Abstract
The aim of the present study was to assess the long-term clinical, functional, and radiographic outcome of direct repair of spondylolysis using cerclage wire fixation according to Scott in young patients with symptomatic spondylolysis or low-grade isthmic spondylolisthesis as compared to the outcome after uninstrumented posterolateral in situ fusion. Twenty-five out of 28 patients of the direct repair group (89%) and 23 out of 28 of the fusion group (82%) were available for follow-up examination. The assessment by independent observers included a structured interview (Oswestry questionnaire [ODI], visual analogue scale, SRS questionnaire), a clinical examination, functional testing, plain radiography, and MRI. The groups were comparable as to the mean age at operation (18.2 vs. 16.2 years.), the follow-up time (14.8 vs. 15.0 years), and the amount of preoperative slip (7.2 vs. 13.1%). The mean ODI and SRS total scores were significantly better in the fusion group (4.3 [0–16] and 96 [57–117]) as compared to the direct repair group (11.4[0–52] and 87[53–107]; P=0.02 and P=0.011, respectively). In functional testing, both groups reached normal values for abdominal and back muscle strength. The lumbar spine flexion and extension ROM was decreased in both groups showing no statistical difference between the groups. Significant progressive narrowing of the olisthetic disc was detected on the plain radiographs after direct repair. On the flexion-extension radiographs, in the direct repair group, the mobility in the lytic/olisthetic segment was decreased in comparison to normal values from the literature. The mobility at the level above the operated segment was decreased in the direct repair group as compared to the fusion group (P=0.057). On T2-weighted MR images in the direct repair group, the signal intensity of the disc below the affected vertebra was decreased in 17/23 (74%) patients. There was no difference between the groups in the nucleus signal intensity of the adjacent disc above the operated segment. No association between the disc degeneration on MRI and the outcome of the patients could be established. In the direct repair group the following complications were seen: transient nerve root irritation (2), superficial infection (1), UTI (1); in the fusion group the complications were: subcutaneous seroma (2) and UTI (1). There were six re-operations, cerclage removal(4), conversion into segmental fusion(2) in the direct repair group, and one re-operation, instrumented respondylodesis, in the fusion group. In conclusion, the results of direct repair of the spondylolysis using cerclage wire fixation according to Scott were very satisfactory in 76% of the patients after a mean follow-up of 14.8 years. After direct repair, the ODI deteriorated with time leading to a clinically moderate but statistically significant difference in favour of segmental fusion. Lumbar spine mobility was decreased after direct repair. Secondary segmental instability above the spinal fusion was not detected. The procedure does not seem to be capable of preventing the olisthetic disc from degeneration. The theoretical benefits of direct repair could not be proven.
Keywords: Spondylolysis, Isthmic spondylolisthesis, Operative treatment, Direct repair, Posterolateral fusion, Comparative study
Introduction
In 1968, Kimura from Japan published a method for treatment of spondylolysis by direct repair of the defect using bone graft and postoperative plaster immobilisation [22 cited in 25]. Subsequently, this procedure was adopted by several authors for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis. Different kinds of internal fixation were added to avoid postoperative immobilisation [9, 16, 20, 25, 30, 31]. In the majority of cases satisfactory results have been achieved as stated in numerous retrospective reports [8, 16, 18, 20, 30, 31]. In an earlier comparative study after a mean follow-up time of 54 months (in the following called “first follow-up”), the authors failed to show any differences in the subjective, clinical, and functional outcome when compared to the results of uninstrumented posterolateral fusion [42]. The purpose of this study is to re-investigate the same groups of patients after a minimum follow-up of 11 years with special emphasis on the functional outcome and the condition of the discs.
Patients and methods
During the years 1986–1991, 28 patients underwent the direct repair procedure of the pars defect according to Scott [43] at the authors’ institution. The details of the operative procedure and the postoperative regimen have been described in an earlier article [42]. In 26 of the 28 patients, the indication for operation was symptomatic spondylolysis or low-grade isthmic spondylolisthesis causing persistent pain interfering with daily activities despite a minimum of 6-month non-operative treatment, including modification of sports activities, stabilising abdominal and back muscle exercises, and in some patients a soft brace. Two patients under 10 years of age were pain-free before the operation. The indication for operation in these patients was secondary lumbar scoliosis related to a slip of 25%. All 28 patients were invited for a follow-up examination.
In addition, 28 patients who had undergone uninstrumented posterolateral fusion for symptomatic low-grade isthmic spondylolisthesis were invited as a control group. The study design was a retrospective comparison of two cohorts. The study groups were matched for age, gender, percentage of preoperative slip, and postoperative follow-up interval.
At the time the operations were performed, no strict protocol was applied at the authors’ institution concerning the operation methods for low-grade isthmic spondylolisthesis. The choice between segmental fusion and direct repair was made by the operating surgeon based on personal preference. Segmental fusion was performed in all age groups. There was, however, an upper age limit of 30 years for the direct repair patients. Standing ap- and lateral radiographs were taken routinely. No additional preoperative investigations, such as flexion-extension radiographs, pars injections, discography, or MRI, were performed for patient selection.
The clinical records and preoperative radiographs as well as the results of the first follow-up investigation were available from all patients.
In the direct repair group, 25 of the 28 patients (89%) and 23 of the 28 patients (82%) in the segmental fusion group accepted the invitation for a follow-up. The level of the defect was L5 in 20, L4 in 3, and L3 in 2 patients of the direct repair group. In the segmental fusion group it was L5 in all 23 patients. Nineteen patients had L5–S1 fusions; in the remaining four patients fusion was performed from L4 to S1. The demographic data of the patients are given in Table 1.
Table 1.
The mean age of the patients, gender distribution, follow-up time, and operated spinal segments in the two treatment groups
| Direct repair (n=25) | Segmental fusion (n=23) | P value | ||
|---|---|---|---|---|
| Mean (SD) age, at the time of surgery | 18.2 (6.0) | 16.1 (2.7) | NS | |
| Mean (SD) age, at the final follow-up | 33.1 (5.8) | 31.1 (2.8) | NS | |
| Male/female | 9/16 | 10/13 | NS | |
| Follow-up time | ||||
| Mean (SD) | 14.8 (0.9) | 15.0 (2.3) | NS | |
| Range | (11–16) | (13–19) | ||
| Segmental fusion | ||||
| L5–S1 | 19 (83%) | |||
| L4–S1 | 4 (17%) | |||
| Direct repair | ||||
| L3 | 2 (8%) | |||
| L4 | 1 (4%) | |||
| L5 | 22 (88%) | |||
NS Not significant
Informed consent was obtained from all participants. The permission to perform this study was given by the Ethics Committee of the hospital district where the study was conducted.
The follow-up investigation was carried out by independent observers (TL, VR, IH). It included questionnaires, a physical examination, functional testing, radiography, and MR imaging.
