Abstract
The surface expression and regulated endocytosis of kainate (KA) receptors (KARs) plays a critical role in neuronal function. PKC can modulate KAR trafficking, but the sites of action and molecular consequences have not been fully characterized. Small ubiquitin-like modifier (SUMO) modification of the KAR subunit GluK2 mediates agonist-evoked internalization, but how KAR activation leads to GluK2 SUMOylation is unclear. Here we show that KA stimulation causes rapid phosphorylation of GluK2 by PKC, and that PKC activation increases GluK2 SUMOylation both in vitro and in neurons. The intracellular C-terminal domain of GluK2 contains two predicted PKC phosphorylation sites, S846 and S868, both of which are phosphorylated in response to KA. Phosphomimetic mutagenesis of S868 increased GluK2 SUMOylation, and mutation of S868 to a nonphosphorylatable alanine prevented KA-induced SUMOylation and endocytosis in neurons. Infusion of SUMO-1 dramatically reduced KAR-mediated currents in HEK293 cells expressing WT GluK2 or nonphosphorylatable S846A mutant, but had no effect on currents mediated by the S868A mutant. These data demonstrate that agonist activation of GluK2 promotes PKC-dependent phosphorylation of S846 and S868, but that only S868 phosphorylation is required to enhance GluK2 SUMOylation and promote endocytosis. Thus, direct phosphorylation by PKC and GluK2 SUMOylation are intimately linked in regulating the surface expression and function of GluK2-containing KARs.
Kainate (KA) receptors (KARs) are highly expressed and widely distributed throughout the mammalian CNS and are involved in processes ranging from neuronal development and differentiation to neurodegeneration and neuronal cell death. Within individual neurons, KARs are present at the presynaptic terminal, where they modulate both inhibitory and excitatory neurotransmitter release; at the postsynapse, where they mediate excitatory neurotransmission; and at extrasynaptic sites, where they modulate neuronal excitability (1–4).
KARs are composed of tetrameric combinations of five possible subunits (GluK1–GluK5), and their functional expression is dynamically regulated by a complex interplay of exocytosis, endocytosis, and recycling. In hippocampal neurons, GluK2-containing KARs internalize to early endosomes after KA or NMDA stimulation and are sorted into either recycling or degradative pathways, depending on the endocytic stimulus. KA application causes a PKC-dependent internalization of KARs that are targeted to lysosomes, whereas NMDA receptor activation evokes a Ca2+-, PKA-, and PKC-dependent endocytosis of KARs, with subsequent reinsertion back into the plasma membrane (5). In dorsal root ganglion neurons, GluK1-containing KARs internalize via a PKC-dependent mechanism (6), and PKC also participates in KAR trafficking in the perirhinal cortex, where it is involved in NMDA receptor-independent KAR LTD (7). PKC phosphorylation of GluK2 at S846 and S868 was recently reported to regulate GluK2 progress through the biosynthetic pathway and endocytosis from the plasma membrane (8).
Posttranslational protein modification by small ubiquitin-like modifier (SUMO) is another key determinant of KAR trafficking (9). SUMO-1 is a 97-aa protein that can be covalently attached to lysine residues in target proteins by an enzymatic pathway analogous to the ubiquitin pathway (10, 11). In hippocampal neurons, SUMOylation of GluK2 at a single C-terminal lysine residue (K886) occurs at the plasma membrane in response to direct agonist activation and leads to their rapid internalization. Mutation of K886 or reduction of GluK2 SUMOylation using the SUMO-specific protease sentrin-specific protease 1 (SENP-1) prevents KA-induced KAR endocytosis. In addition, KAR-mediated excitatory postsynaptic currents are decreased by infusion of SUMO-1 and enhanced by infusion of sentrin-specific protease 1 (9, 12).
Although SUMOylation of GluK2 is known to be required for agonist-induced endocytosis, how receptor activation leads to SUMOylation is unclear. Because phosphorylation can regulate SUMOylation of other substrates (10, 13), and given the PKC dependence of KA-induced GluK2 endocytosis (5, 8), we investigated whether PKC activity is required for SUMO-1–dependent GluK2 endocytosis, and if so, whether direct phosphorylation of GluK2 mediates this effect.
