Abstract
Brassinosteroids (BRs) are plant hormones that regulate growth and development. They share structural similarities with animal steroids, which are decisive factors of sex determination. BRs are known to regulate morphogenesis and environmental stress responses, but their involvement in sex determination in plants has been only speculative. We show that BRs control sex determination in maize revealed through characterization of the classical dwarf mutant nana plant1 (na1), which also feminizes male flowers. na1 plants carry a loss-of-function mutation in a DET2 homolog—a gene in the BR biosynthetic pathway. The mutant accumulates the DET2-specific substrate (24R)-24-methylcholest-4-en-3-one with a concomitant decrease of downstream BR metabolites. Treatment of wild-type maize plants with BR biosynthesis inhibitors completely mimicked both dwarf and tasselseed phenotypes of na1 mutants. Tissue-specific na1 expression in anthers throughout their development supports the hypothesis that BRs promote masculinity of the male inflorescence. These findings suggest that, in the monoecious plant maize, BRs have been coopted to perform a sex determination function not found in plants with bisexual flowers.
Keywords: steroid reductase, 5α-steroid reductase, propiconazole, uniconazole
Sex determination in plants is a complex and dynamic process that requires the coordination of gene activities, environmental conditions, and hormones. In contrast to most animal species, the vast majority of plants are bisexual (1). They produce flowers that contain male (staminate) as well as female (pistillate) organs. Monoecious plants such as maize, however, develop unisexual flowers (called florets in grasses) located in separate inflorescences on the same plant (2). This spatial separation of sexes in maize was instrumental for the development and rapid advancement of hybrid seed technology during the 1930s (3). Male and female organs in bisexual flowers are in close proximity, which favors inbreeding. In contrast, monoecy through sex determination promotes outcrossing and allows controlled pollination for efficient hybrid seed production. Hybrid plants often exhibit heterosis (hybrid vigor), which contributes to increases in yield and disease resistance (3).
In maize, the staminate inflorescence commonly referred to as “tassel” forms at the tip of the terminal shoot internode. The female inflorescence—termed “ear”—bears only pistillate florets and is produced at the tips of lateral branches. Unisexual maize florets are derived from initially bisexual floral meristems. During flower development, differential hormone levels initiate the arrest and abortion of stamen primordia in ears and of pistil primordia in tassels, respectively (2, 4, 5).
The influence of the phytohormone gibberellic acid (GA) on sex determination in maize is illustrated by the formation of functional anthers in the ear of the GA-deficient dwarf mutants (6). Furthermore, exogenous GA application or environmental conditions that increase endogenous levels of GA result in the opposite phenotype of pistil development in the tassel (7, 8). This phenotype is referred to as “tasselseed” (ts). Six heritable ts mutants—three recessive (ts1, ts2, ts4) and three dominant (Ts3, Ts5, Ts6)—were reported in maize (6). Cloning of the ts1 gene revealed that it encodes a class 2 lipoxygenase involved in an early step of jasmonic acid (JA) biosynthesis. The ts phenotype of both ts1 and ts2 mutants is partially complemented by exogenous application of JA, suggesting that TS2 may also function in JA metabolism (9). Recently, ts4 and Ts6 were also molecularly characterized and shown to function on the level of transcriptional regulation and not in hormone biosynthesis. TS6 is an APETALA2-like transcription factor whose expression is controlled by ts4, which encodes a microRNA (10). Despite these diverse sex determination mechanisms, a common feature of all six ts mutants is their normal plant height (6).
Here, we report the genetic and molecular analysis of a ts mutant that is also severely compromised in height and overall plant architecture. We show that its defect is caused by mutation of a 5α-steroid reductase—an important enzyme of the brassinosteroid (BR) biosynthesis pathway. Furthermore, our data indicate that the sex determination process in maize tassels is controlled by BRs.
Results and Discussion
Genetic and Molecular Analysis of na1.
The ts–dwarf mutant was isolated from an F2 population of a plant with active Mutator (Mu) transposons. To explore its inheritance in detail, we introgressed the mutant into various maize inbred backgrounds. In F2 populations of these crosses, the dwarf and ts traits cosegregated as a single locus in each progeny evaluated. Although the expression of the dwarf phenotype was relatively consistent, the expressivity of the ts phenotype varied significantly among mutant segregants in different genetic backgrounds (Table 1). None of the tall plants in any F2 population exhibited a ts phenotype. These observations suggest that the dwarf and ts phenotypes are in fact caused by the same mutation.
Table 1.
The feminization of na1 mutant tassels is influenced by the genetic background
| Inbred | F2 families | WT height plants | na1-like dwarfs | P |
ts |
|
| WT height plants | na1-like dwarfs | |||||
| B73 | 4 | 236 | 62 | 0.09 | 0 | 34 |
| A188 | 4 | 167 | 49 | 0.43 | 0 | 35 |
| A619 | 4 | 174 | 50 | 0.35 | 0 | 45 |
| Mo20W | 5 | 230 | 73 | 0.71 | 0 | 4 |
A ts phenotype was recorded if more than five florets of a tassel were feminized. Plants were counted as dwarfs if they resembled the na1-1 architecture. Nondirectional P values shown were calculated with a Chi-square test of independence for a 3:1 segregation of tall and dwarf plants, respectively.
