SUMMARY
Breast cancer patients often develop locoregional or distant recurrence years after mastectomy. Understanding the mechanism of metastatic recurrence after dormancy is crucial for improving the cure rate for breast cancer. Here, we characterized a bone metastasis dormancy model to show that aberrant expression of vascular cell adhesion molecule 1 (VCAM-1), in part dependent on the activity of the NFκB pathway, promotes the transition from indolent micrometastasis to overt metastasis. By interacting with the cognate receptor integrin α4β1, VCAM-1 recruits monocytic osteoclast progenitors and elevates local osteoclast activity. Antibodies against VCAM-1 and integrin α4 effectively inhibit bone metastasis progression and preserve bone structure. These findings establish VCAM-1 as a promising target for the prevention and inhibition of metastatic recurrence in bone.
INTRODUCTION
One mysterious feature of metastases is that distant relapse can occur many years after successful primary tumor removal and clinically disease-free survival (Aguirre-Ghiso, 2007). The latency before distant metastasis relapse is defined as metastasis dormancy. Understanding the mechanism of dormancy and its reactivation has important clinical implications for controlling metastatic progression and maintaining patients in a disease-free state (Chambers et al., 2002; Goss and Chambers, 2010). In preclinical models, cancer can remain dormant either as quiescent cells (cellular dormancy) or as indolent small clusters that maintain balanced proliferation and death (tumor mass dormancy) (Aguirre-Ghiso, 2007). Various possible mechanisms of dormancy have been suggested based on studies done in preclinical models, including inefficient angiogenesis, antibody- or T cell-mediated immune surveillance, lack of proliferative signals, and the activity of metastasis suppressor genes and microRNAs, although the extent to which these mechanisms reflect clinical dormancy is unclear (Aguirre-Ghiso, 2007; Goss and Chambers, 2010). Clinical dormancy in patients has been extensively studied in breast cancer. Time distribution analyses of both mortality and recurrence showed an early polynomial-like curve and a late persistent rate for up to more than 20 years (Demicheli et al., 1996). Interrupted and prolonged dormancy was proposed to explain the bimodal pattern (Demicheli, 2001), yet with little molecular insight.
Postoperative distant recurrence arises invariably from disseminated tumor cells (DTCs), which are often found in the bone marrow of breast cancer patients without any clinical sign of metastasis (Braun et al., 2005; Klein, 2009). Bone metastasis is a frequent complication of breast cancer and is often accompanied by debilitating bone fracture, severe pain, nerve compression and hypercalcemia (Weilbaecher et al., 2011). Bone metastasis is characterized by the intricate interaction between tumor cells and bone microenvironment. In breast cancer, continuous expansion of osteolytic bone metastasis is driven by the “vicious cycle” of tumor-dependent activation of bone-degrading osteoclasts and bone stroma-dependent stimulation of tumor malignancy (Weilbaecher et al., 2011). Therefore, identification of tumor-derived osteoclastogenic factors may provide new potential therapeutic targets. Currently, it is unknown whether molecules involved in the vicious cycle are also important for driving the transition from indolent micrometastasis to overt metastasis in bone. This lack of understanding can be largely explained by the paucity of appropriate animal models that mimic the metastatic relapse process. Here, we report the establishment of a dormancy-reactivation model of breast cancer bone metastasis. Using this model, we linked osteoclast activation with the switch from micrometastasis to osteolytic macrometastasis, and identified vascular cell adhesion molecule-1 (VCAM-1) as a key regulator of this process.
