Abstract
Brain inflammation is a double-edged sword: it is required for brain repair in acute damage, whereas chronic inflammation and autoimmune disorders are neuropathogenic. Certain pro-inflammatory cytokines and chemokines are closely related to cognitive dysfunction and neurodegeneration. Representative anti-inflammatory cytokines, such as interleukin (IL)-10, can suppress neuroinflammation and have significant therapeutic potentials in ameliorating neurodegenerative disorders, such as Alzheimer’s disease (AD). Here, we show that adeno-associated virus (AAV) serotype 2/1 hybrid-mediated neuronal expression of the mouse IL-10 gene ameliorates cognitive dysfunction in APP+PS1 bigenic mice. AAV2/1 infection of hippocampal neurons resulted in sustained expression of IL-10 without its leakage into the blood, reduced astro/microgliosis, enhanced plasma amyloid-β peptide (Aβ) levels, and enhanced neurogenesis. Moreover, increased levels of IL-10 improved spatial learning as determined by the radial arm water maze. Finally, IL-10-stimulated microglia enhanced proliferation but not differentiation of primary neural stem cells in the co-culture system, while IL-10 itself had no effect. Our data suggest that IL-10 gene delivery has a therapeutic potential for a non-Aβ-targeted treatment of AD.
INTRODUCTION
Accumulating evidence supports the idea that activated glial cells play a significant role in the pathogenesis of multiple psychiatric and neurological disorders, such as autism, depression, multiple sclerosis, and Alzheimer’s disease (AD).1–4 Amyloid-β peptide (Aβ) processed from β-amyloid precursor protein (APP) is a component of senile plaque, which triggers the accumulation of astro/microglia that produce pro-inflammatory factors, such as cytokines and chemokines, leading to neuroinflammation.5 Glial cells generally maintain brain homeostasis and plasticity, as well as provide neuroprotection for functional recovery from traumatic injuries.6 However, chronic inflammation or autoimmune-related dysfunction of glial cells may promote loss of synapses and neurogenesis, cognitive/motor dysfunction, and eventually neurodegeneration.7, 8 Therefore, prevention of detrimental neuroinflammation is an alternative therapeutic target for treating neurologic disorders.
Recent advancements in immunotherapeutic studies of AD include, but are not limited to, extensive animal and human studies on Aβ vaccination therapy, passive Aβ immunotherapy, non-Aβ-related therapies with non-steroidal anti-inflammatory drugs, and other types of anti-inflammatory compounds.9, 10 Due to the failure of Aβ vaccination therapy in clinical trials,11 alternative non-Aβ immunotherapies have been investigated. Among them, glatiramer acetate (GA) immunization with specific adjuvants can ameliorate AD pathogenesis in the APP mouse brain.12 GA immunotherapy leads to improved cognitive function, enhanced neurogenesis, and reduced beta-amyloidosis in APP and APP+presenilin-1 (PS1) mice.12–14 In an experimental allergic encephalomyelitis (EAE) model, GA-treated animals develop GA-specific T suppressor cells, which are characterized as T helper 2/3 (Th2/3) type cells secreting anti-inflammatory cytokines such as interleukin (IL)-4 and IL-10.15
IL-10 is a pleiotropic cytokine and inhibits the synthesis and release of pro-inflammatory cytokines such as tumor necrosis factor-α, IL-1β, 6, 8, and 12.16, 17 IL-10 also attenuates the lipopolysaccharide (LPS)-induced expression of pro-inflammatory cytokines, suppresses caspase-3-mediated neuronal apoptosis,18,19 and reduces LPS-induced neurotoxicity through the inhibition of NADPH oxidase activity.20 Moreover, virus-mediated expression of IL-10 in laterally hemisectioned spinal cords promotes neuronal survival and improves motor function, both of which are associated with activation of glycogen synthase kinase 3-β, Akt, and STAT3.21 In IL-10-deficient mice, peripheral infection of LPS causes a prominent cognitive deficit as compared to wild-type mice.22 These findings support the concept that IL-10 has therapeutic potential to ameliorate neuroinflammation, cognitive dysfunction, and neurodegeneration. In fact, blockage of transforming growth factor (TGF)-β signaling or a nasal vaccination with Protollin resulted in elevated IL-10 transcript concomitant with reduced beta-amyloidosis in APP mice.14, 23 However, IL-10 has never been directly tested for its effect on AD animal models. We therefore proposed that sustained expression of IL-10 might attenuate AD pathogenesis.
Adeno-associated virus (AAV)-mediated gene delivery has several advantages over other virus-based gene therapies. First, there is a minimal immune response in the host as compared to the responses observed with adenovirus or herpes simplex virus infections.24, 25 Second, it is not restricted to proliferating cells and does not show spontaneous tumorigenesis as found with retroviral vectors. Third, it has long-term transgene expression that persists for over one year after vector administration in the adult retina and brain.26, 27, 28 Finally, most of the human population has already been infected with wild-type, asymptomatic AAV. An AAV1-derived gene delivery system is superior to AAV2 for brain gene delivery due to its global gene expression as compared to AAV2’s gene expression.29 For this study, we have employed an AAV serotype 2/1 hybrid recombinant gene delivery system consisting of AAV2 inverted terminal repeats and AAV1 Rep and Cap genes (AAV2/1) to induce the neuronal expression of murine IL-10 in mouse hippocampi.30 We analyzed the effect of IL-10 expression in double transgenic mice expressing familial AD mutants of APP and PS1 (APP+PS1 Tg),31, 32 which exhibit accelerated Aβ deposition and memory impairment as compared to APP Tg mice.33
RESULTS
AAV2/1-mediated expression of mouse IL-10 in the brain
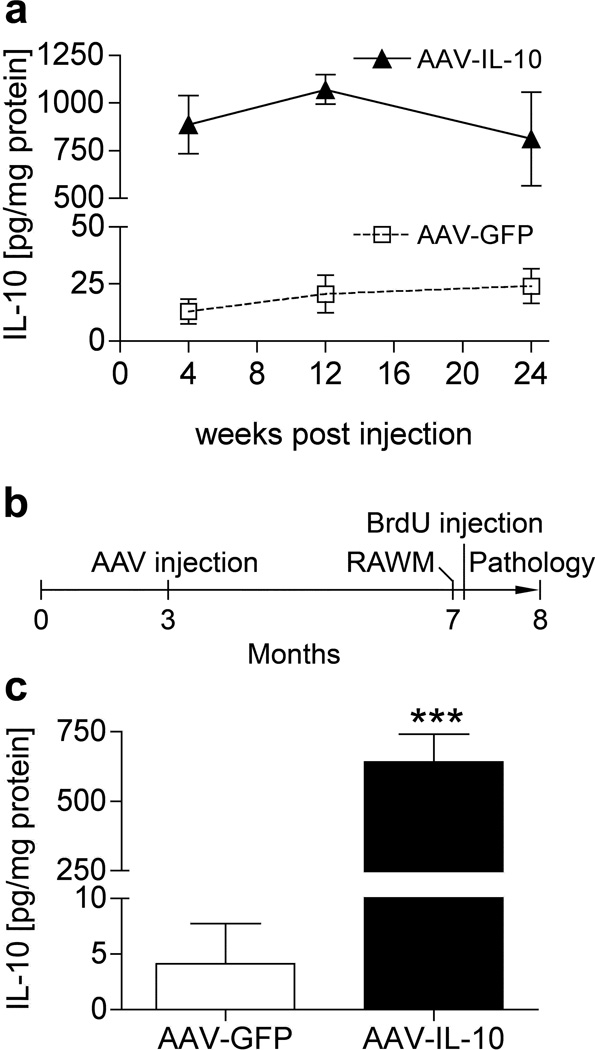
We first tested the time course of recombinant IL-10 protein expression after stereotaxic hippocampal injection of AAV2/1 virus expressing IL-10 (AAV-IL-10) or green fluorescent protein (AAV-GFP). We have previously observed long-term neuronal expression of recombinant proteins in the mouse brain using this system.30 We determined the appropriate number of viral particles (VP) by dose-response of the AAVs in non-Tg mice using AAV-IL-10 or negative control AAV-GFP, and determined the titer as 3 × 109 VP for the hippocampal injection study. The AAV injections themselves did not induce gliosis in the mouse brains.30 Injection of 3 × 109 VP of AAV-IL-10 produced IL-10 expression at 886.9 ± 152.6, 1072 ± 77.6, and 811.7 ± 246.4 pg/mg at 4, 12, and 24 weeks, respectively, which is significantly higher than AAV-GFP-injected mice (13.0 ± 5.5 to 24.2 ± 7.6 pg/mg of IL-10 over the same time period, Figure 1a), thus demonstrating long-term expression of the recombinant protein for up to 6 months. In addition, sham-injected non-Tg mice show similar IL-10 levels as AAV-GFP-injected non-Tg mice (data not shown). To understand the effect of regulatory cytokine IL-10 on gliosis and beta-amyloidosis, we injected AAV-IL-10 or control AAV-GFP virus (1.5 × 109 VP/µl, 1µl per hippocampus) into bilateral hippocampal regions of APP+PS1 mice at 3 months of age with neuropathological analyses at 8 months of age (Figure 1b). At that point, we confirmed the expression level of IL-10 in the hippocampus of APP+PS1 mice injected with AAV viruses (Figure 1c). AAV-IL-10 injection produced significantly higher amounts of IL-10 than AAV-GFP (641.5 ± 100.3 and 4.1 ± 3.6 pg/mg IL-10, respectively, Figure 1c). We also confirmed no increase in circulating IL-10 levels in plasma between AAV-IL-10 and AAV-GFP groups 5 months after intracranial injection (14.2 ± 9.4 and 12.6 ± 10.8 pg/ml plasma, respectively), suggesting that the tightly sealed blood brain barrier prevents leakage of recombinant IL-10 from the brain parenchyma.