Here we show that two PKC sites in the C-terminus of GluK2, S846 and S868, are phosphorylated in response to KA. In both in vitro assays and in COS-7 cells, phosphomimetic mutation of the S868 PKC site increased GluK2 SUMOylation. Treatment of neurons with the PKC activator phorbol 12-myristate 13-acetate (PMA) also increased GluK2 SUMOylation, mimicking the effect of KA. Finally, mutation of S868, but not of S846, to a nonphosphorylatable alanine abolished the KA-evoked increase of GluK2 SUMOylation, inhibited the SUMO-1–dependent reduction of KA-evoked KAR currents, and blocked KA-induced GluK2 endocytosis in neurons. Our results suggest a model in which agonist binding to GluK2 leads to PKC activation and phosphorylation of S868, which in turn leads to an increase in SUMOylation and receptor endocytosis.
Results
GluK2 Is Phosphorylated by PKC at S846 and S868 in Response to KA.
Phosphorylation has been implicated in GluK2 endocytosis, but direct agonist-induced phosphorylation of GluK2 in neurons has not been reported. Here we used virally expressed YFP-myc-GluK2 in neurons (9) and found that KA (20 μM, 5 min) caused robust phosphorylation (Fig. 1A). Importantly, the fraction of total endogenous or virally expressed WT YFP-myc-GluK2 expressed at the plasma membrane was similar under resting conditions, and their levels of endocytosis in response to KA stimulation were also similar (Fig. S1; ref. 14). Given that PKC in particular has been proposed as an important regulator of GluK2 trafficking (5, 8), we tested whether KA-induced GluK2 phosphorylation could be abolished by inhibition of PKC. GluK2 phosphorylation in response to KA was significantly reduced in the presence of the PKC inhibitor chelerythrine compared with KA alone (Fig. 1A), suggesting that GluK2 is phosphorylated by PKC after KA stimulation. To identify the phosphorylation sites on GluK2, we mutated the previously described PKC phosphorylation sites S846 and S868 (8) to nonphosphorylatable alanine residues. The resulting S846A and S868A showed reduced phosphorylation compared with WT, suggesting that both of these sites are phosphorylated in response to KA stimulation (Fig. 1B).
Fig. 1.
KA stimulation leads to PKC phosphorylation of GluK2 and PKC-dependent SUMOylation of GluK2 in neurons. (A) Representative blot showing the levels of GluK2 phosphorylation after KA stimulation. Neurons expressing YFP-myc-GluK2 were stimulated with 20 μM KA for 5 min in the presence or absence of the PKC inhibitor chelerythrine, immunoprecipitated with anti-myc antibody (sheep), and probed with anti–phospho-Ser/Thr and then with anti-myc antibody (mouse). The graph shows the normalized YFP-myc-GluK2 phosphorylation data. *P < 0.05; n = 4. (B) Mapping of GluK2 phosphorylation sites using S846A and S868A nonphosphorylatable mutants. The experiment was performed identically to that shown in A. The graph compares GluK2 WT and phospho-null mutants, either control or KA-stimulated, normalized to YFP-myc-GluK2 WT. *P < 0.05; n = 5. (C) (Left) representative anti-GluK2 Western blot showing non-SUMOylated (lower band) and SUMO-modified (upper band) GluK2 in 18 DIV neurons after stimulation with 20 μM KA, 1 μM PMA, or both KA and PMA. (Right) Representative blot showing the effects of 20 μM KA, 5 μM chelerythrine, or KA after preincubation with chelerythrine. The graphs show the ratio of SUMOylated to non-SUMOylated GluK2 normalized to the nontreated control. *P < 0.05; (Left) n = 15; (Right) n = 7.
PKC Activity Regulates GluK2 SUMOylation in Neurons.
