Abstract
Variation in the masticatory behavior of hunter-gatherer and agricultural populations is hypothesized to be one of the major forces affecting the form of the human mandible. However, this has yet to be analyzed at a global level. Here, the relationship between global mandibular shape variation and subsistence economy is tested, while controlling for the potentially confounding effects of shared population history, geography, and climate. The results demonstrate that the mandible, in contrast to the cranium, significantly reflects subsistence strategy rather than neutral genetic patterns, with hunter-gatherers having consistently longer and narrower mandibles than agriculturalists. These results support notions that a decrease in masticatory stress among agriculturalists causes the mandible to grow and develop differently. This developmental argument also explains why there is often a mismatch between the size of the lower face and the dentition, which, in turn, leads to increased prevalence of dental crowding and malocclusions in modern postindustrial populations. Therefore, these results have important implications for our understanding of human masticatory adaptation.
Keywords: diet, phenotypic plasticity, mastication, skull
One of the major differences categorizing human populations is variation in subsistence strategies and related paramasticatory behavior. A shift from a primarily hunting and gathering strategy to one based on extensive horticulture or animal husbandry is known to have occurred independently on several occasions in human prehistory, yielding a correlated shift in settlement pattern, demography, population expansion, and social reorganization (e.g., 1, 2). Given the wider cultural changes associated with increased food processing and, therefore, consumption of a more homogeneous and softer diet in agriculturalists, it has been hypothesized that the dietary changes associated with agriculture are likely to have had an important effect on the form of the cranium and mandible (e.g., 3–5). Although localized studies comparing hunter-gather and farming populations in Nubia (6), South America (7), the Ohio Valley (8), and the southern Levant (9) have found some support for an associated change between the masticatory apparatus and the initial transition to agriculture, it is currently unclear what effect agriculture has had on global patterns of human mandibular variation when compared against other wider microevolutionary factors, such as gene flow, migration, and natural selection. Hence, this study represents a global comparative analysis of the effects of subsistence strategy on modern human mandibular variation.
In recent decades, it has become clear that the majority of modern human cranial shape variation is congruent with a null model of neutral evolution, with relatively few morphological regions being subject to diversifying selection (e.g., 10–19). However, there appear to be two major exceptions to this general pattern. Aspects of facial morphology, and particularly nasal morphology, are likely to have been subject to diversifying natural selection in response to climatic conditions (11, 20–22), which would explain why facial shape is correlated with climate when cold-adapted populations are included (13, 18). Second, it has been found (14, 15) that global patterns of mandibular variation do not follow a model of neutral evolution.
If the null model of evolutionary neutrality can be rejected for global patterns of human mandibular variation, alternative nonneutral hypotheses must be considered. One of the most obvious alternative models is that agricultural populations will experience different biomechanical or selective pressures on mandibular shape than hunter-gatherers, such that modifications have occurred either via phenotypic plasticity or natural selection. Previous morphometric studies (23, 24) found some geographical patterning in mandibular morphology, as well as a signal of climatic and/or masticatory plasticity. However, hunter-gatherer and agricultural populations have never explicitly been compared at a global level to evaluate the likely role of subsistence economy in the evolution of the mandible. Here, this hypothesis is tested by comparing (using Mantel tests) pairwise population distance matrices based on mandibular shape data (Fig. 1) against distance matrices based on neutral genetic, geographical, climatic, and subsistence data (characterized in four ways). To provide a baseline against which to evaluate these results, the same analyses were also repeated for the cranium and subsets of the cranium (Fig. 2) believed to be related to masticatory function (palatomaxilla, zygotemporal, and temporal lines) and those previously shown to fit a neutral model of variation (cranial vault and chondrocranium). In all cases, the data were collected on the same individuals representing 11 globally distributed populations (Table 1), of which six were categorized as agriculturalist and five as hunter-gatherer (Table 2).
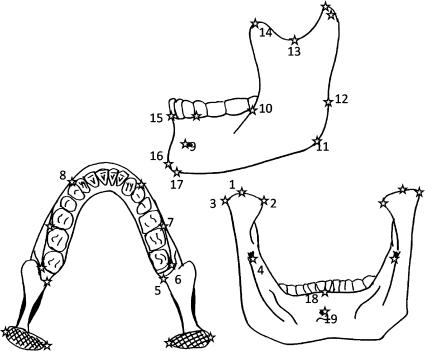
Fig. 1.
Configuration of 33 mandibular landmarks. Bilateral landmarks: 1, condyle tip; 2, condylion medial; 3, condylion lateral; 4, mandibular foramen (superior); 5, alveolus (posterior); 6, M3 (lateral-posterior); 7, M1-M2 (lateral); 8, canine-P3 (lateral); 9, mental foramen (anterior); 10, ramus (anterior and in line with alveolus); 11, gonion; 12, ramus (posterior and in line with alveolus); 13, sigmoid notch; 14, coronion. Midline landmarks: 15, infradentale; 16, pongonion; 17, gnathion; 18, mandibular orale; 19, linguale.
