Introduction
In 2010, an estimated 739,930 women were newly diagnosed with cancer. In approximately 9.4% of cases, patients were under 45 years of age [1, 2]. Although the probability of developing invasive cancer under 39 years of age is relatively low for women, the number of survivors at reproductive age are reasonably high, owing to notable improvements since 1975 in the relative 5-year survival rates as a result of earlier diagnosis and improved treatments [2]. Moreover, the 5-year relative survival rate among children under 19 years of age has improved from 61.7% for patients diagnosed between 1975 and 1977 to 82.6% for patients diagnosed between 2001 and 2007 [1]. The improved survival rate has resulted in more focus on patient quality of life, including the ability to preserve fertility as well as survival. Consequently, the demand for fertility preservation in cancer patients has increased in recent years.
Although the American Society of Clinical Oncology (ASCO) and American Society of Reproductive Medicine (ASRM) consider sperm and embryo cryopreservation as established procedures for fertility preservation [3, 4], ASRM recently authorized the support of “oocyte cryopreservation as a fertility preservation strategy for women with cancer and other illnesses requiring treatment that pose a serious threat to their future fertility because they may have no other viable option” [5]. Along with the advancement of oocyte cryopreservation technology, ovarian tissue cryopreservation is becoming recognized as a valid strategy, with more than 13 live births reported to date using this technique [6]. However, a number of researchers have expressed concerns about the possible risk of reintroducing cancer cells [7–9]. Additionally, this procedure requires oophorectomy to obtain ovarian tissue, and has a major drawback in that amputation of 50% of the ovarian reserve results in risk of accelerating the process of premature ovarian failure after surgery or treatment. Therefore, the procedure should only be performed on female patients undergoing treatment with a distinct risk of infertility [10]. Oocyte cryopreservation is an attractive strategy to preserve fertility in women, since no surgery is required and there is no risk of cancer cell contamination. In particular, in hematopoietic cancer patients who are single and wish to use their future spouse’s sperm, oocyte cryopreservation is the only way to preserve fertility. Here, we have reported a successful pregnancy and delivery with vitrified-warmed oocytes stored over 9 years, following intracytoplasmic sperm injection (ICSI) and embryo transfer to the patient’s own uterus without a gestational carrier after bone marrow transplantation (BMT) for chronic myeloid leukemia (CML) conditioning with high-dose cyclophosphamide and fractional total body irradiation (TBI) of 1,200 cGy.
Case report
In September 2001, a 22 year-old single woman with CML requested oocyte cryopreservation before BMT, which was approved by the Institutional Review Board of CHA Medical Center. The patient continued taking hydroxyurea for 4 months from June 2001 onwards. Starting on the third day of the cycle, controlled ovarian hyperstimulation was carried out with 6,900 IU of urinary gonadotropin (Metrodin (4,875 IU), Serono, Switzerland; Humegon (2,025 IU), Organon, Holland), and the level of luteinizing hormone (LH) monitored without using gonadotropin-releasing hormone to prevent premature LH surge. When the two follicles were over 18 mm in diameter, ovulation was triggered with 10,000 IU of human chorionic gonadotropin (hCG) (Profasi, Serono, Switzerland). Transvaginal oocyte retrieval was carried out 34 h after hCG administration. Seven oocytes were retrieved and vitrified using the previously described electron microscope grid method [11]. Cumulus-oocyte complexes were pre-equilibrated for 2.5 min in 2 mL of Dulbecco’s phosphate-buffered saline (Gibco BRL, Grand Island, NY) supplemented with 1.5 M of ethylene glycol (E-9129; Sigma, St. Louis, MO) and 10% (vol.vol) fetal bovine serum (Gibco BRL) at 37°C. Oocytes were then placed for the final equilibration in the same volume of Dulbecco’s phosphate-buffered saline supplemented with 5.5 M of ethylene glycol, 1.0 M of sucrose, and 10% fetal bovine serum for 20 s. In the meantime, two to three oocytes were mounted on an electron microscope grid (Gilder, Westchester, PA) using a fine pipette and excess cryoprotectant solution was removed with the underlying sterilized filter paper. The grids containing oocytes were