The isolation of this ts–dwarf from a Mu–active population made it likely that the causative mutation was induced by a Mu insertion. Using a PCR-based approach designed to selectively amplify DNA fragments flanking the sites of Mu insertions (SAIFF) (11), we identified a PCR product that cosegregated with the mutant phenotype but was absent in DNA from wild-type siblings. Molecular analysis of this PCR product revealed 497 bp of flanking sequence neighboring a Mu8 element. The Mu–flanking sequence matched part of the first exon of a gene sharing homology to DE-ETIOLATED2 (DET2) from Arabidopsis thaliana. DET2 is one of the best-characterized enzymes of the BR biosynthetic pathway in Arabidopsis, and it catalyzes the 5α-reduction of (24R)-24-methylchloest-4-en-3-one to (24R)-24-methyl-5α-cholestan-3-one (12, 13). BRs are plant-specific steroidal hormones that control cell elongation, photomorphogenesis, and vascular differentiation. Pronounced dwarfism is a hallmark of mutants deficient in BR biosynthesis or signaling (14–16). ZmDET2 shares 49% and 74% identity with DET2 and OsDET2 homologs, respectively. Phylogenetic analysis revealed that ZmDET2 has a predicted 5α-steroid reductase domain and conserved binding sites for cofactors and the steroid substrate (Figs. S1 and S2).
To confirm that a mutation in the maize ortholog of DET2 is responsible for the mutant phenotype, we obtained five lines from the Maize Genetics Cooperation Stock Center (Maize COOP), which exhibited dwarf and ts phenotypes. One mutant, nana plant1 (na1), first isolated in 1922, represents one of the three classical dwarf types of maize (17–19). The mechanism underlying the na1 phenotype has remained elusive even after decades of extensive investigation (20, 21). Interestingly, na1 maps to the region of the Zmdet2 locus on chromosome 3L (22). Genetic complementation tests confirmed that the COOP line na1-2 and our mutant (na1-1) are allelomorphic (Fig. S3). Subsequent sequencing of the na1 allele in na1-2 revealed a point mutation that results in a premature stop codon truncating NA1 by 100 amino acids (Fig. 1L). Moreover, we isolated two additional alleles of na1 (na1-3 and na1-4) via targeted transposon mutagenesis with Mu (23). Both alleles had Mu insertions in the na1 gene (Fig. 1L and Table S1).
Fig. 1.
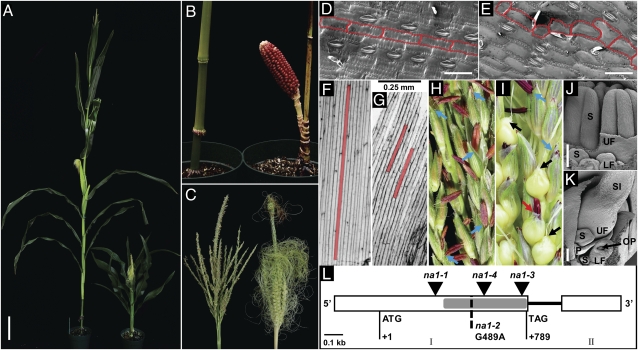
Morphological and molecular features of na1 mutants. (A) Mature wild-type (Left) and na1 (Right) plants. (B) Close-up of lower stem internodes of wild-type (Left) and na1 (Right) siblings. (C) Staminate wild-type (Left) and feminized na1 (Right) tassels. (D and E) Scanning electron microscopy (SEM) of leaf epidermal cells of wild-type (D) and na1 (E). Cell boundaries have been highlighted in red. (F and G) Light microscopy of mesocotyl epidermis cells of seedlings grown for 6 d in the dark. Red boxes illustrate single cells of wild-type (F) and na1 (G) mutants. (H and I) Close-up of wild-type (H) and na1 (I) tassel florets. Blue arrows, staminate florets; black arrows, pistillate florets; red arrow, floret with anthers, as well as female secondary sexual characteristics. (J and K) SEM of tassel spikelets. (J) Staminate wild-type spikelet with three developing stamen primordia in upper floret (UF) and lower floret (LF). (K) na1 mutant tassel spikelet with developing pistil in UF. OP, ovule primordium; P, palea; S, stamen; SI, silk. (L) Structure of na1 mutant alleles. Mu insertions are shown as inverted triangles. G to A substitution in na1-2, resulting in a premature STOP codon, is indicated by a dashed line. Exons I and II are indicated as open boxes; intron is a solid black line. A gray rectangle shows the predicted 5α-steroid reductase domain. ATG and TAG indicate start and stop codons, respectively (Table S1). [Scale bars: A, 20 cm; D and E, 75 μm; F and G, 250 μm; J and K, 1 mm (note different-sized scale bars)].
Morphometric Analysis of na1 Mutants.