Subjective outcome
Subjective outcome was assessed using the Oswestry questionnaire [14] and the SRS questionnaire [12, 17, 28]. The Oswestry questionnaire evaluates subjective low-back disability. According to Fairbank et al. [14], the index shows the degree of low-back disability, graded as follows: 0–19 = minimal disability; 20–39 = moderate disability; 40–59 = severe disability; and ≥60 = crippled. Low-back pain and radiating leg pain was measured using the visual analogue scale (VAS). The SRS questionnaire contains 24 questions, which gives a maximum score of 120, meaning a highly satisfied and asymptomatic patient [12, 17, 28]. The questionnaires were mailed to the patients along with the invitation to participate in the study. The questionnaires were completed by the patients at home and returned at the follow-up visit. The answers were verified during the physical examination.
Clinical examination
The height in centimetres (cm) and the weight in kilograms (kg) were measured, and the body mass index (BMI) was calculated (weight [kg]/(height [m2]). The finger-tip/floor distance during maximal flexion of the spine in the standing position with the knees fully extended was measured in centimetres (cm). The straight leg-raising test (SLR) was performed when the patient was supine; the result was considered negative when the leg was raised over 60° without causing any back pain or radiating pain below the knee. The muscle strength for great toe extension and ankle extension and flexion (normal/decreased/absent) was tested as was the skin sensitivity (normal/decreased/absent) in the dermatomes of L3-S1.
Functional testing
Functional tests, spinal mobility, and trunk muscle strength measurements were carried out by the same physiotherapist. Spinal mobility was determined by measuring lumbar flexion and extension in degrees with a goniometer and trunk side-bending with a tape measure from the fingertips on the thigh to the knee joint in centimetres [2]. The individual spinal mobility measurements were graded as abnormal when the values were two standard deviations below the mean of the age- and gender-adjusted reference values. The non-dynamometric trunk strength was evaluated by repetitive sit-up, arch-up, and squatting tests [3]. The repetition rate was one per 2–3 s. The movement was repeated as many times as possible at a comfortable but constant rate; the maximum repetition was 50 times. The results of the trunk strength measurement were scored from 1 (poor) to 5 (excellent). The result was poor when it was one standard deviation or more below the mean of the age- and gender-adjusted normal population values and excellent when it exceeded the mean by one standard deviation or more [3].
Radiography
The amount of vertebral slip was measured from the standing lateral radiographs of the lumbar spine using the method by Laurent and Einola [23] as the quotient between the sagittal displacement and the sagittal length of the slipped vertebral body expressed in percent.
The narrowing of the lytic/olisthetic disc was calculated as the quotient of height of the affected disc and the height of the disc two levels above and expressed in percent.
To study the range of segmental motion, and to judge the quality of the spondylodesis in the fusion group, the sagittal angulatory and translatory motion of the operated segment(s) and the two adjacent segments above was measured from the flexion-extension radiographs using the method of Putto and Tallroth [36]. A segmental motion of 3° or more was the criterion for non-union in the fusion group. The bony healing of the isthmic defect after direct repair was assessed from the standing lateral radiographs and the flexion-extension films and classified as solid, uncertain or pseudarthrosis. No oblique films were taken at the last follow-up to minimise the radiation exposure of the patients.
MR imaging
MR examinations were performed on 18 patients with a 1.0T and on 26 patients with a 1.5T superconducting imager (Siemens Magnetom Expert 1T and Siemens Magnetom Symphony 1.5T, Siemens AG, Erlangen, Germany). Four patients, two in each group, were not willing to participate due to claustrophobia. A local coil was used in all studies. T1-weighted spin echo and T2-weighted fast spin echo sequences were used in the sagittal direction with a slice thickness of 4 mm. T1-weighted images were acquired in the coronal plane. In addition, T1-weighted axial images were obtained at the level of each intervertebral disc. The slices were angled perpendicular to the long axis of the canal.
To minimise the risk of an intra- and interobserver error [37] all MR images were read together at least twice by two of the authors (VR and PT). The evaluation was based on a consensus decision, and, in borderline cases, the milder option was chosen.
A visible decrease in the signal intensity of the intervertebral discs on the T2-weighted images was noted. The discs were classified as normal (bright), speckled or black [7, 27]. The disc height was assessed and it was considered narrowed if a decrease of more than 25% from the expected height of the disc was noted. The signal intensity of the medulla and cauda equina was assessed. The size of the spinal canal from Th12 to S1 was evaluated on an arbitrary scale from 1 to 5: (1) increase in cerebrospinal fluid (CSF) around the medulla/cauda equina, (2) normal CSF, (3) decrease in cerebrospinal fluid (CSF) around the medulla/cauda equina, (4) no CSF around the medulla/cauda equina, and (5) medullar compression [38].
The size of the neural foramen and any compression of the nerve root from Th12 to S1 were evaluated from the T1-weighted images on an arbitrary scale from 1 to 3: (1) normal, (2) mild to moderate narrowing of the neural foramen and partial obliteration of the perineural fat but no impingement of the nerve root, and (3) severe narrowing of the neural foramen and obliteration of the perineural fat with impingement of the nerve root.
The status of the psoas and back muscles was evaluated from the T1-weighted axial images on an arbitrary scale from 1 to 3: (1) normal, (2) mild atrophy, and (3) severe atrophy. The back muscles, i.e. the erector spinae and multifidus, were estimated together. The evaluation was conducted in two ways: by visually comparing the cross-section of the muscles with the diameter of the nearby vertebral body and by assessing the amount of high signal-intensity streaks, representing fat in the muscle mass [33, 34]. The back muscles were evaluated at the level of both the L3 and the L5 vertebra.
The results are given as mean and standard deviation (SD) or range. The statistical comparisons were performed using the Mann–Whitney test and the χ2-test. The correlations were calculated by Spearman’s rank correlation test. The P values less than 0.05 were considered significant.
Results
The mean BMI in the direct repair group was 25 kg/m2 (range, 19–38 kg/m2) and in the segmental fusion group 26 kg/m2 (range 19–34 kg/m2). The mean finger–floor distance was 7 cm (range 0–40) and 1 cm (range 0–27), respectively. The SLR test provoked pain in three patients in the direct repair group and in one patient in the fusion group. However, all patients were able to raise their leg up to 90°. One patient in the fusion group had decreased muscle strength of the great toe extension as well as ankle extension and flexion. Another patient had abnormal L4 dermatome skin sensation in the fusion group, but none had such sensation in the direct repair group.
Subjective outcome
At the final follow-up, the mean Oswestry score was 11.4 (0–52) in the direct repair group and 4.3 (0–16) in the fusion group (P=0.02). At the first follow-up, no significant difference had been detected between the groups [42]. Six patients in the direct repair group, but none in the fusion group had ODI more than 20 (P=0.012). Their mean age at operation was higher than the mean age of the whole group (24 vs.18 years.). There were no statistically significant differences between the groups in the VAS for low-back pain and leg pain (Table 2).
