activity in the lungs of WT mice.
Fig. 6.
Response to VILI is comparable in D1KO and WT mice. (A) BAL protein content in WT and D1KO mice exposed to VILI compared with SB animals. Differences between groups are indicated. *P < 0.05. (B) H&E staining of a lung section of a WT mouse and a D1KO mouse SB and after exposure to VILI. Histology of VILI-exposed WT and D1KO mice shows higher polymorphonuclear cell infiltration and hyaline membrane formation with no differences between the two groups (n = 3 animals per group). (C) Fold changes of VILI biomarker genes (IL-6, IL-1β, Cxcl1, TNF-α, and Cxcl2) by qPCR in WT and D1KO mice exposed to VILI relative to SB animals as control. *P < 0.05 vs. WT mice. (D) Fold change of Hsp1, Serpina 3G, Mmp9, and Retlnγ by qPCR in WT (n = 8) and D1KO (n = 6) (gray bars) mice exposed to VILI relative to SB animals as control.
Hypothyroidism and Susceptibility to VILI.
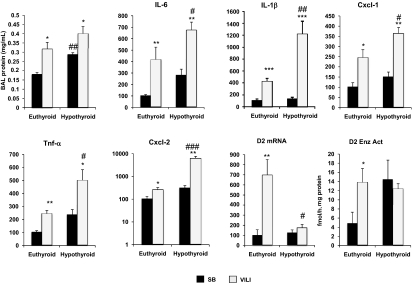
D2KO mice are more susceptible to VILI than WT mice, but we did not detect differences in serum TH concentrations between WT and D2KO mice. To investigate whether TH deprivation in the lung after VILI reproduced the findings in the D2KO mice, globally hypothyroid mice were exposed to VILI. Induction of hypothyroidism was confirmed by serum T3, T4, and rT3 expression at low levels or below the level of detection. TSH levels in SB mice and mice with VILI were markedly elevated (15,848 ± 3,020 and 24,481 ± 1,594 mU/L, respectively; P < 0.05).
Hypothyroid SB mice lung had increased BAL protein content compared with euthyroid SB mice (Fig. 7). A similar trend was found in hypothyroid VILI mice, but it did not reach significance (0.4 ± 0.03 vs. 0.31 ± 0.01 mg/mL) (Fig. 7). Hypothyroid VILI-treated WT mice had marked elevations in lung chemokines and cytokine levels compared with euthyroid animals (Fig. 7) reported as a ratio, untreated as 100: 676.9 ± 67.8 vs. 417.5 ± 108.52 for IL-6 (P < 0.05); 1,215.51 ± 218.8 vs. 423.7 ± 53.7 for IL-1β (P < 0.01); 365.6 ± 27.7 vs. 149.5 ± 25.2 for Cxcl1 (P < 0.05); 501.8 ± 81.2 vs. 244.1 ± 25.1 for TNF-α (P < 0.05); and 6,370 ± 1,267 vs. 254.1 ± 60.3 for Cxcl2 (P < 0.001). On the basis of chemokine and cytokine induction, hypothyroid mice had increased susceptibility to VILI.
Fig. 7.
Hypothyroid WT mice have increased susceptibility to VILI. BAL protein content in hypothyroid mice compared with euthyroid animals exposed to VILI. VILI evoked significant elevations in BAL protein levels in euthyroid mice (*P < 0.05) and hypothyroid mice (*P < 0.05; ##P < 0.01 vs. SB euthyroid mice). mRNA levels of the VILI biomarker genes IL-6, IL-1β, Cxcl1, TNF-α, and Cxcl by qPCR in euthyroid (n = 8) and hypothyroid (n = 6) mice SB and after VILI. Lung D2 mRNA and enzymatic activity (Enz Act) in euthyroid and hypothyroid mice after VILI compared with SB animals as control. n = 6 animals per group. Values shown are mean ± SEM. *P < 0.05 vs. SB; **P < 0.01 vs. SB; ***P < 0.001 vs. SB; #P < 0.05 vs. euthyroid VILI; ##P < 0.01 vs. euthyroid VILI; ###P < 0.001 vs. euthyroid VILI.
Hypothyroidism did not modify the expression of D2 mRNA in the lungs of SB WT mice (Fig. 7), but, as expected, we observed a significant increase in D2 enzymatic activity (Fig. 7). In contrast to the results observed in euthyroid animals, exposure to VILI did not change the already high D2 enzymatic activity of hypothyroid animals.
