Abstract
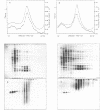
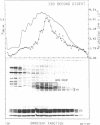
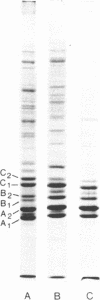
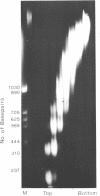
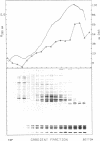
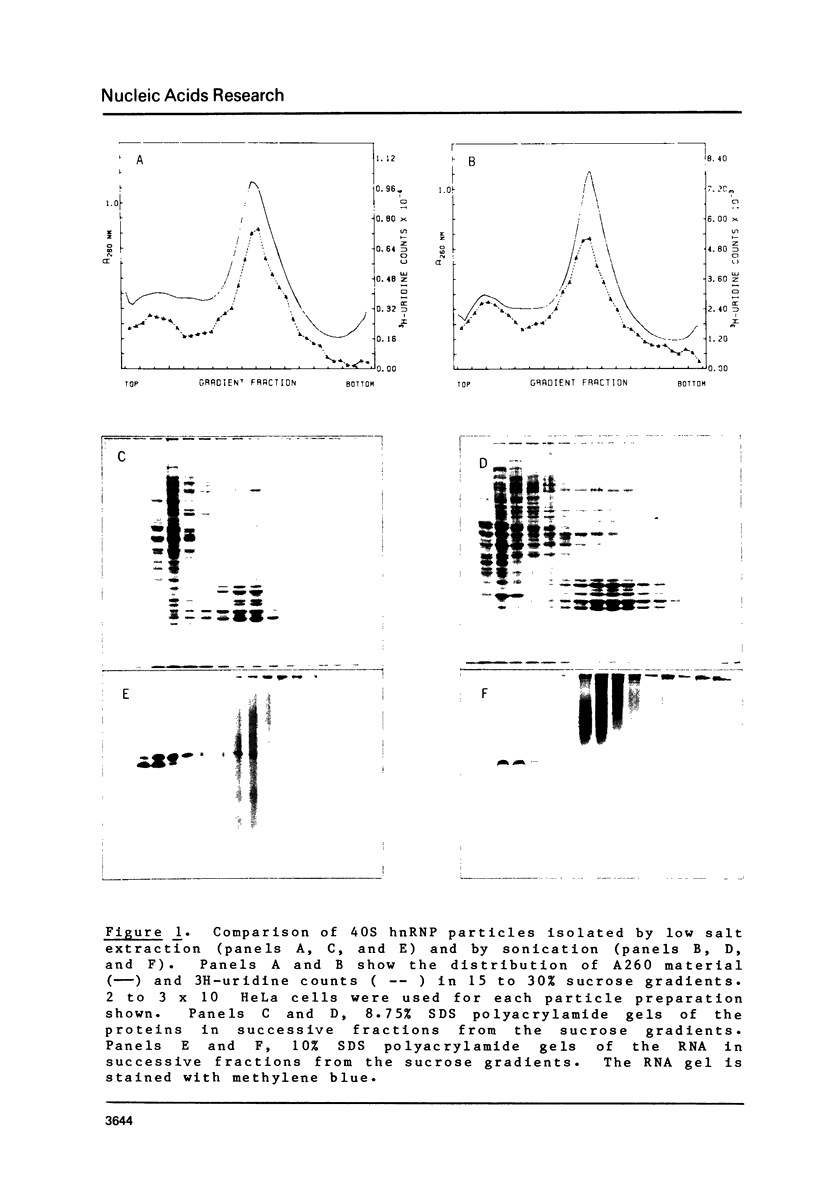
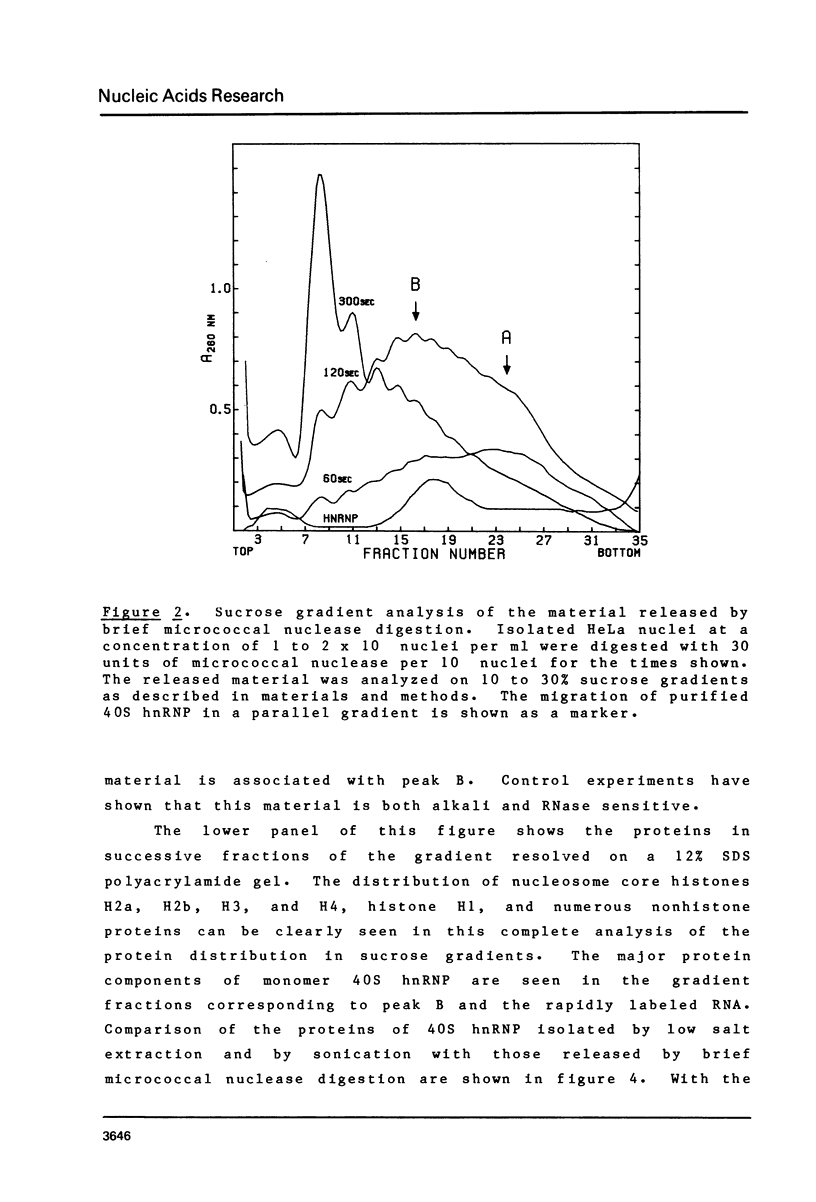
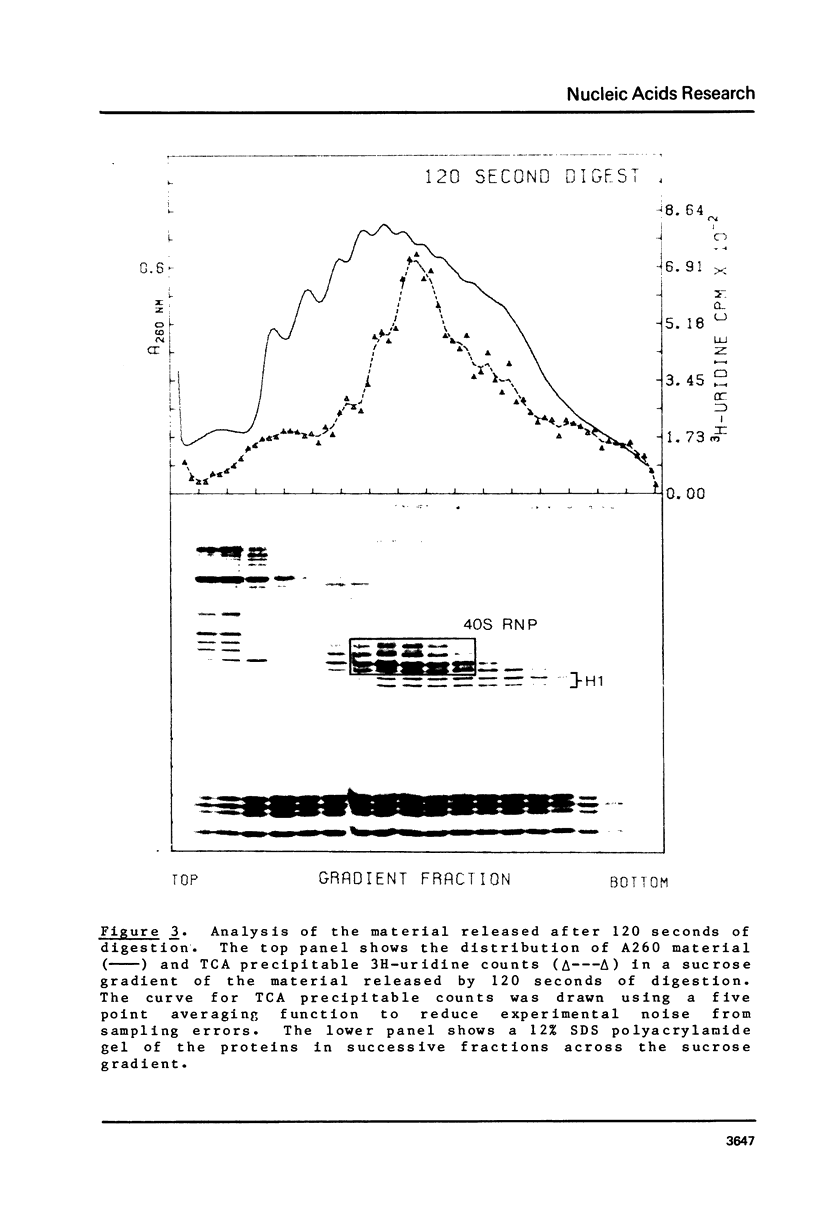
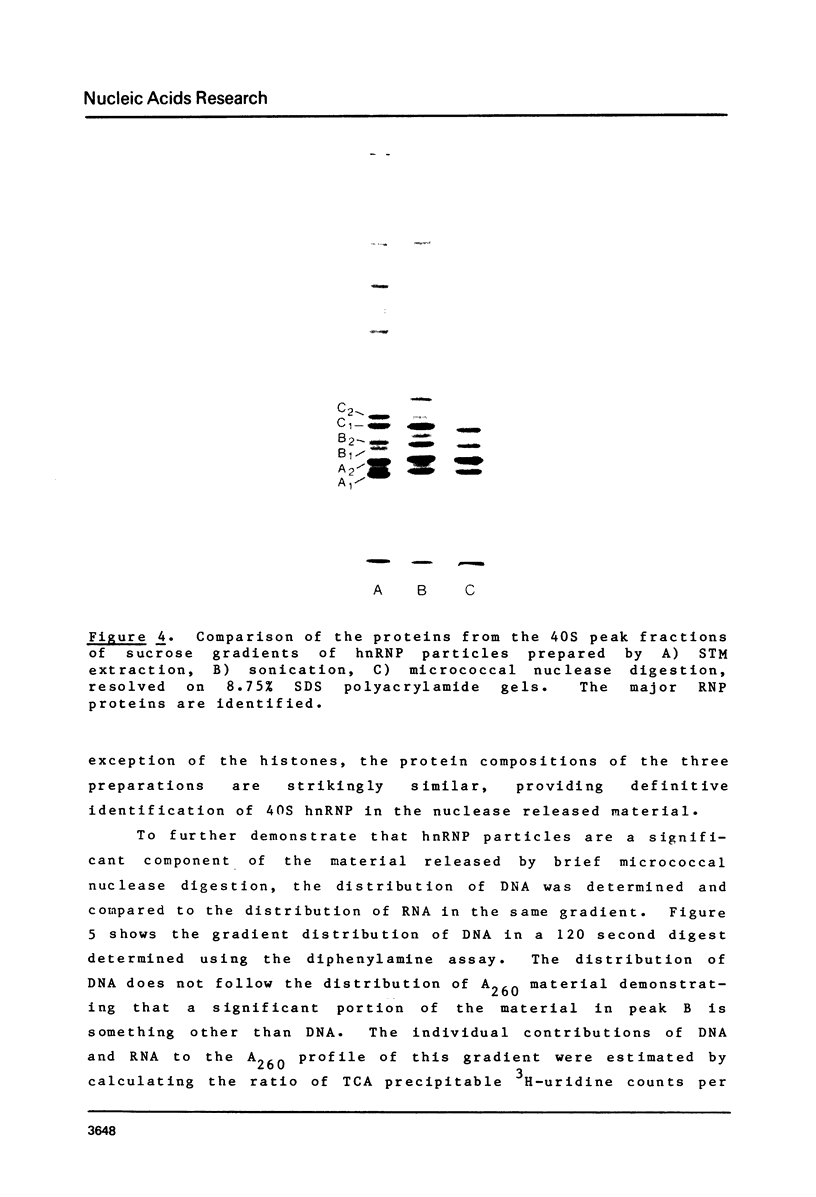
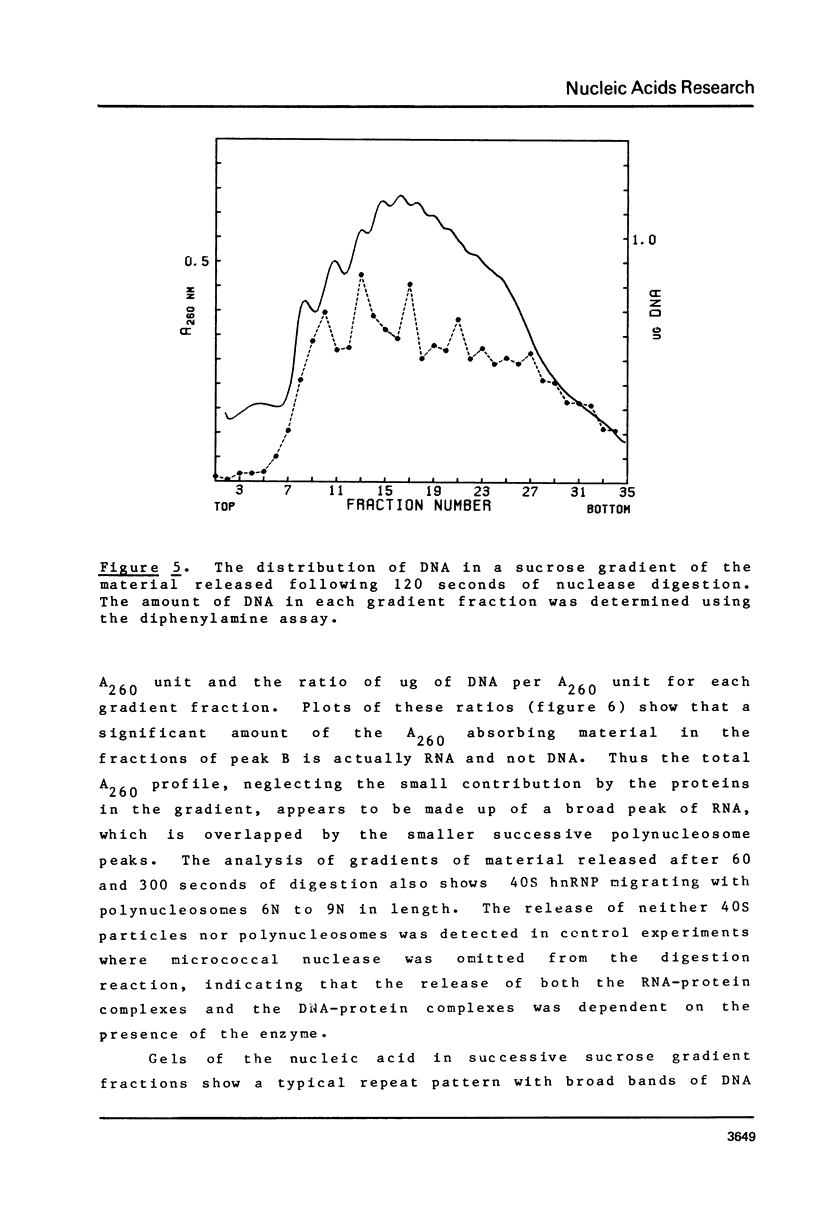
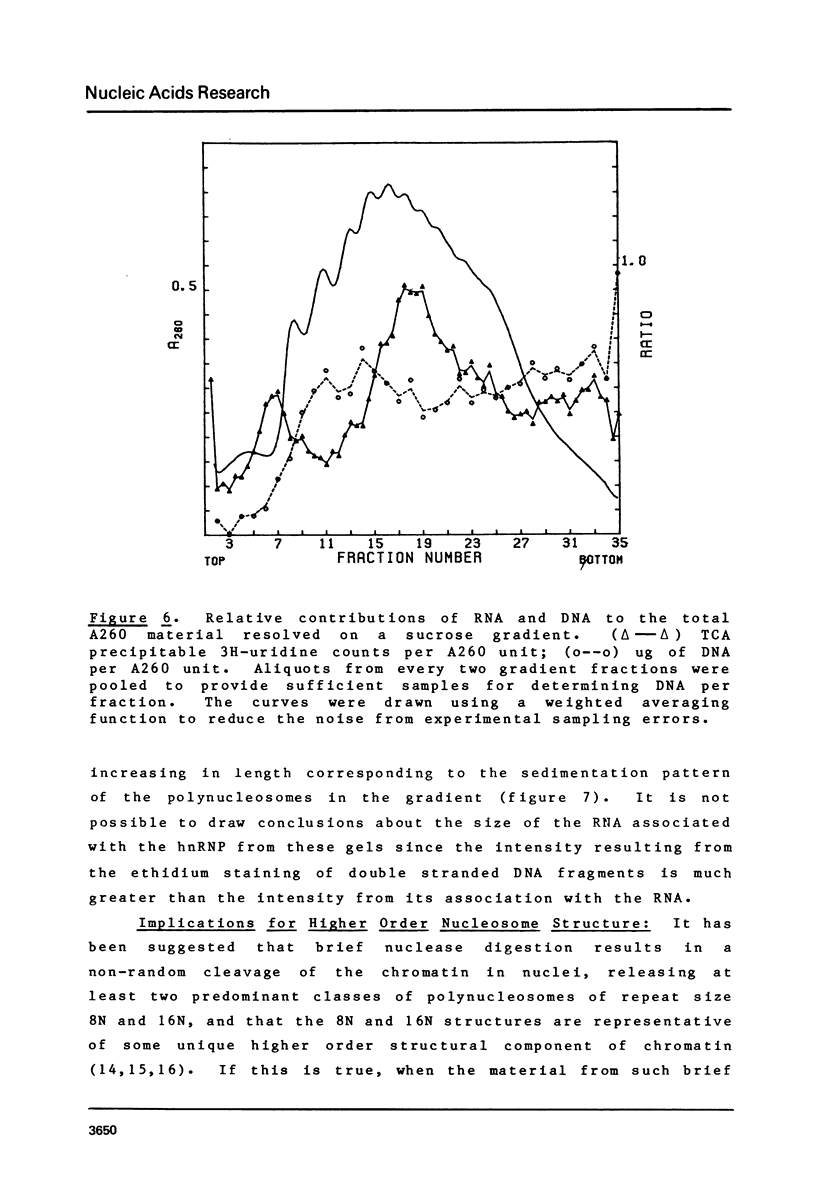
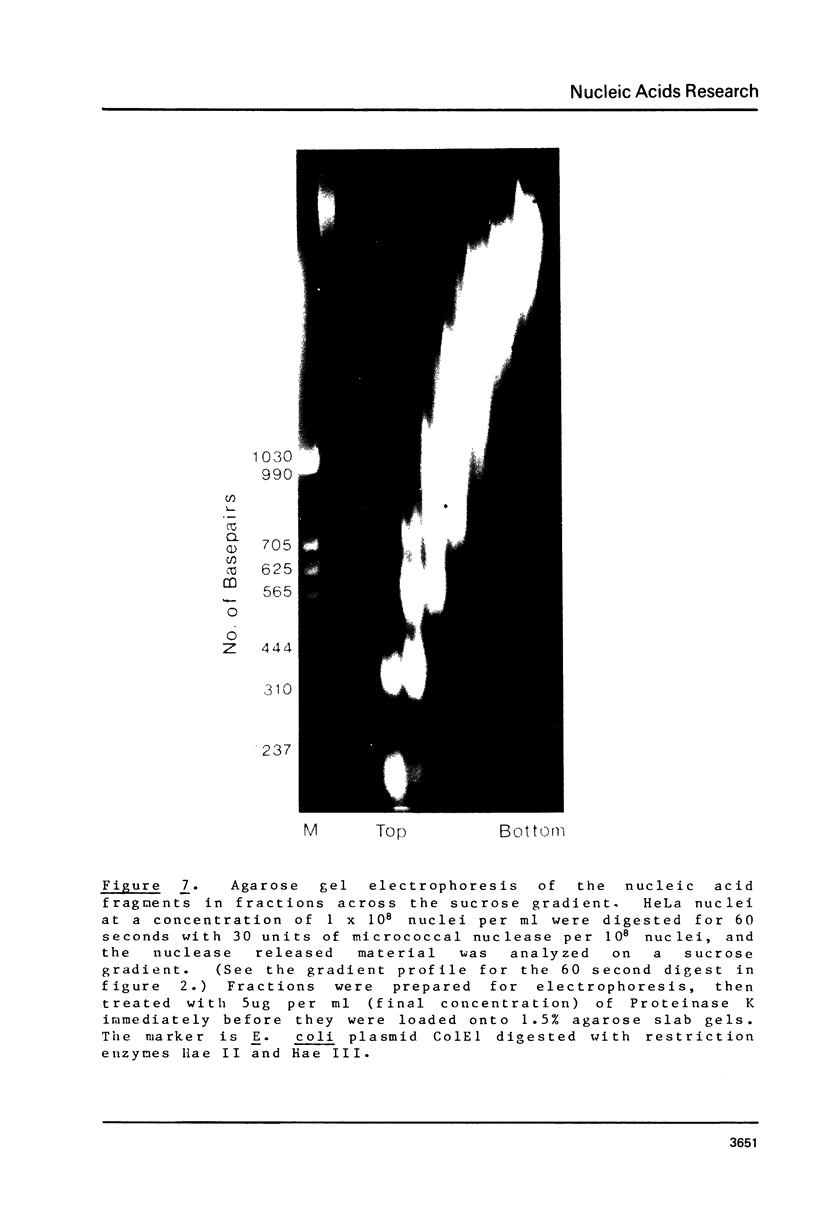
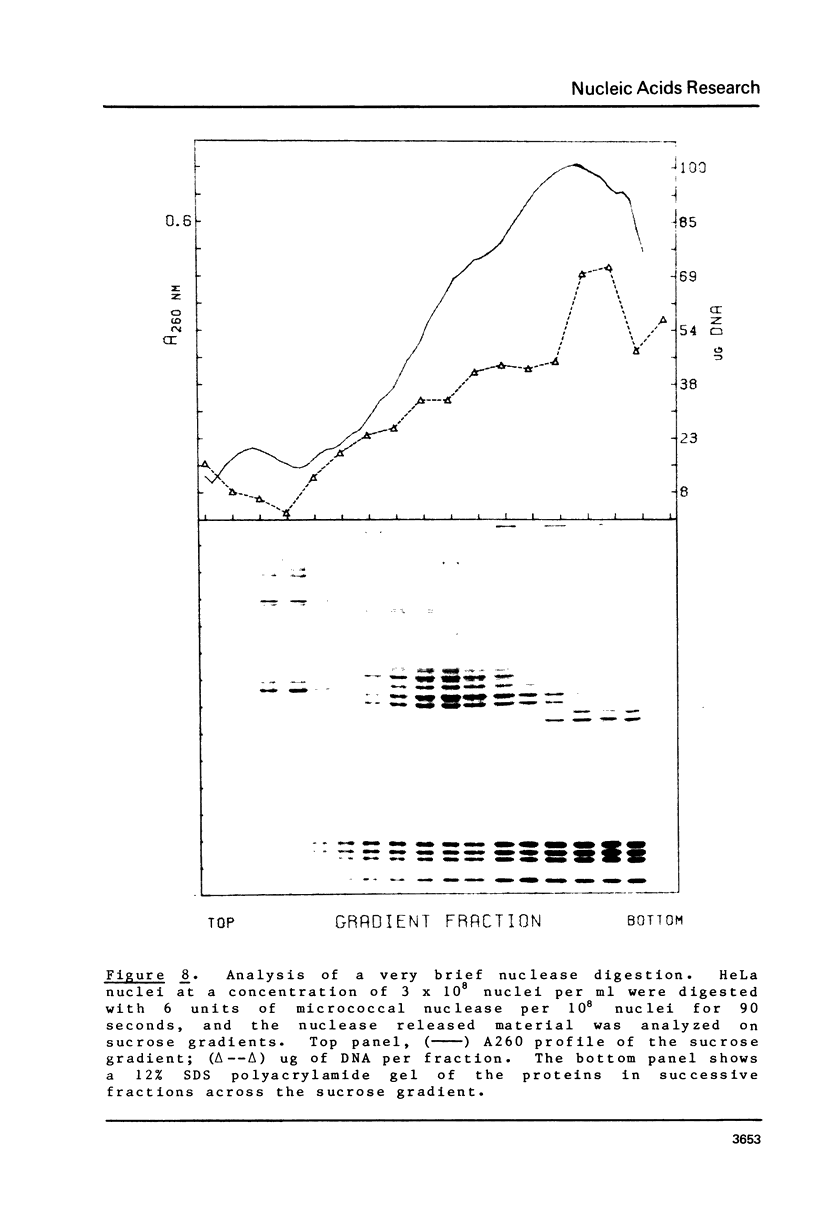
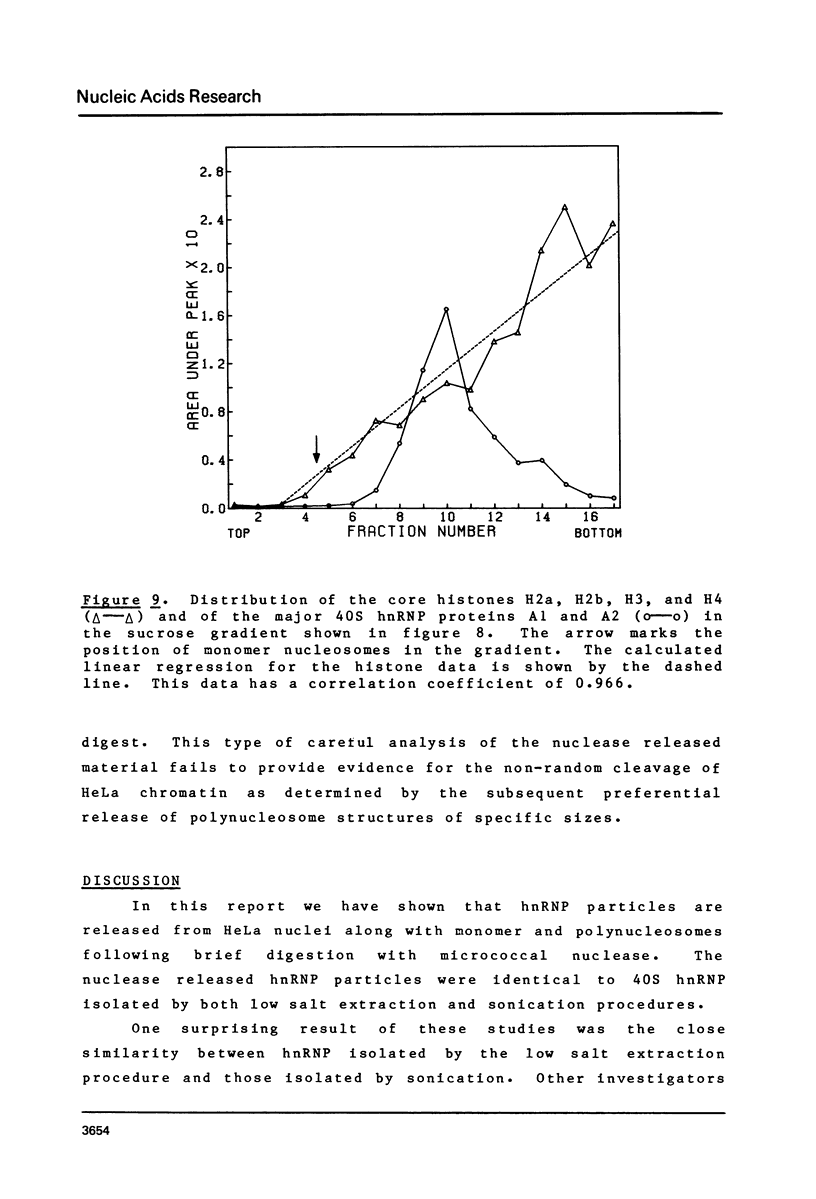