Figure 1. AAV-mediated somatic gene transfer of IL-10 and GFP.
(a) Non-tg mice at 3 months of age were stereotactically injected with AAV-IL-10 or AAV-GFP into the hippocampi at total 3×109 viral particles (VP) in 2µl (1µl per hippocampus), and sacrificed 4, 12, and 24 weeks post-injection. The amount of IL-10 in hippocampal protein extract was quantified by IL-10 ELISA. Sustained expression of IL-10 was observed after hippocampal injection of AAV-IL-10, but not AAV-GFP. (b) Experimental design for AAV-IL-10 treatment in APP+PS1 mice. Bilateral hippocampal injection of AAV-GFP or AAV-IL-10 was performed at 3 months of age. Mice were subjected to a 2-day RAWM task at 7 months of age and intraperitoneally injected with BrdU five times for 2.5 days 21 days before they were sacrificed at 8 months of age. (c) IL-10 expression in the hippocampus at 8 months of age. APP+PS1 mice were stereotactically injected with AAV-GFP or IL-10 at 3 months of age. Bars represent mean ± SEM (n = 3 per group). *** denotes P <0.001 as determined by Student’s t-test.
IL-10 suppresses gliosis in APP+PS1 Tg mice
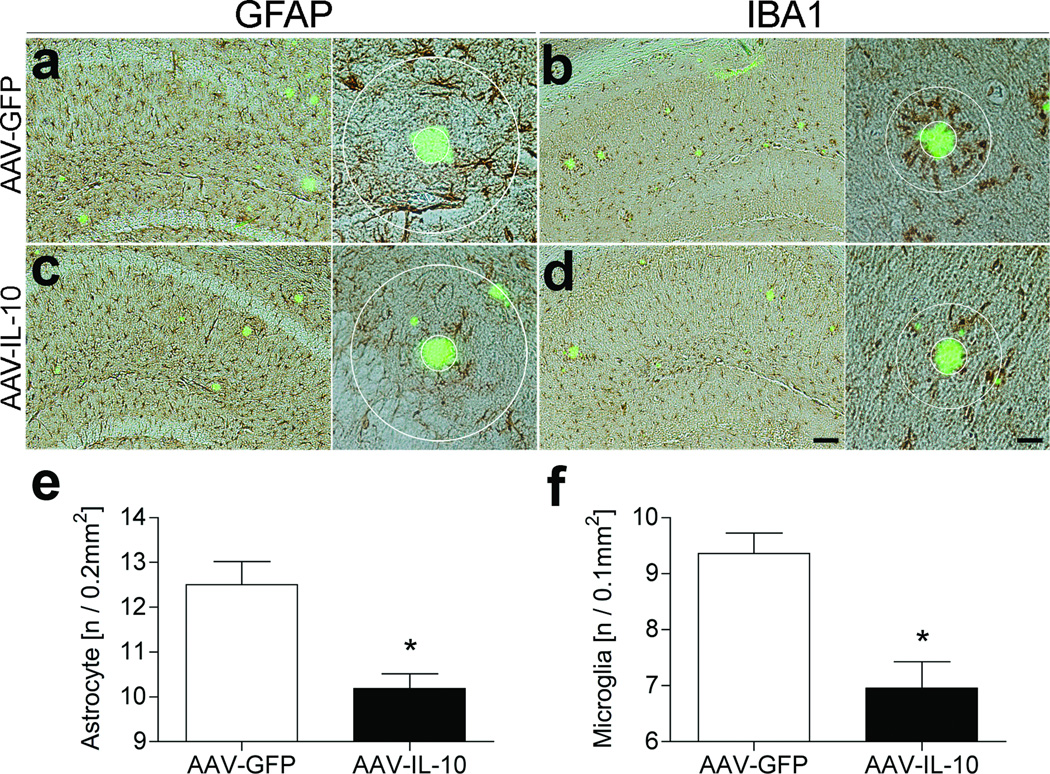
Aβ aggregation and deposition causes reactive gliosis and subsequent neuroinflammation in transgenic AD mouse models.34–36 Thus, we examined the effect of IL-10 on astro/microgliosis in the AAV-injected APP+PS1 mice at the endpoint of the study. AAV-IL-10 injection significantly reduced astrogliosis (18.6 % reduction vs. AAV-GFP-injected group, Figure 2a, c, e) and microglial accumulation around thioflavin-S (TS)+ compact plaques (25.7 % reduction vs. AAV-GFP-injected group, Figure 2b, d, f). These results suggest that IL-10 can significantly suppress glial accumulation induced by TS+ plaques in APP+PS1 mice.
Figure 2. Gene delivery of IL-10 suppresses glial inflammation in APP+PS1 mice.
(a–d) APP+PS1 mice injected with AAV-GFP (a, b) or AAV-IL-10 (c, d) at 3 months of age were sacrificed at 8 months of age. The hippocampal frozen sections were immunostained for GFAP (astrocyte; a, c) or IBA1 (microglia; b, d), and counterstained by TS. Scale bars represent 200µm in low magnification (left, ×40) and 40µm in high magnification (right, ×400). (e, f) Quantification of GFAP (e) or IBA1 (f) positive cells found within the circle surrounding TS-positive Aβ plaques. Radii of outer concentric circles in GFAP-positive cells were 100µm greater than the inner circles that surrounded the compact plaques (a, c), and 50µm greater in IBA1-positive cells (b, d). Bars represent mean ± SEM (n = 5 per group, 10 sections per brain). * denotes P <0.05 as determined by Student’s t-test.
