Abstract
Objective
To evaluate predictive role of day–3 serum anti-Müllerian hormone (AMH) levels and antral follicle count (AFC) in ovarian hyperstimulation syndrome (OHSS) in patients undergoing IVF/ICSI cycles.
Materials and methods
Forty-one women with moderate/severe OHSS and 41 age matched women without OHSS were compared to evaluate the predictive value of certain risk factors for OHSS. AFC, and E2, FSH, LH, AMH, inhibin-B levels measured on day 3 of the menstrual cycle before controlled ovarian hyperstimulation.
Results
Mean FSH was significantly lower (p < 0.0001); and mean LH, AFC and AMH were significantly higher in women with OHSS compared to women without OHSS (p = 0.049, p < 0.0001 and p < 0.0001, respectively). There was no significant difference in inhibin B (p = 0.112) and estradiol (p = 0.706) between the groups. The ROC area under curve (AUC) for AMH presented the largest AUC among the listed risk factors. AMH (AUC = 0.87) and AFC (AUC = 0.74) had moderate accuracy for predicting OHSS while Inhibin B (AUC = 0.58) and LH (AUC = 0.61) had low accuracy. The cut-off value for AMH 3.3 ng/mL provided the highest sensitivity (90%) and specificity (71%) for predicting OHSS. It’s positive (PPV) and negative predictive values (NPV) were 61% and 94%, respectively. The cut-off value for AFC was 8 with 78% sensitivity, 65% specificity, 52% PPV and 86% NPV.
Conclusion
Measurement of basal serum AMH and AFC can be used to determine the women with high risk for OHSS.
Keywords: Antimullerian hormone, Ovarian hyperstimulation syndrome, Antral follicle count
Introduction
Ovarian hyperstimulation syndrome (OHSS) is a severe complication observed in some patients undergoing controlled ovarian hyperstimulation (COH) during in vitro fertilization (IVF) [1]. OHSS is characterized by an increase in ovarian size associated with a dramatic increase in vascular permeability causing ascites and eventually amore severe and possibly lethal form of infertility requiring hospitalization in otherwise healthy women [2].
The specific risk factors for OHSS include young age, lean habitus, polycystic ovarian syndrome (PCOS), the presence of multiple small and intermediate follicles, and excessively high levels of serum estradiol (E2) on the day of HCG (>4,000 pg/mL) [3–5]. However, prediction of OHSS prior to COH in an individual IVF cycle using only age and body mass index (BMI) remains a difficult task [6]. Monitoring the serum E2 level and number of follicles has been effective in reducing the incidence of OHSS, but these are determined near the completion of COH [7, 8].
If it would be possible to accurately predict OHSS before starting ART cycle, a better alternative would be chosen for COH with lesser risks for the patients, such as using GnRH antagonists for pituitary suppression and GnRH agonists to trigger oocyte maturation. Therefore fewer cycles would be canceled.
Recently, serum anti-müllerian hormone (AMH) levels have been reported to better reflect the level of ovarian reserve than other markers such as basal serum FSH, inhibin B levels and antral follicle count (AFC). OHSS has been reported to be associated with high serum levels of AMH prior to COH. Patients with cancelled cycles due to a high risk of OHSS had serum AMH levels in the highest quartile, and basal serum AMH levels could be utilized effectively to predict OHSS [9–13].
The aim of this study was to determine the value of serum AMH levels and AFC as predictors of OHSS in patients undergoing a first cycle of COH for IVF/ICSI cycles.
Material and methods
Subjects
Patients undergoing IVF/ICSI cycles in Istanbul University, Cerrahpasa School of Medicine, Department of Obstetrics and Gynecology, IVF Unit between January 2008 and January 2010 were retrospectively reviewed to evaluate the predictive value of certain risk factors including age, body mass index (BMI), AFC, and E2, FSH, LH, AMH, and inhibin B levels measured on day 3 of the menstrual cycle for OHSS before COH. During this period 695 patients were treated by IVF/ICSI and 41 of 695 women had moderate or severe OHSS at their first cycle. This study group was compared to 41 age matched women who were at their first cycle and did not have OHSS.