Discussion
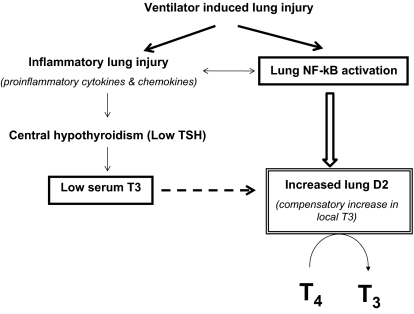
Our results show that VILI increased lung immunoreactive D2 and its enzymatic activity. Furthermore we demonstrate that D2 up-regulation likely is protective, because D2KO mice had more severe lung injury. One interpretation is that in VILI, induction of D2 may be adaptive to the circulating low T3 levels, generating more T3 locally. Alternatively, D2 could be up-regulated in response to the local injury as part of an inflammatory pathway involving NF-κB (Fig. 7). NF-κB, an important mediator of immune and inflammatory responses, has been strongly implicated in ALI (46–51). Evidence supports the notion that NF-κB induces transcription of human and rat D2 gene expression, and, indeed, a potent NF-κB–binding site has been demonstrated in the human D2 gene (52, 53).
Interestingly in humans with sepsis and sepsis-associated ALI, D2 is associated with susceptibility to ALI (35). European Americans carrying the G allele of the D2-coding SNP (rs225014, Thr92Ala) were protected against sepsis-associated ALI. Studies addressing functional consequences of the Thr92Ala SNP are still unclear (54). Peeters et al. (55) showed that the Thr92Ala substitution does not change the kinetics of the transiently expressed D2 enzyme in vitro. One in vivo study demonstrated decreased D2 velocity in skeletal muscle and thyroid tissue in subjects homozygous for the 92Ala allele as result of a decreased maximum enzyme velocity (56). However, two subsequent studies found much lower levels of D2 activity (two orders of magnitude) in human skeletal muscle (57, 58), calling these findings into question. Discrepant results can be explained by the complex process of D2 regulation, which involves both transcriptional and posttranscriptional mechanisms. An additional possibility is that the positive findings of the genetic studies that found Thr92Ala to be associated with different pathological conditions are based on linkage with another locus. However, genetic analysis in a homologous amino acid position of D2 in mice failed to demonstrate polymorphism.
The induction of D2 by mechanical ventilation in WT mice may constitute a protective mechanism against VILI by compensating for the local reduction of TH signaling as shown in critical illness (58, 59). In D2KO mice exposed to VILI, the down-regulation of D1 would decrease T3 production, whereas the increase in D3 would inactivate TH. Given these effects, plus the natural absence of D2, the total outcome would be the failure to maintain active T3 locally and possibly a relatively hypothyroid status of the lung of D2KO compared with WT mice. All the factors mentioned above suggest that severe lung damage might be associated with active TH deprivation in lung. Hypothyroidism can aggravate lung injury by impeding the clearance of alveolar fluid, resulting in persistent hypoxia, enhanced cell damage, reduced cell proliferation, altered alveolar macrophage populations, and depressed antioxidant defense systems (60). Consistently, airspace T3 rapidly stimulates the clearance of alveolar fluid in rat lungs with and without lung injury (61) by regulating Na-K-ATPase activity (62). Decreased serum TH levels have been associated with worsening pulmonary function in lung injury or during sepsis (63); in agreement with these observations, administration of TH to patients with respiratory distress syndrome and sepsis improves lung compliance (64). In agreement with these studies, we demonstrated that hypothyroidism worsens lung damage after VILI. We did not measure the TH content of the lung tissue during VILI directly because the contribution of T3 from extracellular edema made measurement of the true TH content difficult. However, the evidence presented here demonstrates that the profile of inflammatory gene expression in the VILI-exposed D2KO lung is similar to that in the hypothyroid lung. Consistently, treatment of D2KO mice with l-T3 was able to reverse the profiles of expression of the proinflammatory markers by reducing them and increasing the protective markers. Taken together, our data suggest that the lungs of D2KO mice are relatively hypothyroid during the induction of ALI and that T3 treatment is protective against inflammatory lung injury.