IL-10 has no effect on Aβ deposition but enhances plasma Aβ in APP+PS1 Tg mice
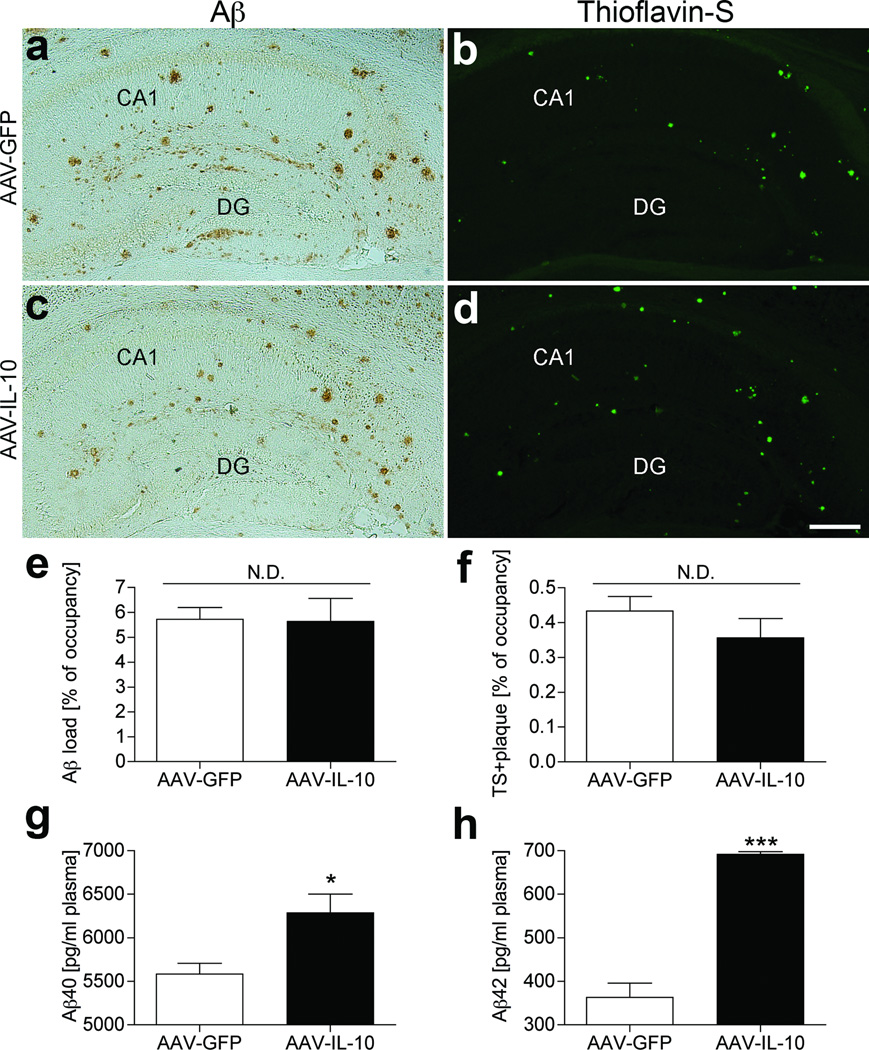
We examined if IL-10-mediated suppression of astro/microgliosis is associated with beta-amyloidosis (Figure 3a–d). Unexpectedly, both hippocampal total Aβ load and TS+ compact plaques were unchanged between AAV-GFP and AAV-IL-10 groups (5.7 ± 0.5 % and 5.6 ± 0.9% of occupancy for Aβ load, 0.4 ± 0.0% and 0.4 ± 0.1% of occupancy for TS+ plaques, respectively, Figure 3e, f), suggesting no direct effect of IL-10 on beta-amyloidosis in the brain. Although we examined the effect of AAV-IL-10 on the generation of Aβ oligomer species by SDS-PAGE and immunoblotting, there was no significant difference between groups (Supplementary Figure S1). In addition, IL-10 treatment of primary cultured neurons expressing recombinant APP Swedish mutant by infection of recombinant adenovirus has no effect on Aβ production, suggesting that IL-10 has no effect on Aβ synthesis (data not shown).
Figure 3. Aβ deposition in the hippocampal region of gene-delivered APP+PS1 mouse brain.
(a–d) Frozen sections of AAV-GFP (a, b) or AAV-IL-10 (c, d) injected APP+PS1 mice were immunostained with anti-Aβ antibody (a, c) and counterstained by TS for compact plaque (b, d). Scale bar, 200µm. (e, f) Total Aβ load (e) and TS-positive area (f) in hippocampal region were quantified in AAV-GFP or AAV-IL-10 injected APP+PS1 mice. Bars represent mean ± SEM (n = 5 per group, 10 sections per brain). N.D. denotes no significant difference. (g, h) The level of Aβ40 (g) or Aβ42 (h) in plasma was measured by ELISA. Bars represent mean ± SEM (n = 5). * or *** denote P <0.05 or 0.001 as determined by Student’s t-test.
Next, we confirmed plasma Aβ levels in AAV-injected APP+PS1 mice using both Aβ40 and Aβ42 ELISA. Plasma Aβ40 and Aβ42 levels were significantly increased in AAV-IL-10-injected APP+PS1 mice as compared to AAV-GFP-injected APP+PS1 mice [6,288 ± 212.1 (IL-10) and 5,584 ± 123.3 (GFP) pg/ml plasma: 12.5 % increase of Aβ40 and 691.8 ± 6.3 (IL-10) and 363.5 ± 32.1 (GFP) pg/ml plasma: 47.5 % increase of Aβ42, Figure 3g, h]. These results suggest that over-expression of IL-10 promotes Aβ clearance from the brain to the vascular circulation, although its effect did not alter the overall Aβ deposition in the brain.
Enhanced neurogenesis in AAV-IL-10-injected APP+PS1 mice
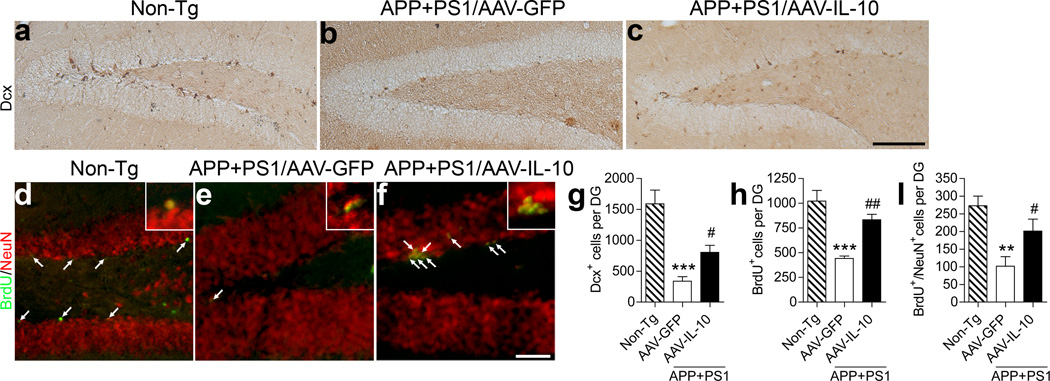
Previous reports showed that GA immunotherapy significantly enhanced neurogenesis in the subgranular zone (SGZ) of the dentate gyrus,13, 37 which is associated with increased IL-10 expression in the brain by infiltration of GA-specific T cells as well as T cell-associated microglia and astrocytes.38 Therefore, we examined the effect of AAV-IL-10 on neurogenesis and neuronal differentiation. We examined expression of doublecortin (Dcx), a marker for newly generated premature neurons,39 in the SGZ (Figure 4a–c, g). We observed significantly reduced numbers of Dcx+ cells in the SGZ of APP+PS1 mice injected with AAV-GFP [338.2 ± 74.4, 21.1% of non-Tg mice (1591 ± 222.7), Figure 4a, b, g], which is consistent with a previous report.40 However, the AAV-IL-10-injected APP+PS1 mice showed significantly increased Dcx+ cells in the SGZ (805.7 ± 112.3, 50.6 % of non-Tg mice, Figure 4b, c, g), suggesting enhanced neuronal differentiation or overall neuronal proliferation in response to IL-10 expression. To determine the effect of IL-10 on neuronal proliferation and differentiation, the animals were temporally injected with BrdU to track cell proliferation three weeks prior to euthanasia, and the neuronal differentiation of BrdU-incorporated cells in the SGZ was evaluated by immunofluorescence of BrdU and NeuN, a differentiated neuronal marker (Figure 4d–i, h). The number of BrdU+ and BrdU+/NeuN+ cells was significantly increased in the AAV-IL-10-injected APP+PS1 mice [total BrdU+ cells: 833.3 ± 54.8 (AAV-IL-10) and 443.0 ± 22.3 (AAV-GFP), which were 81.5 and 43.3 % of non-Tg mice (1022 ± 107.6); BrdU+/NeuN + cells: 201.2 ± 34.1 (AAV-IL-10) and 101.6 ± 27.5 (AAV-GFP), which were 73.6 and 37.2 % of non-Tg mice (273.2 ± 27.3), Figure 4h,i]. However, the percent BrdU+/NeuN+ cells over total BrdU+ cells was unchanged between AAV-GFP and AAV-IL-10-injected APP+PS1 mice (data not shown), suggesting that IL-10 enhances neural stem cell proliferation but not differentiation.
Figure 4. Gene delivery of IL-10 enhances neurogenesis in SGZ of APP+PS1 mice.