Criteria for inclusion were a written informed consent for the IVF/ICSI treatment, a patient age of 40 maximum, a basal FSH (day 3) level of <15 mIU/mL, body mass index of 18–30 kg/m2; normal prolactin and thyroid stimulating hormone (TSH) values, normal gynecological ultrasound, and cervical smear. Exclusion criteria for an IVF/ICSI treatment were acute infectious diseases, systemic illnesses, and known hypothalamic, pituitary and surrenal disorders. The local ethics committee approved the study.
Diagnosis of OHSS
The criteria for classification of OHSS defined by Navot et al. [3] were used to assess the relative severity of OHSS. Moderate OHSS was characterized by abdominal distension and ascites and ovarian size of 8–12 cm on ultrasonography. Severe OHSS consisted of clinical ascites with or without pleural effusion, oedema, oliguria, hematocrit levels >45%, white blood cell count >15,000/mm3, a serum creatinine of 1.0–1.5 mg/mL, and abnormal liver function tests. Only those patients with a moderate or severe OHSS were classified as having a positive case of OHSS. Diagnosis of moderate OHSS was made by the presence of abdominal distension and ascites and ovarian size of 8–12 cm on ultrasonography. Diagnosis of severe OHSS was made by presence of one of the clinical (clinical ascites with or without pleural effusion, oedema, oliguria), or laboratory (hematocrit levels >45%, white blood cell count >15,000/mm3, a serum creatinine of 1.0–1.5 mg/mL, and abnormal liver function tests) criteria in a patient with moderate OHSS.
Study protocol
On day 2–3 of a spontaneous cycle within 3 months of commencing ovarian stimulation, blood samples for assay of E2, FSH, LH, AMH and inhibin B levels were obtained. All patients received GnRH agonist leuprolide acetate (1 mg/day sc Lucrin®, Abbott-France Pharmaceuticals, France) beginning on the 21st day the of previous cycle. Leuprolide acetate was reduced to 0.5 mg/day and gonadotropin 150–225 IU (Menogon®, Ferring, Istanbul; Gonal F®, Merck Serono, Istanbul; or Puregon®, Schering Plough, Istanbul) were initiated on the third day of menstruation based on age, body mass-index (BMI) and basal FSH value.
COH was monitored by transvaginal sonography, and then the dose of gonadotropin was adjusted according to the follicle size and number. When three or more follicles reached >18 mm, both the gonadotropin and agonist injections were stopped and either 10,000 IU of hCG (Pregnyl®, Schering Plough, Istanbul) or rechCG (Ovitrelle®, Merck Serono, Istanbul) was given.
Egg collection was planned 34–36 h after hCG injection, followed by embryo transfer 72 h after oocyte retrieval. Luteal phase was supported with progesterone (200 mg administered vaginally three times daily Progynex®, Koçak, Istanbul, or Crinone gel® 8%, Merck Serono, Istanbul) or by 100 mg progesterone injection IM daily (Progynex® ampoule, Koçak, Istanbul) until the day of the pregnancy test 15 days after the embryo transfer.
Hormone assays
AMH. All samples were assayed in duplicate using the AMH/MIS enzyme-linked immunosorbent assay kit (Diagnostic Systems Lab, Webster, Texas, USA). The sensitivity of the assay was 0.017 ng/mL. The intra- and inter- assay variations were <5% and <8%, respectively.
Inhibin B. All samples were assayed in duplicate using a commercial ELISA kit (Diagnostic Systems Lab, Webster, Texas, USA). The sensitivity of the assay is 7 pg/mL. The intra- and interassay variations were <6% and <8%, respectively.
FSH, Estradiol, and LH. These were measured by Chemiluminescent Microparticle Immunoassay (Architect Abbott Lab, IL, USA).
Statistical analysis
Results were expressed as mean±SD, frequency, and percentages. One sample Kolmogorov-Smirnov test and histogram graphics were used to show the distribution. Categorical characteristics of patients were compared with χ2 test. Independent Samples T test and Mann Whitney U tests were used for comparison of numeric variables.