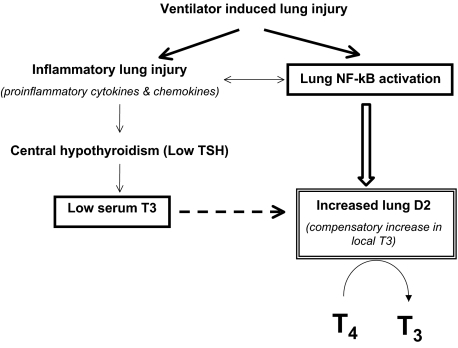
In summary, the rapid induction of D2 enzymatic activity after mechanical ventilation constitutes a protective mechanism against VILI through local production of T3. This response likely compensates for the local reduction of bioactive TH signaling, which may serve to dampen the inflammatory response to VILI. This work supports a role for D2 in the regulation of response to acute lung injury (Fig. 8).
Fig. 8.
Model of lung D2 induction by VILI. VILI is characterized by increases in inflammatory cytokine expression and activation of mediators of immune and inflammatory responses such as NF-κB. The proinflammatory cytokines increase hypothalamic D2 (17) and cause central hypothyroidism that might be responsible for the low serum T3. The induction of D2 in the lung by mechanical ventilation might be adaptive to the low circulating levels of T3 or in response to the local activation of NF-κB. The dashed arrow represents the posttranslational effect of T3 on D2.
Methods
Experimental Animals.
All experiments were approved by the Animal Care and Use Committee of the University of Chicago. D2KO and D1KO male mice were generated and propagated as described (45, 65). Mice were 80–100 d of age at the time of analyses and were housed under standard conditions with free access to food and water.
Induction of Hypothyroidism and Treatment with l-T3.
TH deprivation was induced in 12–15 male WT mice by feeding with a low-iodine diet for 15 d (Harlan Teklad). On day 15, animals receive an i.p. injection of 10 mU bovine TSH and, 2 h later, an injection of 131I (150 mCi, in 0.2 mL of saline). Four weeks after injection, VILI was applied to hypothyroid mice.
In a separate group of experiments, D2KO mice were treated with l-T3 (2 μg/100 g body weight) every day for 5 d. Two hours after the last injection the mice were allocated randomly to the VILI group (six mice) or SB controls (six mice).
Model of VILI.
Groups of mice (WT, n = 8 mice per group; D2KO, n = 6 mice per group; D1KO, n = 6 mice per group; hypothyroid WT mice, n = 6 mice per group) were allocated randomly to either the SB or VILI treatment as previously described (36, 37). Mechanical ventilation experiments were performed in age-matched males after anesthesia with inhaled isofluorane followed by i.p. ketamine/xylazine. Mice were intubated (20-gauge catheter) and placed on mechanical ventilation (Harvard Apparatus) at room air, tidal volume of 40 mL/kg, 65 breaths/min, and positive end expiratory pressure of 0 cm H2O for 4 h, as previously described (36). Ventilated animals were monitored with intermittent blood pressure, arterial blood gas, and body temperature monitoring to ensure adequate perfusion and received 8 mL/kg 0.9% saline at the initiation of ventilation and 2 h after the onset of ventilation. Deep anesthesia was maintained with ketamine/xylazine, and animals were placed in a heated pad throughout the experiment. Control mice were allowed to breathe spontaneously for 4 h under same anesthesia and intubation treatment.
BAL.
BAL fluid was recovered as we previously described (66) to determine protein levels. Briefly, mice underwent BAL of both lungs with HBSS (1 mL per mouse). Lavage samples were centrifuged at 500 × g on a refrigerated microcentrifuge for 20 min. The supernatant was aliquoted for protein assays using the Bio-Rad DC Protein Assay (Bio-Rad Laboratories).
Histology and Immunohistochemistry.