We next investigated whether modulation of PKC activity affected GluK2 SUMOylation in neurons. We treated neurons with KA, PMA, or the PKC inhibitor chelerythrine and probed the resulting immunoblots with anti-GluK2 antibody to directly compare the intensity of the non-SUMOylated GluK2 band with the higher molecular weight SUMO-conjugated GluK2 band on the same blot. Both KA (20 μM, 20 min) and PMA (1 μM, 20 min) significantly increased GluK2 SUMOylation in cultured neurons, and their effects were not cumulative (Fig. 1C). Preincubation of neurons with the PKC selective inhibitor chelerythrine (5 μM, 20 min) reduced basal GluK2 SUMOylation and completely prevented the KA-evoked increase in GluK2 SUMOylation, suggesting that PKC phosphorylation is sufficient to facilitate GluK2 SUMOylation (Fig. 1C).
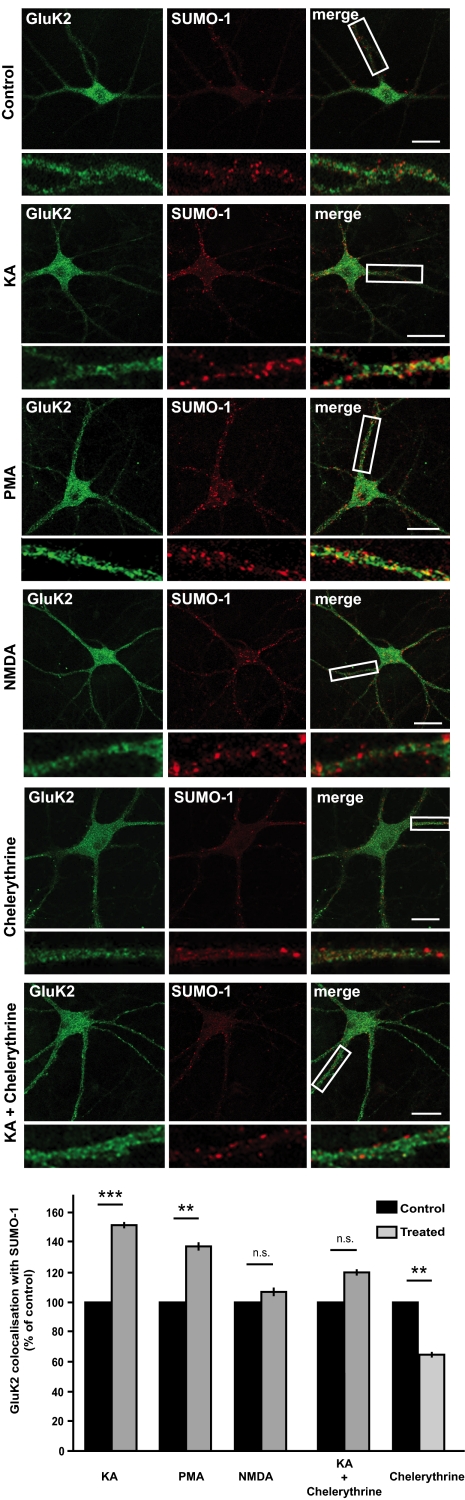
PKC Activation Increases Colocalization of GluK2 and SUMO-1 in Neurons.
Both KA and NMDA cause GluK2 internalization (5), but only receptors endocytosed in response to KA colocalize with SUMO-1 (9). Here we extend those findings to show that the KA-evoked increase in colocalization is prevented by chelerythrine, and that activation of PKC by PMA is sufficient to increase colocalization of SUMO-1 and GluK2 in neurons (Fig. 2). Interestingly, chelerythrine alone causes a decrease in SUMO-1 and GluK2 colocalization compared with control, consistent with basal PKC activity regulating GluK2 SUMOylation. These results, together with those shown in Fig. 1, indicate that PKC-mediated phosphorylation of GluK2 precedes SUMOylation, consistent with phosphorylation acting as a trigger for the subsequent SUMO conjugation and GluK2 internalization.
Fig. 2.