Receiver operating characteristic (ROC) curve analysis and comparison of area under curves (AUC) were performed to determine cut-off values of FSH, inhibin B, AMH, LH, age, gonadotropin dose and AFC for the prediction of OHSS and to calculate their sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the prediction of OHSS. The standard AUC definitions were as follows: AUC = 1 indicates a perfect test, AUC > 0.9 indicates high accuracy and AUC between 0.7 and 0.9 indicates moderate accuracy. Analyses were performed in Statistical Package for the Social Sciences (SPSS) 17.0 and Medcalc 11.4.4.0 (free trial). A p value of <0.05 was considered statistically significant.
Results
Of 695 IVF ICSI cycles, 41 (5.8%) were complicated by OHSS and among 41 cycles 11 were severe OHSS. These 41 women with moderate or severe OHSS were compared to 41 age-matched women who underwent IVF/ICSI in the same period and did not have OHSS.
Table 1 summarizes patients’ demographics; mean basal E2, FSH, LH, AMH, inhibin B levels, AFC and treatment characteristics. There was no significant difference between the two groups with regard to different gonadotropin types. Gonadotropin dose was significantly lower in women with OHSS compared to women without OHSS (1891 ± 875 U vs. 2595 ± 1331 U, p = 0.003).
Table 1.
Patients’ demographics, mean basal E2, FSH, LH, AMH, inhibin B levels, AFC and treatment characteristics
| OHSS present (n = 41) | OHSS absent (n = 41) | P value | |
|---|---|---|---|
| Age (year) mean±SD | 29.3 ± 4.6 | 29.3 ± 4.6 | 1.000 |
| BMI (kg/m2) mean±SD | 24.4 ± 4.2 | 25.0 ± 3.9 | 0.188a |
| Gonadotropin dose (U) mean±SD | 1891 ± 875 | 2595 ± 1331 | 0.003a |
| Gonadotropin type n(%) | |||
| HMG | 15 (37%) | 16 (39%) | 0.153c |
| recFSH | 26 (63%) | 25 (61%) | |
| AMH (ng/mL) mean±SD; | 6.9 ± 3.9 | 2.9 ± 2.0 | <0.0001b |
| FSH (mIU/mL) mean±SD; | 5.0 ± 1.8 | 6.9 ± 3.8 | <0.0001b |
| LH (mIU/mL) mean±SD;) | 4.7 ± 3.0 | 3.8 ± 2.6 | 0.049b |
| Inhibin B (pg/mL) mean±SD | 97.9 ± 60.8 | 80.7 ± 51.5 | 0.112a |
| E2 (pg/mL) mean±SD; | 41.8 ± 29.4 | 43.6 ± 22.9 | 0.706b |
| AFC mean±SD; | 12.9 ± 6.8 | 8.2 ± 6.0 | <0.0001b |
| Number of pregnancies n(%) | 15 (37.5%) | 18 (43%) | 0.77c |
AMH anti-müllerian hormone; BMI body-mass index; E2 Estradiol; FSH follicle stimulating hormone; GnRH gonadotropin releasing hormone; HMG human menopausal gonadotropin; LH luteinizing hormone. NS non-significant
aIndependent samples t test (2 tailed),
bMann Whitney U test,
cChi square test
We found no significant difference between the two groups regarding mean BMI (p = 0.188), day 3 E2 (p = 0.706) and inhibin B (p = 0.112).
Mean FSH was significantly lower in women with OHSS compared to women without OHSS (5 ± 1.8 vs. 6.9 ± 3.8; p < 0.0001). Mean AMH and AFC was significantly higher in women with OHSS compared to women without OHSS (6.9 ± 3.9 vs. 2.9 ± 2; p < 0.0001 and 12.9 ± 6.8 vs. 8.2 ± 6.0; p < 0.0001, respectively). LH was very slightly higher in women with OHSS compared to women without OHSS (4.7 ± 3.0 vs. 3.8 ± 2.6, p = 0.049).