Table 2.
Results of the subjective assessment by the patients using Oswestry disability index (ODI), visual analogue scale (VAS), and SRS questionnaire
| Direct repair (n=25) | Segmental fusion (n=23) | P value | |
|---|---|---|---|
| Mean Oswestry index (range) | |||
| First follow-up | 7.6 (0–27) | 8.6 (0–19) | NS |
| Last follow-up | 11.4 (0–52) | 4.3 (0–16) | 0.02 |
| Oswestry index at the last follow-up | |||
| 20–40 | 5 patients | None | |
| >40 | 1 patient | None | |
| Mean VAS (range, mm) | |||
| First follow-up—LBP | 18 (0–84) | 18 (0–70) | NS |
| First follow-up—leg pain | 8.9 (0–50) | 7.2 (0–74) | NS |
| Last follow-up—LBP | 22.8 (0–76) | 15.5 (0–45) | NS |
| Last follow-up—leg pain | 14.1 (0–74) | 4.0 (0–28) | NS |
| Mean SRS score (range) | |||
| Last follow-up | 87.6 (53–107) | 96.5 (57–117) | 0.011 |
NS Not significant
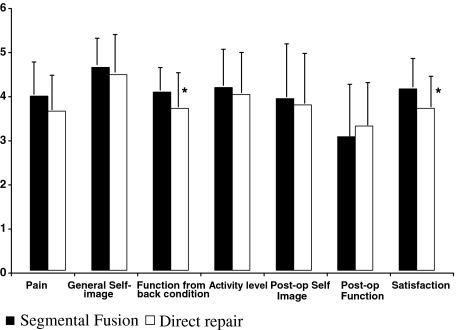
The average SRS total score was significantly higher in the fusion group as compared to the direct repair (Table 2). The results of the seven main domains of the SRS questionnaire are shown in Fig. 1. The patients in the fusion group had significantly higher mean values for function from back condition and satisfaction domains than the patients in the direct repair group (P =0.052 and P=0.024, respectively). Two patients in both groups reported back pain often or very often on the SRS questionnaire (SRS question 6). Sixteen (64%) patients in the direct repair group and 20 (87%) in the fusion group were extremely or quite satisfied with the results of surgery (SRS question 22; P=0.066). Similarly, 21 (84%) and 22 (96%) patients, respectively, would choose surgery definitely or probably again with the same diagnosis (SRS question 24).
Fig. 1.
Results of the seven domains of the SRS questionnaire. Values are means and SD. The patients in the fusion group had significantly higher values for function from back pain and satisfaction domains than the patients in the direct repair group (P<0.05)
Complications
The following complications occurred in the direct repair group: two cases of transient nerve root irritation, one superficial infection, and one urinary tract infection. In the fusion group, two subcutaneous seroma and one urinary tract infection were seen.
There were six re-operations in the direct repair group: cerclage removal in four patients (two due to discomfort, two due to young age) and segmental fusion due to ongoing symptoms in two patients. One patient had a pseudarthrosis, and in the other patient the isthmic defect was healed. In the fusion group there was one re-operation. Instrumented respondylodesis was performed because of symptomatic pseudarthrosis.
Functional testing
The results of the trunk strength and spinal mobility measurements are shown in Table 3. The mean values of the non-dynamometric trunk strength measurements were above the mean of the reference values in both groups. There were no significant differences in the trunk strength measurements between the two treatment groups. Spinal mobility was decreased markedly in both groups showing no significant difference between the groups.
Table 3.
Trunk strength and spinal mobility measurements at the last follow-up
| Mean trunk strength measurement scores (SD) | Direct repair (n=25) | Segmental fusion (n=23) | P value |
|---|---|---|---|
| Sit-up | 3.6 (1.3) | 3.3 (1.2) | NS |
| Arch-up | 4.1 (1.2) | 4.2 (1.2) | NS |
| Squatting | 3.7 (1.3) | 4.2 (1.2) | NS |
| Abnormala spinal mobility measurement results (%) | |||
| Lumbar flexion | 8 (32%) | 5 (21%) | NS |
| Lumbar extension | 6 (24%) | 6 (26%) | NS |
| Trunk side-bending | 3 (12%) | 1 (4%) | NS |
NS Not significant
aBelow the mean—2 SD of the age- and sex-adjusted normal population values [50]
Radiography
The results of the measurements from the standing lateral radiographs are presented in Tables 4 and 5. The mean slip percentage decreased during the follow-up in both groups. None of the patients presented significant (>10 percentage units) slip progression in the direct repair or in the fusion group. But a significant further progression of disc space narrowing in the affected segment was detected, as had been already seen at the first follow-up in comparison to the preoperative values. Healing of the lytic defect in the direct repair group was acertained in nine (43%) patients. In 11 (52%) patients, healing was deemed questionable, and in one patient a clear pseudarthrosis was detected.
Table 4.
The amount of the vertebral slip preoperatively and at follow-up measured from standing lateral radiographs
| Direct repair (n=25) | Segmental fusion (n=23) | |
|---|---|---|
| Mean vertebral slip (SD) [%] | ||
| Preoperatively | 7.2 (8) | 13.1 (4) |
| Range | (0–31) | (5–23) |
| Last follow-up | 3.0 (7) | 5.6 (7) |
| Range | (0–24) | (0–23) |
| Slip progression (>10 percentage units) | None | None |
Table 5.
The mean relative disc height (in percent) of the lytic/olisthetic level in comparison to the L3/L4 disc preoperatively, at the first follow-up, and at the last follow-up
| Direct repair (n=25) Disc height (SD) [% of L3/L4] |
Segmental fusion (n=23) Disc height (SD) [% of L3/L4] |
|
|---|---|---|
| Preoperatively | 103 (19) | 93 (20) |
| First follow-up | 92 (20)* | 78 (20) |
| Last follow-up | 82 (18)* | 63 (31) |
*Paired t test, P=0.018
On the flexion-extension radiographs, the mean flexion-extension mobility in the three lowermost lumbar segments was decreased in both treatment groups when compared to the values from the first follow-up study (Table 6). The average flexion-extension motion of the operated segment in the direct repair group showed a decrease from 13.2 to 9.9° being clearly below the normal values. The mobility in the segment above the direct repair was lower than that above the segmental fusion (11.2 vs. 15.2°). This difference did not reach a statistical significance in contrast to a significant difference at the first follow-up examination. A pseudarthrosis was diagnosed in two patients of the fusion group resulting in a fusion rate of 89%. The results of the measurements of sagittal translatory motion during flexion-extension are presented in Tables 7 and 8. There are no significant differences in the mean values between the two groups (Table 7). In comparison to the mean values reported by Tallroth et al. [50, 51], both treatment groups show a higher number of segments in the normal range of sagittal translation, i.e. in the range of measurement inaccuracy (<3 mm; Table 8).