To characterize VILI-mediated alterations in lung morphology, left lungs (three animals per group) were excised from the mainstem bronchus at death and placed immediately in formalin overnight, followed by embedding in paraffin for histological evaluation by H&E staining as previously described (66). For analysis of D2 protein localization, the lung samples were deparaffinized and rehydrated by passage through graded alcohols to water and were placed in a pressure cooker with citrate buffer, pH 6.0, for 1 min after reaching boiling temperature to retrieve antigenic sites masked by formalin fixation. The tissue sections then were placed in 0.5% vol/vol hydrogen peroxide/methanol for 10 min to inactivate endogenous peroxidase activity; were blocked for 20 min with normal horse serum (Vectastain Elite ABC Kit; Vector Laboratories); and subsequently were incubated for 1 h at room temperature with the primary rabbit D2 antibody (working dilution: 1/100) or with the preimmune serum as a negative control stain. The antibody to D2 was directed against the highly conserved peptide EVRSWLEKNFSKR, residues 253–265 near the C terminus of the protein (67), which was not included in the deleted region of the D2KO mouse. The primary antibody was detected using the ImmPress polymerized reporter enzyme staining system (ImmPRESS reagent kit; Vector Laboratories), according to the manufacturer's specifications. The immunostaining was visualized with the Vector novaRed substrate kit for peroxidase (Vector Laboratories), following the manufacturer's specifications. Counterstaining was performed with hematoxylin (Vector hematoxilin QS nuclear counterstain; Vector Laboratories). Each sample was graded (n = 3 per group) from 0 to 3+ in different locations covering the pulmonary epithelium (including airway epithelium and type II pneumocytes), the pulmonary endothelium, and inflammatory cell infiltrates.
Measurement of Specific mRNA Content In Lung by qPCR.
Total RNA was isolated from whole lungs of VILI-challenged and SB mice (six to eight mice per group) for expression profiling as previously described (36). Transcript levels of Serpina 3G, Mmp9, Hsp1, Remlγ, mouse chemokines and cytokines IL-1β, IL-6, Cxcl2, Cxcl1, and TNF-α, and deiodinases D1, D2, and D3 were measured by qPCR in 96-well microtiter plates with an ABI Prism 7700 Sequence Detector System (Applied Biosystems). Amplification of the housekeeping gene RNA polymerase II was used as an internal control. The oligonucleotide primers were designed to cross introns (Table S1). Experimental protocols were based on the manufacturer's recommendation using the TaqMan Gold RT-PCR Core Reagents Kit (Applied Biosystems). Experimental parameters were 48 °C for 30 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A relative quantitative method was used to analyze changes in gene expression in a given sample relative to an untreated control sample, and specific mRNA transcript levels were expressed as fold difference, except for hypothyroid mice, for which the values are shown relative to WT euthyroid mice as 100.
Measurement of THs and TSH Concentrations in Serum.
Blood was collected to measure serum TH and TSH concentration levels at baseline, after induction of hypothyroidism, and after 4 h of VILI exposure or SB (control). Serum TSH was measured in 50 μL of serum using a sensitive, heterologous, disequilibrium double-antibody precipitation RIA, and results were expressed in bioassayable TSH units (68). Serum T4 and T3 concentrations were measured by antibody-coated tube RIAs (Siemens Medical Solutions) using 25 and 50 μL of serum, respectively. rT3 was measured in 35 μL of serum by RIA using reagents from Adaltis Italia. All samples were analyzed individually for each mouse. Because of the previously reported suppression of THs with ketamine/xylazine (69), comparisons between the ventilated and SB animals were made with both groups treated with the anesthetic.
D2 Enzymatic Activity.
D2 enzymatic activity was assessed as described (70) with the following modifications: 100 μg protein of tissue homogenates in 100 μL reaction mixture containing 0.1 M phosphate buffer (pH 7), 1 mM EDTA, 20 mM DTT, 1 mM propylthiouracil, 100,000 cpm [125 I] T4, and 2 nM unlabeled T4 were incubated at 37 °C for 1 h. Saturating levels of unlabeled T3 (1 μM) were added to the reaction mixture to inhibit the D3 enzyme.
Statistical Analysis.
Values are reported as mean ± SEM except for qPCR cytokine and chemokine expression data of D1KO and D2KO mice, which are presented as mean fold change ± SEM. Statistical analysis was performed with Student's t test.
Supplementary Material
Acknowledgments
We thank Donald L. St. Germain and Valerie Anne Galton (Dartmouth Medical School) for providing the mice deficient in D1 and D2 and Antonio C. Bianco, (University of Miami Miller School of Medicine) for providing antibodies to mouse D2. This work was supported in part by the National Institutes of Health Grants 4R37-DK15070, DK20595, and HL 50894 and by the Esformes and Abrams endowments at the University of Chicago.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 19465.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109926108/-/DCSupplemental.