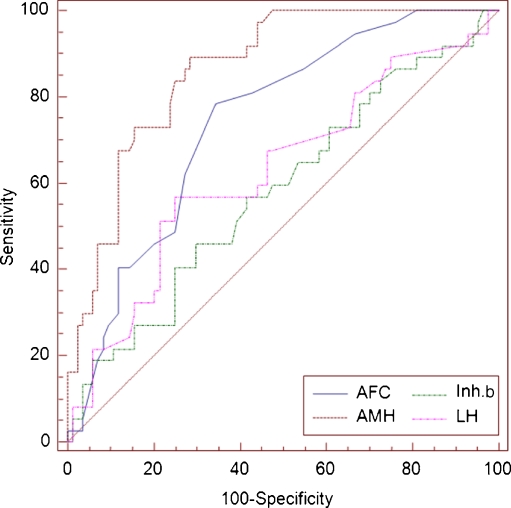
Table 2 shows the results of ROC for the sensitivity, specificity, PPV, and NPV of the AMH, AFC, inhibin B and LH in predicting the risk of OHSS. The ROC area under curve (AUC) for AMH presented the largest AUC among the listed risk factors; and the AUC for AMH was significantly larger than the corresponding values for AFC, LH, and inhibin B (Fig. 1). The ROC-AUC of inhibin B, LH and FSH were non-significant (ROC-AUC: 0.58; ROC-AUC: 0.61; ROC AUC: 0.34; respectively).
Table 2.
Comparison of ROC-AUC, sensitivity, specificity, NPV, and PPV of various markers for prediction of OHSS
| AUC (95% CI) | cut-off value | Sensitivity (95% CI) | Specificity (95% CI) | NPV | PPV | |
|---|---|---|---|---|---|---|
| AMH | 0.87 (0.80–0.92) | 3.3 | 0.90 (0.77–0.97) | 0.71 (0.61–0.81) | 0.94 (0.84–0.98) | 0.61 (0.47–0.73) |
| AFC | 0.74 (0.66–0.82) | 8 | 0.78 (0.62–0.89) | 0.65 (0.54–0.76) | 0.86 (0.74–0.93) | 0.52 (0.39–0.65) |
| Inhibin B | 0.58 (0.49–0.67) | 83.5 | 0.57 (0.40–0.72) | 0.58 (0.47–0.69) | 0.75 (0.63–0.85) | 0.38 (0.25–0.51) |
| LH | 0.61 (0.52–0.69) | 4.2 | 0.54 (0.37–0.69) | 0.75 (0.64–0.84) | 0.77 (0.66–0.85) | 0.51 (0.36–0.66) |
| FSH | 0.34 (0.23–0.44) | 5.17 | 0.51 (0.32–0.55) | 0.67 (0.54–0.71) | 0.68 (0.61–0.74) | 0.48 (0.30–0.57) |
AMH antimullerian hormone; AFC antral follicle count; LH, luteinizing hormone; ROC-AUC Receiver Operating Characteristic - Area Under Curve; NPV negative predictive value; PPV positive predictive value; FSH follicle stimulating hormone
Fig. 1.
The ROC area under curves for prediction of OHSS
The ROC-AUC for AMH was 0.87 with a 95% CI (0.80–0.92). The cut-off value for AMH 3.3 ng/mL was determined with the highest sensitivity (90%) and specificity (71%) for predicting OHSS and its PPV and NPV were 61% and 94%, respectively. Comparison of sensitivity, specificity, NPV, and PPV of different AMH threshold values for prediction of OHSS are presented in Table 3.
Table 3.