Brief digestion of HeLa nuclei with mirococcal nuclease releases monomer hnRNP particles as well as monomer and polynucleosomes. Sucrose gradient analysis of the nuclease released material reveals a series of small A260 peaks overlapping a more predominant peak in the 40S region of the gradient. Analysis of the proteins, DNa, and RNA in successive gradient fractions has confirmed that the smaller peaks are monomer and polynucleosomes, and that the larger peak is 40S hnRNP. Like 40S particles isolated by low salt extraction or by sonication, the nuclease released particles are composed of rapidly labeled RNA associated with a group of non-histone proteins the most predominant of which are the 32,000-44,000 MW proteins previously identified as core hnRNP proteins. These results provide further evidence that 40S hnRNP particles exist as discrete structural components of larger in vivo ribonucleoprotein complexes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Bhorjee J. S., Pederson T. Nonhistone chromosomal proteins in synchronized HeLa cells. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3345–3349. doi: 10.1073/pnas.69.11.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt T. R., Jump D. B., Smulson M. E. Nucleosome periodicity in HeLa cell chromatin as probed by micrococcal nuclease. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1628–1632. doi: 10.1073/pnas.76.4.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Howard E. F. Small nuclear RNA molecules in nuclear ribonucleoprotein complexes from mouse erythroleukemia cells. Biochemistry. 1978 Aug 8;17(16):3228–3236. doi: 10.1021/bi00609a009. [DOI] [PubMed] [Google Scholar]
- Hozier J., Renz M., Nehls P. The chromosome fiber: evidence for an ordered superstructure of nucleosomes. Chromosoma. 1977 Jul 18;62(4):301–317. doi: 10.1007/BF00327030. [DOI] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- LeStourgeon W. M., Beyer