Colocalization between SUMO-1 and GluK2 increases after PKC activation. Effects of NMDA, KA, KA plus chelerythrine, chelerythrine, and PMA on the colocalization of SUMO-1 and GluK2 in hippocampal neurons. Note the increased colocalization (yellow in the composite images) after PKC activation. The KA-induced increase in colocalization is abolished by the PKC inhibitor chelerythrine. The boxes in the composite images indicate the magnified areas of dendrites (shown in the lower panels). Green represents GluK2; red, SUMO-1; yellow, colocalization. The graph shows colocalization of GluK2 and SUMO-1 in dendrites as the percentage of GluK2 immunoreactivity colocalizing with the SUMO-1 signal. **P < 0.01; ***P < 0.001. Data are from three independent experiments each analyzing 10–15 cells with at least three regions of interest per cell. (Scale bar: 20 μm.)
PKC Phosphorylation Enhances SUMOylation of GluK2 in Vitro.
To confirm and augment the results obtained in neurons, we studied the effect of PKC phosphorylation on in vitro SUMOylation of GluK2. After incubation with PKC, purified GST-tagged GluK2 C-terminus [GST-CT-GluK2(WT)] displayed significantly increased in vitro SUMOylation (Fig. 3A). To define the PKC phosphorylation site(s) responsible for this effect, we mutated S846 and S868 to nonphosphorylatable alanine or phosphomimetic aspartic acid residues and subjected these GST fusion proteins to in vitro SUMOylation. Both GST-CT-GluK2(S868D) and GST-CT-GluK2(S846D) phosphomimetic mutants exhibited enhanced SUMOylation; however, SUMOylation of the non-PKC-phosphorylatable S846A and S868A mutants was similar to that of WT (Fig. S2).
Fig. 3.
SUMOylation of GluK2 PKC phospho-mutants. (A) SUMOylation of nonphosphorylated and PKC-phosphorylated GST-CT-GluK2. Purified proteins were phosphorylated in vitro with PKC and then subjected to in vitro SUMOylation. The graph shows GST-CT-GluK2 SUMOylation after phosphorylation by PKC. In each lane, the intensity of the upper (SUMO-modified) band was divided by the intensity of the lower (nonmodified) band. *P < 0.05, n = 5. (B) SUMOylation of GluK2 in COS-7 cells. (Left) GluK2(WT) SUMOylation in cells coexpressing GluK2, Ubc9, and SUMO-1. (Middle and Right) SUMOylation levels for serine 868 and 846 mutants, respectively. The graph shows mutant GluK2 SUMOylation in COS-7 cells, normalized to the WT control. ***P < 0.001; n = 8–10 per condition.
S868D Phosphomimetic Mutation Increases GluK2 SUMOylation in COS-7 Cells.
We cotransfected COS-7 cells with full-length YFP-myc-GluK2 mutants, YFP-tagged SUMO-1, and FLAG-Ubc9 to enhance SUMOylation and then performed immunoblotting for GluK2 (Fig. 3B). As expected (9), the non-SUMOylatable K886R showed no SUMO conjugation. GluK2(S868D) displayed increased SUMOylation compared with GluK2(WT). However, SUMOylation of GluK2(S846D) was not significantly different from that of WT or the nonphosphorylatable mutants. Thus, although GluK2(S846D) showed enhanced SUMOylation in vitro, these data suggest that phosphorylation of this site is not functionally relevant for regulating the SUMOylation of full-length GluK2 in cells.
Reduced Surface Expression of S846D and S868D GluK2 Mutants.
We next assessed the surface expression of WT and mutant GluK2 in COS-7 cells under basal conditions (Fig. S3). GluK2(K886R) and the non–PKC-phosphorylatable mutants S846A and S868A displayed levels of surface expression comparable to that seen for GluK2(WT). In contrast, surface expression of the PKC site phosphomimetic mutants S846D and S868D was significantly reduced. Because SUMOylation of GluK2(S846D) was not enhanced in COS-7 cells (Fig. 3B), reduced surface expression of the S846D mutant was at first glance unexpected. However, these results are consistent with a recent report showing that phosphorylation at either or both of these PKC sites reduces endoplasmic reticulum (ER) exit and surface expression (8). Therefore, to explore whether the decreased surface expression of S846D and S868D is SUMOylation-dependent, we generated non-SUMOylatable (K886R) variants of the GluK2 S846D and S868D mutants and assessed their surface expression in COS-7 cells. The surface expression levels of the non-SUMOylatable variants of the phosphomimetic mutants were no different from those of the S846D and S868D single mutants, suggesting that the reduced surface expression observed is attributable solely to the previously reported ER retention of these mutants (8), and not to phosphorylation-mediated changes in GluK2 SUMOylation (Fig. S4).