Table 6.
Segmental sagittal angulatory motion of the lower lumbar spine as measured from flexion-extension radiographs at the first and last follow-up
| Level | Follow-up | Direct repair (n=22)a Flex/ext (SD)[drs] (range) |
Segmental fusion (n=23)b Flex/ext (SD)[drs] (range) |
P value | Normal valuesc Mean [drs] (Range) |
|---|---|---|---|---|---|
| L3–4 | First | 16.2(4) (11–34) |
16.9(4) (8–26) |
NS | 15 |
| Last | 14.5(5) (7–25) |
15.3(4) (7–21) |
NS | (6–17) | |
| L4–L5 | First | 12.2(4) (4–20) |
17.5(8) (8–29) |
0.006 | 16 |
| Last | 10.4(5) (0–19) |
15.2(8) (0–30) |
0.057 | (9–21) | |
| L5-S1 | First | 13.2(6) (0–23) |
1.4(4) (0–14) |
0.000 | 17 |
| Last | 9.9(8) (0–28) |
0.7(2) (0–11) |
0.000 | (10–24) |
NS Not significant
aOnly L5 cases
bIn four cases with L4–S1 fusion L2–L3, L3–L4, and L4–L5 were measured
cAccording to White and Panjabi [56]
Table 7.
The mean values of segmental sagittal translatory motion of the lower lumbar spine as measured from flexion-extension radiographs at the last follow-up
| Level | Direct repair (n=22)a Translation (SD)[mm] (range) |
Segmental fusion (n=23)b Translation (SD)[mm] (range) |
P value |
|---|---|---|---|
| L3–L4 | 1.5 (1.1) (0–5) |
1.4 (1.0) (0–5) |
NS |
| L4–L5 | 1.1 (0.9) (0–4) |
1.7 (1.5) (0–6) |
NS |
| L5–S1 | 0.6 (6) (0–3) |
0 (0) (0) |
0.002 |
NS Not significant
aOnly L5 cases
bIn four cases with L4–S1 fusion L2–L3, L3–L4, and L4–L5 were measured
Table 8.
Segmental sagittal translatory motion of the lower lumbar spine as measured from flexion-extension radiographs at the last follow-up in comparison to normal values (expressed in percent of segments)
| L3–L4 | L4–L5 | L5–S1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| mm | Direct repair (n=22)b | Fusion (n=23)c | Normala (n=56) | Direct repair (n=22)b | Fusion (n=23)c | Normala (n=56) | Direct repair (n=22)b | Fusion (n=23)c | Normala (n=56) |
| <3 | 92 | 96 | 62 | 96 | 83 | 54 | 96 | 100 | 76 |
| 3 | 4 | - | 9 | – | 9 | 9 | 4 | – | 2 |
| 4 | – | - | 14 | 4 | – | 9 | – | – | 14 |
| 5 | –4 | 4 | 13 | – | 4 | 25 | – | – | 4 |
| 6 | – | – | 2 | – | 4 | 3 | – | – | 2 |
| 7 | – | – | – | – | – | - | – | – | 2 |
aNormal values for symptom-free adults according to Tallroth et al. [50]
bOnly L5 cases
cIn four cases with L4–S1 fusion L2–L3, L3–L4, and L4–L5 were measured
There was no correlation between the radiographic findings and the clinical outcome. Pseudarthrosis or questionable bony healing did not have any influence on the result in the direct repair group or fusion group.
MR imaging
The results of the assessment of the intervertebral discs on the T2-weighted MR images are presented in Table 9. In the direct repair group, the signal intensity of the disc below the affected vertebra was normal in six (26%) patients. The disc space was narrowed in 13 (57%) cases. Above the direct repair, disc signal intensity was normal in 16 (70%) patients. Three (13%) of the intervertebral disc spaces were narrowed. In the fusion group, two (8%) discs inside the fusion area had a normal signal. Narrowed intervertebral spaces were noted in 23 (85%) discs. At one level above the fusion, normal signal intensity was found in 15 (71%) discs.
Table 9.
Nucleus signal intensity of the lower lumbar discs on T2-weighted MR images at the last follow-up (in percent of discs)
| Direct repair (n=23, %) | Segmental fusion (n=21, %) | ||
|---|---|---|---|
| L3-4 | Normal | 65 | 76 |
| Speckled | 21 | 10 | |
| Black | 14 | 14 | |
| L4–L5 | Normal | 70 | 71 |
| Speckled | 21 | 5 | |
| Black | 9 | 24 | |
| L5–S1 | Normal | 26 | 8 |
| Speckled | 43 | 48 | |
| Black | 31 | 44 | |
The MR images showed normal signal intensity of the spinal cord in all patients with no signs of atrophy of the medulla or cauda equina. In the direct repair group, three patients (four levels) showed decrease of CSF around the cauda equina (gr. 2) at the L2–L3, L3–L4 or L4–L5 levels, but none had a spinal stenosis. In contrast, the spinal canal was exceptionally wide in two (13%) of the patients at the level of the slip (L5). In the fusion group, five patients (six levels) showed a decrease of CSF around the cauda equina (gr. 2) at the L1–L2, L2–L3, L3–L4 or L4–L5 levels. There was no patient with spinal stenosis above or inside the fusion area. The spinal canal was exceptionally wide in five (24%) of the patients at the level of the slip (L5).
Narrowing of the neural foramina was not observed in any patient of the direct repair group. In contrast, in the fusion group, mild or moderate narrowing of the neural foramina without compression of the nerve root was noted in two (10 %) patients bilaterally at the disc level L5–S1. None of the patients had severe narrowing of the foramen or obliteration of the perineural fat with impingement of the nerve root or any clinical nerve root symptoms.
Visual assessment of the T1-weighted MR images revealed degeneration of the muscles in 14 (61%) of the patients in the direct repair group and in 6 (23%) patients in the control group. Back muscles contained high signal intensity fatty streaks within the muscle mass. The atrophied psoas muscles showed a diminished cross-section area, but fat was not detected within the muscle. The MRI findings did not correlate with the clinical outcome.
Discussion
Segmental spinal fusion is the traditional method for treatment of symptomatic isthmic spondylolisthesis in patients not responding to non-operative measures. It is a safe procedure with a high success rate and few complications [6, 15, 23, 29, 39, 44–48].
The rational for the direct repair procedure, introduced first by Kimura from Japan [22 cited in 25], is to avoid the drawbacks of fusion. The aim is to save a spinal motion segment [16, 21] in order to retain lumbar spine mobility and to restore normal anatomy [26, 35, 58]. However, at least theoretically, it would be possible to achieve a restitutio ad integrum [19] only in cases with a spondylolysis without a vertebral slip. As this procedure is recommended and used by the majority of authors also for patients with an isthmic slip up to 25%, in those cases the affected motion segment will not be biomechanically normal even after a successful bony healing of the pars defect. Besides, if there is a slip present there must also be structural changes in the annulus fibrosus.