VCAM-1 is a member of the transmembrane immunoglobulin (Ig) superfamily (Osborn et al., 1989). Proteolytic shedding of VCAM-1 also generates a soluble form of VCAM-1 (Garton et al., 2003). The predominant receptor for VCAM-1 is integrin α4β1 (i.e. very late antigen-4, VLA-4), which is expressed by many cell types of the hematopoietic lineage, including B and T lymphocytes, monocytes, eosinophils, and basophils (Carter and Wicks, 2001). VCAM-1 is expressed by cytokine-activated endothelial cells (Osborn et al., 1989) and VCAM-1-α4β1 binding plays an important role in mediating leukocyte adhesion and transendothelial migration during inflammation (Springer, 1994), which may be the underlying mechanism for VCAM-1 function in rheumatoid arthritis (Carter and Wicks, 2001) and early atherosclerosis (Cybulsky et al., 2001). Aberrant expression of VCAM-1 in cancer cells was documented in preclinical models as well as patient samples of gastric cancer (Ding et al., 2003), renal cell carcinoma (Lin et al., 2007) and breast cancer (Chen et al., 2011). However, it is unknown whether tumor-derived VCAM-1 has any functional role in breast cancer metastasis to bone. Combining the power of functional genomics and a multiphoton imaging technique, ex vivo imaging bone metastasis (EviBoM), we discovered a role of VCAM-1 in promoting the outgrowth of indolent bone micrometastasis and established VCAM-1 as a promising target for preventing metastatic recurrence in bone.
RESULTS
Identification of VCAM-1 as a crucial activator of indolent bone micrometastasis
We previously used an in vivo selection strategy to derive bone-metastatic variants of the MDA-MB-231 breast cancer cell line in order to identify bone metastasis genes (Kang et al., 2003). Dilution cloning of the parental MDA-MB-231 population revealed a small percentage of pre-existing highly bone metastatic cells that overexpress the bone-metastasis gene signature, including genes such as CXCR4, IL11, CTGF, MMP1, and OPN (Kang et al., 2003). Consistent with the functional importance of these genes, single cell-derived populations (SCPs) of MDA-MB-231 that lack the expression of the bone metastasis gene signature (e.g., SCP3, 4, 6, 21) were incapable of generating any X-ray detectable bone metastases within 100 days after injection (Figure 1A–B, also see Figure 5B in Kang et al., 2003). However, after more than 4 months, a small percentage (~10%) of mice injected with SCP6 developed overt bone metastasis. Cell lines derived from such a bone lesion (e.g., PD1, post-dormancy generation 1) possess drastically increased metastatic potential (Figure 1A–C). Close monitoring of mice injected with luciferase-labeled SCP6 by the more sensitive bioluminescence imaging (BLI) revealed repeated failure of micrometastases in bone to expand to become overt macrometastases (Figure S1A, highlighted by yellow circles), in contrast to the rapid development of osteolytic lesions by highly bone metastatic lines such as SCP25 (Figure S1A). This long “lead time” before the infrequent overt bone metastasis formation (Figure S1A, red circle) from indolent micrometastasis is similar to the clinical observation of metastatic dormancy and subsequent relapse. From the bone metastases formed by PD1, second generation sublines (PD2) were isolated (Figure 1A). Most of the PD2 sublines (PD2A, B, C, D, and E) metastasize to bone as efficiently as PD1, whereas one subline, PD2R, significantly lost its metastatic potential and was therefore considered as a partial revertant to the dormant state (Figure 1B, C). These near-isogenic sublines that were derived from a common progenitor but had dramatic differences in metastatic abilities represented an ideal cohort to identify crucial drivers of outgrowth from indolent bone micrometastasis.
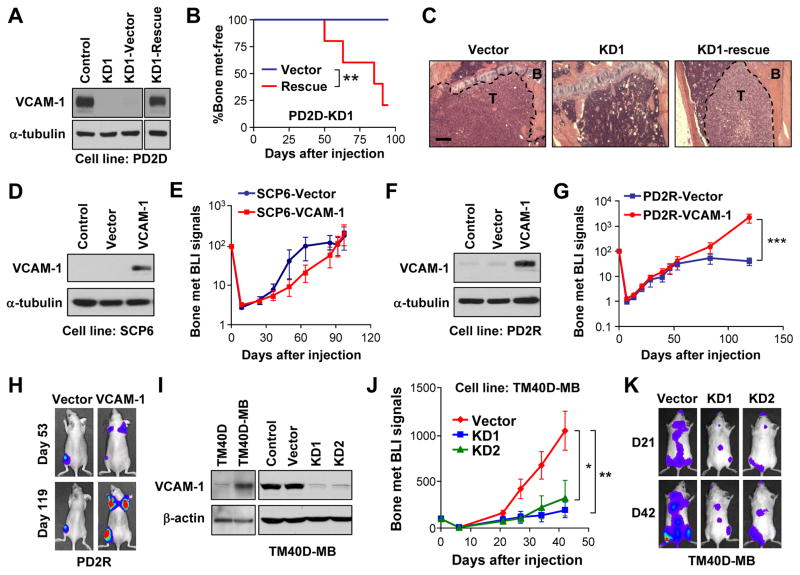
Figure 1. Identification of VCAM-1 as a bone metastasis gene in a mouse model of metastatic dormancy and activation.