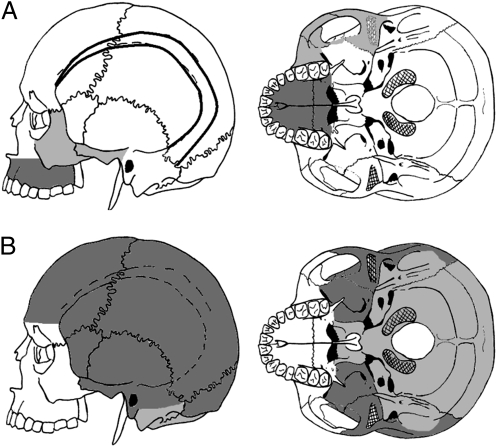
Fig. 2.
Cranial subsets tested (with numbers of landmarks in parentheses) for comparison with mandibular data. (A) Regions of the cranium related to masticatory function: temporal lines (dark lines) (25), zygotemporal (light gray) (22), and palatomaxilla (dark gray) (21). (B) Regions of the cranium not related to masticatory function: chondrocranium (light gray) (39) and cranial vault (dark gray) (51). A full anatomical description of all landmarks used is provided in Table S2.
Table 1.
Matched global population data used
| Population | Subsistence economy* | Classical genetics† | Morphology: Museum collected | Cranial (n) | Mandible (n) | Geographical coordinates |
| Ibo | Ibo | Yoruba/Ibo | NHM | 30 | 30 | 7.5, 5.0 |
| Central African | Mbuti | Biaka | NHM, MH | 21 | 19 | 4.0, 17.0 |
| San | Kung San | San | NHM, MH, AMNH, NHMW, DC | 31 | 23 | −21.0, 20.0 |
| Chinese (Han) | Chekiang | Han Chinese | NHMW | 30 | 27 | 32.5, 114.0 |
| Japanese | Japanese | Japanese | MH | 30 | 30 | 38.0, 138.0 |
| Mongolian | Khalka/Chahar | Mongol | MH | 30 | 30 | 45.0, 111.0 |
| Italian | Romans | Italians | NHMW | 30 | 19 | 46.0, 10.0 |
| Hawikuh | Pima | Pima | SNMNH | 30 | 30 | 33.5, −109.0 |
| Alaskan Inuit | Nunamiut | Alaskan Inuit | AMNH | 30 | 30 | 69.0, −158.0 |
| Greenland Inuit | Greenland | Greenland Inuit | SNMNH | 30 | 30 | 70.5, −53.0 |
| Australian | Aranda | Aborigine | DC | 30 | 27 | −22.0, 126.0 |
| Total | 322 | 295 |
AMNH, American Museum of Natural History (New York); DC, Duckworth Collection (Cambridge, United Kingdom); MH, Musée de l'Homme (Paris); NHM, Natural History Museum (London); NHMW, Das Naturhistorische Museum, Wien (Vienna); SNMNH, Smithsonian National Museum of Natural History (Washington).
*Data collated from the Corrected Ethnographic Atlas, available online at http://eclectic.ss.uci.edu/ (44).
†Data collated from Cavalli-Sforza et al. (35).
Table 2.
Quantitative data on subsistence economy collated for each population
| Population | Predominant subsistence economy | Gathering | Hunting | Fishing | Animal husbandry | Agriculture | Milking |
| Ibo | Extensive agriculture | 0–5% | 0–5% | 0–5% | 6–15% | 86–100% | 1 |
| Italians | Intensive agriculture | 0–5% | 0–5% | 16–25% | 16–25% | 56–65% | 2 |
| Japanese | Intensive agriculture | 0–5% | 0–5% | 6–15% | 6–15% | 76–85% | 1 |
| Chinese | Intensive agriculture | 0–5% | 0–5% | 6–15% | 6–15% | 76–85% | 1 |
| Mongolian | Mostly pastoralism | 0–5% | 6–15% | 0–5% | 76–85% | 6–15% | 2 |
| Hawikuh | Intensive agriculture | 26–35% | 6–15% | 6–15% | 0–5% | 46–55% | 1 |
| Biaka/Mbuti | Mostly hunting | 26–35% | 66–75% | 0–5% | 0–5% | 0–5% | 1 |
| San | Mostly gathering | 76–85% | 16–25% | 0–5% | 0–5% | 0–5% | 1 |
| Alaskan | Mostly hunting | 6–15% | 66–75% | 16–25% | 0–5% | 0–5% | 1 |
| Greenland | Mostly fishing | 6–15% | 16–25% | 66–75% | 0–5% | 0–5% | 1 |
| Australian | Mostly gathering | 56–65% | 36–45% | 0–5% | 0–5% | 0–5% | 1 |
For each category of subsistence dependence, the following ordinal scale was used: 0 = 0–5%, 1 = 6–15%, 2 = 16–25%, 3 = 26–35%, 4 = 36–45%, 5 = 46–55%, 6 = 56–65%, 7 = 66–75%, 8 = 76–85%, 9 = 86–100%. The predominant subsistence economy assigned in the Ethnographic Atlas database was used to assign populations into two groups: agriculturalist/pastoralist and hunter/gatherer/fishers. For the milking variable, 0 = missing, 1 = little/none, 2 = more often than sporadically.