immediately plunged into liquid nitrogen, and a cryovial cap and goblet were used for placement of the grid. Oocytes were then stored for 109 months before warming. The patient received HLA-matched, non-reactive sibling BMT in January 2002 after conditioning therapy consisting of cyclophosphamide (60 mg/kg) and TBI at a dose of 1,200 cGy in three fractions. Graft-versus-host disease prophylaxis was performed with cyclosporine A. Engraftment was swift, and the post-transplant clinical course was uneventful. She became completely amenorrheic after BMT and a serial hormonal test revealed hypergonadotrophic hypogonadism. Prior to BMT, follicle stimulating hormone (FSH), LH and estradiol (E2) level of 3.3 IU/L, 3.0 IU/L and 42.8 pg/ml were estimated. One year after BMT, secondary premature ovarian failure was diagnosed (FSH 79.64 IU/L, LH 58.78 IU/L and E2 5.03 pg/ml). The patient was on hormone replacement therapy (HRT) after BMT as of March 2003. After 8 years of transplantation, she got married. In July 2010, the patient was considered free of disease at the age of 31, and requested warming of oocytes. The serum FSH and anti-Mullerian hormone (AMH) levels of the patient were 92.23 mIU/mL and 0.14 ng/mL, respectively. For warming, the vitrified grids were sequentially transferred to culture dishes containing 2 mL of Dulbecco’s phosphate buffered saline supplemented with 1.0, 0.5, 0.24, 0.125, or 0 m of sucrose and 10% (vol.vol) fetal bovine serum at intervals of 2.5 min at 37°C. After warming, five of seven oocytes survived. Two were arrested at the meiosis I stage without release of the first polar body. Intracytoplasmic sperm injection was performed on the three matured oocytes. All were fertilized and developed into eight cell embryos, which were ranked as grade 2 (embryos are graded from 1 through to 5, 1 being the highest quality) (Fig. 1). Two embryos were transferred into the uterine cavity under transabdominal ultrasound guide at Day 3. Endometrial thickness on the day of transfer was 10 mm. The remaining embryo was cultured for two more days, but did not develop into blastocysts, thus discarded. Eleven days after embryo transfer, the serum β-hCG level was recorded as 198.97 mIU/mL. A viable intrauterine pregnancy was confirmed by transvaginal ultrasonography at a gestational age of 7 weeks. During antenatal care, integrated test evaluation and ultrasonography did not reveal any anomalies. At a gestational age of 35 weeks and 3 days, severe preeclampsia developed, and cesarean section was performed. The pregnancy resulted in the live birth of a healthy boy, weighing 2,410 g, with an Apgar score of 7 at 1 min and 9 at 5 min. He was discharged after 11 days, and has not been reported with any diseases or abnormalities as yet.
Fig. 1.
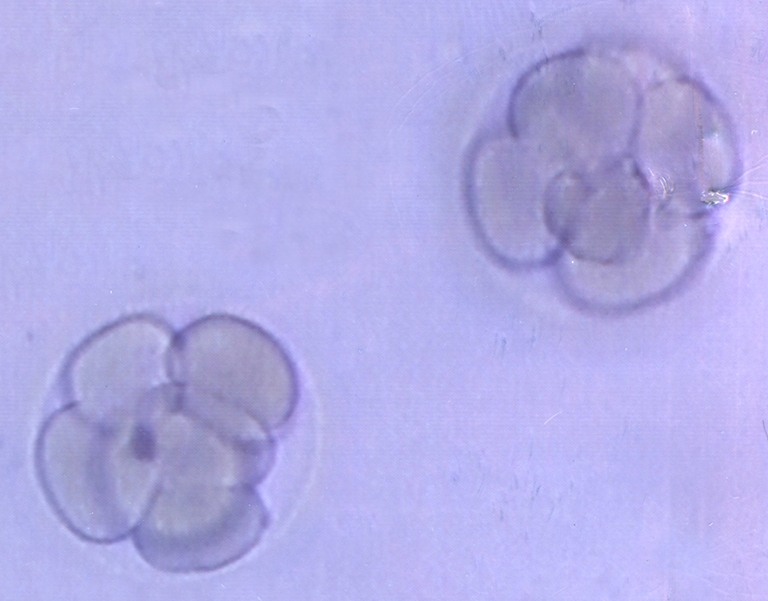
Image of embryos transferred of a CML patient
Discussion
Counseling for fertility preservation is important for patients within the reproductive age group that are faced with cancer treatment. At present, embryo cryopreservation is considered the sole standard fertility preservation method in females by the American Society of Clinical Oncology (ASCO) and American Society of Reproductive Medicine (ASRM) [3, 4]. Oocyte cryopreservation and ovarian tissue cryopreservation are other possible options for fertility preservation. Although the results of autotransplantation of frozen-thawed ovarian tissue harvested before chemo-radiotherapy have improved enormously and become a feasible method, several concerns on the safety of ovarian tissue transplantation in cancer patients have been documented [7–9]. There is a potential risk that frozen-thawed ovarian tissue might harbors malignant cells inducing recurrence of the disease after reimplantation. In cases of leukemia, malignant cells have been identified in the cryopreserved ovarian cortex [8, 9]. Additionally, Rosendahl et al. [9] suggested that transplantation of frozen-thawed ovarian cortex into women treated for leukemia is associated with a risk of reintroducing the disease. Therefore, oocyte cryopreservation is the first option for patients with chronic myeloid leukemia who have no current partners and wish to use a future partner’s sperm. Low effectiveness of oocyte cryopreservation is a major limitation, but studies have reported improved pregnancy outcomes [12–16]. Noyes et al. [15, 16] showed that delivery rate using vitrified oocytes is comparable to that of conventional in vitro fertilization using fresh oocytes, with an ongoing/delivered pregnancy rate of 57%.