(A) Relationship of parental SCP6 and post-dormancy (PD) sublines. Sublines with strong bone-metastatic ability were color-coded in red and sublines with weak bone-metastatic ability were color-coded in green. (B-C) Kaplan-Meier curve of bone metastasis development of different cell lines with BLI of representative mice. N=10. (D) Supervised clustering of samples using genes differentially expressed between weak (green) and strong (red) bone-metastatic cell lines. Genes highlighted in red were functionally tested. (E) Differential expression of VCAM-1 in parental and PD sublines detected by western blot. (F) VCAM-1 KD in PD2D as detected by western blot. (G) Kaplan-Meier curve of bone metastasis development of the indicated PD2D cells. N=8. **p < 0.01, #p > 0.1 by log-rank test. (H) Representative BLI of mice from (G) on Day 63 and Day 112. (I) Representative X-radiographs with arrows pointing to osteolytic lesions. See also Figure S1 and Table S1.
To ensure the lineage relationships between different sublines derived in this study and the parental MDA-MB-231, we analyzed genomic copy number changes by array comparative genomic hybridization (CGH) (Figure S1B). PD1 and SCP6 shared very similar chromosomal profiles to MDA-MB-231, suggesting that the dramatic differences in their metastatic abilities are not likely the result of gross genomic alterations or accidental contaminations from other cell lines.
Because the highly aggressive bone metastatic ability of the PD sublines is similar to that of the MDA-MB-231 variants previously isolated from in vivo selection (Kang et al., 2003), we first tested whether the PD sublines activated the same bone metastasis signature (Kang et al., 2003). Surprisingly, none of the previously described bone metastasis genes was found to be upregulated in the PD sublines (Figure S1C-D), suggesting that an independent mechanism was developed by the PD cells to acquire the bone metastatic ability. Microarray profiling revealed differentially expressed genes between the highly metastatic PD1/PD2 lines and the weakly metastatic lines (SCP3, SCP4, SCP6, MDA-MB-231, and PD2R) (Figure 1D-E and Table S1). Several genes overexpressed in PD1/PD2 lines have been implicated in breast cancer, including IL1B (Jin et al., 1997), TFPI2 (Wojtukiewicz et al., 2003), MAGEB2 (Lurquin et al., 1997), KLRC1 (Minn et al., 2005), and VCAM-1 (Minn et al., 2005). However, none of them has been previously linked to bone metastasis.
To determine whether one or more of these five genes contributed to metastasis, we used short hairpin RNAs (shRNAs) to stably silence each gene in a representative strongly metastatic PD line, PD2D. Knockdown (KD) of IL1B, TFPI2, MAGEB2 or KLRC1 had no effect on bone metastasis (Figure S1E). Three different VCAM-1 shRNAs showed different KD efficiency (Figure 1F). While KD1 and KD2 significantly reduced VCAM-1 expression, KD3 did not reduce VCAM-1 level (Figure 1F) and thus served as an additional negative control. The two efficient knockdowns significantly abolished the bone metastatic ability (Figure 1G-H). X-radiography (Figure 1I) showed the osteolytic nature of the bone lesions formed by control cells and the attenuation of this phenotype in the KD cells. To further confirm the specificity of VCAM-1 KD, VCAM-1 expression was rescued in the KD1 subline by stably expressing a shRNA-resistant VCAM-1 coding sequence (Figure 2A). When inoculated in vivo, the rescue cells showed recovered metastatic potential (Figure 2B, C). Collectively, these results suggest that VCAM-1 is essential for the acquired osteolytic bone metastatic ability in the PD lines.
Figure 2. Essential role of VCAM-1 in bone metastasis.