Results
Overall, the results (Table 3) show that the global pattern of mandibular morphology strongly reflects the dichotomous distinction between “hunter-gatherer” and “agricultural/pastoralist” subsistence economy, irrespective of the specific geographical location or population history of each population. In comparisons of morphology and genetics, only the mandible and the palatomaxilla were not significantly correlated with genetic patterns, supporting the notion (14, 15) that the mandible does not reflect neutral population history. Given that the palatomaxilla is morphologically integrated with the mandible via dental occlusion, it is not unexpected that it follows a similar nonneutral pattern. Perhaps surprisingly, the mandible does pattern geographically, although the relationship between mandibular and geographical distance is much weaker (r = 0.44) than for the cranium (r = 0.72). Nicholson and Harvati (24) also found a geographical patterning in their analysis of modern human mandibular variation, which they interpreted as being related to climatic effects as well as population history. All morphological regions, except for the temporal lines, were significantly correlated with climate, although these correlations disappeared once neutral genetic distance was controlled for. This supports previous studies (e.g., 16, 19) suggesting that climatically driven diversifying selection has played a relatively minor role in generating global patterns of cranial variation. However, aspects of facial variation associated with thermoregulation were deliberately not tested here. Thus, the results do not negate the possibility of climatically driven natural selection on facial morphology in cold-adapted populations (e.g., 11, 18, 20–22).
Table 3.
Results of Mantel and partial Mantel tests performed
| Nonmasticatory |
Masticatory |
|||||||
| Cranium* | Chondro | Vault | Mandible | Palatomax | Zygotemp | Templines | ||
| Mantel tests | ||||||||
| Genetics | 0.61 (0.002) | 0.54 (0.001) | 0.62 (0.001) | 0.23 (0.149) | 0.18 (0.179) | 0.50 (0.004) | 0.38 (0.010) | |
| Geography | 0.72 (0.001) | 0.79 (0.001) | 0.63 (0.001) | 0.44 (0.010) | 0.19 (0.188) | 0.70 (0.001) | 0.52 (0.002) | |
| Climate | 0.49 (0.002) | 0.26 (0.007) | 0.38 (0.016) | 0.38 (0.009) | 0.32 (0.021) | 0.38 (0.015) | 0.20 (0.166) | |
| Subsistence 1† | 0.15 (0.166) | 0.23 (0.075) | 0.19 (0.091) | 0.31 (0.032) | 0.32 (0.024) | 0.22 (0.097) | 0.09 (0.453) | |
| Subsistence 2† | 0.13 (0.340) | 0.12 (0.388) | 0.20 (0.122) | 0.25 (0.041) | 0.28 (0.036) | 0.11 (0.395) | 0.02 (0.893) | |
| Subsistence 3† | 0.26 (0.052) | 0.25 (0.080) | 0.30 (0.022) | 0.37 (0.008) | 0.31 (0.031) | 0.27 (0.058) | 0.12 (0.375) | |
| Subsistence 4† | 0.19 (0.100) | 0.22 (0.079) | 0.23 (0.057) | 0.32 (0.012) | 0.30 (0.037) | 0.22 (0.093) | 0.07 (0.565) | |
| Partial Mantel tests (genetics controlled for) | ||||||||
| Climate | 0.39 (0.018) | 0.10 (0.486) | 0.24 (0.071) | 0.33 (0.033) | 0.28 (0.051) | 0.26 (0.061) | — | |
| Subsistence 1† | — | — | — | 0.31 (0.017) | 0.32 (0.034) | — | — | |
| Subsistence 2† | — | — | — | 0.26 (0.045) | 0.28 (0.040) | — | — | |
| Subsistence 3† | — | — | 0.31 (0.021) | 0.36 (0.014) | 0.30 (0.029) | — | — | |
| Subsistence 4† | — | — | — | 0.31 (0.017) | 0.30 (0.040) | — | — | |
| Partial Mantel test (genetics, climate, and geography controlled for) | ||||||||
| Subsistence 3† | 0.31 (0.045) | 0.34 (0.032) | 0.34 (0.028) | 0.38 (0.009) | 0.28 (0.036) | 0.31 (0.032) | 0.09 (0.477) | |
Correlation coefficients (P values in parentheses) for Mantel and partial Mantel test comparisons of morphological distance matrices and genetic, geographical, climatic, and subsistence distance matrices. Nonsignificant results (P > 0.05 for full, P > 0.017 for 3-way partial, and P > 0.010 for 5-way partial Mantel tests) are shown in bold. Chondro, chondrocranium (basicranium); Palatomax, palate and maxilla region; Templines, insertions of the temporalis muscles; Zygotemp, zygomatic and temporal region (Fig. 2 and Table S2).
*Cranium refers to the full cranial configuration, including the vault, face, and base.
†Subsistence 1, binary matrix; Subsistence 2, quantitative data (Table 2); Subsistence 3, hunting and fishing treated as single variable; Subsistence 4, horticulture and animal husbandry treated as single variable.
Irrespective of how differences in subsistence economy were quantified, the mandible and the palatomaxilla were significantly correlated with subsistence, whereas the remaining regions of the cranium were not. In the case of the third subsistence matrix (where hunting and fishing were treated equally), the vault was also significantly correlated, but this disappeared once population history was controlled for. Similarly, the relationship between the palatomaxilla and subsistence economy disappeared once population history was controlled for. In contrast, the mandible remained correlated with subsistence economy, even following Bonferroni correction. The only exception to this was in the case of subsistence matrix 2, where hunting and fishing are treated separately, suggesting that this categorization of subsistence creates artificial differences that do not actually affect the morphology of the mandible. Moreover, in contrast to all cranial regions tested, a five-way partial Mantel test (α = 0.01) between mandibular variation and the most strongly correlated matrix of subsistence difference (matrix 3) was significant (r = 0.38, P = 0.009), demonstrating that mandibular distance remains significantly correlated with subsistence even when the potentially confounding effects of genetics, geography, and climate are all controlled for (Table 3).