As reported previously, we commenced oocyte cryopreservation with vitrification in 1997, and demonstrated that oocyte vitrification could be effectively applied to assisted reproductive technology [11, 17]. In August 1999, we succeeded in delivering the first healthy male baby, weighing 2,900 g, in our clinic [17], and thereafter started recommending oocyte vitrification as a tool for fertility preservation of cancer patients. In total, 41 cancer patients visited our clinic for fertility preservation from 1999 onwards. Among the three patients requesting warming of oocytes, this case is the first report of a successful delivery. Generally, most patients subjected to TBI use gestational carriers for pregnancy [18], due to problems, such as miscarriage, midtrimester pregnancy loss, preterm birth, and low birthweight [19]. However, bearing in mind the few successful pregnancies reported after TBI and BMT [20–22], we decided not to use a gestational carrier. In our case, preterm birth was due to severe preeclampsia and not preterm labor. We suggest that uterine function after irradiation can be preserved for pregnancy, if there is adequate hormonal replacement therapy, and we recommend that fertility specialists consider using the patient’s own uterus without routine employment of a gestational carrier. Although several studies have focused on oocyte cryopreservation for cancer patients [13, 23–25], the outcomes of transfer using cryopreserved oocytes have rarely been documented [18, 26] owing to the limited number of cancer patients that return for pregnancy. Our patient agreed to participate in an oocyte cryopreservation protocol for cancer patients using the vitrification method in 2001. To our knowledge, the present case is the first successful delivery by a cancer patient with vitrified-warmed oocytes. The period of vitrification of oocytes to warming took about 9 years, because the patient got married after confirmation of complete remission from leukemia. As far as we aware, this case represents the longest cryostorage period of vitrified-warmed oocytes leading to successful delivery of a healthy offspring by a patient that recovered from chronic myeloid leukemia. Thus, oocytes of cancer patients stored over the long-term can successfully develop to the in vitro cleavage stage and result in a live birth. This example of a successful pregnancy via oocyte banking should be a positive sign of hope for patients subjected to cancer treatments with potential risk of infertility. We additionally anticipate that oncologists and fertility specialists will cooperate more actively with fertility preservation, and suggest that individualized approaches of single or combined fertility preservation techniques should be applied for all cancer patients wishing to preserve fertility.
Acknowledgement
This study was supported by a grant (A084923) of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea.
Conflict of interest M.K Kim has nothing to disclose. D.R Lee has nothing to disclose. J.E Han has nothing to disclose. Y.S Kim has nothing to disclose. W.S Lee has nothing to disclose. J.W Kim has nothing to disclose. H.J Won has nothing to disclose. T.K Yoon has nothing to disclose.
Footnotes
Capsule Successful pregnancy and delivery were achieved in a chronic myeloid leukemia patient using vitrified-warmed oocytes stored for 9 years after allogenic bone marrow transplantation.
