Abstract
Purpose
To assess the reliability of trypan blue (TB) and calcein AM/ethidium homodimer-1 (CaAM/EthD-1) staining to evaluate the viability of fresh and thawed human ovarian follicles.
Methods
Isolated follicles from fresh and thawed cortex were stained using TB versus CaAM/EthD-1 methods (n = 10 patients). Measurements were performed by two independent observers. The reliability was evaluated by intraclass correlation coefficient (ICC) and the differences between paired measurements were tested by the Wilcoxon test.
Results
Inter-observer reliability was excellent for each method. Nevertheless, it was even better with the TB method (ICC = 0.83) compared with CaAM/EthD-1 (ICC = 0.75). Moreover, the ICCs for viability measurements using the two methods were good for each observer (observer 1: ICC = 0.49; observer 2: ICC = 0.40).
Conclusion
Compared with CaAM/EthD-1, TB appears to be more reliable as a staining method for follicle viability evaluation. TB staining is a quick and useful method, complementary to histological analysis for quality control in ovarian tissue cryopreservation.
Keywords: Human ovarian tissue, Fresh/thawed isolated follicles, Viability staining, Reliability, Trypan blue, Calcein AM/ethidium homodimer-1
Introduction
Recent advances in cancer treatment have significantly improved the long-term survival rate of young patients. However many women find themselves facing the prospect of losing their fertility after aggressive chemotherapy and/or radiotherapy [1]. By retrieving and cryopreserving ovarian tissue prior to the initiation of cancer treatment, it is now possible to offer the option of restoring fertility. To date, 15 healthy babies have been born worldwide after orthotopic transplantation of frozen/thawed ovarian tissue [2–13]. Despite these encouraging results, a dramatic depletion of ovarian follicles has been reported after transplantation and has affected long-term graft functionality. This severe follicular loss can be caused by direct cryoinjuries to the follicles due to a suboptimal freezing/thawing process, but mainly by ischemic damage during revascularization of the graft after transplantation [14, 15]. Histological examination is an important tool to evaluate the morphology of frozen/thawed follicles before transplantation. However, it’s time-consuming and requires special skills and equipment. Furthermore, histological evaluation is not instructive regarding follicle viability. To evaluate the potential of viable follicles before grafting, a reliable and rapid method is essential. Previous studies reported mainly two methods to measure the viability of these follicles: trypan blue (TB) and calcein AM/ethidium homodimer-1 (CaAM/EthD-1) [16–19]. TB is a vital dye which is only able to enter cells with compromised membranes and color them blue [16, 17]. Cell-permeant CaAM is enzymatically converted into calcein in the cytoplasm of living cells, turning these fluorescent green. EthD-1, on the other hand, enters cells with damaged membranes and takes on a bright red fluorescence upon binding to nucleic acids [18, 19]. Therefore this work aimed to determine the most appropriate and reliable method for measuring follicles viability (TB versus CaAM/EthD-1) to assess the quality of ovarian cryopreservation. For this purpose, we analyzed the inter-observer and inter-method reliabilities of TB and CaAM/EthD-1 staining on the one hand and the impact of the freezing/thawing procedure on the viability of ovarian follicles by these two staining methods on the other hand.
Materials and methods
All products were purchased from Sigma-Aldrich (France), unless otherwise indicated.
Ovarian tissue collection and cryopreservation
The study was approved by the regional research ethics committee (Comité Consultatif des Personnes se Prêtant à la Recherche Biomédicale d’Auvergne). Ovarian cortical samples from 10 patients were collected during endoscopic surgery for benign cysts, after obtaining informed consent to participate in the study. The mean age of the women was 29.7 ± 7.5 years (mean ± SD). The specimens were immediately immersed in “medium A” at 4°C and transported to the laboratory on ice. “Medium A” was composed of: NaCl (94.7 mmol/l), KCl (4.8 mmol/l), MgSO4 (0.8 mmol/l), NaH2PO4 (1.0 mmol/l), NaHCO3 (25.0 mmol/l), CaCl2 (1.8 mmol/l), sodium lactate (21.3 mmol/l), sodium pyruvate (0.3 mmol/l), D-glucose (5.5 mmol/l), L-glutamine (25.0 mmol/l), taurine (0.5 mmol/l), and 0.5% of human serum albumin (Vitrolife Sweden AB, Sweden) [20]. Slices of about 1 cm² in area and 1 mm in thickness each were cut. For each staining assay, one fresh tissue piece per patient was used before freezing for viability analysis. Other tissue pieces were frozen.