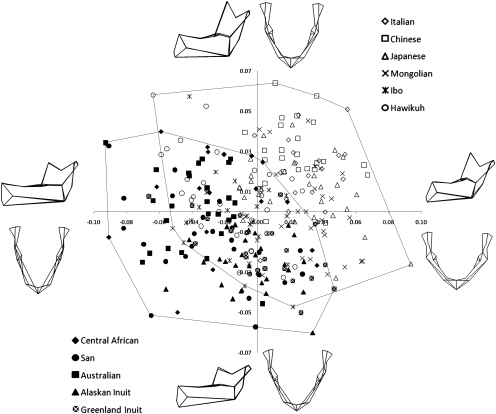
Fig. 3 illustrates the major mandibular shape variation associated with differences in subsistence economy. The first and second principal components (PCs), which, together, account for almost 33% of the total shape variation, effectively distinguish between agriculturalists (open symbols) and nonagriculturalists (closed symbols). Despite overlap among individual population samples, agriculturalist populations have relatively shorter and broader mandibles with taller and more angled rami and coronoid processes, whereas hunter-gatherer populations have longer and narrower mandibles with short and upright rami and coronoid processes.
Fig. 3.
Shape changes on PC1 and PC2 (21.4% and 11.1% variance explained, respectively) associated with major differences in subsistence economy. Agriculturalist populations (open symbols) have relatively short and broad mandibles with a tall, angled ramus and coronoid process, whereas hunter-gatherer populations (closed symbols) have relatively long and narrow mandibles with a short, upright ramus and coronoid process.
Discussion
The results demonstrate that global patterns of human mandibular shape reflect differences in subsistence economy rather than neutral population history. This suggests that as human populations transitioned from a hunter-gatherer lifestyle to an agricultural one, mandibular shape changed accordingly, effectively erasing the signal of genetic relationships among populations. However, some areas of the cranium functionally associated with chewing still predominantly reflect genetic population history rather than variation in masticatory behavior. In contrast, the palatomaxilla region, which is tightly integrated with the mandible, follows a similar pattern as the mandible, although the relationship with masticatory behavior is weaker overall. Importantly, what this suggests is that masticatory pressure acts preferentially on the mandible rather than the maxillary region, with the maxilla altering in relation to the mandible to retain effective dental occlusion. Therefore, despite considerable integration between the mandible and the skull (25, 26), the results presented here suggest that the mandible can evolve independently (e.g., 27).
These analyses raise a number of important questions that require further testing. First, are the shape differences observed between the agriculturalist and hunter-gatherer populations attributable to phenotypic (i.e., developmental) (28) plasticity or natural selection? Second, what exactly is the disruptive or selective pressure on mandibular shape? For practical and ethical reasons, the first of these questions is difficult to test in humans, but an experiment comparing hyraxes (29) found that those raised on softer, more processed food items experienced ∼10% less growth in the mandible, lower face, and zygomatic region than the group raised on fresh, unprocessed food. This supports the idea (4, 5) that it is the biomechanical properties of mastication (with hunter-gatherers presumably experiencing longer and more intensive bouts of chewing than agriculturalists) that is the selective force. Previous localized comparisons of hunter-gatherer and farming populations (6–9) found significant changes in the masticatory apparatus occurring within a relatively short time period, suggesting developmental plasticity or rapid selection.
Reduced duration and intensity of mastication are also thought to be linked to the higher prevalence of dental crowding and malocclusions in postindustrial urban populations (30), whereby inadequate chewing stresses generate insufficient strain for mandibular and maxillary growth in relation to overall tooth size (31). Congruently, a comparison of two populations with high and low dental attrition (32) suggested that intense masticatory activity leads to a more anteriorly rotated mandible, yielding a longer corpus, shorter ramus, and greater overall prognathism. This is also supported by studies of malocclusions identified in nonhuman primates raised on unnaturally soft diets (e.g., 33). The results obtained here are also congruent with this idea, whereby one of the major shape differences found was that agriculturalists had shorter mandibles overall, with reduced alveolar and corpus lengths, thereby diminishing the space for complete dental eruption. This would also furnish important insights into potential biomechanical measures for the prevention of common orthodontic problems experienced by many modern urban postindustrial societies (e.g., ref. 5, p. 278). Additional study into the incidence of dental crowding and malocclusions in modern hunter-gatherer populations is required to further our understanding of the interplay between masticatory stress, mandibular form, and dental eruption patterns.
Although much of the existing literature would predict that global patterns of mandibular variation reflect the intensity and duration of mastication, there are other potential explanations that should be considered. Given that modern humans all partake in substantial food processing and cooking, it is not entirely clear that all hunter-gatherer populations necessarily experience more intensive masticatory regimes than all agriculturalists. However, although appropriate soft or liquid weaning foods are available cross-culturally, hunter-gatherer populations consistently breastfeed for longer (e.g., 34), thereby delaying the onset of full masticatory behavior in young children. Whether this may have a correlated effect on the ontogeny of mandibular growth in different subsistence groups also requires further investigation.