(A) Rescued VCAM-1 expression in the VCAM-1-KD PD2D subline as detected by western blot. (B) Kaplan-Meier representation of bone metastasis relapse by control and VCAM-1-rescued cell lines in (A). N=6. **p < 0.01 by log-rank test. (C) H&E staining of tibia showing presence or absence of overt metastatic tumors (T) in bone (B) by different tumor cells. Scale bar 200μm. (D) Ectopic overexpression of VCAM-1 in SCP6 as detected by western blot. (E) BLI curves of in vivo bone metastasis assay with SCP6 variants. Data represent mean ± SD. N=6. No time point showed statistic significance by Mann-Whitney test. (F) Ectopic overexpression of VCAM-1 in PD2R as detected by western blot. (G) BLI curves of bone metastasis development by control and VCAM-1-overexpressing PD2R cells. Data represent mean ± SEM. N=10. ***p < 0.001 by Mann-Whitney test. (H) Representative BLI of mice in (G). (I) Endogenous expression of VCAM-1 in the murine cell line TM40D and the subline TM40D-MB, and VCAM-1 KD in TM40-MB, as detected by western blot. (J) BLI curves of bone metastasis development by the indicated TM40D-MB cells. Data represent mean ± SEM. N=10. *p < 0.05, **p < 0.01 by Mann-Whitney test. (K) Representative BLI of mice in (J) on Day 21 and Day 42. See also Figure S2.
Acquisition of VCAM-1 expression by in vivo evolution
Escape from metastasis dormancy was hypothesized to occur through adaptive changes of micrometastases (Weinberg, 2008), although this argument has not been supported by evidence collected from a mouse model with a natural history of metastatic progression from dormancy. Most previous metastasis models enriched subpopulations with inherently stronger metastatic potential in a cell line through in vivo selection (Kang et al., 2003; Minn et al., 2005). Without the in vivo evolution of dormancy, this approach is unlikely to find genes critical for activation of overt bone metastasis from dormant micrometastases. To evaluate our model in this aspect, we investigated whether VCAM-1 upregulation was a newly evolved event during the outgrowth from micrometastasis of SCP6. Highly abundant VCAM-1 protein was detected in aggressive PD lines with localization restricted to cell membrane (Figure S2A, B), allowing flow cytometric analysis of VCAM-1+ cells. VCAM-1 expression pattern was examined exhaustively in SCP6 by FACS analysis of 8.7×106 cells (Figure S2C). Only 17 cells were stained positive. This extremely low percentage of VCAM-1+ cells were determined to be false positive when the sorted VCAM-1+ cells were expanded into a larger population and VCAM-1 expression was reexamined (Figure S2C). Therefore, we conclude that VCAM-1 upregulation most likely evolved in vivo during the natural metastasis progression from indolent micrometastasis to overt macrometastasis.
Validation of the pro-metastatic role of VCAM-1 in other bone metastasis models
To further evaluate whether VCAM-1 is sufficient for bone metastasis, we overexpressed it in SCP6 and the revertant line PD2R. While ectopic expression of VCAM-1 was insufficient to confer bone metastatic ability to SCP6 (Figure 2D-E), it was sufficient to promote the outgrowth of bone metastases formed by PD2R, which otherwise failed to further expand 50 days after injection (Figure 2F-H). This result indicates the initial conversion from micrometastases to overt macrometastases required more than VCAM-1 upregulation in the genetic background of SCP6. Nevertheless, VCAM-1 overexpression alone is sufficient to rescue the metastatic property of PD2R, which is more closely related to PD1.
To test whether VCAM-1 function in promoting bone metastasis was restricted to MDA-MB-231, we utilized other bone metastasis models. Recently, the TM40D murine mammary tumor cell line was subjected to in vivo selection to establish the highly bone metastatic TM40D-MB subline. Interestingly, VCAM-1 was one of the fourteen genes that were found to be significantly overexpressed in TM40D-MB (Li et al., 2008) (Figure 2I). We silenced VCAM-1 expression in TM40D-BM and observed dramatic reduction of bone metastasis (Figure 2I-K).