Despite the fact that many authors cite the aforementioned theoretical benefits to justify the direct repair procedure, surprisingly little work has been done to verify these benefits and to investigate the influence on the outcome of the patients, i.e. to compare the outcome of direct repair to that of fusion which should be the “bottom line” [1]. There are no randomised controlled trials available. To the knowledge of the present authors, only one non-randomised retrospective comparative study addressing this question has so far been published [42]. In this study, the authors failed to show any benefit of direct repair in comparison to uninstrumented posterolateral fusion in young patients after a mean follow-up time of 54 months. Dai et al [11] published a study presenting two groups of patients, one treated by direct repair only and the other by direct repair and one- or two-level facet joint fusion. They did not find any significant difference in the outcome between the groups. The patients, however, were allocated to the two treatment groups according to the degree of disc degeneration on preoperative MRI, i.e. they were not comparable from the beginning. Thus, the study cannot be seen as a comparative study answering the actual question.
The limitations of the present study are obvious. The study is retrospective and non-randomised. The study groups were not homogeneous. In the direct repair group, there were two patients with L3 lysis and one with L4 lysis. Four patients of the fusion group had fusions L4–S1 instead of L5-S1. This was taken into consideration during the radiographic measurements (Tables 6, 7, 8). Processing the subjective outcome data after exclusion of the odd cases aforementioned did not change the results significantly, within the groups or in comparisons between the groups. The total number of the patients was small, and some preoperative data was not available (Oswestry score, VAS, SRS score, flexion-extension radiographs, MRI). The follow-up interval, however, was longer than that in any other series published before, and the follow-up rate (89 and 82%, respectively) is sufficiently high. The assessment of the patients was performed by independent observers (spine surgeons, radiologists, physiotherapists). The data from the first follow-up investigation of the same groups of patients were also available allowing the assessment of the influence of time on the results.
The average outcome, assessed by the Oswestry score, VAS, and SRS questionnaire, was very satisfactory in both treatment groups. This is in accordance with figures from the literature reporting on success rates from 58 to 91% [4–6, 9, 13, 15, 18, 23, 29, 35, 39, 43, 53, 58]. The criteria for outcome measurement, however, vary considerably between different studies, which makes direct comparison impossible. We found in our series a deterioration of the Oswestry score and the VAS in the direct repair group between the first and the final follow-up investigation. This led to a moderate, but statistically significant, difference in favour of fusion. A common feature of the six patients with an Oswestry score >20 at the final follow-up was their higher age at operation 17–29 years (mean 23 years). The mean age of the remaining patients of the group was 16 years. The figures are, however, too small to draw any conclusions concerning an upper age limit for this procedure. Besides, among the patients with a good result, six out of 19 were aged between 19 and 28 years. Bradford and Iza [8], and Nicol and Scott [31] found better results in patients less than 30 years of age. Hefti et al. [18] stated that the results are poorer in patients over 20 years of age. According to Ivanic et al. [19], young age at operation is a predictor of favourable outcome. They had more poor results in patients over 20 years of age. In the report of Tonino and van der Werf [54] 6 out of 12 patients were over 30 years of age at operation. All 6 showed a good result after a mean follow-up of 10 years. Kakiuchi [21] operated on patients with a mean age of 32 (12–60) years. He did not find any influence of age and reported an excellent result even in a 60-year-old male. The patients operated on by Dai et al. [11] were aged from 15 to 56 years. They state that they do not see any reason to set an upper age limit, as they rely on the preoperative MR assessment of the discs. The actual influence of age on the result of the procedure is difficult to assess. Up till now, there is no reliable evidence from the literature for setting an upper age limit. The incidence of back pain in the general population is rising with age. Furthermore the probability of a pain source outside the lytic/olisthetic segment is higher, the older the patient is.
In the SRS score, which was used for the first time for evaluation of direct repair, the results of fusion seemed to be superior to direct repair concerning function, pain, and patient satisfaction. The deterioration of the results in some of the patients of the direct repair group indicates that the time factor may also have influence on the results. As the disc below the slipped vertebra is usually damaged [40, 41], it is possible that the progression of the degenerative process is responsible for this decline.
The earlier differences, however, were not reflected in the measurements of the trunk strength and spinal mobility. The trunk strength was better than normal in both groups. The spinal mobility was equally decreased in both groups. It is surprising, since direct repair should spare spinal mobility. The explanation for the decrease in mobility may be due to progressive disc degeneration. This assumption is supported by our radiographic results to be discussed later. Another factor decreasing spine mobility may be scar formation. The direct repair operation according to Scott was performed through a midline approach with bilateral exposure of the posterior structures down to the base of the transverse processes. It obviously causes more iatrogenic damage to the soft tissues compared to posterolateral fusion using Wiltse’s paraspinal muscle split approach [57]. The difference was clearly seen in the assessment of the musculature from MR images. We could not find any data of this observation in the literature. Large soft tissue damage during direct repair could be prevented by using minimally invasive operative techniques.
Both operation methods were capable of preventing further slip progression, independently of the quality of bony healing. This is not surprising, as the risk of progression in low-grade slips is usually minimal. Significant progression of disc space narrowing in the affected segment in the direct repair group can only be interpreted as progression of disc degeneration. This may be due to the fact that the discs had been damaged already preoperatively. Disharmonic motion and instability around all three axes as demonstrated by Olsson et al. [52] in spondylolisthesis could be another reason. We did not find any comparable data of this phenomenon in the literature. It supports, however, our suspicion that the direct repair procedure is not capable of restoring the affected segment to normal. Loss of disc space height in the fused segment is seen as atrophy due to absence of motion necessary to maintain normal disc metabolism.
The reliable assessment of bony healing after direct repair from plain lateral radiographs (lateral and/or oblique) is impossible. A fissure-like pseudarthrosis not being in line with the central X-ray beam will usually remain undiscovered. In addition, metal implants obscure the view. For reasons of radiation protection we did not obtain oblique radiographs or CT images which would have improved the accuracy of the judgement. As a result, we have a considerable proportion of cases classified as “uncertain”, several of them possibly being unilateral non-unions. The figures from the literature on bony healing should be judged with special caution as far as they are based on plain radiographs. A pseudarthrosis rate of 11% in the fusion group is acceptable and in accordance with earlier reports.
Contradictory opinions have been published on the effect of non-union on the final outcome. According to Nicol and Scott [31], Debnath et al. [13], Wu et al. [58], and Dai et al. [11] pseudarthrosis is related to a poor or fair result. Hefti et al. [18], Johnson and Thompson [20], and Pellisé et al. [35] could not prove this relationship. In our patients non-union did not jeopardise the result, in the direct repair group or in the fusion group. The only explanation we found is that the operation, even if the fusion does not heal, leads to pain reduction due to stabilisation of the segment.