Conclusions
The consistency of the results obtained here, irrespective of how subsistence was quantified, attests to the strength of the conclusions that can be drawn from these data. The change from a hunter-gatherer economy to one based on animal and/or plant domesticates had a dramatic effect on the shape of the human mandible, effectively erasing the signature of past population history. Although it still remains to be proven conclusively whether this change is attributable to masticatory stress, weaning behavior, or other demographic factors, it appears to act in a correlated fashion globally, causing a consistent shift toward a shorter, broader mandible. Therefore, these results yield important insights into the evolution of human masticatory adaptation as well as having implications for our understanding of modern clinical phenomena, such as the relatively high incidence of dental crowding and malocclusions in postindustrial populations.
Materials and Methods
Materials.
Matched genetic, morphological, geographical, climatic, and subsistence data were collated for each of 11 globally distributed human populations (Table 1). Genetic data comprised classical marker polymorphisms frequency data for 64 alleles representing 12 loci (35) (Table S1).
The morphological data comprised configurations of 3D landmarks taken on the cranium and the mandible of museum specimens representing each of the 11 genetic populations. All specimens measured were anatomically complete adults with fully fused sphenooccipital synchondroses and were sexed by the author using standard osteological techniques (36). The morphological and genetic data were matched based on provenance, state of preservation, chronology, and information on linguistic or ethnic affiliation. A total of seven anatomical configurations were captured using a Microscribe 3DX digitizer (eMicroscribe) and analyzed separately: (i) 33 landmarks representing the mandible (Fig. 1), (ii) 151 landmarks representing the entire cranium, (iii) 51 landmarks representing the cranial vault, (iv) 39 landmarks representing the chondrocranium (basicranium), (v) 25 landmarks representing the insertions of the right temporalis muscle (superior and inferior temporal lines), (vi) 22 landmarks representing the shape of the right “zygotemporal,” and (vii) 21 landmarks representing the “palatomaxilla” (Fig. 2). With the exception of the temporal lines, the other cranial regions have been described in detail in previous publications (16–18), and anatomical descriptions of all landmarks can be found in Table S2. The cranial vault and chondrocranium are not thought to be affected by masticatory function and have been shown to reflect population history reliably (13–15, 17, 18). The remaining three cranial subsets are involved in masticatory function, and are therefore included as a baseline against which to compare the results for the mandible. Experiments have shown that regions of the primate cranium, such as the temporomandibular joint (TMJ) (e.g., 37) and those related to the insertion of the major jaw adductor muscles (e.g., 38–40), experience high strains during mastication. Thus, masticatory-related behavior is most likely to have an impact on the palate and lower maxillary region bearing the upper dentition; the TMJ; and the attachment sites of the temporalis, masseter, and pterygoid muscles. Therefore, the zygotemporal delineates the size of the temporal fossa, the shape of the TMJ, and the attachment sites for the masseter muscles, whereas the palatomaxilla consists of the palate and the lower maxilla bearing the upper dentition (17). The temporal line configuration consists of three anatomical landmarks representing the end points of the temporal lines [temporal line (anterior) and the points of intersection between the squamous suture and the temporal lines] and 22 equally spaced semilandmarks. Curves of semilandmarks were captured for the superior and inferior temporal lines separately by digitizing one 3D coordinate every 4 mm. Curves were subsequently resampled for equal numbers of evenly spaced semilandmarks (41) using Resample.exe software (http://www.nycep.org/nmg/programs.html). Twelve semilandmarks were sampled for the superior temporal line, and 10 were sampled for the inferior temporal line. All landmark configurations were tested for intraobserver error following the partial superimposition method (42) and were deemed acceptable if individual landmark error was ≤1 mm.
Geographical data comprised latitude and longitude coordinates provided for the genetic samples matched for each population (35). Climatic data (16) comprised annual minimum, maximum, and mean values for each of four climatic variables [temperature (°C), precipitation (mm/d), vapor pressure (hPa), and cloud cover (%)] collated from the Intergovernmental Panel on Climate Change database (www.ipcc-data.org) (43) (values of all climatic data used are presented in Table S3).
Data on subsistence economy (Table 2) were collated from the updated Ethnographic Atlas available electronically and online as coded files at http://eclectic.ss.uci.edu/ (44). Variables 1–5 relate to the percentage dependence by each society on gathering, hunting, fishing, animal husbandry, and agriculture. In addition, data for variable 41 “Milking of Domestic Animals” was collated as the sixth quantitative variable. The 11 populations were categorized into two main groups, “Agricultural/Pastoralist” and “Hunter-Gatherer-Fishers,” based on qualitative variable 42 (“Overall Subsistence Economy”). The closest match in the Ethnographic Atlas was obtained for each of the genetic/morphological populations, taking geographical, ethnic, and linguistic information into account. In cases where there were two or more equally well-matched samples, they always had identical subsistence codes for the six quantitative variables used.
Geometric Morphometrics.
Each of the seven individual morphological landmark configurations was subjected to generalized Procrustes analysis (GPA), tangent space projection, and principal components analysis (PCA) in Morphologika 2.5 (http://life.bio.sunysb.edu/morph/soft-3d.html) (45). In the case of the temporal lines configuration, only anatomical landmarks were used in the GPA (41). Following the method used by Roseman and Weaver (12), the resultant PC scores required to explain 95% of the total variance were used as input variables for generating morphological distance matrices (Table S4), because this has been shown previously (16–18) to reflect the overall anatomical complexity of morphology, rather than the initial size of the landmark configuration per se.