References
- 1.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 2.Alkemade A. Central and peripheral effects of thyroid hormone signalling in the control of energy metabolism. J Neuroendocrinol. 2010;22(1):56–63. doi: 10.1111/j.1365-2826.2009.01932.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 4.Gogakos AI, Duncan Bassett JH, Williams GR. Thyroid and bone. Arch Biochem Biophys. 2010;503(1):129–136. doi: 10.1016/j.abb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Ness GC. Thyroid hormone. Basis for its hypocholesterolemic effect. J Fla Med Assoc. 1991;78:383–385. [PubMed] [Google Scholar]
- 6.Ansari MA, de Mello DE, Devaskar UP. Effect of prenatal glucocorticoid on fetal lung ultrastructural maturation in hyt/hyt mice with primary hypothyroidism. Biol Neonate. 2000;77(1):29–36. doi: 10.1159/000014192. [DOI] [PubMed] [Google Scholar]
- 7.Bizzarro MJ, Gross I. Effects of hormones on fetal lung development. Obstet Gynecol Clin North Am. 2004;31:949–961, xii. doi: 10.1016/j.ogc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Keijzer R, et al. Expression of thyroid hormone receptors A and B in developing rat tissues; evidence for extensive posttranscriptional regulation. J Mol Endocrinol. 2007;38:523–535. doi: 10.1677/jme.1.02125. [DOI] [PubMed] [Google Scholar]
- 9.Boelen A, et al. Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid. 2008;18:1095–1103. doi: 10.1089/thy.2008.0090. [DOI] [PubMed] [Google Scholar]
- 10.Escobar-Morreale HF, Obregón MJ, Hernandez A, Escobar del Rey F, Morreale de Escobar G. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology. 1997;138:2559–2568. doi: 10.1210/endo.138.6.5212. [DOI] [PubMed] [Google Scholar]
- 11.Ohba K, Yoshioka T, Muraki T. Identification of two novel splicing variants of human type II iodothyronine deiodinase mRNA. Mol Cell Endocrinol. 2001;172:169–175. doi: 10.1016/s0303-7207(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 12.Wagner MS, et al. Hypothyroidism induces type 2 iodothyronine deiodinase expression in mouse heart and testis. J Mol Endocrinol. 2003;31(1-2):541–550. doi: 10.1677/jme.0.0310541. [DOI] [PubMed] [Google Scholar]
- 13.Escobar-Morreale HF, Obregón MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedraza PE, Obregon MJ, Escobar-Morreale HF, del Rey FE, de Escobar GM. Mechanisms of adaptation to iodine deficiency in rats: Thyroid status is tissue specific. Its relevance for man. Endocrinology. 2006;147:2098–2108. doi: 10.1210/en.2005-1325. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Dulchavsky SA, Dutta S. Effects of triiodothyronine (T3) supplementation upon ozone-induced lung injury. Free Radic Res Commun. 1993;18:299–308. doi: 10.3109/10715769309147497. [DOI] [PubMed] [Google Scholar]
- 16.Türe M, Memiş D, Kurt I, Pamukçu Z. Predictive value of thyroid hormones on the first day in adult respiratory distress syndrome patients admitted to ICU: Comparison with SOFA and APACHE II scores. Ann Saudi Med. 2005;25:466–472. doi: 10.5144/0256-4947.2005.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: An update. J Endocrinol. 2010;205(1):1–13. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Perez A, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf) 2008;68:821–827. doi: 10.1111/j.1365-2265.2007.03102.x. [DOI] [PubMed] [Google Scholar]
- 19.Chopra IJ. Clinical review 86: Euthyroid sick syndrome: Is it a misnomer? J Clin Endocrinol Metab. 1997;82:329–334. doi: 10.1210/jcem.82.2.3745. [DOI] [PubMed] [Google Scholar]
- 20.Peeters RP, et al. Increased thyroxine sulfate levels in critically ill patients as a result of a decreased hepatic type I deiodinase activity. J Clin Endocrinol Metab. 2005;90:6460–6465. doi: 10.1210/jc.2005-0866. [DOI] [PubMed] [Google Scholar]
- 21.Peeters RP, et al. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab. 2005;90:6498–6507. doi: 10.1210/jc.2005-1013. [DOI] [PubMed] [Google Scholar]
- 22.Arem R, et al. Reduced tissue thyroid hormone levels in fatal illness. Metabolism. 1993;42:1102–1108. doi: 10.1016/0026-0495(93)90266-q. [DOI] [PubMed] [Google Scholar]