(a–c) Representative images of Dcx staining in the dentate gyrus of non-Tg (a) or APP+PS1 mice injected with AAV-GFP (b) or AAV-IL-10 (c) into the hippocampus at 3 months of age and i.p. injected with BrdU 3 weeks prior to the euthanasia at 8 months of age. Scale bar, 200µm. (d–f) Immunofluorescence of BrdU (green) and NeuN (red) in the dentate gyrus of non-Tg (d) or APP+PS1 mice injected with AAV-GFP (e) or AAV-IL-10 (f). Scale bar, 100µm. (g, h, i) Quantification of Dcx+ (g), BrdU+ cells (h), or BrdU+NeuN+ cells (i) in SGZ. Bars represent mean ± SEM (n = 5 per group, 10 sections per brain). ** or *** denotes P <0.01 or 0.001 versus non-Tg, and # or ## denotes P < 0.05 or 0.01 versus AAV-GFP group, as determined by one-way ANOVA and Newman–Keuls post-test.
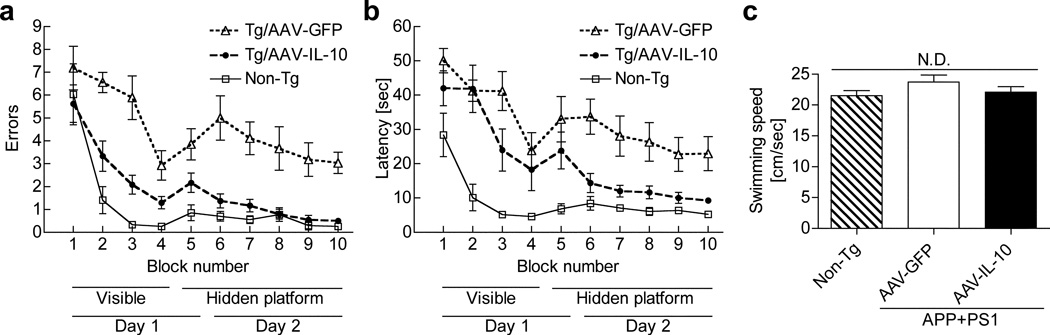
IL-10 improves spatial cognitive dysfunction in APP+PS1 mice
Enhanced neurogenesis and reduced neuroinflammation strongly suggests a potential effect of IL-10 on cognitive function of APP+PS1 mice. For that purpose, we injected AAV-IL-10 or control AAV-GFP virus into bilateral hippocampal regions of APP+PS1 mice at 3 months of age (Figure 1b), and subjected the mice to a radial arm water maze (RAWM) task according to the established protocol (Figure 5a, b).30 Un-injected age-matched non-Tg mice served as a positive control group for the spatial learning task. Day 1 consisted of four visible platform tests (T1–4), followed by one hidden platform test (T5) after a short-term (30 min) break. Day 2 consisted of five hidden platform tests (T6-T10) with a short-term break (30 min) between T9 and T10. Error counts at T7-T9 reflect established memory acquisition, and those at T6 and T10 reflect short-term memory recall after overnight (T6) or 30-min breaks (T10). The age-matched non-Tg mouse group modeled the wild type learning curve. In contrast, AAV-GFP-injected APP+PS1 groups showed significantly higher errors and latency than the non-Tg group throughout the trials, indicating impaired spatial memory acquisition and recall. On the other hand, the AAV-IL-10-injected groups showed significantly reduced error numbers and latency compared to the AAV-GFP-injected or un-injected group, indicating secured memory acquisition and recall in this experimental paradigm. The average swimming speeds in the open water were unchanged among the 3 tested groups, ruling out the possibility of differences in their swimming abilities distorting the data (Figure 5c).
Figure 5. Gene delivery of IL-10 improves memory function of APP+PS1 mice.
(a, b) APP+PS1 mice received bilateral hippocampal injections of AAV-GFP or AAV-IL-10 at 3 months of age and were tested by the 2-day RAWM task at 7 months of age. Non-tg serves as a positive control for the spatial learning task. The compiled average errors (a) and latency (b) are shown. Bars represent mean ± SEM (n = 9 per group). P-values for the variances between Tg/AAV-GFP vs. Tg/AAV-IL-10 are < 0.0001 for time, column factor, and < 0.001 for subject matching (for errors) and < 0.0001 for time, column factor, and subject matching (for latency) as determined by two-way ANOVA and Bonferroni tests. (c) Measurement of average swimming speed of animals. Average swimming speed through the training was equivalent on all mice groups. N.D. denotes no significant difference, as determined by one-way ANOVA and Newman–Keules post-test. Error bars represent SEM (n = 9 per group).
To understand if enhanced spatial learning is correlated with enhanced synaptic transmission, we also examined the expression levels of NR2B in both total and Tyr 1472 phosphorylated forms by Western blotting. There was no difference in the expression of either form between two groups (data not shown), suggesting that AAV-IL-10-induced improvement in cognitive function is independent from NR2B upregulation in this model.
IL-10 enhances neural stem cell proliferation via microglia in vitro
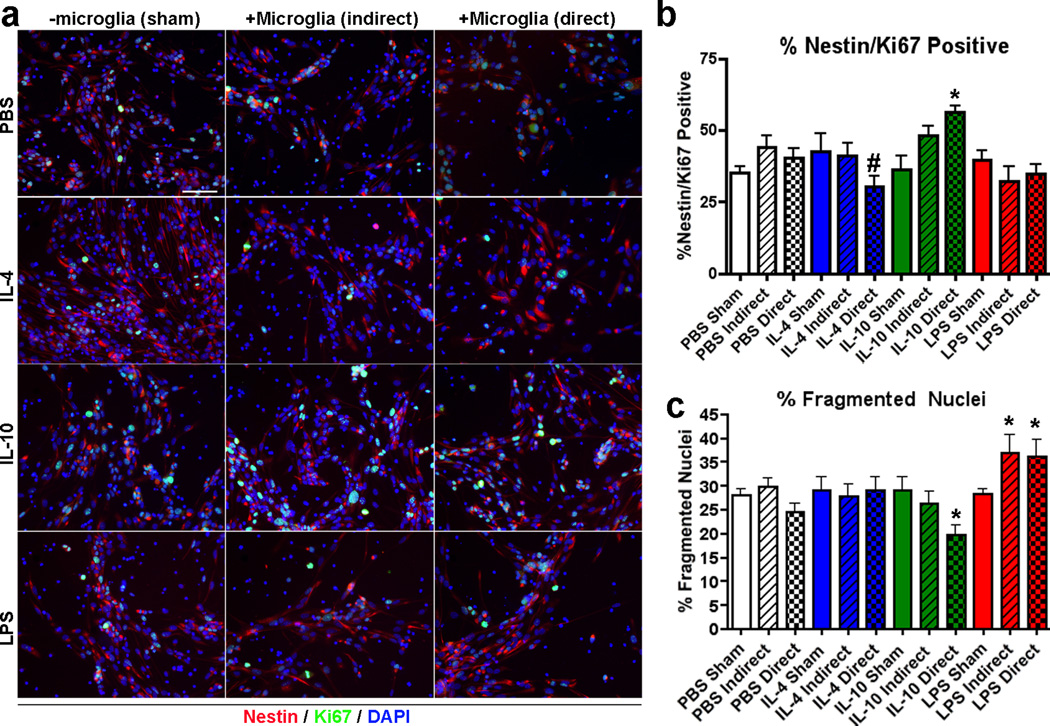
It has been previously reported that microglia activation alters the relative levels of neurogenesis.41 To understand the effect of IL-10 on neurogenesis, we have examined if IL-10 has direct or indirect effects on neuronal stem cell proliferation and differentiation in three different tissue culture conditions in the presence or absence of primary cultured mouse microglia using two different co-culture systems in vitro. The rationale of exploring the microglial/neuronal interactions is that both neuronal and microglial cell types express IL-10 receptor while its expression is negligible in astrocytes (Supplementary Figure S2). Thus, IL-10 may have both direct and indirect effects on neurogenesis by stimulating the two cell types. Mouse primary microglia were isolated from P0 pups, and mouse neuronal stem cells were isolated from E13.5 embryonic brains accordingly. To compare the differential effects of classically and alternatively activated microglial, microglia were pre-treated with IL-4, IL-10 (alternative activators, 10 ng/ml), or lipopolysaccharide (LPS, classical activator, 100 ng/ml) for 4 hr, followed by co-culture with dissociated mouse neuronal stem cells via either an indirect (transwell) or direct co-culture system in cell proliferation media for 3 days. Control stem cell cultures were also stimulated with cytokines only in the absense of microglia. Mitotic neuronal stem cells were identified by immunofluorescence staining of Nestin+/Ki67+/Dapi+ cells (Figure 6a), in which proliferation was significantly enhanced or reduced by direct co-culture with IL-10- or IL-4-treated microglia as compared to PBS-treated microglia (56.2 ± 2.3, 30.53 ± 3.6, and 40.3 ± 3.3% in IL-10, IL-4, and PBS-treated microglia direct co-culture groups, respectively, Figure 6b). On the other hand, there was also a significantly higher number of apoptotic cells as induced by either LPS-treated microglia (36.2 ± 3.4 and 36.9 ± 3.6%, respectivery) as compared to direct or indirect co-culture with PBS-treated microglia group (24.4 ± 1.8 and 29.7 ± 1.9%, respectively, Figure 6c). The number of apoptotic cells was significantly lowered by direct co-culture with IL-10-treated microglia (19.8 ± 1.9%, Figure 6c).