Sexual Dimorphism.
Given that the mandible is sexually diagnostic in humans (e.g., 46), an assessment of the likely impact of sexual dimorphism was performed. The configuration centroid sizes for males and females were found to be significantly different (two-tailed t test, P < 0.0001), with male mandibles significantly bigger than female mandibles. First, a form analysis was performed in Morphologika 2.5 whereby, following GPA, configurations are rescaled by their centroid sizes before conducting the PCA (Fig. S1). A second PCA was also performed using the Procrustes shape variables and adding log centroid size as another variable (Fig. S2). In both cases, there was a statistically significant distinction between male and female mandibles on the first PC (accounting for over 30% of the total variation in each case). However, there was no significant difference (two-tailed t test, P = 0.576) between male and female mandibles on the first PC in the shape-only analysis (Fig. S3), suggesting that removing isometric scaling from the analysis also removes the effect of sexual dimorphism related to size. Subsequently, all analyses were carried out on the size-adjusted data.
Population Matrices.
Pairwise population distance matrices were calculated for each of the five data types. A genetic D-matrix was generated from the allele frequency data in RMAT 1.2 software (available from John Relethford), following the model described by Harpending and Ward (47). Morphological D-matrices were generated separately for the entire cranium, mandible, vault, chondrocranium, palatomaxilla, zygotemporal, and temporal lines, from the PC data using RMET 5.0 (http://konig.la.utk.edu/relethsoft.html), following the model of Relethford and Blangero (48). In each case, matrices were computed under the conservative assumption of complete heritability (i.e., h2 = 1) because of the lack of population-specific estimates of heritability for the morphological regions in question.
Geographical distances among all populations were calculated as great circle distances in kilometers, based on the haversine (49). The following waypoints were used to connect continents to provide a more realistic estimate of the pairwise geographical distance among populations; Cairo, Egypt (30.0, 31.0; exit/entry Africa); Istanbul, Turkey (41.0, 28.0, exit/entry Europe); Phnom Penh, Cambodia (11.0, 104.0, exit/entry Oceania); Anadyr, Russia (64.0, 177.0, exit/entry New World); and Prince Rupert, Canada (54.0, −130.0, exit/entry North America). A pairwise climatic distance matrix was constructed from the 12 climatic variables (Table S3) using Euclidean distances in PAST 1.7 (50).
Pairwise differences in subsistence were calculated in four ways. In the first instance, a simple binary matrix of differences in subsistence economy was created whereby if two populations were the same (i.e., both agriculturalist-pastoralist or both hunter-gather-fisher), it was scored as 1; if they were different, it was scored as 0. Thereafter, three pairwise difference matrices were computed based on the square root of the sum of the squared differences between values for each of the six quantitative variables shown in Table 2. For the second matrix, each of the six variables was taken at face value. For the third matrix, hunting and fishing were treated as a single variable in order not to create artificial differences between predominantly fishing and hunting communities, which might otherwise have very similar masticatory behavior (e.g., Greenland and Alaskan Inuit). For the fourth matrix, as well as hunting and fishing being treated equally, agriculture and animal husbandry were treated as a single variable to reduce the distances between horticulturalists and pastoralists (e.g., Mongolians and other agriculturalists).
Mantel Tests.
Given that matrices violate the statistical assumptions of traditional correlation tests, all population matrices were statistically compared using Mantel tests (51), where P values are assigned through a randomization test with 10,000 permutations (52). In the first instance, the correlation between each of the seven morphological regions and the genetic, geographical, climatic, and subsistence matrices was assessed. Thereafter, partial Mantel tests (53) were performed to control for the confounding effect of shared ancestry (genetics) when assessing the strength of correlation between morphology and the potential selective forces of climate and subsistence (16). In addition, the relationship between the morphological matrices and subsistence was tested, controlling for genetics, geography, and climate simultaneously. All Mantel tests were performed in PASSaGE 1.1 (http://www.passagesoftware.net), and the critical alpha level was set at α = 0.05. Following the method of Roseman (11), Bonferroni correction was applied to partial Mantel tests (i.e., α = 0.017, α = 0.010).
Supplementary Material
Acknowledgments
I thank Lia Betti, Metin Eren, Stephen Lycett, and two anonymous reviewers for invaluable comments relating to this research. Many thanks to John Relethford for making available his RMAT and RMET software and to all the museum curators who generously gave access to the collections in their care.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission. T.D.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113050108/-/DCSupplemental.