Freezing/thawing procedure
The freezing medium, “medium B”, consisted of “medium A” without CaCl2 and supplemented with HEPES (21.8 mmol/l), glycine (50.0 mmol/l), propanediol (3.0 mol/l) and raffinose (0.05 mol/l) [20]. The “medium B” was added in 3 steps to “medium A” which contained ovarian slices, to a final dilution of 1:1 (v/v) under gentle agitation. After 15 min of equilibration at 4°C, each slice immersed in the cryoprotective solution was transferred to a 1.8 ml cryovial (Nunc, Fisher Bioblock Scientific, France) and loaded in a programmable freezer (Minicool 40 PC, Air liquide, France). The cooling rate was 2°C/min from 4°C to −11°C, temperature at which nucleation was induced by semi-automatic seeding. Then, the temperature was lowered to −40°C at a rate of 2°C/min and to −150°C at a rate of 10°C/min. Finally the cryovials were plunged into liquid nitrogen for storage.
For the thawing process, cryovials were rapidly immersed in a 37°C water bath for 5 min and the cryoprotective solution containing the specimens was diluted in 2 steps of 5 min by “medium A” under gentle agitation. The tissue slices were washed twice with “medium A” before proceeding with the quality analysis. For each staining assay, one thawed tissue piece per patient was used for viability analysis.
Follicle isolation and viability assessment
For each patient (n = 10), fresh and thawed ovarian slices were sectioned into uniform-sized pieces of 2 × 2 mm (length x width) and 1 mm thickness, placed in “medium A” supplemented with 200 UI/ml type Ia collagenase and incubated at 37°C for 1 h with gentle agitation. Enzyme activity was inhibited by the addition of an equal volume of cold phosphate buffered saline medium supplemented with 0.3% of Bovine Serum Albumin. The suspension was filtered using a 70 μm nylon filter (BD Falcon™, BD, USA) and centrifuged at 100 g for 5 min at 4°C. The pellet was further processed as described below. We performed our analysis on “small” isolated follicles (primordial and primary) as they constitute the main source of follicles in the ovary and are a major factor in human fertility potential.
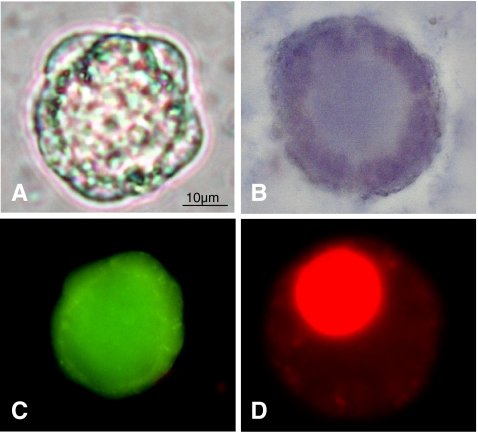
For TB staining, recovered follicles were incubated for 10 min at room temperature with 0.4% final trypan blue. Dead cells were stained blue, and live ones were unstained (Fig. 1A, B). CaAM/EthD-1 staining was performed by incubating isolated follicles with 2 μmol/L calcein AM and 5 μmol/L ethidium homodimer (Live/Dead® Viability/Cytotoxicity kit; Invitrogen, France) for 45 min at 37°C in the dark [21]. Nonfluorescent cell-permeant calcein-AM enters the cell and is cleaved by ubiquitous esterases in living cells, producing calcein which is well retained within live cells and gives an intense uniform green fluorescence (excitation/emission ~495/515 nm). Ethidium homodimer-I enters cells with damaged membranes and then binds to nucleic acids, resulting in a 40-fold enhancement of fluorescence and producing a bright red fluorescence in dead cells (excitation/emission ~495/635 nm). A conventional fluorescein longpass filter, by which calcein AM and ethidium homodimer could be viewed simultaneously, was used (Fig. 1C, D). The viability of each sample was simultaneously assessed by two independent observers (an experienced laboratory technician and a PhD student) using light microscopes for TB staining or epifluorescence microscopes for CaAM/EthD-1 staining. For each staining method, a follicle was classified as dead if the oocyte and/or >50% of granulosa cells were dead (Fig. 1). Partially or completely denuded oocytes were excluded. In order to avoid a potential inter- and intra-patient bias due to a variable number of analyzed follicles, for each patient we assessed the viability of a fixed amount of follicles: 100 follicles before and after freezing/thawing, both after TB and CaAM/EthD-1 staining.