Figure 6. Classical/Alternative activation of microglia alter neuronal stem cell proliferation.
(a) Immunofluorescence at 20× magnification of dissociated mouse neuronal stem cells co-cultured with microglia pre-skewed with either IL-4, IL-10, or LPS using an indirect (transwell) or direct co-culture system (Nestin, a stem cell marker, in red, Ki67, a mitotic marker, in green, and Dapi, a nuclear marker, in blue). Sham group (-microglia) has treatment of ligand without microglia. (b, c) Quantification of % Nestin+/Ki67+/Dapi+ cells (b) or % fragmented nucleus (c) over total Dapi+ cells in the different treatment groups (n=10 fields of view per group). Bars represent mean ± SEM. * or # indicates p <0.05 vs. PBS-treated group of the same microglia co-culture condition or IL-10-treated direct co-culture group as determined by ANOVA and Tukey’s post-hoc.
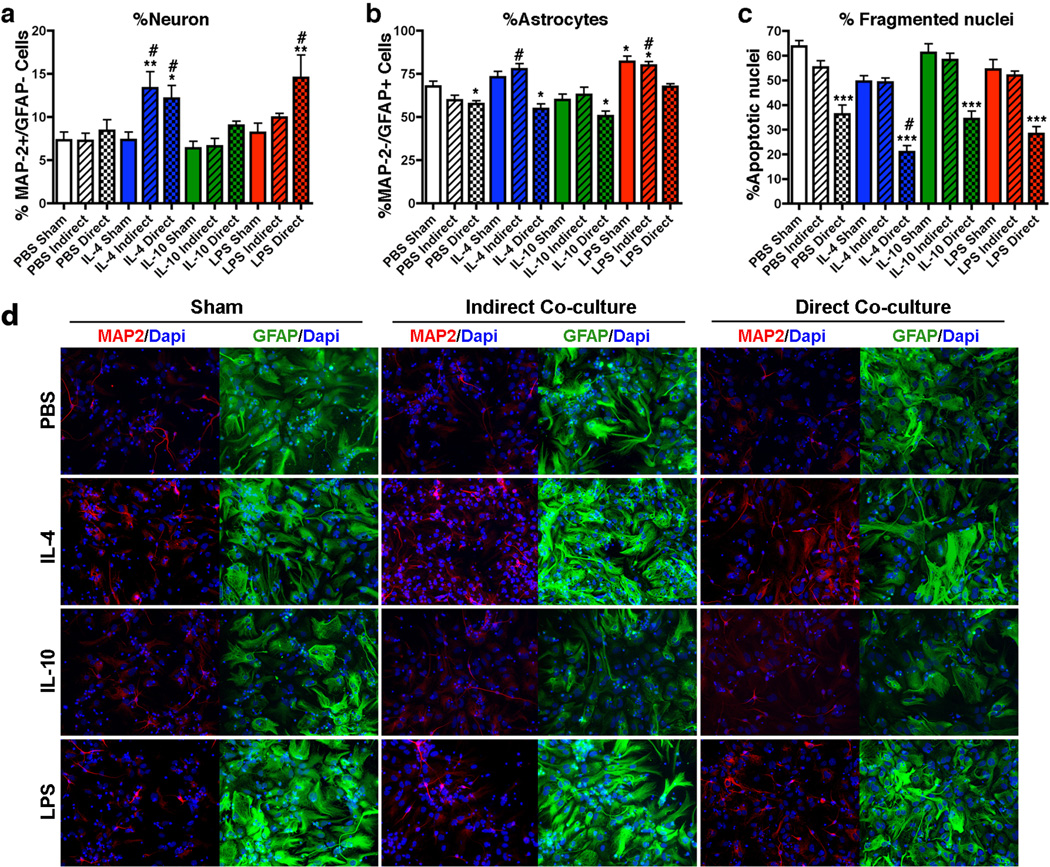
Using a similar experimental design, we performed differentiation of neuronal stem cells in differentiation media with co-cultured microglia pre-skewed with LPS, IL-4, or IL-10. Since the neuronal stem cells had not been differentiated into neuronal progenitor cells, cell differentiation was mainly astrogliogenic (Figure 7b). However, we found significantly greater number of MAP-2+/GFAP− cells (neuronal differentiation) when co-cultured with IL-4-treated microglia with or without direct contact (21.4 ± 2.8 and 23.8 ± 1.9%, respectively), and with LPS-treated microglia with direct contact (26.0 ± 2.2%) as compared to direct or indirect co-cultured groups with the PBS-treated microglia (18.2 ± 1.3 and 17.1 ± 1.9%, respectively (Figure 7a). The number of astroglia (GFAP+/MAP-2−) was also significantly reduced in all microglia direct co-culture groups except direct co-culture group with LPS-treated microglia (67.5 ± 1.7% vs. 67.8 ± 2.9% in PBS Sham group), and enhanced in LPS sham and indirect co-culture with LPS or IL-4-treated microglia (LPS sham 82.1 ± 3.1%, LPS indirect 79.9 ± 2.3%, IL-4 indirect 77.7 ± 3.2%, and PBS indirect 59.8 ± 2.8%, Figure 7b). Unlike LPS and IL-4 treatments, IL-10 sham and co-culture with IL-10-treated microglia (either direct or indirect manner) had no effect on differentiation. Importantly, direct co-culture with microglia specifically showed an anti-apoptotic effect in the differentiation condition, which is strengthened in IL-4-treated microglia (PBS sham 63.7 ± 2.4%, PBS direct co-culture 36.3 ± 3.7%, IL-4 direct co-culture 21.0 ± 2.5%, IL-10 direct co-culture 34.3 ± 3.2%, LPS direct co-culture 28.3 ± 3.0%, Figure 7c). This indicates that resident microglia are either anti-apoptotic or pro-phagocytic in the neuron differentiation conditions, which may be one of the mechanisms responsible for the pro-neurogenic output. These data collectively indicate that IL-4-treated microglia are more neurogenic and enhance overall stem cell differentiation, LPS-treated microglia and LPS itself are astrogliogenic, and IL-10-treated microglia enhances neural stem cell proliferation but have no effect on differentiation. These data demonstrate the beneficial effect of IL-10 gene delivery on APP+PS1 mice for suppression of plaque-associated gliosis, enhanced neurogenesis via enhanced stem cell proliferation, and improved memory formation.
Figure 7. Classical/Alternative activation microglia alter neuronal stem cell differentiation.
(a, b, c) Quantification of % Map-2+/GFAP− neurons (a), % Map-2−/GFAP+ astrocytes (b) or % Apoptotic nuclei (c) over total Dapi+ cells in different treatment groups (n=10 fields of view per group). (d) Immunofluorescence of dissociated mouse neuronal stem cells co-cultured with microglia pre-skewed with either IL-4, IL-10, or LPS using a transwell (indirect) or direct co-culture system in differentiating conditions (Map-2, a neuronal marker, in red, GFAP, an astrocyte marker, in green, and Dapi, a nuclear marker, in blue). Sham group (-microglia) has treatment of ligand without microglia. Bars represent mean ± SEM. * or # indicates p <0.05 vs. PBS Sham or PBS treated group of the same microglia co-culture condition group as determined by ANOVA and Tukey’s post-hoc. *** indicates p <0.05 vs. all directly co-cultured groups as determined by ANOVA and Tukey's post-hoc.