References
- 1.Diamond J, Bellwood P. Farmers and their languages: The first expansions. Science. 2003;300:597–603. doi: 10.1126/science.1078208. [DOI] [PubMed] [Google Scholar]
- 2.Gignoux CR, Henn BM, Mountain JL. Rapid, global demographic expansions after the origins of agriculture. Proc Natl Acad Sci USA. 2011;108:6044–6049. doi: 10.1073/pnas.0914274108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen C. Bioarchaeology: Interpreting Behavior from the Human Skeleton. Cambridge, MA: Cambridge Univ Press; 1997. [Google Scholar]
- 4.Lieberman DE. Speculations about the selective basis for the modern human craniofacial form. Evol Anthropol. 2008;17:55–68. [Google Scholar]
- 5.Lieberman DE. The Evolution of the Human Head. Cambridge, MA: Harvard Univ Press; 2011. [Google Scholar]
- 6.Carlson DS, Van Gerven DP. Masticatory function and post-Pleistocene evolution in Nubia. Am J Phys Anthropol. 1977;46:495–506. doi: 10.1002/ajpa.1330460316. [DOI] [PubMed] [Google Scholar]
- 7.González-José R, et al. Functional-cranial approach to the influence of economic strategy on skull morphology. Am J Phys Anthropol. 2005;128:757–771. doi: 10.1002/ajpa.20161. [DOI] [PubMed] [Google Scholar]
- 8.Paschetta C, et al. The influence of masticatory loading on craniofacial morphology: A test case across technological transitions in the Ohio valley. Am J Phys Anthropol. 2010;141:297–314. doi: 10.1002/ajpa.21151. [DOI] [PubMed] [Google Scholar]
- 9.Pinhasi R, Eshed V, Shaw P. Evolutionary changes in the masticatory complex following the transition to farming in the southern Levant. Am J Phys Anthropol. 2008;135:136–148. doi: 10.1002/ajpa.20715. [DOI] [PubMed] [Google Scholar]
- 10.Relethford JH. Global patterns of isolation by distance based on genetic and morphological data. Hum Biol. 2004;76:499–513. doi: 10.1353/hub.2004.0060. [DOI] [PubMed] [Google Scholar]
- 11.Roseman CC. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc Natl Acad Sci USA. 2004;101:12824–12829. doi: 10.1073/pnas.0402637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roseman CC, Weaver TD. Multivariate apportionment of global human craniometric diversity. Am J Phys Anthropol. 2004;125:257–263. doi: 10.1002/ajpa.10424. [DOI] [PubMed] [Google Scholar]
- 13.Harvati K, Weaver TD. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1225–1233. doi: 10.1002/ar.a.20395. [DOI] [PubMed] [Google Scholar]
- 14.Smith HF. Which cranial regions reflect molecular distances reliably in humans? Evidence from three-dimensional morphology. Am J Hum Biol. 2009;21:36–47. doi: 10.1002/ajhb.20805. [DOI] [PubMed] [Google Scholar]
- 15.Smith HF. The role of genetic drift in shaping modern human cranial evolution: A test using microevolutionary modeling. Int J Evol Biol. 2011;2011:145262. doi: 10.4061/2011/145262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Cramon-Taubadel N. Congruence of individual cranial bone morphology and neutral molecular affinity patterns in modern humans. Am J Phys Anthropol. 2009a;140:205–215. doi: 10.1002/ajpa.21041. [DOI] [PubMed] [Google Scholar]
- 17.von Cramon-Taubadel N. Revisiting the homoiology hypothesis: The impact of phenotypic plasticity on the reconstruction of human population history from craniometric data. J Hum Evol. 2009b;57:179–190. doi: 10.1016/j.jhevol.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 18.von Cramon-Taubadel N. The relative efficacy of functional and developmental cranial modules for reconstructing global human population history. Am J Phys Anthropol. 2011;146:83–93. doi: 10.1002/ajpa.21550. [DOI] [PubMed] [Google Scholar]
- 19.Betti L, Balloux F, Amos W, Hanihara T, Manica A. Distance from Africa, not climate, explains within-population phenotypic diversity in humans. Proc Biol Sci. 2009;276:809–814. doi: 10.1098/rspb.2008.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franciscus RG, Long JC. Variation in human nasal height and breadth. Am J Phys Anthropol. 1991;85:419–427. doi: 10.1002/ajpa.1330850406. [DOI] [PubMed] [Google Scholar]
- 21.Hubbe M, Hanihara T, Harvati K. Climate signatures in the morphological differentiation of worldwide modern human populations. Anat Rec (Hoboken) 2009;292:1720–1733. doi: 10.1002/ar.20976. [DOI] [PubMed] [Google Scholar]
- 22.Noback ML, Harvati K, Spoor F. Climate-related variation of the human nasal cavity. Am J Phys Anthropol. 2011;145:599–614. doi: 10.1002/ajpa.21523. [DOI] [PubMed] [Google Scholar]
- 23.Humphrey LT, Dean MC, Stringer CB. Morphological variation in great ape and modern human mandibles. J Anat. 1999;195:491–513. doi: 10.1046/j.1469-7580.1999.19540491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholson E, Harvati K. Quantitative analysis of human mandibular shape using three-dimensional geometric morphometrics. Am J Phys Anthropol. 2006;131:368–383. doi: 10.1002/ajpa.20425. [DOI] [PubMed] [Google Scholar]
- 25.Bastir M, Rosas A, Kuroe K. Petrosal orientation and mandibular ramus breadth: Evidence for an integrated petroso-mandibular developmental unit. Am J Phys Anthropol. 2004;123:340–350. doi: 10.1002/ajpa.10313. [DOI] [PubMed] [Google Scholar]