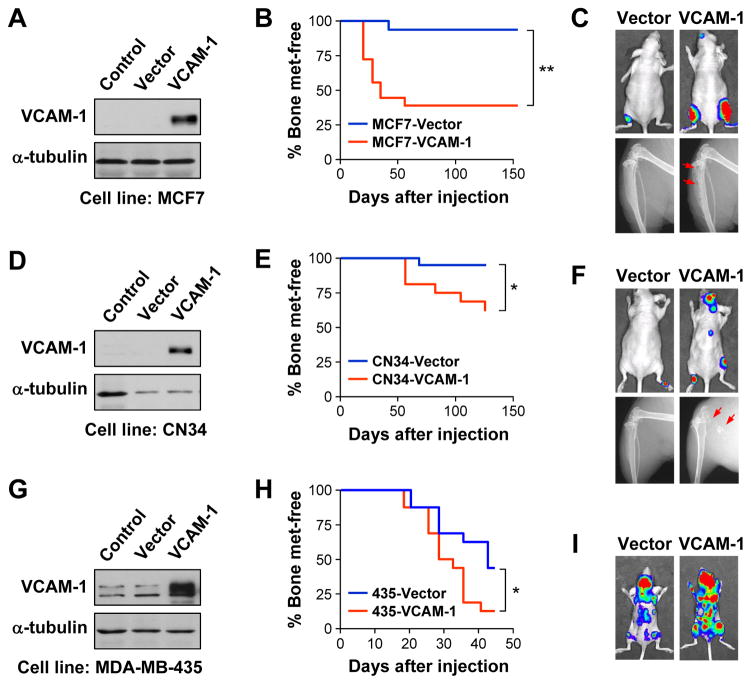
We further tested the pro-metastatic function of VCAM-1 in several human breast cancer cell lines with mild bone metastatic abilities. MCF7 is a estrogen receptor (ER)-positive cell line that generates slowly growing bone metastases in less than 20% of inoculated mice (Lu et al., 2009). Ectopic expression of VCAM-1 in MCF7 led to formation of osteolytic bone metastases in 69% of mice (Figure 3A–C). Two ER-negative cancer cell lines were tested in addition to the MDA-MB-231 derivatives (also an ER− model). CN34 was recently isolated from pleural effusion and showed marginal potential to form bone metastasis (Zhang et al., 2009). VCAM-1 overexpression substantially increased the osteolytic bone metastasis of CN34 cells (Figure 3D-F). Finally, MDA-MB-435, coexpressing both epithelial and melanocytic markers (Chambers, 2009), contains discernable level of VCAM-1 (Figure 3G) and displays mild aggressiveness to bone (Lu et al., 2009). VCAM-1 overexpression further enhanced bone colonization efficiency of MDA-MB-435 (Figure 3H-I). It should be noted that the pro-metastasis effect of VCAM-1 overexpression in CN34 was less dramatic compared with MCF7 and MDA-MB-435, probably reflecting the requirement for other genes in CN34 to colonize the bone more efficiently. Taken together, we conclude that VCAM-1 has a general pro-metastatic function in bone metastasis.
Figure 3. Pro-metastatic activity of VCAM-1 in additional bone metastasis models.
(A) Ectopic overexpression of VCAM-1 in MCF7 as detected by western blot. (B) Kaplan-Meier curve of bone metastasis development of control and overexpression MCF7 cells. N=10. **p < 0.01 by log-rank test. (C) Representative BLI (Day 125) and X-radiographs (Day 154) of mice from (B). Arrows point to osteolytic lesions. (D) Ectopic overexpression of VCAM-1 in CN34 as detected by western blot. (E) Kaplan-Meier curve of bone metastasis development of control and VCAM-1 overexpressing CN34 cells. N=10. *p < 0.05 by log-rank test. (F) Representative BLI (Day 125) and X-radiographs (Day 154) of mice from (E). Arrows point to osteolytic lesions. (G) Ectopic overexpression of VCAM-1 in MDA-MB-435 as detected by western blot. (H) Kaplan-Meier curve of bone metastasis development of control and VCAM-1-overexpressing MDA-MB-435 cells. N=10. *p < 0.05 by log-rank test. (I) Representative BLI (Day 42) of mice from (H).