DISCUSSION
The beneficial effects of IL-10 have been attributed to IL-10-mediated anti-inflammatory responses including decreased glial activation and pro-inflammatory cytokine production.42 In the CNS, IL-10 has therapeutic effects in models of stroke,43 EAE,44 Parkinson’s disease,45 and traumatic or excitotoxic spinal cord injuries.46, 47 In this study, we have shown for the first time that AAV-IL-10 treatment of APP+PS1 mice can suppress astro/microgliosis and restore impaired spatial learning and neurogenesis. In addition, although IL-10 gene delivery did not reduce beta-amyloidosis in the brain, vascular transport of Aβ was enhanced. These findings suggest the possibility of IL-10 having significant therapeutic potential in clinical trials and may partially explain the molecular mechanism of the beneficial effect of GA-mediated immunotherapy on animal models of AD.
A recent study demonstrated that blocking TGF-β signaling with a dominant-negative TGF-β receptor driven by a CD11c promoter results in elevated IL-10 transcripts concomitant with reduced beta-amyloidosis in APP mice.23 In this study, however, IL-10 stimulation did not suppress Aβ deposition in the mouse brain. We have previously reported differences in Aβ clearance in IL-10 and other cytokine-treated human monocyte-derived macrophages (MDM).48 IL-10 is inefficient in Aβ42 degradation and may potentially increase the Aβ42/Aβ40 ratio, which is critical in Aβ deposition in APP+PS1 mice.33, 48, 49 Moreover, IL-10 increases abortive secretion of Aβ from MDM after its phagocytosis, and TGF-β has a synergistic effect with IL-10 in enhancing the secretion of Aβ40, which is a dominant species in Tg2576 APP mice. This may be a reason why IL-10 expression did not reduce Aβ burden in the mouse brain.48
Secretion of Aβ aggregates enhances Aβ deposition or clearance to the vascular system, which may contribute to the elevated plasma Aβ40 and 42 levels in the AAV-IL-10-injected APP+PS1 mice as compared to the AAV-GFP-treated APP+PS1 mice. One possible mechanism of this phenomenon may be that infiltrated macrophages and /or resident microglia are stimulated by IL-10 to promote Aβ secretion after they phagocytose the Aβ, which then increases the brain-to-blood efflux in cerebral vessels, probably mediated by low-density lipoprotein (LDL) receptor and LDL-related protein 1.50 Thus, it is possible that increased secretion of Aβ from the brain into the blood decreases Aβ deposition in the brain at later time points. Taken together, these results also indicate that the beneficial effects of IL-10 are not beta-amyloidosis-related.
One anti-inflammatory effect of peripherally administrated GA in EAE models is its ability to induce Th2/3 cells, which increase expression of IL-10 in the brain.38 Subcutaneous injection of GA has been shown to promote neurogenesis, including cell proliferation, migration, and differentiation.37 A number of studies show a significant correlation between learning and memory formation and neurogenesis that is mostly associated with elevated neurotrophic factors and their function in neuronal protection, survival, and synaptic plasticity.51, 52 Thus, neurogenesis in the GA-treated EAE model might be associated with increased IL-10. In this study, a direct introduction of AAV-IL-10 into the mouse hippocampus significantly restored the number of Dcx+, BrdU+, and BrdU+NeuN+ neurons in the SGZ of APP+PS1 mice to those of age-matched non-Tg mice. Our in vivo study indicates that IL-10 has proliferative effects on neural stem cells in the SGZ. This point was demonstrated in our in vitro microglia/neural stem cell co-culture system showing that IL-10-stimulated microglia, but not IL-10 alone, enhanced proliferation but not differentiation of neural stem cells. IL-4-stimulated microglia, on the other hand, enhanced neural differentiation and astrogliogenesis to some extent, while LPS also was astrogliogenic and LPS-treated microglia was neurogenic in a direct co-culture system. One limitation of the experimental design is that LPS-treated microglia show classic activation in the acute phase, which will be resolved and shifted to an alternative activation status at later time points. Since our co-culture incubation period is 7 days, the LPS-treated microglia exhibited both classic and alternative activation statuses, which may reflect its neurogenic effect in our experiment. Similar findings were also reported in a rat primary culture model.53 To the best of our knowledge, our work is the first to show that neuronal expression of IL-10 can directly enhance neurogenesis. IL-10 also enhances survival of normal human B cells by increased expression of the anti-apoptotic protein Bcl-2.54, 55 IL-10 expression promotes neuronal survival after spinal cord injury by upregulation of Bcl-2 and Bcl-xl and activation of Akt.21 Therefore, IL-10 may potentially mediate neurogenesis and neuronal survival via similar pathways in the hippocampus of APP+PS1 mice.
While hippocampal-dependent spatial learning and memory has been linked to neurogenesis, to date the RAWM task itself has not been shown to be directly dependent on neurogenesis. Given the involvement of neurogenesis in a wide number of spatial learning tasks, it is quite likely that enhanced neurogenesis contributes to the enhanced performance of AAV-IL-10-injected APP+PS1 mice in the RAWM test. In accord, AAV-IL-4-injected APP+PS1 mice show enhanced neurogenesis with enhanced spatial learning as determined by the RAWM test.56
In summary, we demonstrated for the first time that neuronal expression of the anti-inflammatory cytokine IL-10 suppresses astro/microgliosis and restores the neurogenesis and spatial learning of APP+PS1 mice. This work also suggests that anti-inflammatory cytokines can potentially function as neuromodulators or neurohormones in the brain and indicates a novel approach to treating neurodegenerative disorders through anti-inflammatory signaling cascades.
MATERIALS AND METHODS
Animals
All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee of University of Nebraska Medical Center and Laboratory Animal Safety Committee at Boston University School of Medicine. Tg2576 mice expressing the Swedish mutation of human APP695 were obtained from Drs. G. Carlson and K. Hsiao-Ashe through Mayo Medical Venture.31 PS1 mutant mice (M146L line 6.1) were provided by Dr. K. Duff through University of South Florida and maintained as PS1 transgene homozygotes.32 Generation of APP+PS1 bigenic mice and genotyping were previously described.30 Age-matched non-tg mice in the B6/129 F1 strain (Jackson laboratory, Bar Harbor, ME) were maintained by intercrossing in the same facility.
AAV-IL-10 gene construction, AAV1/2 hybrid virus generation, and purification
To construct an AAV vector for expressing the mouse IL-10 gene, PCR primers (5’-AAAGGATCCATGCCTGGCTCAGCACTGCTATG-3’ and 5’-AAAACTCGAGTCAATACACACTGCAGGTGTTTTAG-3’) and a cDNA template (ATCC pCD-SRa-TF115) were used for proof-reading PCR amplification of the 576 bp cDNA region. The PCR amplicon was digested with Bam HI-Xho I and subcloned into the corresponding restriction sites of pAAV2-MCS-WPRE to develop pAAV2-MCS-WPRE-IL-10.30 The PCR-amplified region was entirely DNA sequenced prior to AAV virus generation. For AAV-GFP, pGFP vector was used (provided by R. Klein).57 Recombinant AAV virus expressing IL-10 or GFP was generated, purified, and titrated as described.30
Stereotaxic injection
AAV viruses were bilaterally injected into mouse hippocampi as described.30 Briefly, mice at 3 months of age received i.p. injection of ketamine/xylazine anesthesia (100 mg kg−1 ketamine and 20 mg kg−1 xylazine). After mice were immobilized in a stereotaxic apparatus (Stoelting, Wood Dale, IL), a linear skin incision was made over the bregma, and a 1-mm burr hole was drilled in the skull 2.1 mm posterior and 1.8 mm lateral to the bregma on both sides using a hand-held driller. 2 µl of saline containing AAV-GFP or AAV-IL-10 (total 3 × 109 VP, 1µl per hippocampus) was injected into the hippocampus 1.8 mm below the surface of the skull using a 10-µl Hamilton syringe.