References
- 1.Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, et al. SEER cancer statistics review, 1975–2008. Bethesda: National Cancer Institute; 2010. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 4.The Practice Committee of the American Society for Reproductive Medicine; Practice Committee of the Society for Assisted Reproductive Technology Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90(5 Suppl):S241–6. doi: 10.1016/j.fertnstert.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 5.The Practice Committee of the American Society for Reproductive Medicine ASRM Practice Committee response to Rybak and Lieman: elective self-donation of oocytes. Fertil Steril. 2009;92:1513–4. doi: 10.1016/j.fertnstert.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–50. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 7.Bittinger SE, Nazaretian SP, Gook DA, Parmar C, Harrup RA, Stern CJ. Detection of Hodgkin lymphoma within ovarian tissue. Fertil Steril. 2011;95(2):803. doi: 10.1016/j.fertnstert.2010.07.1068. [DOI] [PubMed] [Google Scholar]
- 8.Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra’anani H, et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23(5):1007–13. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 9.Rosendahl M, Andersen MT, Ralfkiaer E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94(6):2186–90. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Rosendahl M, Andersen CY, Ernst E, Westergaard LG, Rasmussen PE, Loft A, et al. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow-up study. Hum Reprod. 2008;23(11):2475–83. doi: 10.1093/humrep/den248. [DOI] [PubMed] [Google Scholar]
- 11.Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2003;79(6):1323–6. doi: 10.1016/S0015-0282(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 12.Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remohi J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. 2008;89(6):1657–64. doi: 10.1016/j.fertnstert.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Grifo JA, Noyes N. Delivery rate using cryopreserved oocytes is comparable to conventional in vitro fertilization using fresh oocytes: potential fertility preservation for female cancer patients. Fertil Steril. 2010;93(2):391–6. doi: 10.1016/j.fertnstert.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 14.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril. 2009;92(2):520–6. doi: 10.1016/j.fertnstert.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Noyes N, Knopman J, Labella P, McCaffrey C, Clark-Williams M, Grifo J. Oocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytes. Fertil Steril. 2010;94(6):2078–82. doi: 10.1016/j.fertnstert.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet. 2010;27(8):495–9. doi: 10.1007/s10815-010-9434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon TK, Chung HM, Lim JM, Han SY, Ko JJ, Cha KY. Pregnancy and delivery of healthy infants developed from vitrified oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2000;74(1):180–1. doi: 10.1016/S0015-0282(00)00572-0. [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Brown SE, Nguyen K, Reddy V, Brubaker C, Winslow KL. Live birth after the transfer of human embryos developed from cryopreserved oocytes harvested before cancer treatment. Fertil Steril. 2007;87(6):1469. doi: 10.1016/j.fertnstert.2006.07.1546. [DOI] [PubMed] [Google Scholar]
- 19.Critchley HO, Wallace WH. Impact of cancer treatment on uterine function. J Natl Cancer Inst Monogr. 2005;34:64–8. doi: 10.1093/jncimonographs/lgi022. [DOI] [PubMed] [Google Scholar]
- 20.Chao HT, Wang PH, Yuan CC, Lee WL. Successful pregnancy in a woman with acute myeloid leukemia treated with high-dose whole-body irradiation. J Reprod Med. 1998;43(8):703–6. [PubMed] [Google Scholar]
- 21.Matias K, Matias C, Teixeira H, Freire AD, Azevedo A. Successful pregnancy following busulfan and cyclophosphamide conditioning and allogeneic bone marrow transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant. 2008;14(8):944–5. doi: 10.1016/j.bbmt.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang WS, Tzeng CH, Hsieh RK, Chiou TJ, Liu JH, Yen CC, et al. Successful pregnancy following very high-dose total body irradiation (1575 cGy) and bone marrow transplantation in a woman with acute myeloid leukemia. Bone Marrow Transplant. 1998;21(4):415–7. doi: 10.1038/sj.bmt.1701106. [DOI] [PubMed] [Google Scholar]
- 23.Klock SC, Zhang JX, Kazer RR. Fertility preservation for female cancer patients: early clinical experience. Fertil Steril. 2010;94(1):149–55. doi: 10.1016/j.fertnstert.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Porcu E, Fabbri R, Damiano G, Fratto R, Giunchi S, Venturoli S. Oocyte cryopreservation in oncological patients. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S14–6. doi: 10.1016/j.ejogrb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Porcu E, Fabbri R, Damiano G, Giunchi S, Fratto R, Ciotti PM, et al. Clinical experience and applications of oocyte cryopreservation. Mol Cell Endocrinol. 2000;169(1–2):33–7. doi: 10.1016/S0303-7207(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 26.Porcu E, Venturoli S, Damiano G, Ciotti PM, Notarangelo L, Paradisi R, et al. Healthy twins delivered after oocyte cryopreservation and bilateral ovariectomy for ovarian cancer. Reprod Biomed Online. 2008;17(2):265–7. doi: 10.1016/S1472-6483(10)60204-0. [DOI] [PubMed] [Google Scholar]