Therapeutic targeting of VCAM-1 or cognate receptor by antibodies
To evaluate the therapeutic benefit of targeting VCAM-1 using a more clinically applicable approach, we used a previously developed monoclonal antibody (clone P3C4) capable of blocking VCAM-1 binding activity (Dittel et al., 1993). To test the anti-metastatic activity of the antibody, mice were treated 10 days after the intracardiac injection of PD2D cells to mimic adjuvant therapy. Mice treated with control IgG developed aggressive bone metastases that grew continuously, whereas those treated with anti-VCAM-1 only developed a low level of bone metastasis burden that reached a plateau within 50 days (Figure 4A-B). Dramatically diminished metastasis was also achieved when antibody was applied following an early adjuvant protocol (Figure S3A-C). To evaluate whether VCAM-1 antibody can inhibit established metastases, mice were treated with antibody 62 days after cell injection, and again demonstrated efficacy in impeding metastasis growth (Figure 4C).
Figure 4. Antibody therapy targeting VCAM-1 and integrin α4 inhibits bone metastasis.
(A) BLI curves of bone metastasis development by PD2D cells in nude mice with control IgG or anti-VCAM-1 (clone P3C4) treatment started from Day 10. Data represent mean ± SEM. N=6. *p < 0.05 by Mann-Whitney test. (B) Representative BLI of mice treated with IgG or anti-VCAM-1 antibodies. (C) BLI curves of bone metastasis progression by PD2D cells with control IgG or anti-VCAM-1 treatment started from Day 62. BLI signals were normalized to Day 62 to facilitate comparison. Data represent mean ± SEM. N=6. *p < 0.05 by Mann-Whitney test. (D) BLI curves of bone metastasis development by PD2D cells with control IgG or anti-α4 (clone PS/2) treatment started from Day 0 until Day 21. Data represent mean ± SEM. N=10. **p < 0.01 by Mann-Whitney test. (E) Representative BLI of mice treated with IgG or anti-α4. (F) X-radiography of PD2D-injected mice treated with anti-VCAM-1 (A) or anti-α4 (D) at the end of experiments. Arrows point to osteolytic lesions. See also Figure S3.
VCAM-1 is a ligand for integrin α4β1 and only binds weakly to integrin α4β7 (Springer, 1994). Because neither SCP6 nor PD cells express α4 (Figure S3D), in vivo VCAM-1-integrin interaction, if existent, is expected to be between tumor and murine host cells. Since α4 is the common integrin subunit, we chose to use a previously developed neutralizing monoclonal antibody against murine α4, clone PS/2 (Miyake et al., 1991). As expected, mice injected with PD2D and treated with anti-α4 showed less bone metastasis formation and better preserved bone structure (Figure 4D–E).
VCAM-1 promoting osteoclast activation in bone metastasis
As highly bone-metastatic PD cells do not possess a significantly higher proliferation rate than the dormant SCP6 and weakly metastatic PD2R cells in vitro (Figure S4A), we investigated the potential mechanism for VCAM-1 in conferring the growth advantage of PD cells in the bone microenvironment. The “vicious cycle” in bone metastasis, mediated by the activation of bone-degrading osteoclasts, has been well known to play a central role in determining the osteolytic nature and aggressiveness of breast cancer bone metastasis (Weilbaecher et al., 2011). We hypothesized that VCAM-1 promotes bone metastasis through activating osteoclasts, thereby instigating the establishment of the vicious cycle. First, we examined osteoclast activity in IgG or antibody-treated bone metastases (adjuvant therapy protocol) by staining the osteoclast marker tartrate resistant acid phosphatase (TRAP). TRAP+ osteoclasts were abundantly enriched in the bone metastases formed by PD2D in the IgG treated mice (Figure 5A). Other part of the bone did not have elevated number of TRAP+ osteoclasts, suggesting a local osteoclast-inducing milieu by PD2D cells (Figure S4B). Dramatically weaker osteoclast activity was observed in metastases treated with anti-VCAM-1 or anti-α4 (Figure 5A and S5B). Metastases formed by PD2R with ectopic VCAM-1 overexpression also contained numerous TRAP+ osteoclasts (Figure S4C).
Figure 5. VCAM-1 promotes osteoclast activation by direct interaction with pre-osteoclasts.