- 26.Rosas A, Bastir M, Alarcón JA, Kuroe K. Thin-plate spline analysis of the cranial base in African, Asian and European populations and its relationship with different malocclusions. Arch Oral Biol. 2008;53:826–834. doi: 10.1016/j.archoralbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Preuschoft H, Witzel U. Biomechanical investigations on the skulls of reptiles and mammals. Senckenb Lethaea. 2002;82:207–222. [Google Scholar]
- 28.Holmes MA, Ruff CB. Dietary effects on development of the human mandibular corpus. Am J Phys Anthropol. 2011;145:615–628. doi: 10.1002/ajpa.21554. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman DE, Krovitz GE, Yates FW, Devlin M, St Claire M. Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. J Hum Evol. 2004;46:655–677. doi: 10.1016/j.jhevol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Corruccini RS. An epidemiologic transition in dental occlusion in world populations. Am J Orthod. 1984;86:419–426. doi: 10.1016/s0002-9416(84)90035-6. [DOI] [PubMed] [Google Scholar]
- 31.Kaifu Y, Kasai K, Townsend GC, Richards LC. Tooth wear and the “design” of the human dentition: A perspective from evolutionary medicine. Am J Phys Anthropol. 2003;46(Suppl 37):47–61. doi: 10.1002/ajpa.10329. [DOI] [PubMed] [Google Scholar]
- 32.Varrela J. Effects of attritive diet on craniofacial morphology: A cephalometric analysis of a Finnish skull sample. Eur J Orthod. 1990;12:219–223. doi: 10.1093/ejo/12.2.219. [DOI] [PubMed] [Google Scholar]
- 33.Corruccini RS, Beecher RM. Occlusal variation related to soft diet in a nonhuman primate. Science. 1982;218:74–76. doi: 10.1126/science.7123221. [DOI] [PubMed] [Google Scholar]
- 34.Sellen DW, Smay DB. Relationship between subsistence and age at weaning in “preindustrial” societies. Hum Nat. 2001;12:47–87. doi: 10.1007/s12110-001-1013-y. [DOI] [PubMed] [Google Scholar]
- 35.Cavalli-Sforza LL, Menozzi P, Piazza A. The History and Geography of Human Genes. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 36.Buikstra JE, Uberlaker DH. Standards for Data Collection from Human Skeletal Remains. 1994 Arkansas Archaeological Survey Report No. 44 (Arkansas Archaeological Survey, Fayetteville, AK) [Google Scholar]
- 37.Hylander WL. Experimental analysis of temporomandibular joint reaction force in macaques. Am J Phys Anthropol. 1979;51:433–456. doi: 10.1002/ajpa.1330510317. [DOI] [PubMed] [Google Scholar]
- 38.Herring SW. In: The Skull. Hanken J, Hall BK, editors. Chicago: Chicago Univ Press; 1993. pp. 153–206. [Google Scholar]
- 39.Hylander WL, Johnson KR. In vivo bone strain patterns in the zygomatic arch of macaques and the significance of these patterns for functional interpretations of craniofacial form. Am J Phys Anthropol. 1997;102:203–232. doi: 10.1002/(SICI)1096-8644(199702)102:2<203::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Kupczik K, et al. Assessing mechanical function of the zygomatic region in macaques: Validation and sensitivity testing of finite element models. J Anat. 2007;210:41–53. doi: 10.1111/j.1469-7580.2006.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNulty KP. In: Modern Morphometrics in Physical Anthropology. Slice DE, editor. New York: Kluwer; 2005. pp. 349–373. [Google Scholar]
- 42.von Cramon-Taubadel N, Frazier BC, Lahr MM. The problem of assessing landmark error in geometric morphometrics: Theory, methods, and modifications. Am J Phys Anthropol. 2007;134:24–35. doi: 10.1002/ajpa.20616. [DOI] [PubMed] [Google Scholar]
- 43.New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim Res. 2002;21:1–25. [Google Scholar]
- 44.Gray JP. A corrected ethnographic atlas. World Cultures. 1999;10(1):24–85. [Google Scholar]
- 45.O'Higgins P, Jones N. Facial growth in Cercocebus torquatus: An application of three-dimensional geometric morphometric techniques to the study of morphological variation. J Anat. 1998;193:251–272. doi: 10.1046/j.1469-7580.1998.19320251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coquerelle M, et al. Sexual dimorphism of the human mandible and its association with dental development. Am J Phys Anthropol. 2011;145:192–202. doi: 10.1002/ajpa.21485. [DOI] [PubMed] [Google Scholar]
- 47.Harpending HC, Ward RH. In: Biochemical Aspects of Evolutionary Biology. Nitecki M, editor. Chicago: Univ of Chicago Press; 1982. pp. 213–256. [Google Scholar]
- 48.Relethford JH, Blangero J. Detection of differential gene flow from patterns of quantitative variation. Hum Biol. 1990;62:5–25. [PubMed] [Google Scholar]
- 49.Sinnott RW. Virtues of the haversine. Sky Telescope. 1984;68:159. [Google Scholar]
- 50.Hammer O, Harper DAT, Ryan PD. PAST: Palaeontological statistics package for education and data analysis. Palaeontol Electronica. 2001;4:1–9. [Google Scholar]
- 51.Mantel NA. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27:209–220. [PubMed] [Google Scholar]
- 52.Smouse PE, Long JC. Matrix correlation analysis in anthropology and genetics. Yearb Phys Anthropol. 1992;35(S15):187–213. [Google Scholar]
- 53.Smouse PE, Long JC, Sokal RR. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst Zool. 1986;35:627–632. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.