Bromodeoxyuridine (BrdU) administration and tissue preparation
The cell-proliferation marker BrdU was intraperitoneally injected (50 mg kg−1 of body weight) twice daily every 12 h for 2.5 days (5 total injections) to label proliferating cells as described.13 Three weeks after the first BrdU injection, mice were deeply anesthetized with isoflurane, blood samples were collected, and then mice were transcardially perfused with 25 ml of ice-cold PBS. The brain was rapidly removed, the left hemisphere was immediately frozen in dry ice for biochemistry, and the right hemisphere was immersed in freshly depolymerized 4% paraformaldehyde in 1x PBS for 48 hours at 4°C and cryoprotected by successive 24-hour immersions in 15% and 30% sucrose in 1x PBS. Fixed, cryoprotected brains were frozen and sectioned coronally using a Cryostat (Leica, Bannockburn, IL) with sections collected serially and stored at −80°C before performing immunohistochemistry.
Immunohistochemistry and immunofluorescence
Immunohistochemistry was performed as described previously.30 Briefly, sections were incubated using specific antibodies to identify pan-Aβ (rabbit polyclonal antibody, pAb, 1:100, Invitrogen, Carlsbad, CA), GFAP (astrocyte marker, rabbit pAb, 1:2000, DAKO, Carpinteria, CA), IBA1 (microglia marker, rabbit pAb, 1:1000, Wako, Richmond, VA), and doublecortin (premature neuronal marker, Dcx, goat pAb, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with Envision Plus (DAKO) for Aβ, GFAP and IBA1 staining, or biotin-conjugated anti-goat seconday IgG and Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) for Dcx staining. Immunodetection was visualized using 3,3'-diaminobenzidine (Vector Laboratories, Burlingame, CA). Compact plaques were stained with 1% thioflavin-S (TS, Sigma-Aldrich, St. Louis, MO) in 50% ethanol. For immunofluorescence, sections were incubated with anti-BrdU (6 µg ml−1) (mouse mAb, Roche Diagnostics, Indianapolis, IN), followed by incubation with Alexa Fluor®488-conjugated anti-mouse IgG (1:1000, Invitrogen, Carlsbad, CA). The sections were then incubated with biotin-conjugated anti-NeuN (neuronal marker, mouse mAb, 1:500, Millipore, Billerica, MA), followed by incubation with Streptavidin-Alexa Fluor®568 (1:1000) (Invitrogen, Carlsbad, CA). For quantification analysis, the areas of Aβ loads and TS-positive plaques were analyzed using image analysis software (ImageJ, NIH, Bethesda, MD) at 300-µm intervals in ten 30-µm thickness coronal sections from each mouse. Five brains per group were analyzed. The number of GFAP-positive astrocytes and IBA1-positive microglia around Aβ plaques in the hippocampus was counted at 300-µm intervals in ten 30-µm thickness coronal sections from each mouse. Three TS-positive plaques were randomly chosen per section, and five mouse brains per group were analyzed (30 plaques per animal) by counting the number of astrocytes and microglia surrounding the plaques according to the published method.30 The number of Dcx+ and BrdU+NeuN+ cells in the subgranular zone (SGZ) of the dentate gyrus were calculated by a stereological method based on the Cavalieri principle as described.58 Ten 30-µm thickness coronal sections at 300-µm intervals from each mouse were used for the quantification. For immunocytochemistry a similar approach was utilized as previously reported.59 Briefly, cells fixed with 4% paraformaldehyde were permeabilized with 1% Triton-X in PBS and were incubated in primary antibody for GFAP (1:500), Map2 (mouse mAb, 1:500) Ki67 (proliferation marker rabbit pAb, 1:500, Novus USA, Littleton, CO), or Nestin (stem cell filament marker, rat mAb, 1:50, Ames, IA) followed by incubation with secondary antibodies which includes Alexa Fluor®546-conjugated anti-mouse IgG, or Alexa Fluor®546-conjugated anti-rat IgG and Alexa Fluor®488-conjugated anti-rabbit IgG, (all at 1:500, Invitrogen).
Protein Extraction and Enzyme-Linked Immunosorbent Assay (ELISA)
Hippocampal tissues were homogenized in solubilization buffer (50 mM Tris, pH 7.5, 100 mM NaCl, 2 mM ethylenediamine tetraacetic acid-Na, 1% Triton X-100, and protease inhibitor cocktail, all from Sigma, St. Louis, MO) and centrifuged at 14,000 × g for 20 minutes at 4°C. The protein concentration of the supernatant was quantified by BCA assay kit (Pierce, Rockford, IL). IL-10 was measured using commercially available enzyme immunoassays (mouse IL-10 ELISA set, BD OptEIA™, BD Biosciences, San Jose, CA) according to the manufacturer’s instructions. The concentrations of Aβ40 and Aβ42 in plasma were quantified using commercially available ELISA kits (Invitrogen, Carlsbad, CA) and following the manufacturer's protocols.
Two-day radial arm water maze
The radial arm water maze task was run as described previously with minor modifications.30 Animals were introduced into the perimeter of a circular water-filled tank 110 cm in diameter and 91 cm in height (San Diego Instruments, San Diego, CA) with triangular inserts placed in the tank to produce six swim paths radiating out from a central area. Spatial cues for mouse orientation were present on the walls of the tank. At the end of one arm, a 10 cm circular plexiglass platform was submerged 1 cm deep—hidden from the mice. On day one, 15 trials (12 trials with visible platform followed by 3 trials with hidden platform) were run in five blocks of 3. A cohort of 4 mice was run sequentially for each block. After each 3-trial block, a second cohort of mice was run creating an extended rest period before mice were exposed to the second block. The target arm location remained constant for a given mouse throughout the test. Each trial lasts 1 min and an error is scored each time the body of the mouse, excluding tail, enters the wrong arm, enters the arm with the platform but does not climb on it, or does not make a choice for 20 s. Each trial ends when the mouse climbs onto and remains on the hidden platform for 10 seconds. The mouse is given 20 s to rest on the platform between each trial. On day two, the mice were run in exactly the same manner as day one except that the platform was hidden for all trials. Between blocks 4 and 5 on day 1 and blocks 9 and 10 on day two, an additional break of 30 minutes was given to each mouse to test short-term memory recall. The errors on each block were averaged and used for statistical analysis. All the animal behaviors were recorded by Ethovision System 3.1 (Noldus Information Technology, Inc., Leesburg, MA) and a CCD camera suspended 170 cm above the liquid surface.
Primary culture of mouse microglia and neural stem cells
Primary culture of mouse microglia was prepared from newborn wild type P0 pup as described 36, 48, 60. Cultures were incubated for 7 days in complete DMEM (Dulbecco’s modified essential media, 10% fetal bovine serum, and 1x penicillin/streptomycin, Invitrogen) + 10 ng ml−1 monocyte colony stimulating facor and 10 ng ml−1 GM-CSF (Abzyme, Newton, MA). Primary neural stem cell cultures were prepared as previously described.59 Briefly, cortices were dissected from wild type pups at E14 and placed in cold HBSS + 2% glucose (Invitrogen). Tissue was triturated and mechanically dissociated with a micropipette in 1mL of complete proliferation media [Proliferation Media, Proliferation Supplement (both from Stem Cell Technologies, Vancouver, BC, Canada), 20ng ml−1 EGF (Stem Cell Technologies), and 1x penicillin/streptomycin (Invitrogen)] and filtered through a cell strainer (70-µm pore size, Millipore, Billerica, MA), followed by plating on 24-well tissue culture plates. Cells were cultured as neurospheres in complete proliferation media for 7 days until spheres reached between 100–150 µm in diameter. Neurospheres were collected and mechanically dissociated by trituration in complete proliferation media and then plated onto poly-D-lysine and laminin (both from Sigma-Aldrich) coated coverslips (18-mm in diameter) at a density of 125,000 cells/well. Cells were incubated for four hours in proliferation media and then media was changed to either fresh proliferation media (for the proliferation study), or differentiation media (Neurobasal media, B27, and 1x penicillin/streptomycin, Invitrogen, for the differentiation study). Microglia were stimulated with murine IL-4 (10 ng ml−1, Abzyme), murine IL-10 (10ng ml−1, Abzyme), lipopolysaccharide (LPS, 100 ng ml−1, E.coli 055:B5 Sigma-Aldrich), or PBS on either transwell inserts (0.4 µm pore size, Millipore) or in polypropylene tubes (Falcon 2059) in either proliferation or differentiation media for four hours after the initial plating of the neural stem cells. A sham experiment was also conducted in which cytokines in media alone were incubated in snap tubes for the same amount of time. After four hours, microglia on transwell inserts or direct cultures were added to the neural stem cells. The cells were then allowed to incubate for 3 days (proliferation) or 7 days (differentiation) at which point they were fixed in 4% paraformaldehyde in PBS, and subjected to immunocytochemistry.