- 23.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 24.Ranieri VM, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: A randomized controlled trial. JAMA. 1999;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremblay LN, Miatto D, Hamid Q, Govindarajan A, Slutsky AS. Injurious ventilation induces widespread pulmonary epithelial expression of tumor necrosis factor-alpha and interleukin-6 messenger RNA. Crit Care Med. 2002;30:1693–1700. doi: 10.1097/00003246-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Boelen A, et al. Contribution of interleukin-12 to the pathogenesis of non-thyroidal illness. Horm Metab Res. 2004;36(2):101–106. doi: 10.1055/s-2004-814219. [DOI] [PubMed] [Google Scholar]
- 28.Boelen A, et al. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. J Endocrinol. 2004;182:315–323. doi: 10.1677/joe.0.1820315. [DOI] [PubMed] [Google Scholar]
- 29.Boelen A, Platvoet-ter Schiphorst MC, Bakker O, Wiersinga WM. The role of cytokines in the lipopolysaccharide-induced sick euthyroid syndrome in mice. J Endocrinol. 1995;146:475–483. doi: 10.1677/joe.0.1460475. [DOI] [PubMed] [Google Scholar]
- 30.Chow CC, Mak TW, Chan CH, Cockram CS. Euthyroid sick syndrome in pulmonary tuberculosis before and after treatment. Ann Clin Biochem. 1995;32:385–391. doi: 10.1177/000456329503200406. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami M, Usami I, Kuroki H, Goto M. [Thyroid hormones in patients with clinical stable pneumoconiosis] Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:1215–1219. [PubMed] [Google Scholar]
- 32.Okutan O, Kartaloglu Z, Onde ME, Bozkanat E, Kunter E. Pulmonary function tests and thyroid hormone concentrations in patients with chronic obstructive pulmonary disease. Med Princ Pract. 2004;13(3):126–128. doi: 10.1159/000076950. [DOI] [PubMed] [Google Scholar]
- 33.Scoscia E, et al. Low triiodothyronine (T3) state: A predictor of outcome in respiratory failure? Results of a clinical pilot study. Eur J Endocrinol. 2004;151:557–560. doi: 10.1530/eje.0.1510557. [DOI] [PubMed] [Google Scholar]
- 34.Wawrzyńska L, Sakowicz A, Filipecki S. [Euthyroid sick syndrome in patients with respiratory failure] Pneumonol Alergol Pol. 1996;64(Suppl 2):193–199. [PubMed] [Google Scholar]
- 35.Ma S-F, et al. Deiodinase 2 (Dio2) is a novel candidate gene which confers susceptibility and severity in acute lung injury and ventilation-induced lung injury in European Americans. Am J Respir Crit Care Med. 2010;181:A1020. [Google Scholar]
- 36.Hong SB, et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer NJ, et al. GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. FASEB J. 2009;23:1325–1337. doi: 10.1096/fj.08-119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh Y, et al. Heat shock response decreases endotoxin-induced acute lung injury in rats. Respirology. 1999;4:325–330. doi: 10.1046/j.1440-1843.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Yan Y, Salnikow K, Kluz T, Costa M. Nickel-induced down-regulation of serpin by hypoxic signaling. Toxicol Appl Pharmacol. 2004;194(1):60–68. doi: 10.1016/j.taap.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson D, Hill DL. Reconstruction after rotational motion. Magn Reson Med. 2003;49(1):183–187. doi: 10.1002/mrm.10333. [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- 43.Pesce JT, et al. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao XH, et al. Distinct roles of deiodinases on the phenotype of Mct8 defect: A comparison of eight different mouse genotypes. Endocrinology. 2011;152:1180–1191. doi: 10.1210/en.2010-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider MJ, et al. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15:2137–2148. doi: 10.1210/mend.15.12.0740. [DOI] [PubMed] [Google Scholar]
- 46.Jacobson JR, et al. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 47.Leeper-Woodford SK, Detmer K. Acute hypoxia increases alveolar macrophage tumor necrosis factor activity and alters NF-kappaB expression. Am J Physiol. 1999;276:L909–L916. doi: 10.1152/ajplung.1999.276.6.L909. [DOI] [PubMed] [Google Scholar]