Fig. 1.
Viability assessment of small isolated human ovarian follicles using trypan blue (TB) (A,B) and calcein AM/ethidium homodimer-1 (CaAM/EthD-1) (C,D) staining. Live follicle (1A): unstained, and dead follicle (1B): blue stained with TB dye exclusion test (light microscope; original magnification × 400). Live follicle (1C): green fluorescent, and dead follicle (1D): red fluorescence with the test using two fluorescent probes: CaAM/EthD-1 (epifluorescence microscope; original magnification × 400)
Statistical analysis
Differences between paired measurements were tested by the Wilcoxon signed-rank test [22]. This test was performed on the viability measurements made by the two different observers for each staining method (inter-observer reliability) in the same sample; on the viability measurements made by each observer for each staining method: TB versus CaAM/EthD-1 (inter-method reliability) in the same sample; and on average viability measurements made by the two observers respectively for fresh and thawed follicles from the same patient for each staining method (for the assessment of the impact of the freezing/thawing procedure on the viability of ovarian follicles). A 0.05 type I error was set as the significant level. After the above-mentioned discordance test, intraclass correlation coefficients (ICC) were computed according to Shrout and Fleiss [23] to assess the reliability of the different observers using measurements from the same sample. The formula used is as follows: 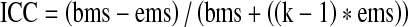 , where bms (between mean squares) and ems (error mean squares) are obtained from an ANOVA table, k being the number of observations per subject (k = 2). These coefficients were based on the usual values to characterize poor (ICC < 0.4), good (0.4 ≤ ICC < 0.75) and excellent reliability (0.75 ≤ ICC < 1) [24–26]. As recommended by the standard method reference of Bland and Altman [27, 28], the results of the inter-observer and method reliability analysis are respectively shown in Figs. 2 and 3 by drawing up the plots of difference (ordinate) against the mean of the measurements (abscissa). The presentation of the 95% limits of agreement is to allow visual judgment of how well two methods of measurement agree. All statistical analyses were performed using SAS for Windows® v9.2 (SAS institute inc., Cary, NC, USA).
, where bms (between mean squares) and ems (error mean squares) are obtained from an ANOVA table, k being the number of observations per subject (k = 2). These coefficients were based on the usual values to characterize poor (ICC < 0.4), good (0.4 ≤ ICC < 0.75) and excellent reliability (0.75 ≤ ICC < 1) [24–26]. As recommended by the standard method reference of Bland and Altman [27, 28], the results of the inter-observer and method reliability analysis are respectively shown in Figs. 2 and 3 by drawing up the plots of difference (ordinate) against the mean of the measurements (abscissa). The presentation of the 95% limits of agreement is to allow visual judgment of how well two methods of measurement agree. All statistical analyses were performed using SAS for Windows® v9.2 (SAS institute inc., Cary, NC, USA).
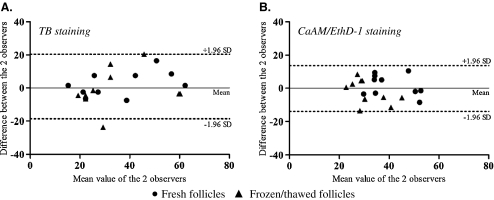
Fig. 2.
Inter-observer reliability for TB (A) and CaAM/EthD-1 staining (B) (Bland and Altman method). Viability measurement was performed by two observers (observer 1 and observer 2) on fresh and thawed isolated follicles (n = 10 patients). The solid line locates the mean and the dashed lines the upper and lower limits of the 95% fluctuation interval of the difference between the two observers
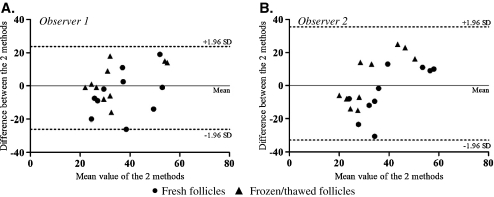
Fig. 3.