A. L., Christensen M. E., Walker B. W., Poupore S. M., Daniels L. P. The packaging proteins of core hnRNP particles and the maintenance of proliferative cell states. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):885–898. doi: 10.1101/sqb.1978.042.01.090. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm D. B., Sommerville J. The structure of chromosome-derived ribonucleoprotein in oocytes of Triturus cristatus carnifex (Laurenti). Chromosoma. 1974;48(2):137–158. doi: 10.1007/BF00283960. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Levey A., Ozarslan S., Quinlan T., Swift H., Urbas L. Some properties of RNA:protein complexes from the nucleus of eukaryotic cells. Cold Spring Harb Symp Quant Biol. 1974;38:921–932. doi: 10.1101/sqb.1974.038.01.094. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., McCarty K. S. Rapidly labeled messenger ribonucleic acid-protein complex of rat liver nuclei. J Biol Chem. 1968 Oct 25;243(20):5377–5384. [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- REDDI K. K. Action of micrococcal phosphodiesterase on tobacco mosaic virus nucleic acid. Nature. 1958 Nov 8;182(4645):1308–1308. doi: 10.1038/1821308a0. [DOI] [PubMed] [Google Scholar]
- REDDI K. K. Degradation of tobacco mosiac virus nucleic acid with micrococcal phosphodiesterase. Biochim Biophys Acta. 1959 Nov;36:132–142. doi: 10.1016/0006-3002(59)90077-0. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Seifert H., Scheurlen M., Northemann W., Heinrich P. C. Low molecular weight RNAs as components of nuclear ribonucleoprotein particles containing heterogeneous nuclear RNA. Biochim Biophys Acta. 1979 Aug 29;564(1):55–66. doi: 10.1016/0005-2787(79)90188-6. [DOI] [PubMed] [Google Scholar]
- Strätling W. H., Müller U., Zentgraf H. The higher order repeat structure of chromatin is built up of globular particles containing eight nucleosomes. Exp Cell Res. 1978 Dec;117(2):301–311. doi: 10.1016/0014-4827(78)90144-1. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Divilliers G., Jacob M. Rearrangements in the course of ribonuclease hydrolysis of pre-messenger ribonucleoproteins. A warning. Eur J Biochem. 1979 Apr;95(3):593–606. doi: 10.1111/j.1432-1033.1979.tb13000.x. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Jacob M. Effects of sodium chloride and pancreatic ribonuclease on the rat-brain nuclear particles: the fate of the protein moiety. Eur J Biochem. 1974 Aug 15;47(1):129–137. doi: 10.1111/j.1432-1033.1974.tb03676.x. [DOI] [PubMed] [Google Scholar]