GluK2(S868A) Is Not SUMOylated in Response to KA Activation.
We next examined whether the KA-induced increase in GluK2 SUMOylation depends on phosphorylation at S868. We virally expressed YFP-myc-GluK2 in cultured cortical neurons, pulled down recombinant GluK2 using GFP-Trap beads (ChromoTek), and probed the blots for SUMO-1 (Fig. S5A). KA stimulation increased the amount of SUMO-1–modified WT GluK2, but did not affect the levels of SUMOylation of the nonphophorylatable mutant S868A (Fig. S5B).
GluK2(S868A) Does Not Colocalize with SUMO-1 After KA Stimulation.
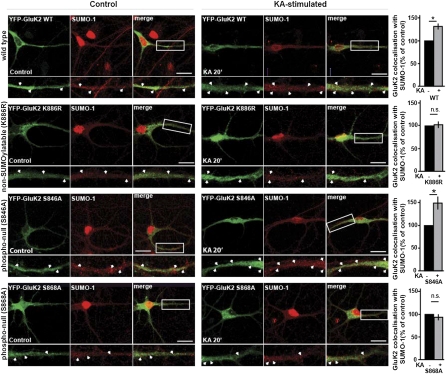
Because agonist activation results in internalization of WT GluK2 and colocalization with SUMO-1 (9), we examined the colocalization of virally expressed nonphosphorylatable mutants of GluK2 with endogenous SUMO-1 in neurons after KA stimulation. At 20 min after KA application, GluK2(S846A) displayed increased colocalization with SUMO-1 that was not significantly different from that of WT GluK2. However, the extent of colocalization between SUMO-1 and GluK2(S868A) or the non-SUMOylatable GluK2(K886R) did not change after KA stimulation (Fig. 4). Again, these results suggest that the KA-induced increase in GluK2 SUMOylation requires previous phosphorylation of S868, but not of S846.
Fig. 4.
Increased YFP-myc-GluK2 and SUMO-1 colocalization after KA stimulation is prevented by blocking S868 phosphorylation. Colocalization of virally expressed YFP-myc-GluK2 with endogenous SUMO-1 in control or KA-treated (20 μM, 20 min) cultured hippocampal neurons. Note that the KA-induced increase in colocalization (indicated in yellow in the composite images) is prevented by the K886R (non-SUMOylatable) and S868A (phospho-null) mutations, but not by the S846A mutation. The boxes in the composite images indicate the magnified areas (shown in the lower panels). Green indicates YFP-myc-GluK2; red, SUMO-1; yellow, colocalization. The graphs show the percentage of YFP-myc-GluK2 signal colocalizing with SUMO-1 immunoreactivity, with data for each mutant normalized to its respective control. *P < 0.05; n = 3 independent experiments, with 8–12 cells analyzed per condition. (Scale bar: 20 μm.)
Effects on Functional KAR Responses in HEK293 Cells.
Infusion of SUMO-1 into neurons causes a rapid decrease in synaptic KAR excitatory postsynaptic current amplitude due to the internalization of KARs, whereas infusion of nonconjugatable SUMO-1-ΔGG has no effect (9). We recapitulated these electrophysiological experiments using YFP-myc-GluK2 expressed in HEK293 cells. Consistent with the neuronal results, the amplitude of the KA-evoked responses decreased after infusion of SUMO-1 (49% ± 8%), but not after infusion of SUMO-1-ΔGG (100% ± 13%) (Fig. 5A). Because this system lacks native receptors, it allowed us to assess the functional properties of non–PKC-phosphorylatable GluK2 mutants. Robust KA-evoked currents were recorded in cells expressing GluK2(S868A), which, unlike GluK2(WT) and GluK2(S846A), were not significantly decreased by infusion of SUMO-1. These data indicate that PKC phosphorylation at S868 is a permissive step required for maximal SUMOylation of GluK2 and agonist-evoked internalization of functional KARs.