Inter-method reliability TB and CaAM/EthD-1: Bland and Altman plot of each measurement performed by observer 1 (A) and observer 2 (B). Staining with TB and CaAM/EthD-1 was performed on fresh and thawed isolated follicles (n = 10 patients). The solid line locates the mean and the dashed lines the upper and lower limits of the 95% fluctuation interval of the difference between the two observers
Results
Inter-observer reliability for TB and CaAM/EthD-1 staining of follicles
Viability analysis with the TB test, performed on fresh and thawed follicles, showed an average of 35.1% ± 14.7% and 36.0% ± 17.6% viable follicles respectively for observer 1 and 2 (n = 10 patients). For CaAM/EthD-1, average percentages of viable follicles were 36.2% ± 10.4% for observer 1 and 36.0% ± 9.3% for observer 2 (n = 10). A comparative study showed there was no significant difference between viability measurements made by each observer either for TB (p = 0.78) or for CaAM/EthD-1 (p = 0.97) staining performed on fresh and thawed follicles. The Bland and Altman plot for inter-observer reliability is presented in Fig. 2. Inter-observer reliability was excellent for each staining method. Nevertheless, it was even better with the TB method (ICC = 0.83) in comparison with CaAM/EthD-1 (ICC = 0.75).
Inter-method reliability for follicle viability measurement between TB and CaAM/EthD-1 staining
For each observer, no statistically significant difference appeared between viability measurements with TB versus CaAM/EthD-1 staining performed on fresh and thawed follicles (observer 1: p = 0.79; observer 2: p = 0.99) (n = 10). The ICCs for viability measurements by the two methods were good for each observer (observer 1: ICC = 0.49; observer 2: ICC = 0.40). Figure 3 shows Bland and Altman plots regarding inter-method reliability for the two observers.
Impact of the freezing/thawing procedure on the viability of ovarian follicles
For the 10 patients studied, a significantly lower percentage of live follicles was observed from thawed ovarian tissue compared with those from fresh tissue, after CaAM/EthD-1 (32.6% ± 9.6% versus 39.7% ± 10.4%, p = 0.02) staining, while no significant difference appeared after TB staining (34.8% ± 15.2% versus 36.2% ± 16.3%).
Discussion
Transplantation of frozen/thawed ovarian tissue is a promising strategy to restore fertility in cancer patients [1]. However, the freezing/thawing procedure and neovascularization of the graft can lead to significant follicular loss limiting the longevity of transplants [29]. An unequivocal evaluation of viable follicles in thawed ovarian cortical slices is therefore required prior to transplantation. This experiment was designed to assess the reliability of trypan blue (TB) and calcein AM/ethidium homodimer-1 (CaAM/EthD-1) staining to evaluate the viability of fresh and frozen/thawed follicles. To our knowledge, this is the first study which analyses the inter-observer and inter-method reliability of these two staining on a human ovarian model. This work aimed to determine the most appropriate and reliable method for measuring follicle viability and the most suitable method for routine clinical application.
In our study we collected ovarian samples surrounding benign cysts. This type of biopsy is a promising source of ovarian tissue for research purposes since morphological and functional patterns are similar to those of the normal ovarian cortex [20, 30]. Follicle isolation was performed by the collagenase IA enzyme method because it provides a suitable and non-deleterious means of recovering large numbers of small follicles from the human ovarian cortex [31]. In comparison with mechanical methods, the use of enzymes is less time-consuming since follicle isolation can be achieved within 1 h and no special training is required. Collagenase IA enzymatic digestion was preferred to Liberase treatment in order to optimize the number of fully isolated follicles [16]. However, recent studies showed that this purified enzyme blend allowed a reproducible isolation of small good quality follicles from human ovarian tissue [16, 32]. It could be interesting in the future to carry out a comparative study between the two staining methods, TB versus CaAM/EthD-1, on follicles isolated using the Liberase enzymatic treatment.