The results of the segmental flexion-extension motion measurements from the lateral radiographs were interesting and in some extent unexpected. They showed that mobility decreased in all segments in both treatment groups if compared to the first follow-up. This overall trend may be attributed to aging. In the direct repair group, the mean range of flexion-extension motion in the operated segment as well as in the segment above was clearly below the normal values seen in the literature [56]. The reasons may be progressive disc degeneration and scar formation, as mentioned earlier. The segmental motion above the posterolateral fusion corresponded to normal motion for the L4–L5 level. No significant difference could be found in the measurements of the translatory motion in this segment either. Secondary hypermobility or so-called adjacent segment instability due to fusion could not be demonstrated, at least not yet at this point of time after a mean postoperative follow-up of 15 years. We were not able to find any data of this in the literature for comparison.
The assessment of the condition of the intervertebral discs on MRI did not reveal marked changes if compared to the first follow-up. The disc below the direct repair was dehydrated in 74% (at the first follow-up 84%), above the direct repair in 30% (vs. 58%), and above the fusion in 29% (vs. 36%) of the cases. No spinal stenosis was seen on MRI. Mild narrowing of the neural foramina L5–S1 was registered in 10% of the fusion patients, but in none of the direct repair group.
Thus, the MRI investigation did not show any differences of clinical importance between the groups. Signs of disc degeneration were present, but, as in our earlier investigation, they were not correlated to pain or to the outcome of the patients.
For patient selection, preoperative assessment using pars injections [8, 21, 49, 58] discography [55] or MRI [10, 11, 35] has been recommended.
Bradford and Iza [8] suggested pars infiltration with a local anaesthetic for localisation of the source of pain and defining the appropriate operative procedure. Suh et al. [49] concluded that this technique is safe and reliable in predicting a successful outcome. Due to the small number of patients and a short follow-up, the value of this statement is limited. Wu et al. [58] performed direct repair in 93 patients who had pain relieve after pars injection and a negative bone scan. They achieved excellent or good results in 91% after an average follow-up of 30 months. Kakiuchi [21] operated on 16 patients who had temporary pain relief after pars injection. The rate of excellent results after 25 months follow-up in his series was 88%. We have not used this method, as, to our knowledge, the predictive value has not been reliably demonstrated. The pars defect is not a closed compartment. In many cases it is a fibrocartilaginous structure, and in some cases, there may be a small gap as in an atrophic pseudarthrosis. But there is no empty space to inject fluid into it. In addition, the positioning of the tip of the needle using p.a. image intensifier control as recommended may not be optimal in every case. The anaesthetic will always spread around and may reach the nearby facet joint capsule, the nerve root, and possibly even the nervous structures of the outer annulus. Considering the complexity of the nerve supply of these different anatomic structures situated very close to each other, we doubt that a pars injection is really selective, i.e. affecting only the pars interarticularis.
Van der Werf et al. [55] reported on 12 patients who underwent direct repair after having a normal discography. Ten patients showed a good result at a 10-year follow-up. In their recent study on the same group of patients they explain that their low percentage of disc degeneration preoperatively may be partly due to the fact that their criteria for disc degeneration on discography were “at that time not as exact as they are today”[54]. Ten good results out of 12 patients (83%) is in the range of good results achieved by many authors without performing preoperative discography. Thus, in our opinion, discography as an invasive procedure for deciding preoperatively whether direct repair can or cannot be performed should be considered with great caution.
Kakiuchi [21] reported on 16 patients, 10 of whom (62%) had clear signs of disc degeneration below the slipped vertebra on preoperative MR images. After a mean follow-up of 25 months, 13 (81%) were symptom-free regardless of the degree of degeneration on the MR scans before operation. The remaining three patients showed major improvement.
Dai et al. [11] performed direct repair alone only in patients who had no or mild disc degeneration on MRI judged by signal intensity on T2-weighted images. Patients with moderate and severe disc degeneration received additional fusion. They achieved favourable results in both groups. However, their results after a mean follow-up of 50 months were substantially no better than those reported by other authors. As they did not have a proper control group, their statement on the value of preoperative MRI for the choice of the procedure does not seem justified.
The degree of disc degeneration in the lumbar spine measured as change in the nucleus signal intensity is related to age. Degeneration seen on MRI may be symptomatic or asymptomatic [52]. MRI does not allow distinguishing between a painful and pain-free degenerated disc. Degeneration is more common in individuals with isthmic spondylolisthesis than in normal controls [10, 41]. Disc degeneration correlates with the duration of clinical symptoms in isthmic spondylolisthesis. But no correlation could be established between the severity of clinical symptoms and the degree of degeneration on MRI [10]. Besides, dehydrated discs below the affected vertebra are found also in totally asymptomatic individuals with mild isthmic slip [42].
The present study as well as the first follow-up of the same groups of patients failed in establishing any correlation between the disc findings on MRI at the follow-up and outcome. Consequently, we are not convinced that preoperative MRI would be of any help for patient selection based on the state of hydration of the intervertebral discs.
Kimura [22 cited in 25] and Dai et al. [11] did not use any internal fixation. Their patients had postoperative plaster immobilisation or bed rest until bony healing. Buck [9] introduced the direct screw method to avoid postoperative immobilisation. Numerous other fixation methods have been invented in order to improve stability. They are mainly combinations of screws, rods, plates, wires, and hooks. To the knowledge of the present authors, the only paper comparing different metal implant constructs in clinical cases is the publication by Wu et al. [58]. They were not able to find any difference in the outcome between Buck screws [9], Morscher’s hook screw [30], and Buck screws augmented with wire. Other authors mainly presented favourable results in the use of their own innovative method without comparing it directly to other fixation methods. We have used the cerclage technique introduced by Scott [43]. Although this wire fixation is mechanically weaker than most of the other constructs, our results are very satisfactory. The question whether internal fixation is necessary at all or which of the constructs should be preferred, cannot be answered due to lack of comparative data.
Conclusion
The results of the direct repair procedure for operative treatment of symptomatic lumbar spondylolysis or mild isthmic spondylolisthesis in young patients using Scott’s technique were very satisfactory in the majority of cases. There was, however, a trend towards worsening of the clinical outcome with time. This could be demonstrated by comparing the results of an earlier follow-up investigation at 54 months to the final follow-up results at, on average, 15 years. The expected theoretical benefits of the direct repair procedure (preservation of lumbar spine motion, protection of the adjacent segment above) could not be proven when compared to a group of patients treated by uninstrumented posterolateral fusion.