- 48.Lentsch AB, Czermak BJ, Bless NM, Ward PA. NF-kappaB activation during IgG immune complex-induced lung injury: Requirements for TNF-alpha and IL-1beta but not complement. Am J Pathol. 1998;152:1327–1336. [PMC free article] [PubMed] [Google Scholar]
- 49.Moine P, et al. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000;13(2):85–91. doi: 10.1097/00024382-200013020-00001. [DOI] [PubMed] [Google Scholar]
- 50.Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: Implications for therapy. Am J Respir Med. 2003;2(3):211–219. doi: 10.1007/BF03256650. [DOI] [PubMed] [Google Scholar]
- 51.Wurfel MM, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med. 2008;178:710–720. doi: 10.1164/rccm.200803-462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fekete C, et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: Implications for the nonthyroidal illness syndrome. Endocrinology. 2004;145:1649–1655. doi: 10.1210/en.2003-1439. [DOI] [PubMed] [Google Scholar]
- 53.Zeöld A, et al. Characterization of the nuclear factor-kappa B responsiveness of the human dio2 gene. Endocrinology. 2006;147:4419–4429. doi: 10.1210/en.2005-1608. [DOI] [PubMed] [Google Scholar]
- 54.Chidakel A, Mentuccia D, Celi FS. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid. 2005;15:899–903. doi: 10.1089/thy.2005.15.899. [DOI] [PubMed] [Google Scholar]
- 55.Peeters RP, et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88:3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 56.Canani LH, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90:3472–3478. doi: 10.1210/jc.2004-1977. [DOI] [PubMed] [Google Scholar]
- 57.Grozovsky R, et al. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-gamma agonists in skeletal myocytes. Endocrinology. 2009;150:1976–1983. doi: 10.1210/en.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mebis L, Langouche L, Visser TJ, Van den Berghe G. The type II iodothyronine deiodinase is up-regulated in skeletal muscle during prolonged critical illness. J Clin Endocrinol Metab. 2007;92:3330–3333. doi: 10.1210/jc.2007-0510. [DOI] [PubMed] [Google Scholar]
- 59.Mebis L, van den Berghe G. The hypothalamus-pituitary-thyroid axis in critical illness. Neth J Med. 2009;67:332–340. [PubMed] [Google Scholar]
- 60.Palmer KC, Mari F, Malian MS. Cadmium-induced acute lung injury: Compromised repair response following thyroidectomy. Environ Res. 1986;41:568–584. doi: 10.1016/s0013-9351(86)80151-7. [DOI] [PubMed] [Google Scholar]
- 61.Bhargava M, et al. Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs. Am J Respir Crit Care Med. 2008;178:506–512. doi: 10.1164/rccm.200709-1429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei J, Nowbar S, Mariash CN, Ingbar DH. Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L762–L772. doi: 10.1152/ajplung.00376.2002. [DOI] [PubMed] [Google Scholar]
- 63.Dulchavsky SA, Bailey J. Triiodothyronine treatment maintains surfactant synthesis during sepsis. Surgery. 1992;112:475–479. [PubMed] [Google Scholar]
- 64.Inan M, et al. Thyroid hormone supplementation in sepsis: An experimental study. Surg Today. 2003;33(1):24–29. doi: 10.1007/s005950300004. [DOI] [PubMed] [Google Scholar]
- 65.Schneider MJ, et al. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- 66.Peng X, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 67.Curcio C, et al. The human type 2 iodothyronine deiodinase is a selenoprotein highly expressed in a mesothelioma cell line. J Biol Chem. 2001;276:30183–30187. doi: 10.1074/jbc.C100325200. [DOI] [PubMed] [Google Scholar]
- 68.Pohlenz J, et al. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: Strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9:1265–1271. doi: 10.1089/thy.1999.9.1265. [DOI] [PubMed] [Google Scholar]
- 69.Alfonso M, Arufe MC, Durán R. Validation of an EIA kit for determination of total thyroid hormones in rat serum. Effects of different anaesthetics. J Physiol Biochem. 1998;54(1):15–21. [PubMed] [Google Scholar]
- 70.Dumitrescu AM, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet. 2005;37:1247–1252. doi: 10.1038/ng1654. [DOI] [PubMed] [Google Scholar]