Fig. 5.
KA-induced GluK2 endocytosis is prevented by blocking S868 phosphorylation. (A) Whole-cell patch clamp recordings of GluK2-expressing HEK293 cells showing a SUMO-1–dependent decrease in KA-evoked currents. Comparison of GluK2 WT, S846A, and S868A using patch solution containing either active (open circles) or inactive (filled circles) SUMO-1. Traces were recorded for 15 min and plotted on the graph normalized to the first minute. Example traces are the average of the first minute (black) and 10–15 min (gray). The graph shows data obtained at 10 min after the first response. Note that SUMO-1 infusion significantly reduced KA-evoked responses mediated by GluK2 WT and S846A, but did not significantly reduce responses mediated by the S868A mutant. (Scale bars: 300 pA and 500 ms). *P < 0.05, ***P < 0.001; n = 5–7. (B) Surface biotinylation of control or KA-treated (20 μM, 20 min) cortical neurons virally expressing YFP-myc-GluK2 WT or mutants. Total (12%) and surface-expressed receptors were detected with anti-GluK2 antibody. The lower panel shows the same blots probed for β-actin. The graph shows quantification of these experiments, with each mutant shown normalized to its control. *P < 0.05; ***P < 0.001; n = 6–10.
Mutation of S868 to Alanine Abolishes KA-Induced Endocytosis of GluK2.
Because the mutation of S868 to alanine is known to prevent the KA-induced increase in GluK2 SUMOylation in neurons and to block the SUMO-dependent rundown of KAR currents in HEK293 cells, we tested whether phosphorylation of S868 is required for KA-induced endocytosis of GluK2-containing KARs in neurons, a process requiring SUMOylation of GluK2. Using surface biotinylation, we found that, similar to the non-SUMOylatable GluK2(K886R) mutant, GluK2(S868A) was not endocytosed after a KA challenge (Fig. 5B). In contrast, in the same experiments, KA caused robust internalization of both GluK2(WT) and GluK2(S846A), confirming that phosphorylation of GluK2 at S868 is necessary for KA-induced SUMOylation and internalization of GluK2-containing KARs in neurons.
Discussion
Phosphorylation regulates the endocytosis of AMPA receptors (15, 16) and NMDA receptors (17). PKC phosphorylation has been shown to influence KAR trafficking and plasticity (5–7), but until recently the roles of specific KAR subunit phosphorylation had not been well defined. While the present study was in progress, Roche and coworkers (8) elegantly demonstrated that PKC directly phosphorylates GluK2 at S846 and S868 to decrease surface expression by reducing ER exit and retarding forward trafficking. That study also showed that phosphorylation of S846 specifically promotes constitutive endocytosis from the cell surface (8). Part of our work here extends those results by demonstrating that GluK2 is directly phosphorylated at both S846 and S868 in response to KA. Because KA application leads to SUMOylation and ultimately endocytosis of GluK2-containing KARs, we wondered whether PKC phosphorylation after KA application acts as a trigger for GluK2 SUMOylation. Consistent with this, we found that KA or PMA treatment caused equivalent, noncumulative increases in GluK2 SUMOylation in neurons, suggesting that both KA and PMA likely act through a common pathway. Inhibiting PKC with chelerythrine reduced levels of GluK2 SUMOylation under both basal and KA-stimulated conditions, consistent with PKC phosphorylation preceding and facilitating SUMO conjugation to GluK2. Both KA and PMA increased colocalization of SUMO-1 and GluK2 in the soma and dendrites of neurons, indicating active recruitment of SUMO-1 to specific locations and also suggesting a facilitatory effect of PKC phosphorylation on GluK2 SUMOylation. In addition, the KA-induced increase SUMO-1 and GluK2 colocalization was prevented by chelerythrine, again suggesting that PKC phosphorylation is a key step in the activity-dependent SUMOylation of KARs. SUMOylation of GST-CT-GluK2(WT) was enhanced by PKC in vitro. Consistent with this, the PKC site phosphomimetic mutants S846D and S868D showed increased levels of SUMOylation in vitro, suggesting that PKC phosphorylation can enhance SUMOylation through direct phosphorylation of GluK2. But because some SUMOylation of both GluK2(WT) and the S846 and S868 phospho-null mutants was detected in these assays, we conclude that although phosphorylation enhances GluK2 SUMOylation, it is not an absolute requirement for SUMOylation to occur.