Statistics
All data were normally distributed and presented as mean values ± standard errors of the mean (SEM). In the case of single mean comparison, data were analyzed by Student’s t-test. In case of multiple mean comparisons, the data were analyzed by one-way ANOVA and Newman-Keuls or Tukey’s post-hoc or two-way repeated measures ANOVA, followed by Bonferroni multiple comparison tests using statistics software (Prism 4.0, Graphpad Software, San Diego, CA). P-values of less than 0.05 were regarded as a significant difference.
Supplementary Material
ACKNOWLEDGEMENT
We thank Drs. K. Hsiao-Ashe for providing Tg2576 mice, K. Duff for providing M146L PS1 mice, R. Klein for pGFP plasmid, University of Pennsylvania Gene Therapy Program for recombinant AAV1 vectors, and Megan Varnum for editing of the manuscript. This work is supported by the Vada Kinman Oldfield Alzheimer’s Research Fund (T.K., T.I.), UNMC Brain Bank Core Fund (T.I.), NIH P01 NS043985 (T.I.), R01 MH083523 (T.I.) and R21 AG032600 (T.I.).
REFERENCES
- 1.Laurence JA, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4(3):206–210. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- 2.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann N Y Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 3.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13(6):501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 4.van der Valk P, Amor S. Preactive lesions in multiple sclerosis. Curr Opin Neurol. 2009;22(3):207–213. doi: 10.1097/WCO.0b013e32832b4c76. [DOI] [PubMed] [Google Scholar]
- 5.Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer's disease. J Cell Mol Med. 2008;12(3):762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Streit WJ. Microglial response to brain injury: a brief synopsis. Toxicol Pathol. 2000;28(1):28–30. doi: 10.1177/019262330002800104. [DOI] [PubMed] [Google Scholar]
- 7.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 8.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 9.van der Most PJ, Dolga AM, Nijholt IM, Luiten PG, Eisel UL. Statins: mechanisms of neuroprotection. Prog Neurobiol. 2009;88(1):64–75. doi: 10.1016/j.pneurobio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Ray B, Lahiri DK. Neuroinflammation in Alzheimer's disease: different molecular targets and potential therapeutic agents including curcumin. Curr Opin Pharmacol. 2009;9(4):434–444. doi: 10.1016/j.coph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Birmingham K, Frantz S. Set back to Alzheimer vaccine studies. Nat Med. 2002;8(3):199–200. doi: 10.1038/nm0302-199b. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel D, Maron R, Burt DS, Weiner HL. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J Clin Invest. 2005;115(9):2423–2433. doi: 10.1172/JCI23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, et al. Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2006;103(31):11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frenkel D, Puckett L, Petrovic S, Xia W, Chen G, Vega J, et al. A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann Neurol. 2008;63(5):591–601. doi: 10.1002/ana.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern JN, Keskin DB, Zhang H, Lv H, Kato Z, Strominger JL. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc Natl Acad Sci U S A. 2008;105(13):5172–5176. doi: 10.1073/pnas.0712131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard M, O'Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12(4):239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 17.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 18.Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10--a role for IL-1 beta? J Neurochem. 2004;88(3):635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- 19.Arimoto T, Choi DY, Lu X, Liu M, Nguyen XV, Zheng N, et al. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol Aging. 2007;28(6):894–906. doi: 10.1016/j.neurobiolaging.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Qian L, Block ML, Wei SJ, Lin CF, Reece J, Pang H, et al. Interleukin-10 protects lipopolysaccharide-induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther. 2006;319(1):44–52. doi: 10.1124/jpet.106.106351. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. IL-10 promotes neuronal survival following spinal cord injury. Exp Neurol. 2009;220(1):183–190. doi: 10.1016/j.expneurol.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav Immun. 2009;23(6):794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, et al. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14(6):681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70(11):8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, Li J, McCown TJ, Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144(1):113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 26.Dudus L, Anand V, Acland GM, Chen SJ, Wilson JM, Fisher KJ, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999;39(15):2545–2553. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 27.Guy J, Qi X, Muzyczka N, Hauswirth WW. Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999;117(7):929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 28.Peel AL, Klein RL. Adeno-associated virus vectors: activity and applications in the CNS. J Neurosci Methods. 2000;98(2):95–104. doi: 10.1016/s0165-0270(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 29.Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77(12):7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyota T, Yamamoto M, Schroder B, Jacobsen MT, Swan RJ, Lambert MP, et al. AAV1/2-mediated CNS gene delivery of dominant-negative CCL2 mutant suppresses gliosis, beta-amyloidosis, and learning impairment of APP/PS1 mice. Mol Ther. 2009;17(5):803–809. doi: 10.1038/mt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 32.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, et al. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 33.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 34.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, et al. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat Neurosci. 2002;5(12):1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 35.He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, et al. Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer's mice. J Cell Biol. 2007;178(5):829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto M, Kiyota T, Horiba M, Buescher JL, Walsh SM, Gendelman HE, et al. Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am J Pathol. 2007;170(2):680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aharoni R, Arnon R, Eilam R. Neurogenesis and neuroprotection induced by peripheral immunomodulatory treatment of experimental autoimmune encephalomyelitis. J Neurosci. 2005;25(36):8217–8228. doi: 10.1523/JNEUROSCI.1859-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci U S A. 2003;100(24):14157–14162. doi: 10.1073/pnas.2336171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, McNeil E, Dressler L, Siman R. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol. 2007;204(1):77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64(1):79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168(1):144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- 43.Dietrich WD, Busto R, Bethea JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. 1999;158(2):444–450. doi: 10.1006/exnr.1999.7115. [DOI] [PubMed] [Google Scholar]
- 44.Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, et al. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009;23(1):92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston LC, Su X, Maguire-Zeiss K, Horovitz K, Ankoudinova I, Guschin D, et al. Human interleukin-10 gene transfer is protective in a rat model of Parkinson's disease. Mol Ther. 2008;16(8):1392–1399. doi: 10.1038/mt.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham KE, McMillen D, Brewer KL. The effects of endogenous interleukin-10 on gray matter damage and the development of pain behaviors following excitotoxic spinal cord injury in the mouse. Neuroscience. 2004;124(4):945–952. doi: 10.1016/j.neuroscience.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Jackson CA, Messinger J, Peduzzi JD, Ansardi DC, Morrow CD. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336(2):173–183. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-beta peptide degradation by human mononuclear phagocytes. J Immunol. 2008;181(6):3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, et al. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27(3):627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu B, Ueno M, Onodera M, Kusaka T, Huang CL, Hosomi N, et al. RAGE, LDL receptor, and LRP1 expression in the brains of SAMP8. Neurosci Lett. 2009;461(2):100–105. doi: 10.1016/j.neulet.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18(2):93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 52.Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 2009;42(5):239–244. doi: 10.5483/bmbrep.2009.42.5.239. [DOI] [PubMed] [Google Scholar]
- 53.Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56(4):412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- 54.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93(1):424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol. 1995;154(9):4341–4350. [PubMed] [Google Scholar]
- 56.Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer's disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24(8):3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein RL, Hamby ME, Sonntag CF, Millard WJ, King MA, Meyer EM. Measurements of vector-derived neurotrophic factor and green fluorescent protein levels in the brain. Methods. 2002;28(2):286–292. doi: 10.1016/s1046-2023(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 58.West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22(2):51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Tsutsumi K, Tokuraku K, Estes KA, Hisanaga SI, Ikezu T. Actin interaction and regulation of cyclin-dependent kinase 5/p35 complex activity. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiyota T, Yamamoto M, Xiong H, Lambert MP, Klein WL, Gendelman HE, et al. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009;4(7):e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.