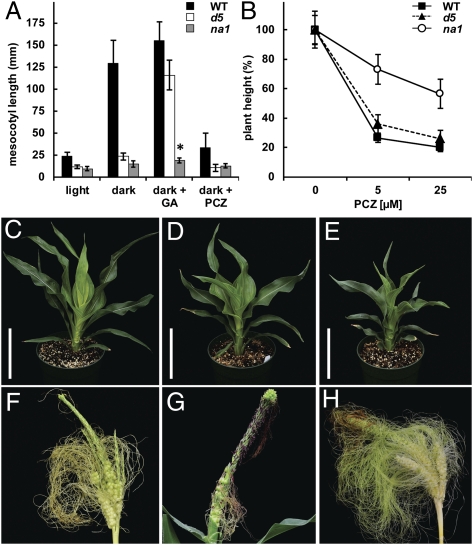
Compared with wild type, na1 mutants only reached approximately one-third in height (Fig. 1A). This decrease in plant height was due to a reduction of internode length and not a reduction in internode number (Fig. 1B). However, not all stem internodes were equally reduced in length. Except for the tassel internode, which consistently showed wild-type length, internode size varied between na1 mutants (Fig. S4A). Leaves of na1 plants were also altered in size and morphology. The leaf width remained largely unaffected, whereas the length of na1 mutant leaves was ∼60% of the wild type, which resulted in a significant change of length-to-width ratio (Fig. S4B). na1 mutant leaves were also positioned more erectly toward the stem and showed twisting around the midrib (Fig. 1A and Fig. S4 C and D). Leaf twisting may be associated with misalignment and altered cell shape of epidermis cells observed in leaves of na1 plants but not in wild type (Fig. 1 D and E). Interestingly, na1 mutants also exhibited altered skotomorphogenesis (seedling development in the dark) with impaired mesocotyl elongation, a characteristic of BR-deficient mutants (Fig. S5) (14–16). As a result of reduced cell elongation, mesocotyls of dark-grown na1 plants reached only 20% of the length of wild-type siblings (Figs. 1 F and G and 2A). Although GA is known to control mesocotyl elongation in etiolated (dark-grown) maize seedlings (24), exogenous GA3 applications did not complement the reduction of mesocotyl length in na1 mutants (Fig. 2A). In addition, wild type and na1-1 grown in the light responded similarly to exogenous applications of GA3 and the GA biosynthesis inhibitor uniconazole (UCZ) (Fig. S4E; ref. 25). These results indicate that the dwarfism of dark- and light-grown na1 plants is independent of GA metabolism or signaling.
Fig. 2.
Phenocopy of na1 mutant characteristics by BR inhibitors. (A) Mesocotyl length of wild type (WT), d5, and na1-1 plants grown in vermiculite in the light or dark for 8 d in the absence or presence of 250 μM GA3 and 50 μM PCZ, respectively. Asterisk indicates significant difference between na1 dark and na1 dark + GA (P = 0.002; n ≥ 10). (B) Height of light-grown WT, d5, and na1-1 plants with and without PCZ. (C and E) Dwarf phenotypes of WT (C) and na1-1 (E) grown for 27 d in soil with 75 μM PCZ. (D) na1-1 control without PCZ. (Scale bars, 20 cm.) (F and H) Tassels of WT (F) and na1-1 (H) plants grown in Turface with 500 μM PCZ. (G) na1-1 control without PCZ. (A and B) Data are means ± SD; n = 10–19 (A) and n = 12 (B).
Tassels of na1 mutants were often partly feminized (Fig. 1C). In addition to staminate and pistillate flowers, na1 tassels contained florets with anthers and female secondary sexual characteristics such as short, thin, and trichomeless glumes without an anthocyanin ring at the base (Fig. 1 H and I) (6, 9). The basic building blocks of maize inflorescences are spikelets, which are produced from ordered rows of spikelet pair meristems. Each spikelet meristem develops an upper floret (UF) and lower floret (LF), which each contains three stamen primordia surrounding a gynoecium. In wild-type tassel spikelets, both gynoecia abort early during development, and stamen initials differentiate into anthers (Fig. 1J) (26). However, in na1 tassel spikelets, one or both florets exhibited pistil maturation with a concomitant arrest of stamen primordia (Fig. 1K).
BR Biosynthesis Inhibitors Phenocopy na1 Mutants.
To test whether a reduction of endogenous BRs causes both the dwarfism and ts phenotype of na1 plants, we treated wild-type and mutant siblings with propiconazole (PCZ), a BR biosynthesis inhibitor (27). Wild type and na1-1 grown in the presence of 5 μM PCZ showed height reductions of 75% and 30%, respectively. This significant difference in PCZ response is not likely due to the already reduced stature of na1 mutants, given that wild-type and the GA-deficient dwarf d5 plants responded similarly to PCZ treatment (Fig. 2B). In addition to the reduced height, PCZ-treated wild types produced short, dark green, and erect leaves that twisted around the midrib, making them effectively indistinguishable from na1 mutants (Fig. 2 C–E). Tassels of PCZ-treated wild types produced viable florets with primary and secondary characteristics of female flowers, predominantly at the base and apical regions (Fig. 2F). The severity and penetrance of the ts phenotype of na1-1 was also enhanced by PCZ (Fig. 2 G and H and Table S2). These findings strongly suggest that both the dwarfism and ts phenotype of na1 mutants are caused by BR deficiency.