Full-length YFP-myc-GluK2(S868D) exhibited increased SUMOylation in COS-7 cells. However, in contrast to the in vitro data, SUMOylation of the S846D mutant was not enhanced. Although the reasons for this effect are unclear, it seems likely that the mechanisms of GluK2 SUMOylation in vivo are more complex than those in vitro. For example, the specificity of SUMOylation in vivo is enhanced by the presence of E3 ligases, which are absent from in vitro assays, potentially explaining the differences between the in vitro and in vivo data.
Consistent with phosphorylation at S868 enhancing SUMOylation, the phospho-null mutation of S868 prevented the KA-stimulated increase in GluK2 SUMOylation, suggesting that phosphorylation at S868 is a requirement for agonist-inducted SUMOylation of GluK2. Although our data indicate that KA application can enhance phosphorylation at both S846 and S868, the observation that the KA-induced increase in GluK2 SUMOylation occurs normally in the S846A mutant suggests that this residue does not play a significant role in enhancing GluK2 SUMOylation after agonist stimulation.
We previously reported that agonist-induced SUMOylation of GluK2 is necessary for agoinst-induced GluK2 endocytosis. Here we show that phosphorylation of GluK2 at S868 after KA stimulation is required for this increase in SUMOylation to occur. Consistent with this, we also show that although infusion of SUMO-1 into HEK293 cells causes a rundown in GluK2 currents, this effect is absent in the S868A mutant. Moreover, the S868A mutant does not undergo agonist-induced endocytosis when expressed in neurons.
Taken together, these results support a model in which KA stimulation of GluK2-containing KARs leads to PKC phosphorylation of GluK2 at S846 and S868. Although the role of S846 phosphorylation in this process is unclear, phosphorylation of S868 enhances SUMOylation of GluK2, ultimately leading to receptor endocytosis. These results reaffirm the complex regulation of GluK2-containing KARs mediated through PKC phosphorylation and support a role for phosphorylation of S868 in the enhancement of GluK2 SUMOylation after agonist treatment, in addition to the intracellular retention of endocytosed/de novo receptors reported previously (8).
In most cases, the mechanisms regulating the temporal and spatial SUMOylation of substrates are unknown. Although identification of increasing numbers of SUMO E3 enzymes likely contributes to the specificity of SUMOylation, emerging evidence suggests that other posttranslational modifications of target proteins may play a role; for example, phosphorylation can either enhance or reduce SUMOylation, depending on the substrate protein (10, 18). Here we show that phosphorylation at S868 promotes GluK2 SUMOylation in vitro. These assays were performed with the E1, Ubc9, and SUMO, but lacked E3s, suggesting that phosphorylation can act to directly recruit the SUMOylation machinery. This finding agrees with the observation that phosphorylation close to SUMOylation motifs can recruit Ubc9 (13) via the negative charge created by the phosphate group binding a cognate basic patch on Ubc9 (19), and suggests that a similar mechanism may exist for GluK2. Nonetheless, it is noteworthy that although a typical phosphorylation-dependent SUMOylation motif (e.g., in MEF-2 or GATA-1) contains a phosphorylated serine residue 5 aa downstream from the target lysine (19), in the case of GluK2, serine 868 is located 18 aa upstream from lysine 886. This might signify that the GluK2 C-terminus folds to form the 3D structure required to interact with the basic path on Ubc9. Alternatively, the increased SUMO-1 conjugation potentially could potentially be mediated through a direct interaction with the E1 enzyme or SUMO-1.