Comparison of sensitivity, specificity, NPV, and PPV of different AMH threshold values for prediction of OHSS
| Cut-off value | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95% CI) |
|---|---|---|---|---|
| 2.29 ng/ml | 100% (91.4–100) | 52.3% (41.2–63.4) | 100 (92–100) | 50.6 (39.2–62) |
| 2.53 ng/ml | 92.6% (80.1–98.5) | 57.1% (45.9–67.9) | 94.1 (83.6–98.8) | 51.4 (39.3–63.3) |
| 3.3 ng/ml | 90% (0.77–0.97) | 71% (0.61–0.81) | 94 (0.84–0.98) | 61 (0.47–0.73) |
| 3.88 ng/ml | 75.6% (59.7–87.6) | 78.5% (68.3–86.8) | 86.8 (77.1–93.5) | 63.3 (48.3–76.6) |
| 4.3 ng/ml | 75.6% (59.7–87.6) | 82.1% (72.3–89.6) | 87.3 (77.9–93.8) | 67.4 (52–80.5) |
| 5.07 ng/ml | 56.1% (39.7–71.5) | 88.1% (79.2–94.1) | 80.4 (70.9–88.0) | 69.7 (51.3–84.4) |
| 10.2 ng/ml | 17% (7.2–32.1) | 100% (95.7–100) | 71.2 (62.1–79.2) | 100 (59.0–100) |
AMH antimullerian hormone; NPV negative predictive value; PPV positive predictive value; OHSS ovarian hyperstimulation syndrome
The ROC-AUC for AFC was 0.74 with a 95% CI (0.66–0.82). The cut-off value for AFC 8 was determined with the highest sensitivity (78%) and specificity (65%) for predicting OHSS and its PPV and NPV were 52% and 86%, respectively.
According to the standard definitions of AUC, AMH (AUC = 0.87) and AFC (AUC = 0.74) had moderate accuracy for predicting OHSS while Inhibin B (AUC = 0.58) and LH (AUC = 0.61) had low accuracy.
Discussion
This study showed that 5.8% of 695 IVF ICSI cycles were complicated by moderate or severe OHSS; 1.5% of those were severe OHSS. These findings are in keeping with the incidence of OHSS (5–9%) that has been reported in the literature; and the severe form of the syndrome may occur in 1–3% [14].
Since the exact pathogenesis of OHSS is poorly understood, its prediction is difficult. OHSS has clear risk factors such as polycystic ovaries, young age, lean habitus, a high number of antral follicles, high E2 on the day of hCG and previous OHSS [15, 16]. But OHSS can occur in the absence of currently recognized risk factors. In our study the mean BMI of the two groups were not different.
The key to prevent OHSS is the recognition of risk factors leading to an individualization of gonadotropin starting dose which should be the minimum dose necessary to achieve the therapeutical goal or usage of a GnRH antagonist for pituitary suppression and an agonist to trigger oocyte maturation. However, accurate prediction of OHSS in an individual IVF cycle is difficult.
AMH is produced by the granulosa cells of primary and preantral follicles, [17, 18] and is a member of the transforming growth factor-ß family synthesized exclusively by the gonads of both sexes. Over the last decade, several studies have examined the clinical usefulness of serum AMH levels as a predictor of ovarian response and pregnancy in ART cycles [12]. The recognition of a dose–response relationship between AMH and ovarian response to FSH leads to the hypothesis that hyperresponse to ovulation induction might result from high AMH. In this context high basal AMH may be associated with an increased risk of developing OHSS. Lee [19] and Nardo [20] have independently calculated a similar performance of AMH for the prediction of hyper response and OHSS.
In the present study we used a cut-off level of day 3 AMH >3.3 ng/mL to determine the risk of OHSS with 90% sensitivity, 71% specificity, 61% PPV, and 94% NPV. We found that AMH is a better predictor than AFC, LH, inhibin B and FSH.