References
- 1.Aebi M, Nazarian S. Editorial. Eur Spine J. 1992;1:141. doi: 10.1007/BF00301303. [DOI] [Google Scholar]
- 2.Alaranta H, Hurri H, Heliövaara M, Soukka A, Harju R. Flexibility of the spine: Normative values of goniometric and tape measurements. Scan J Rehab Med. 1994a;26:147–154. [PubMed] [Google Scholar]
- 3.Alaranta H, Hurri H, Heliövaara M, Soukka A, Harju R. Non-dynamometric trunk performance tests: reliability and normative data. Scan J Rehab Med. 1994b;26:211–215. [PubMed] [Google Scholar]
- 4.Arnold P, Winter M, Scheller G, Konermann W, Rumetsch D, Jani L. Die klinischen und radiologischen Ergebnisse der Isthmusrekonstruktion bei der lumbalen Spondylolyse und der geringgradigen Spondylolisthesis. Z Orthop. 1996;134:226–232. doi: 10.1055/s-2008-1039753. [DOI] [PubMed] [Google Scholar]
- 5.Askar Z, Wardlaw D, Koti M. Scott wiring for direct repair of lumbar spondylolysis. Spine. 2003;28:354–357. doi: 10.1097/01.BRS.0000048496.55167.22. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn JS, Velikas EP. Spondylolisthesis in children and adolescents. J Bone Joint Surg [Br] 1977;59:490–494. doi: 10.1302/0301-620X.59B4.925059. [DOI] [PubMed] [Google Scholar]
- 7.Boden SD, Riew DK, Yamaguchi K, Branch TP, Schellinger D, Wiesel SW. Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg [Am] 1996;78:403–411. doi: 10.2106/00004623-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Bradford DS, Iza J. Repair of the defect in spondylolysis and minimal degrees of spondylolisthesis by segmental wire fixation and bone grafting. Spine. 1985;10:673–679. doi: 10.1097/00007632-198509000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Buck JE. Direct repair of the defect in spondylolisthesis. J Bone Joint Surg [Br] 1970;52:432–437. [PubMed] [Google Scholar]
- 10.Dai LY. Disc degeneration in patients with lumbar spondylolysis. J Spinal Disord. 2000;13:478–486. doi: 10.1097/00002517-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Dai LY, Jia LS, Yuan W, Ni B, Zhu HB. Direct repair of defect in lumbar spondylysis and mild isthmic spondylolisthesis by bone grafting, with or without facet joint fusion. Eur Spine J. 2001;10:78–83. doi: 10.1007/s005860000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Andrea L, Betz RR, Lenke LG, Clements DH, Lowe TG, Merola Haher A. T, Harms J, Huss GK, Blanke K, McGlothlen S. Do radiographic parameters correlate with clinical outcomes in adolescents idiopathic scoliosis. Spine. 2000;25:1795–1802. doi: 10.1097/00007632-200007150-00010. [DOI] [PubMed] [Google Scholar]
- 13.Debnath UK, Freeman BJC, Gregory P, la Harpe D, Kerslake RW, Webb JK. Clinical outcome and return to sport after surgical treatment of spondylolysis in young athletes. J Bone Joint Surg [Br] 2003;85:244–249. doi: 10.1302/0301-620X.85B2.13074. [DOI] [PubMed] [Google Scholar]
- 14.Fairbank JCT, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 15.Freebody D, Bendall R, Taylor D. Anterior transperitoneal lumbar fusion. J Bone Joint Surg [Br] 1971;53:617–627. [PubMed] [Google Scholar]
- 16.Gillet P, Petit M. Direct repair of spondylolysis without spondylolisthesis, using a rod-screw construct and bone grafting of the pars defect. Spine. 1999;24:1252–1256. doi: 10.1097/00007632-199906150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Haher TR, Gorup JM, Shin TM. Results of the Scoliosis Research Society Instrument for evaluation of surgical outcome in adolescent idiopathic scoliosis. A multicenter study of 244 patients. Spine. 1999;24:1435–1440. doi: 10.1097/00007632-199907150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Hefti F, Seelig W, Morscher E. Repair of lumbar spondylolysis with a hook-screw. Int Orthop. 1992;16:81–85. doi: 10.1007/BF00182992. [DOI] [PubMed] [Google Scholar]
- 19.Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook-screw. Spine. 2003;28:255–259. doi: 10.1097/01.BRS.0000042251.62696.A5. [DOI] [PubMed] [Google Scholar]
- 20.Johnson GV, Thompson AG. The Scott wiring technique for direct repair of lumbar spondylolysis. J Bone Joint Surg [Br] 1992;74:426–430. doi: 10.1302/0301-620X.74B3.1587895. [DOI] [PubMed] [Google Scholar]
- 21.Kakiuchi M. Repair of the defect in spondylolysis. J Bone Joint Surg [Am] 1997;79:818–825. doi: 10.2106/00004623-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Kimura M. My method of filling the defect with spongy bone in spondylolysis and spondylolisthesis. Orthop Surg. 1968;19:285–295. [PubMed] [Google Scholar]
- 23.Laurent LE, Einola S. Spondylolisthesis in children and adolescents. Acta Orthop Scand. 1961;31:45–64. doi: 10.3109/17453676108989297. [DOI] [PubMed] [Google Scholar]
- 24.Laurent LE, Österman K. Operative treatment of spondylolisthesis in young patients. Clin Orthop. 1976;117:85–91. [PubMed] [Google Scholar]
- 25.Louis R. Reconstitution isthmique des spondylolyses par plaque et greffes sans arthrodèse. A propos de 78 cas. Rev Chir Orthop. 1988;74:549–557. [PubMed] [Google Scholar]
- 26.Lundin DA, Wiseman D, Ellenbogen RG. Direct repair of the pars interarticularis for spondylolysis and spondylolisthesis. Pediatr Neurosurg. 2003;39:195–200. doi: 10.1159/000072471. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Fujimura Y, Suzuki N, Nishi Y, Nakamura M, Yabe Y, Shiga H. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg [Br] 1998;80:19–24. doi: 10.1302/0301-620X.80B1.7929. [DOI] [PubMed] [Google Scholar]
- 28.Merola A, Haher T, Brkaric M, et al. A multicenter study of the outcomes of the surgical treatment of adolescent idiopathic scoliosis using the scoliosis research society outcome instrument. Spine. 2002;27:2046–2051. doi: 10.1097/00007632-200209150-00015. [DOI] [PubMed] [Google Scholar]
- 29.Möller H (1999) Isthmic spondylolisthesis in adults. Thesis, Karolinska Institutet, Stockholm
- 30.Morscher E, Gerber B, Fasel J. Surgical treatment of spondylolisthesis by bone grafting and stabilization of spondylolysis by means of a hook-screw. Arch Orthop Trauma Surg. 1984;103:175–178. doi: 10.1007/BF00435550. [DOI] [PubMed] [Google Scholar]