Assessment of vital staining using microscopy is a subjective method and no gold standard method exists for measuring follicle viability. Therefore we evaluated the reproducibility of the two staining methods used most often (TB and CaAM/EthD-1) [16–19], by an inter-observer reliability study. Our data showed excellent agreement between the two observers’ results for each staining method. However, a higher ICC was observed for TB compared with CaAM/EthD-1. Therefore TB seems to be the most reliable method. Concerning the inter-method analysis, our results are consistent with previous findings reported in animal studies showing a good reliability between the two staining methods [33–35].
TB staining is used to assess the percentage of viable follicles based on the principle that live cells possess intact cell membranes that exclude TB. The cell-impermeant viability indicator ethidium homodimer-1 is a high affinity nucleic acid stain that is weakly fluorescent until bound to DNA when it emits red fluorescence. Non-fluorescent calcein-AM is converted into intensely fluorescent calcein by cellular esterase, staining live follicles green when the calcein-AM passes through the cell membrane. Therefore, the TB test evaluates one recognized parameter of cell viability: the plasma membrane integrity, while two parameters are assessed with CaAM/EthD-1: integrity of the plasma and nuclear membranes (ethidium homodimer-1) and intracellular esterase activity (calcein-AM). This difference in staining specificities between the two methods could explain why inter-method reliability was not excellent and why the statistical significance was different when analyzing viability of fresh versus thawed follicles.
The percentage of viable follicles reported in our findings appeared lower compared to a previous report by Fauque et al. [17] using TB to assess the viability of follicles enzymatically isolated from human cortex. However, the ovarian samples were obtained from two different clinical contexts: women with polycystic ovary syndrome (PCOS) versus women with benign ovarian cysts and the strategies used to evaluate follicle viability between the two studies were different. These elements could explain the difference in term of follicles viability recovered from fresh tissues between the two studies.
Technically TB staining is very simple to perform. Compared with CaAM/EthD-1, the TB method is less time-consuming and no specialized laboratory equipment is required, while CaAM/EthD-1 requires the use of fluorescence microscopy. These important features encourage the use of the former technique for further optimization of clinical cryopreservation protocols. However, an apparently viable follicle does not necessarily reflect a healthy, functional follicle. It would be prudent to complete this test with morphological analysis of follicles and possibly functional analysis.
In conclusion, we showed by this study that TB and CaAM/EthD-1 staining may be considered as applicable for viability assessment of small isolated human ovarian follicles. Compared with CaAM/EthD-1, TB appears to be a highly reliable method. Moreover it is a quick and useful technique which seems more appropriate for routine analysis as a quality control in ovarian tissue cryopreservation procedures.
Acknowledgements
We would like to thank all the surgical team in the department of gynecology, CHU Estaing, Clermont-Ferrand (France) for providing ovarian tissue for research, Dr Benoît Sion for his critical recommendations on this study and Mrs Elizabeth Petit for language revision of the manuscript. Thanks are also due to Mr Eric Pernet (CICE) for advice and technical assistance.
Conflict of interest statement The authors declare that there are no conflicts of interest.
Footnotes
Capsule Trypan blue appears to be a more reliable staining method for follicle viability evaluation compared with calcein AM/ethidium homodimer-1
Supported by: an industrial doctoral grant (Convention Industrielle de Formation par la Recherche, CIFRE) with the Centre International de Chirurgie Endoscopique (CICE), France (Grant No: 176/2009).
References
- 1.Revel A, Revel-Vilk S. Fertility preservation in young cancer patients. J Hum Reprod Sci. 2010;3:2–7. doi: 10.4103/0974-1208.63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 4.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 5.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 6.Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, Gosden RG. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod. 2008;23:1531–1537. doi: 10.1093/humrep/den032. [DOI] [PubMed] [Google Scholar]
- 7.Piver P, Amiot C, Agnani G, Pech J, Rohrlich PS, Vidal E, et al. Two pregnancies obtained after a new technique of autotransplantation of cryopreserved ovarian tissue. In: 25th Annual Meeting of ESHRE, 28 June – 1 July, 2009. Amsterdam, the Netherlands: Oxford University Press. Hum Reprod. 2009:i15.