Defects in NA1 Greatly Reduced Endogenous BR Levels.
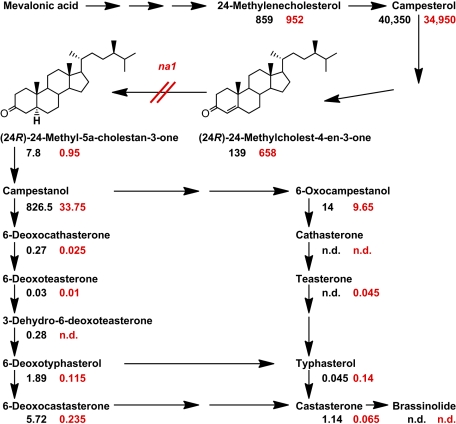
To further support the hypothesis that molecular defects in the na1 gene result in the disruption of BR biosynthesis, we measured the endogenous BR levels in mutant and wild-type shoots. The substrate of the DET2 enzyme, (24R)-24-methylcholest-4-en-3-one, accumulated in na1 mutants to 475% of the wild-type level. In contrast, the concentrations of most downstream intermediates in the BR biosynthetic pathway were drastically reduced in na1 plants (Fig. 3), consistent with NA1 acting as a 5α-steroid reductase.
Fig. 3.
Quantitative analysis of endogenous BR intermediates in 4-wk-old wild-type and na1-1 plants. BR levels (nanograms per gram of fresh weight) measured in pools (n = 10) of wild-type (black) and na1 (red) shoots are shown below each compound. Data points represent means of two independently grown experiments. To calculate the means, data points below the detection limit were set to 0. Detailed results for each replica are shown in Table S3. n.d., not detected.
na1 Is Expressed in Anthers Throughout Their Development.
There is currently no evidence for long-distance transport of BRs; however, their extracellular reception implies short-distance transport and that BRs act at or close to the site(s) of their synthesis (28). We therefore hypothesized that na1 may be expressed in tassel florets. To address this question, we used full-length antisense cDNA as a probe to assess the expression of na1 in developing tassels of wild-type and na1 siblings using in situ hybridization. na1 transcripts were detected in stamens throughout their development, including the locule chambers of wild-type tassel florets (Fig. 4 A–F). In addition, we observed na1 transcript accumulation in the outer cell layer(s) of the carpel primordia, either coincident or before abortion (Fig. 4 A–C). No transcripts were detected in tassel inflorescences of na1 mutants (Fig. 4 G and H). These results suggest that BRs play a role in the promotion of stamen maturation in tassels.
Fig. 4.
na1 expression pattern. (A–H) RNA in situ hybridization targeting full-length na1 cDNA (purple). (A–F) Wild-type tassel inflorescence spikelets. (G and H) Tassel inflorescence spikelets of na1-1. C, carpel primordium; G, glume; GR, gynocial ridge; LO, locule chamber; P, palea; S, stamen or stamen primordium. (I) qRT-PCR of organ-specific na1 expression in B73. S, 10-d-old seedling; R, 10-d-old root; YL, young leaf blade; EL, expanded leaf blade; M, midrib of expanded leaf; ST, top 0.5- to 1-cm-long internode at the stage of tassel sex determination; TX, developing tassel of 0.5- to 3-cm length; TA, tassel at anthesis. Data represent means ± SD relative to seedling (set to 1); n = 3 or 4 biological replicates with each 3 technical replicates.
In addition to the ts phenotype, na1 mutants are affected in development of stems and leaves. We performed quantitative RT-PCR (qRT-PCR) to analyze the specific expression of na1 in these affected organs. na1 transcripts were detected in all organs analyzed. The highest transcript accumulation was found in tassels at the stage of sex determination (0.5–3 cm in length) and in elongating stems (29) (Fig. 4I). In comparison, the lowest expression of na1 was found in leaves with <5% of the mRNA levels of those in tassels (TX) and stems (Fig. 4I).
Sex Determination in Monoecious Maize Inflorescences Is Controlled by Steroid Hormones.
In animals, steroid hormones are a decisive factor for sex determination (30). The human ortholog of DET2—steroid 5α-reductase 2—is required for male sex differentiation because its deficiency causes genetic males to develop as pseudohermaphrodites with feminized organs (31). Plant-specific BRs have been shown to regulate morphogenesis and environmental stress responses, but their involvement in sex determination has been speculative (32–34). Although BR mutants have been reported in several plant species with bisexual flowers, including Arabidopsis, pea, tomato, and rice (ref. 15, Table S4), BR mutants have not been characterized in plants with unisexual flowers. Transgenic down-regulation of ZmDWF1, a maize homolog of the BR biosynthetic enzyme AtDWF1, results in the reduction of plant height, suggesting that the growth-enhancing activity of BRs is conserved in maize (35). We observed that the expected dwarfism of a BR mutant in maize is accompanied by a ts phenotype. Our results implicate an important role of BRs for the promotion of stamen development in tassels. Failure of pistil abortion in na1 mutant tassels might be an indirect result of disrupted stamen development. Alternatively, pistil elimination in tassel florets is initiated in the subepidermal carpel cell layers after Ts2 expression (5). We found na1 transcripts in the outer cell layers of the carpel primordia near the time of their abortion. BRs could therefore also act as a short-range signal from the outer- to the subepidermal carpel layers to trigger pistil primordia degeneration. However, in Arabidopsis, the disruption of gynoecial development in the suess mutant background is enhanced by the lack of BRs (36).