To evaluate the predictive value for OHSS by means of age, BMI, estradiol and AMH levels, a cohort of 262 IVF cycles was investigated by Lee et al. [19], and they found that the ROC of the basal AMH was larger than age and BMI, and works equally well as the number of follicles and estradiol levels on the day of hCG. Basal AMH levels predicted OHSS with a sensitivity of 90.5% and specificity of 81.3%. Interestingly, the cut-off value calculated (3.36 ng/ml) corresponded to the highest quartile (75–100%) of the AMH values in their population, suggesting that hyperresponse and OHSS may be caused by gonadotropin administration to women with enhanced ovarian reserve. In the study of Lee, AMH-AUC for OHSS was 0.902 which is very close to the value (AUC = 0.87) found in our study. In Lee’s study OHSS rate was 8% (21/262) and 19 of 21 OHSS cases were found in 75–100% group. In this group mean BMI was lower compared to our study group (21.2 ± 0.4 vs. 24.4 ± 4.2); this may be a possible explanation for the high OHSS rate, since OHSS is more frequent in lean patients. [3] In Lee’s study, the mean serum AMH level for OHSS patients was significantly higher compared to mean serum AMH level of OHSS patients in our study (n = 21, 5.02 ng/ml vs. n = 41, 6.9 ng/ml, respectively, p < 0.0001); although the same kit was used in both studies. This difference could be attributed to individual characteristics of patients in two groups.
Broer et al. [21] performed a meta-analysis of 9 studies reporting on predictive value of AMH for OHSS and they found that summary estimates of sensitivity and specificity for AMH were 82 and 76%, respectively. In our study, the sensitivity and specificity of AMH were 90% and 71%, respectively. Broer et al. also evaluated 5 studies reporting on predictive value of AFC for OHSS and they found that summary estimates of sensitivity and specificity for AFC were 82 and 80%, respectively. In our study, the sensitivity and specificity of AFC for prediction of OHSS were 78% and 65%, respectively. Broer et al. [21] concluded that the comparison of the summary estimates and ROC curves for AMH and AFC showed no statistical difference. However in our study, AUC of AMH was significantly larger than AUC of AFC (AMH-AUC = 0.87, standard error = 0.0310 vs. AFC-AUC = 0.74, standard error = 0.0451, p = 0.0175).
Considering that PCOS has been associated with high AMH levels, it is logical to conclude that the prevalence of PCOS patients among women with AMH levels in the highest AMH quartile may be increased, thus in part explaining the observed high rate of OHSS in this group of women.
But PCOS is present only in 20% of women undergoing COH and in <20% of patients developing symptoms of impending OHSS, then taking only PCOS like a predictive factor for OHSS is not sufficient [22, 23]. In our study 8.8% of women had ovulatory dysfunction.
Daninger [24] found a significant correlation between baseline ovarian volume and development of OHSS in 101 patients who underwent IVF. In their study, a significant correlation with the baseline number of follicles and development of this syndrome was also found. Other investigators have demonstrated the usefulness of ovarian volume and AFC [25, 26]. On the other hand AFC necessitates skilled ultrasound operators who carefully identify measure, and count ovarian follicles. Also there is a moderate intercycle and interobserver variability in AFC [27].
Unlike AFC measurements, serum AMH assays are not observer-dependent, resulting in less interobserver variability; it may also represent a more sensitive marker of ovarian reserve than AFC. In our study, AFC with a cut off level of 8 can predict OHSS with 78% sensitivity and 65% specificity and its PPV and NPV were 52% and 86%, respectively.
Multiple studies have suggested that early follicular phase serum AMH levels reflected the recruitable pool of antral follicles and serve as a sensitive marker of ovarian reserve. Response to ovarian hyperstimulation will be directly linked to this cohort size [28].
After evaluating several predictive criteria for OHSS, Delvigne has concluded that there were a number of factors including E2 level and the number of follicles [4]. Morris measured E2 levels as a predictive factor and found that all OHSS patients had high E2 levels, but this was not accurate enough to be a predictive factor [29]. We demonstrated availability of a predictive serum marker like AMH that could potentially improve identification of patients at high risk for OHSS and allow us to minimize the incidence of this complication.
Enskog [30] observed a higher inhibin B concentration in OHSS group during the gonadotropin stimulation and also at the day of oocyte retrieval; but only inhibin A was significantly elevated after OHSS onset while inhibin B increased gradually following FSH stimulation and was declining at the time of the LH surge. In the present study, in accord with the results of Moos [31], we found that serum concentration of inhibin B was not found to be a strong predictive factor of OHSS.