- 31.Nicol RO, Scott JHS. Lytic spondylolysis. Repaiur by wiring. Spine. 1986;11:1027–1030. doi: 10.1097/00007632-198612000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Olsson Th, Selvik G, Willner S. Vertebral motion in spondylolisthesis. Acta Radiol (Diagn) 1976;17:861–868. doi: 10.1177/028418517601700614. [DOI] [PubMed] [Google Scholar]
- 33.Parkkola R, Kormano M. Lumbar disc and back muscle degeneration on MRI: correlation to age and body mass. J Spinal Disord. 1992;5:86–92. doi: 10.1097/00002517-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Parkkola R, Rytokoski U, Kormano M. Magnetic resonance imaging of the disc and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18:830–836. doi: 10.1097/00007632-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Pellisé F, Toribio J, Rivas A, García-Fontecha C, Bagó J, Villanueva C. Clinical and CT scan evaluation after direct repair in spondylolysis using segmental pedicular hook fixation. J Spinal Disord. 1999;12:363–267. [PubMed] [Google Scholar]
- 36.Putto E, Tallroth K. Flexion-extension radiographs for motion studies of the lumbar spine. A comparison study of two methods. Spine. 1990;15:107–110. doi: 10.1097/00007632-199002000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Raininko R, Manninen H, Battié MC, Gibbons LE, Gill K, Fisher LD. Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. Spine. 1995;20:1029–1035. doi: 10.1097/00007632-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Remes V, Tervahartiala P, Poussa M, Peltonen J. Thoracic and lumbar spine in diastrophic dysplasia: a clinical and magnetic resonance imaging analysis. Spine. 2001;26:187–195. doi: 10.1097/00007632-200101150-00014. [DOI] [PubMed] [Google Scholar]
- 39.Rombold C. Treatment of spondylolisthesis by posterolateral fusion, resection of the pars interarticularis, and prompt mobilization of the patient. J Bone Joint Surg [Am] 1966;48:1282–1300. [PubMed] [Google Scholar]
- 40.Schlenzka D, Poussa M, Seitsalo S, Österman K. Intervertebral disc changes in adolescents with isthmic spondylolisthesis. J Spinal Disord. 1991;4:344–352. doi: 10.1097/00002517-199109000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Schlenzka D, Seitsalo S, Poussa M, Österman K. Premature disc degeneration: source of pain in isthmic spondylolisthesis in adolescents? J Pediatr Orthop [Part B] 1993;1:153–157. doi: 10.1097/01202412-199201020-00014. [DOI] [Google Scholar]
- 42.Schlenzka D, Seitsalo S, Poussa M, Österman K. Operative treatment of symptomatic lumbar spondylolysis and mild isthmic spondylolisthesis in young patients: direct repair of the defect or segmental spinal fusion? Eur Spine J. 1993;2:104–112. doi: 10.1007/BF00302712. [DOI] [PubMed] [Google Scholar]
- 43.Scott JHS. The Edinburgh repair of isthmic (group II) spondylolysis (abstract) J Bone Joint Surg [Br] 1987;69:491. [Google Scholar]
- 44.Seitsalo S. Operative and conservative treatment of moderate spondylolisthesis in young patients. J Bone Joint Surg [Br] 1990;72:908–913. doi: 10.1302/0301-620X.72B5.2211782. [DOI] [PubMed] [Google Scholar]
- 45.Seitsalo S, Österman K, Poussa M, Laurent LE. Spondylolisthesis in children under 12 years of age: long-term results of 56 patients treated conservatively or operatively. J Pediatr Orthop. 1988;8:516–521. doi: 10.1097/01241398-198809000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Seitsalo S, Schlenzka D, Poussa M, Hyvärinen H, Österman K. Solid fusion vs. non-union in long term follow-up on in situ fusion without internal fixation in symptomatic spondylolisthesis in young patients. Eur Spine J. 1992;1:163–166. doi: 10.1007/BF00301307. [DOI] [PubMed] [Google Scholar]
- 47.Sherman FC, Rosenthal RK, Hall JE. Spine fusion for spondylolysis and spondylolisthesis in children. Spine. 1979;4:59–67. doi: 10.1097/00007632-197901000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Stauffer RN, Coventry MB. Posterolateral lumbar-spine fusion. J Bone Joint Surg [Am] 1972;54:1195–1204. [PubMed] [Google Scholar]
- 49.Suh PB, Esses SI, Kostuik JP. Repair of pars interarticularis defect. The prognostic value of pars infiltration. Spine. 1991;16:S445–S448. doi: 10.1097/00007632-199108001-00027. [DOI] [PubMed] [Google Scholar]
- 50.Tallroth K, Alaranta H, Soukka A. Lumbar mobility in asymptomatic individuals. J Spinal Disord. 1992;5:481–484. doi: 10.1097/00002517-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Tallroth K, Ylikoski M, Landman M, Santavirta S. Reliability of radiographical measurements of spondylolisthesis and extension-flexion radiographs of the lumbar spine. Eur J Radiol. 1994;18:227–231. doi: 10.1016/0720-048X(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 52.Tertti MO, Salminen JJ, Pajanen HEK, Terho PH, Kormano MJ. Low-back pain and disc degeneration in children: a case-control MR imaging study. Radiology. 1991;180:503–512. doi: 10.1148/radiology.180.2.1829844. [DOI] [PubMed] [Google Scholar]
- 53.Tokuhashi Y, Matsuzaki H. Repair of defects in spondylolysis by segmental pedicular screw hook fixation. A preliminary report. Spine. 1996;21:2041–2045. doi: 10.1097/00007632-199609010-00023. [DOI] [PubMed] [Google Scholar]
- 54.Tonino A, Werf G. Direct repair of luimbar spondylolysis. 10-year follow-up of 12 previously reported cases. Acta Orthop Scand. 1994;65:91–93. doi: 10.3109/17453679408993726. [DOI] [PubMed] [Google Scholar]
- 55.Werf JIM, Tonino AJ, Zeegers WS. Direct repair of lumbar spondylolysis. Acta Orthop Scand. 1985;56:378–379. doi: 10.3109/17453678508994351. [DOI] [PubMed] [Google Scholar]
- 56.White AA, Panjabi MM. Clinical biomechanics of the spine. 2. Philadelphia: Lippincott; 1990. pp. 106–1011. [Google Scholar]
- 57.Wiltse LL, Spencer CW. New uses and refinements of the paraspinal approach to the lumbar spine. Spine. 1988;13:696–706. [PubMed] [Google Scholar]
- 58.Wu SS, Lee CH, Chen PQ. Operative repair of symptomatic spondylolysis following a positive response to diagnostic pars injection. J Spinal Disord. 1999;12:10–16. doi: 10.1097/00002517-199902000-00002. [DOI] [PubMed] [Google Scholar]