In conclusion, we propose that steroid-like hormones affect sex determination processes not only in animals, but also in plants. Both classes of organisms use structurally similar signaling molecules, despite greatly different receptor and signal transduction pathways—integral membrane receptors in plants and soluble steroid receptors that translocate from the cytoplasm into the nucleus in animals (37). In maize, the hormones GA, JA, cytokinin, and BR control sex determination in male flowers, but interestingly, only GA has been implicated in the arrest of stamen primordia in ear florets (6, 9, 38, 39). Elucidating the role of BRs in sex determination will advance the basic understanding of hormonal regulation of flowering in plants.
Materials and Methods
Plant Material and Growth Conditions.
Maize plants were grown under greenhouse conditions at 27 °C (day) and 21 °C (night). Unless indicated otherwise, plants were grown in 1:1 (vol/vol) mixtures of Turface Athletics MVP (Profile Products) and Pro-Mix-BX with microrise (Premier Horticulture). Plants were fertilized with 200 ppm Miracle-Gro Excel (Scotts) adjusted to pH 6.0 following manufacturer recommendations.
Genetic Stocks and na1 Alleles.
Maize inbred lines used in this work included A188, A619, B73, and Mo20W. Seeds of d5 were obtained from Carolina Biological Supply. The na1-1 mutant was isolated in 2002 from an F2 population derived from a plant known to contain active Robertson's Mu transposons. Seeds of na1-2 (na*N282; isolated by Gerald Neuffer, University of Missouri, Columbia, MO) used as na1 reference allele, were obtained from Maize COOP (40). The na1-3 and na1-4 alleles were recovered from targeted mutagenesis with Mu as the genetic mutagen and na1-1 as the male tester (23, 41). Approximately 230 ears of Mu-active plants were fertilized with pollen from na1-1 homozygotes. Out of ∼80,000 F1 progeny screened, 9 plants (each from a separate ear) resembled na1 mutants, of which 6 could be introgressed into A619 and B73. Surprisingly, dwarf plants segregated only in two of the F2 populations. Sequencing of the na1 locus in F2 dwarfs from those two lines revealed that they were homozygous for Mu insertions in the first exon of na1 (Table S1).
Cloning of na1.
The SAIFF method used to clone na1 was performed as described (11) with the following changes. Approximately 0.6 μg of genomic DNA from 10 wild-type (A619 × na1-1, BC1 F3), 8 homozygous na1-1 (A619 × na1-1, BC1 F3), and 10 homozygous na1-1 (A188 × na1-1, BC1 F3) plants was used for the restriction digestion with MseI. The primers MuExt22D (5′-CCAACGCCAWSGCCTCYATTTC-3′) and MseExt18 (5′-GTGAACGGTCGATGAGTC-3′) were used in a ratio of 1.5:1, respectively. All PCR products were separated on 2% agarose gels. A PCR product was identified with the +2 selective adaptor primer MseIAAC (5′-GATGAGTCCTGAGTAACC-3′) and the Mu-TIR primer MuInt19 (5′-GCCTCYATTTCGTCGAATC-3′), which cosegregated with all 28 homozygote na1-1, but was absent in wild type.
Candidate Phenotype Screen.
To isolate additional na1 alleles, we screened the MaizeGDB phenotype browser, the Maize Inflorescence Architecture Project database, and RescueMu Database (40, 42, 43). Screen parameters were as follows: (i) dwarfism (≤50% of wild-type height); (ii) stiff, dark green, erect, and twisting leaves; and (iii) feminization of tassel florets (ts). Seeds from five molecularly uncharacterized mutants, which satisfied the criteria, were obtained from the Maize COOP. For genetic complementation tests, pollen from each candidate mutant was used to pollinate na1-1. For four of the candidate lines, only wild-type phenotype was observed in the F1 generation. In contrast, F1 plants derived from crosses of na1-2 with na1-1 showed a combination of dwarf and ts phenotype (Fig. S3).
Chemical Treatments.
Seeds were heat-sterilized for 7 min at 60 °C before planting.
For PCZ (Banner Maxx; Syngenta) treatment, seeds were imbibed for 28 h in paper towels soaked with distilled water containing indicated concentrations of PCZ. Seeds were planted in pots with vermiculite (SunGro Horticulture) and watered with fertilizer solution every 3rd day. PCZ was added at indicated concentrations to the fertilizer solution. After 3 wk, plants were harvested, photographed, and analyzed by using ImageJ software (Version 1.43u; ref. 44). Plant height was determined from the root–shoot transition zone to the highest leaf collar. Turface was used as growth medium for long-term experiments. Growth conditions in Turface were as described above, except that PCZ was administered for 6 wk at indicated concentrations.