In conclusion we found that AMH (AUC = 0.87) and AFC (AUC = 0.74) had moderate accuracy for predicting OHSS while inhibin B (AUC = 0.58) and LH (AUC = 0.61) had low accuracy. A cut-off level of AMH >3.3 ng/mL determines the risk of OHSS at the beginning of COH with 90% sensitivity, 71% specificity, 61% PPV, and 94% NPV. And a cut-off value of AFC >8 in predict OHSS with 78% sensitivity and 65% specificity, 52% PPV, and 86% NPV. AMH measurement and AFC prior to COH can provide useful information to direct the application of mild patient-friendly stimulation protocols in order to avoid OHSS.
Acknowledgements
We would like to thank Sevim Purisa and Penbe Cagatay for her assistance in statistics; Hulya Senol for her assistance in collection of the data.
Conflict of interest None.
Footnotes
Capsule Day–3 serum anti-Müllerian hormone (AMH) levels and antral follicle count (AFC) can predict ovarian hyperstimulation syndrome (OHSS) in patients undergoing IVF/ICSI cycles.
References
- 1.Forman RG, Frydman R, Egan D, Ross C, Barlow DH. Severe ovarian hyperstimulation syndrome using agonists of gonadotropin-releasing hormone for in vitro fertilization: a European series and a proposal for prevention. Fertil Steril. 1990;53:502–509. doi: 10.1016/s0015-0282(16)53348-2. [DOI] [PubMed] [Google Scholar]
- 2.Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8:559–577. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 3.Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril. 1992;58:249–261. doi: 10.1016/s0015-0282(16)55188-7. [DOI] [PubMed] [Google Scholar]
- 4.Delvigne A, Dubois M, Battheu B, Bassil S, Meuleman C, Sutter P, et al. The ovarian hyperstimulation syndrome in in-vitro fertilization: a Belgian multicentric study. II. Multiple discriminant analysis for risk prediction. Hum Reprod. 1993;8:1361–1366. doi: 10.1093/oxfordjournals.humrep.a138261. [DOI] [PubMed] [Google Scholar]
- 5.Schenker JG. Prevention and treatment of ovarian hyperstimulation. Hum Reprod. 1993;8:653–659. doi: 10.1093/oxfordjournals.humrep.a138115. [DOI] [PubMed] [Google Scholar]
- 6.Mathur RS, Joels LA, Akande AV, Jenkins JM. The prevention of ovarian hyperstimulation syndrome. Br J Obstet Gynaecol. 1996;103:740–746. doi: 10.1111/j.1471-0528.1996.tb09867.x. [DOI] [PubMed] [Google Scholar]
- 7.Aboulghar M. Prediction of ovarian hyperstimulation syndrome (OHSS). Estradiol level has an important role in the prediction of OHSS. Hum Reprod. 2003;18:1140–1141. doi: 10.1093/humrep/deg208. [DOI] [PubMed] [Google Scholar]
- 8.Orvieto R. Prediction of ovarian hyperstimulation syndrome. Challenging the estradiol mythos. Hum Reprod. 2003;18:665–667. doi: 10.1093/humrep/deg166. [DOI] [PubMed] [Google Scholar]
- 9.Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Peñarrubia J, Fábregues F, Manau D, Creus M, Casals G, Casamitjana R, et al. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—gonadotropin treatment. Hum Reprod. 2005;20:915–922. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 11.Nakhuda GS, Chu MC, Wang JG, Sauer MV, Lobo RA. Elevated serum müllerian-inhibiting substance may be a marker for ovarian hyperstimulation syndrome in normal women undergoing in vitro fertilization. Fertil Steril. 2006;85:1541–1543. doi: 10.1016/j.fertnstert.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Marca A, Giulini S, Tirelli A, Bertucci E, Marsella T, Xella S, et al. Anti-Müllerian hormone measurement on any day of the menstrual cycle strongly predicts ovarian response in assisted reproductive technology. Hum Reprod. 2007;22:766–771. doi: 10.1093/humrep/del421. [DOI] [PubMed] [Google Scholar]