- 8.Ernst E, Bergholdt S, Jørgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escribá MJ, Simón C, Pellicer A. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93:268.e11–268.e13. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93:2413.e15–2413.e19. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 12.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1097–1103. doi: 10.1093/humrep/der063. [DOI] [PubMed] [Google Scholar]
- 13.Donnez J, Dolmans MM. Preservation of fertility in females with haematological malignancy. Br J Haematol. 2011;154:175–184. doi: 10.1111/j.1365-2141.2011.08723.x. [DOI] [PubMed] [Google Scholar]
- 14.Pegg DE, Jacobsen IA, Armitage WJ, Taylor MJ. Mechanisms of cryoinjury in organs. In: Pegg DE, Jacobsen IA, editors. Organ preservation. Edinbourgh: Churchill Livingstone; 1979. pp. 132–146. [Google Scholar]
- 15.Nugent D, Newton H, Gosden RG, Rutherford AJ. Investigation of follicle survival after human heterotopic grafting. Hum Reprod. 1998;13:23–24. [Google Scholar]
- 16.Dolmans MM, Michaux N, Camboni A, Martinez-Madrid B, Langendonckt A, Nottola SA, Donnez J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum Reprod. 2006;21:413–420. doi: 10.1093/humrep/dei320. [DOI] [PubMed] [Google Scholar]
- 17.Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87:1200–1207. doi: 10.1016/j.fertnstert.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Madrid B, Dolmans MM, Langendonckt AV, Defrère S, Eyck AS, Donnez J. Ficoll density gradient method for recovery of isolated human ovarian primordial follicles. Fertil Steril. 2004;82:1648–1653. doi: 10.1016/j.fertnstert.2004.05.084. [DOI] [PubMed] [Google Scholar]
- 19.Maltaris T, Dragonas C, Hoffmann I, Mueller A, Beckmann MW, Dittrich R. Simple prediction of the survival of follicles in cryopreserved human ovarian tissue. J Reprod Dev. 2006;52:577–582. doi: 10.1262/jrd.18012. [DOI] [PubMed] [Google Scholar]
- 20.Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Déchelotte P, Boucher D, Grizard G. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20:1786–1792. doi: 10.1093/humrep/dei002. [DOI] [PubMed] [Google Scholar]
- 21.Cortvrindt RG, Smitz JE. Fluorescent probes allow rapid and precise recording of follicle density and staging in human ovarian cortical biopsy samples. Fertil Steril. 2001;75:588–593. doi: 10.1016/S0015-0282(00)01754-4. [DOI] [PubMed] [Google Scholar]
- 22.Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 2nd ed. Chapmann & Hall ⁄ CRC, 2000.
- 23.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 24.Smith CAB. On the estimation of intraclasscorrelation. Ann Hum Genet. 1956;21:363–373. doi: 10.1111/j.1469-1809.1972.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 25.Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Shourki MM, Pause CA. Statistical methods for health sciences. 2nd ed. CRC Press LLC, 1999.
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/S0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod. 2005;20:3539–3546. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- 30.Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89:1787–1794. doi: 10.1016/j.fertnstert.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 31.Oktay K, Nugent D, Newton H, Salha O, Chatterjee P, Gosden RG. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67:481–486. doi: 10.1016/S0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 32.Vanacker J, Camboni A, Dath C, Van Langendonckt A, Dolmans MM, Donnez J, Amorim CA. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil Steril 2011 Jun 28. [Epub ahead of print] [DOI] [PubMed]
- 33.Poeschmann M, Fassbender M, Lopes C, Dorresteijn A, Jewgenow K. Viability assessment of primordial follicles of domestic cats to develop cryopreservation protocols for ovarian tissue. Reprod Domest Anim. 2008;43(Suppl. 1):25. [Google Scholar]
- 34.Lopes CA, Santos RR, Celestino JJ, Melo MA, Chaves RN, Campello CC, Silva JR, Báo SN, Jewgenow K, Figueiredo JR. Short-term preservation of canine preantral follicles: effects of temperature, medium and time. Anim Reprod Sci. 2009;115:201–214. doi: 10.1016/j.anireprosci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Merdassi G, Mazoyer C, Guerin JF, Saad A, Salle B, Lornage J. Examination of viability and quality of ovarian tissue after cryopreservation using simple laboratory methods in ewe. Reprod Biol Endocrinol. 2011;9:78. doi: 10.1186/1477-7827-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]