GA3 (Gold Biotechnology) and UCZ (Sumagic; Valent) treatments of light-grown plants began 14 d after germination and continued every 3rd day for 2 wk. A 1-mL solution of 0.005% Silwet L77 (Momentive Performance Materials) with indicated concentrations of GA3 or UCZ was applied into the apical leaf cavity. Control plants were treated with 1 mL of 0.005% Silwet L77. Height was measured from the first (coleoptile) node to the highest leaf collar.
For deetiolation assays, seeds were imbibed as described above with indicated concentrations of PCZ and GA3. They were then planted in trays with vermiculite, watered with identical concentrations of PCZ or GA3, and grown for 7 d at 28 °C and 90% humidity in the dark. Control plants were grown without PCZ or GA3 (Fig. S5). Plants were then harvested and photographed. Mesocotyl length was determined from the root–shoot transition zone to the first node by using ImageJ software (44).
Light Microscopy.
Seeds of segregating homozygous and heterozygous na1-1 siblings (genotyped through PCR at seedling stage) were heat-sterilized for 7 min at 60 °C. Plants were grown in the dark for 6 d at 28 °C and 90% humidity. After harvest, single cell layer epidermal peels were removed from the lower end of the mesocotyl and examined with a Leica Z16-AP0 microscope.
Quantification of Endogenous BR Intermediates.
Shoot tissue of na1-1 and wild-type siblings grown for 4 wk in the greenhouse was harvested and immediately frozen in liquid nitrogen. Before analysis, tissue samples were lyophilized for 3 d at −78 °C and 4 mTorr. Purification and quantification of BRs was performed as described (45).
Statistical Analysis.
Chi-square tests of independence were conducted by using the CHISQ.TEST function in Microsoft Excel. The add-in XL Toolbox (Version 2.72) for Microsoft Excel was used to obtain all other descriptive and comparative statistics in this work.
In Situ Hybridization.
For the na1 probe, a PCR fragment containing the full-length ORF was amplified from B73 cDNA with Na1F (5-TGGAACCAACTGAAGATGCCC-3′) and Na1R (5′-GCCAAGAAGAACGAACCGAAAC-3′) primers. The PCR product was subcloned into the vector pGEM-T Easy (Invitrogen) and sequenced. T7 polymerase (Promega) was used to generate a digoxigenin-labeled antisense probe by in vitro transcription following manufacturer's protocol. In situ hybridizations were performed as described (46), except that the 0.5- to 1-cm-long tassels of B73 and na1-1 were dissected and fixed with FAA (3.7% formaldehyde, 5% acetic acid, 50% ethanol, and 0.5% Triton) for 1 h, followed by hybridization at 60 °C.
RNA Isolation and qRT-PCR.
Total RNA was isolated from different tissues of B73 inbreds as described (47). For qRT-PCR analysis, total RNA was pretreated with DNase I (Amplification grade; Invitrogen), and cDNA was synthesized by using reverse transcriptase (Superscript III; Invitrogen). MOLYBDENUM COFACTOR BIOSYNTHESIS protein (MOL, GRMZM2G067176) was used as internal control (48). na1-specific primers Na1FOR3 5′-AGGCTGAGTTTGCCCATGTT-3′ (300 nM) and Na1REV3 5′-GCAGTCTCGCGCAGCTAATC-3′ (500 nM) and Mol-specific primers MolFOR2 5′-CTGTGTCCTCCGTGCTCCAT-3′ (500 nM) and MolREV2 5′-AGGACTCCCGCATCTCCATA-3′ (500 nM) were designed by using PRIMEREXPRESS software (Applied Biosystems, Invitrogen). All primers showed >90% efficiency at their indicated concentrations. qRT-PCR was performed as described (49) by using the StepOnePlus instrument (Applied Biosystems, Invitrogen).
Scanning Electron Microscopy.
B73 and na1-1 tassels of 0.5- to 1-cm length were fixed in FAA for 1 h at 4 °C and dehydrated in an ethanol series to 100% ethanol. The samples were critical point dried, sputter coated with gold palladium for 45 s, and viewed on an ISI30 SEM scanning electron microscope at an accelerating voltage of 2.0 kV (50). Cryo-SEM was used for imaging of leaf epidermis cells. Small leaf samples were cryofixated in liquid nitrogen and observed in a JEOL 6610LV SEM (JEOL USA) at 5 kV accelerating voltage under vacuum.