- 13.Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles-implications for individualization of therapy. Hum Reprod. 2007;22:2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 14.Vloeberghs V, Peeraer K, Pexsters A, D’Hooghe T. Ovarian hyperstimulation syndrome and complications of ART. Best Pract Res Clin Obstet Gynaecol. 2009;23:691–709. doi: 10.1016/j.bpobgyn.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 16.Fauser BC, Diedrich K, Devroey P. Evian Annual Reproduction Workshop Group 2007. Predictors of ovarian response:progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14:1–14. doi: 10.1093/humupd/dmm034. [DOI] [PubMed] [Google Scholar]
- 17.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti Mullerian hormone. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 18.Visser JA, Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 19.Lee TH, Liu CH, Huang CC, Wu YL, Shih YT, Ho HN, et al. Serum anti- Mullerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2008;23:160–167. doi: 10.1093/humrep/dem254. [DOI] [PubMed] [Google Scholar]
- 20.Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 21.Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 2011;17(1):46–54. doi: 10.1093/humupd/dmq034. [DOI] [PubMed] [Google Scholar]
- 22.Bellver J, Escudero E, Pellicer A. Bilateral partial oophorectomy in the management of severe ovarian hyperstimulation syndrome (OHSS): ovarian mutilating surgery is not an option in the management of severe OHSS. Hum Reprod. 2003;18:1363–1367. doi: 10.1093/humrep/deg285. [DOI] [PubMed] [Google Scholar]
- 23.Tummon I, Gavrilova-Jordan L, Allemand MC, Session D. Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review. Acta Obstet Gynecol Scand. 2005;84:611–616. doi: 10.1111/j.0001-6349.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 24.Danninger B, Brunner M, Obruca A, Feichtinger W. Prediction of ovarian hyperstimulation syndrome by ultrasound volumetric assessment [corrected] of baseline ovarian volume prior to stimulation. Hum Reprod. 1996;11:1597–1599. doi: 10.1093/oxfordjournals.humrep.a019451. [DOI] [PubMed] [Google Scholar]
- 25.Kwee J, Elting ME, Schats R, McDonnell J, Lambalk CB. Ovarian volume and antral follicle count for the prediction of low and hyper responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;15:5–9. doi: 10.1186/1477-7827-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendriks DJ, Kwee J, Mol BW, Velde ER, Broekmans FJ. Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87:764–775. doi: 10.1016/j.fertnstert.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Hansen KR, Morris JL, Thyer AC, Soules MR. Reproductive aging and variability in the ovarian antral follicle count: application in the clinical setting. Fertil Steril. 2003;80(3):577–583. doi: 10.1016/S0015-0282(03)00741-6. [DOI] [PubMed] [Google Scholar]
- 28.Kwee J, Elting MW, Schats R, Bezemer PD, Lambalk CB, Schoemaker J. Comparison of endocrine tests with respect to their predictive value on the outcome of ovarian hyperstimulation in IVF treatment: results of a prospective randomized study. Hum Reprod. 2003;18:1422–1427. doi: 10.1093/humrep/deg205. [DOI] [PubMed] [Google Scholar]
- 29.Morris RS, Paulson RJ, Sauer MV, Lobo RA. Predictive value of serum oestradiol concentrations and oocyte number in severe ovarian hyperstimulation syndrome. Hum Reprod. 1995;10:811–814. doi: 10.1093/oxfordjournals.humrep.a136044. [DOI] [PubMed] [Google Scholar]
- 30.Enskog A, Nilsson L, Brännström M. Peripheral blood concentrations of inhibin B are elevated during gonadotropin stimulation in patients who later develop ovarian OHSS and inhibin A concentrations are elevated after OHSS onset. Hum Reprod. 2000;15:532–538. doi: 10.1093/humrep/15.3.532. [DOI] [PubMed] [Google Scholar]
- 31.Moos J, Rezabek K, Filova V, Moosova M, Pavelkova J, Peknicova J. Comparison of follicular fluid and serum levels of Inhibin A and Inhibin B with calculated indices used as predictive markers of Ovarian Hyperstimulation Syndrome in IVF patients. Reprod Biol Endocrinol. 2009;7:86. doi: 10.1186/1477-7827-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]