In conclusion, we have shown that agonist activation causes PKC phosphorylation of both S846 and S868 in the C-terminus of GluK2. However, only phosphorylation of S868 is required for the KA-induced increase in SUMOylation of K886 that in turn leads to KAR endocytosis. Thus, PKC phosphorylation and SUMOylation are intimately linked to control the surface expression and function of GluK2-containing KARs. These findings illustrate the complexity and refinement of receptor trafficking, provide new insight into the mechanisms that control neuronal function, and identify potential therapeutic targets for intervention in diseases that likely involve KAR dysfunction, such as epilepsy.
Materials and Methods
Rat embryonic dispersed hippocampal (for imaging) or cortical (for biochemistry) neuronal cultures prepared as previously described (20). Proteins were expressed in neurons using attenuated Sindbis virus as described previously (6). Detailed protocols regarding the production of dissociated neuronal cultures, Sindbis virus preparation, Western blotting, phosphorylation analysis, confocal imaging, in vitro phosphorylation/SUMOylation assays, surface biotinylation, immunoprecipitation experiments and electrophysiology can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Professor Ron Hay for the gift of the anti–SUMO-1 antibody and Dr. Stéphane Martin for advice and assistance in the initial stages of this project. Financial support was provided by the Medical Research Council, the European Research Council (to J.M.H.), and the Biotechnology and Biological Sciences Research Council (to J.M.H. and J.R.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111575108/-/DCSupplemental.
References
- 1.Chittajallu R, Braithwaite SP, Clarke VRJ, Henley JM. Kainate receptors: Subunits, synaptic localisation and function. Trends Pharmacol Sci. 1999;20:544–553. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- 2.Isaac JT, Mellor J, Hurtado D, Roche KW. Kainate receptor trafficking: Physiological roles and molecular mechanisms. Pharmacol Ther. 2004;104:163–172. doi: 10.1016/j.pharmthera.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Pinheiro P, Mulle C. Kainate receptors. Cell Tissue Res. 2006;326:457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 5.Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J. 2004;23:4749–4759. doi: 10.1038/sj.emboj.7600483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera R, Rozas JL, Lerma J. PKC-dependent autoregulation of membrane kainate receptors. EMBO J. 2007;26:4359–4367. doi: 10.1038/sj.emboj.7601865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park Y, Jo J, Isaac JT, Cho K. Long-term depression of kainate receptor-mediated synaptic transmission. Neuron. 2006;49:95–106. doi: 10.1016/j.neuron.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Nasu-Nishimura Y, Jaffe H, Isaac JT, Roche KW. Differential regulation of kainate receptor trafficking by phosphorylation of distinct sites on GluR6. J Biol Chem. 2010;285:2847–2856. doi: 10.1074/jbc.M109.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate receptor-mediated synaptic transmission. Nature. 2007;447:321–325. doi: 10.1038/nature05736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–145. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Brain Res Rev. 2010;64:195–212. doi: 10.1016/j.brainresrev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin S, Wilkinson KA, Nishimune A, Henley JM. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat Rev Neurosci. 2007;8:948–959. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hietakangas V, et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA. 2006;103:45–50. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin S, Bouschet T, Jenkins EL, Nishimune A, Henley JM. Bidirectional regulation of kainate receptor surface expression in hippocampal neurons. J Biol Chem. 2008;283:36435–36440. doi: 10.1074/jbc.M806447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158:105–125. doi: 10.1016/j.neuroscience.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Wang JQ, et al. Phosphorylation of AMPA receptors: Mechanisms and synaptic plasticity. Mol Neurobiol. 2005;32:237–249. doi: 10.1385/MN:32:3:237. [DOI] [PubMed] [Google Scholar]
- 17.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossis G, Melchior F. SUMO: Regulating the regulator. Cell Div. 2006;1:13. doi: 10.1186/1747-1028-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohideen F, et al. A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 UBC9. Nat Struct Mol Biol. 2009;16:945–952. doi: 10.1038/nsmb.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirbec H, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.