Supplementary Material
Acknowledgments
We thank H. Maeda, C. Y. Yoo, R. R. Altstatt, and C. Kish for critical reading of the manuscript; S. Takatsuto for deuterium-labeled internal standards; R. Latin for PCZ; R. Eddy, J. Beatty, and their coworkers for help with plant cultivation; D. Petros, N. B. Best, J. S. Budka, A. Maselli, W. S. Peters, and S. Chintamanani for technical assistance; M. G. Neuffer and M. Sachs for technical advice; and the Maize COOP for providing material. This work was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-Aid for Scientific Research (B) 19380069 and 23380066 (to S.F.); Cooperative Research Program for Agricultural Science and Technology Development, Rural Development Administration (Republic of Korea) Grant PJ906910 (to S.C.); Agriculture and Food Research Initiative Grant 2010-65116-20483 (to G.S.C.); National Science Foundation CAREER Integrative Organismal Systems Grant 1054918 (to B.S.); and Purdue College of Agriculture Start-Up Funds (G.S.J. and B.S.). R.W. was supported by a Bilsland Dissertation Fellowship.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. JN020028 (SbDET2), JN020029 (na1-1), JN020030 (na1-2), JN020031 (na1-3), and JN020032 (na1-4)].
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108359108/-/DCSupplemental.
References
- 1.Mitchell CH, Diggle PK. The evolution of unisexual flowers: Morphological and functional convergence results from diverse developmental transitions. Am J Bot. 2005;92:1068–1076. doi: 10.3732/ajb.92.7.1068. [DOI] [PubMed] [Google Scholar]
- 2.Irish EE. Regulation of sex determination in maize. BioEssays. 1996;18:363–369. [Google Scholar]
- 3.Crow JF. 90 years ago: The beginning of hybrid maize. Genetics. 1998;148:923–928. doi: 10.1093/genetics/148.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JC, et al. Cell cycle arrest of stamen initials in maize sex determination. Genetics. 2007;177:2547–2551. doi: 10.1534/genetics.107.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderon-Urrea A, Dellaporta SL. Cell death and cell protection genes determine the fate of pistils in maize. Development. 1999;126:435–441. doi: 10.1242/dev.126.3.435. [DOI] [PubMed] [Google Scholar]
- 6.Dellaporta SL, Calderon-Urrea A. The sex determination process in maize. Science. 1994;266:1501–1505. doi: 10.1126/science.7985019. [DOI] [PubMed] [Google Scholar]
- 7.Nickerson NH. Sustained treatment with gibberellic acid of five different kinds of maize. Ann Mo Bot Gard. 1959;46:19–37. [Google Scholar]
- 8.Rood SB, Pharis RP, Major DJ. Changes in endogenous gibberellin-like substances with sex reversal of the apical inflorescence of corn. Plant Physiol. 1980;66:793–796. doi: 10.1104/pp.66.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta IF, et al. tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science. 2009;323:262–265. doi: 10.1126/science.1164645. [DOI] [PubMed] [Google Scholar]
- 10.Chuck GS, Meeley R, Irish E, Sakai H, Hake S. The maize tasselseed4 microRNA controls sex determination and meristem cell fate by targeting Tasselseed6/indeterminate spikelet1. Nat Genet. 2007;39:1517–1521. doi: 10.1038/ng.2007.20. [DOI] [PubMed] [Google Scholar]
- 11.Muszynski MG, et al. delayed flowering1 Encodes a basic leucine zipper protein that mediates floral inductive signals at the shoot apex in maize. Plant Physiol. 2006;142:1523–1536. doi: 10.1104/pp.106.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi T, et al. Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-En-3-one to (24R)-24-methyl-5α-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 1999;120:833–840. doi: 10.1104/pp.120.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop GJ, Koncz C. Brassinosteroids and plant steroid hormone signaling. Plant Cell. 2002;14(Suppl):S97–S110. doi: 10.1105/tpc.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemhauser JL, Chory J. BRing it on: New insights into the mechanism of brassinosteroid action. J Exp Bot. 2004;55:265–270. doi: 10.1093/jxb/erh024. [DOI] [PubMed] [Google Scholar]
- 17.Hutchison CB. The linkage of certain aleurone and endosperm factors in maize, and their relation to other linkage groups. Cornell Agr Exp Sta Mem. 1922;60:1419–1473. [Google Scholar]
- 18.Li HW. Heritable characters in maize: XLV- nana. J Hered. 1933;24:279–281. [Google Scholar]
- 19.Lindstorm EW. Genetical research with maize. Genetica. 1923;5:327–356. [Google Scholar]
- 20.Van Overbeek J. The growth hormone and the dwarf type of growth in corn. Proc Natl Acad Sci USA. 1935;21:292–299. doi: 10.1073/pnas.21.5.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoemaker RL, Harris RM. Peroxidase activity in the maize stature mutant nana-1. J Ariz Acad Sci. 1975;10:29–32. [Google Scholar]
- 22.Dempsey E. Location of knobs on the genetic maps of chromosomes 6 and 3. Maize Newslett. 1971;45:58–59. [Google Scholar]
- 23.Brutnell TP. Transposon tagging in maize. Funct Integr Genomics. 2002;2:4–12. doi: 10.1007/s10142-001-0044-0. [DOI] [PubMed] [Google Scholar]
- 24.Rood SB, Beall FD, Pharis RP. Photocontrol of gibberellin metabolism in situ in maize. Plant Physiol. 1986;80:448–453. doi: 10.1104/pp.80.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rademacher W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:501–531. doi: 10.1146/annurev.arplant.51.1.501. [DOI] [PubMed] [Google Scholar]
- 26.Cheng PC, Greyson RI, Walden DB. Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am J Bot. 1983;70:450–462. [Google Scholar]
- 27.Sekimata K, et al. A specific and potent inhibitor of brassinosteroid biosynthesis possessing a dioxolane ring. J Agric Food Chem. 2002;50:3486–3490. doi: 10.1021/jf011716w. [DOI] [PubMed] [Google Scholar]
- 28.Symons GM, Ross JJ, Jager CE, Reid JB. Brassinosteroid transport. J Exp Bot. 2008;59:17–24. doi: 10.1093/jxb/erm098. [DOI] [PubMed] [Google Scholar]
- 29.Irish EE, Nelson TM. Development of Tassel seed 2 inflorescences in maize. Am J Bot. 1993;80:292–299. [Google Scholar]
- 30.Bogart MH. Sex determination: A hypothesis based on steroid ratios. J Theor Biol. 1987;128:349–357. doi: 10.1016/s0022-5193(87)80077-2. [DOI] [PubMed] [Google Scholar]
- 31.Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5 α-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, et al. Biochemical characterization of TASSELSEED 2, an essential plant short-chain dehydrogenase/reductase with broad spectrum activities. FEBS J. 2007;274:1172–1182. doi: 10.1111/j.1742-4658.2007.05642.x. [DOI] [PubMed] [Google Scholar]
- 33.Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. Plant Cell. 2004;16(Suppl):S61–S71. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walbot V, Skibbe DS. Maize host requirements for Ustilago maydis tumor induction. Sex Plant Reprod. 2010;23:1–13. doi: 10.1007/s00497-009-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao Y, et al. Functional analysis of ZmDWF1, a maize homolog of the Arabidopsis brassinosteroids biosynthetic DWF1/DIM gene. Plant Sci. 2004;167:743–751. [Google Scholar]
- 36.Nole-Wilson S, Rueschhoff EE, Bhatti H, Franks RG. Synergistic disruptions in seuss cyp85A2 double mutants reveal a role for brassinolide synthesis during gynoecium and ovule development. BMC Plant Biol. 2010;10:198. doi: 10.1186/1471-2229-10-198. 10.1186/1471-2229-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol. 2005;21:177–201. doi: 10.1146/annurev.cellbio.21.090704.151241. [DOI] [PubMed] [Google Scholar]
- 38.Bortiri E, Hake S. Flowering and determinacy in maize. J Exp Bot. 2007;58:909–916. doi: 10.1093/jxb/erm015. [DOI] [PubMed] [Google Scholar]
- 39.Rodo AP, et al. Over-expression of a zeatin O-glucosylation gene in maize leads to growth retardation and tasselseed formation. J Exp Bot. 2008;59:2673–2686. doi: 10.1093/jxb/ern137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence CJ, Schaeffer ML, Seigfried TE, Campbell DA, Harper LC. MaizeGDB's new data types, resources and activities. Nucleic Acids Res. 2007;35(Database issue):D895–D900. doi: 10.1093/nar/gkl1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johal GS, Briggs SP. Reductase activity encoded by the HM1 disease resistance gene in maize. Science. 1992;258:985–987. doi: 10.1126/science.1359642. [DOI] [PubMed] [Google Scholar]
- 42.Sen TZ, et al. MaizeGDB becomes ‘sequence-centric’. Database (Oxford) 2009;2009:bap020. doi: 10.1093/database/bap020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andorf CM, et al. The Locus Lookup tool at MaizeGDB: Identification of genomic regions in maize by integrating sequence information with physical and genetic maps. Bioinformatics. 2010;26:434–436. doi: 10.1093/bioinformatics/btp556. [DOI] [PubMed] [Google Scholar]
- 44.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophot Int. 2004;11:36–42. [Google Scholar]
- 45.Fujioka S, Takatsuto S, Yoshida S. An early C-22 oxidation branch in the brassinosteroid biosynthesis pathway. Plant Physiol. 2002;130:930–939. doi: 10.1104/pp.008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jackson D. in Molecular Plant Pathology: A Practical Approach. In: Bowles DJ, Gurr SJ, McPherson M, editors. Oxford: Oxford Univ Press; 1991. pp. 163–174. [Google Scholar]
- 47.Eggermont K, Goderis IJ, Broekaert WF. High-throughput RNA extraction from plant samples based on homogenisation by reciprocal shaking in the presence of a mixture of sand and glas beads. Plant Mol Biol Rep. 1996;14:273–279. [Google Scholar]
- 48.Sekhon RS, et al. Genome-wide atlas of transcription during maize development. Plant J. 2011;66:553–563. doi: 10.1111/j.1365-313X.2011.04527.x. [DOI] [PubMed] [Google Scholar]
- 49.Orlova I, et al. The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. Plant Cell. 2009;21:4002–4017. doi: 10.1105/tpc.109.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuck G, Meeley RB, Hake S. The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev. 1998;12:1145–1154. doi: 10.1101/gad.12.8.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.