(A) H&E and TRAP staining of hindlimb long bones showing tumor (T, demarcated by dotted line), bone (B) and TRAP+ (red) osteoclasts. Mice were injected with PD2D and samples were obtained from Figure 4F. Scale bar 100μm. (B) In vitro osteoclastogenesis of murine bone marrow co-cultured with different tumor cell monolayers. Mature osteoclasts were quantified as multinucleated TRAP+ (red) cells. Data represent mean ± SD. ***p < 0.001, #p > 0.5 by Student’s t-test and Mann-Whitney test. Scale bar 250μm. (C) RT-PCR showing α4β1 expression in RAW264.7 before as well as after induced differentiation, but not in osteoblast cell lines (MC3T3-E1 and 7F2). Murine bone marrow was used as positive control. (D) Surface expression of α4β1 by RAW264.7 revealed by FACS staining. Scale bar 25μm. (E) RANKL-induced RAW264.7 differentiation on pre-coated plates. Arrows indicate early formation of multinucleated cells. TRAP staining on Day 5 showed morphologically larger osteoclasts in VCAM-1-coated plate. Scale bar 100μm. (F) Expression pattern of known osteoclast differentiation markers in RAW264.7 cultured with or without VCAM-1-coating in the present or absence of RANKL induction. The markers, quantified by qRT-PCR, include known upregulated (Up) and downregulated (Down) genes during osteoclast differentiation. See also Figure S4.
To directly test if VCAM-1 expressed on tumor cells could influence osteoclast differentiation, we co-cultured SCP6 or VCAM-1+ PD cells with primary murine bone marrow cells under conditions inductive of osteoclast differentiation. Significantly more TRAP+ multinucleated osteoclasts formed when co-cultured with PD1 or PD2D as compared to SCP6 (Figure 5B). VCAM-1 KD diminished the ability of PD2D to enhance osteoclastogenesis (Figure 5B). Bone marrow contains a heterogeneous population of cells. VCAM-1 may interact directly with osteoclast progenitors to influence their differentiation, or may instead act through an intermediate cell type, such as osteoblasts (Weilbaecher et al., 2011). To distinguish these two possibilities, we analyzed the expression pattern of α4β1 in two murine osteoblast cell lines (MC3T3-E1 clone 4 and 7F2) and one murine pre-osteoclast cell line (RAW264.7) by RT-PCR (Figure 5C) and flow cytometry (Figure 5D). While β1 was ubiquitously expressed, α4 was only expressed by RAW264.7 (before and after differentiation). Therefore, tumor cells and pre-osteoclasts, given physical proximity, could interact directly via VCAM-1-α4β1 binding. This result allowed us to simplify the analysis of VCAM-1 function in osteoclastogenesis by pre-coating culture plates with recombinant VCAM-1 before seeding RAW264.7. VCAM-1 coating led to earlier multinucleation and eventually larger cell size with more nuclei in mature TRAP+ osteoclasts (Figure 5E).
One obvious explanation for the enhanced multinucleation would be that VCAM-1 induced differentiation. However, when we examined the expression of 19 upregulated and 3 downregulated genes known to be tightly and functionally correlated with osteoclast differentiation (Yang et al., 2008), VCAM-1 coating and BSA coating showed similar pattern during osteoclast differentiation (Figure 5F). In addition, VCAM-1 coating did not change the level of phospho-Src, phospho-ERK or phospho-Akt in RAW264.7 (Figure S4D). Therefore, the enhanced osteoclast multinucleation may simply be the result of more attachment of pre-osteoclasts to a VCAM-1-coated surface and more cell fusion favored by closer cell juxtaposition. Translating this hypothesis in vivo, soluble VCAM-1 (sVCAM-1) and membrane-bound VCAM-1 on tumor cells may recruit and arrest more osteoclast progenitors to provide an osteoclastic niche.
VCAM-1-mediated attraction of osteoclast progenitors
sVCAM-1 produced by PD cells was readily detectable in conditioned medium as well as in serum from tumor-bearing mice (Figure S4E), suggesting a potential role of VCAM-1 in recruiting circulating osteoclast precursors to promote osteoclastogenesis. We tested this hypothesis by first showing that recombinant VCAM-1 is a chemoattractant for pre-osteoclasts by a standard checkerboard analysis (Figure S5A). VCAM-1-α4β1 binding was essential for chemoattraction (Figure 6A) and sVCAM-1 secreted by tumor cells was able to attract pre-osteoclasts (Figure 6B). Secondly, we demonstrated significantly higher adhesion rate of RAW264.7 cells to VCAM-1+ PD2D cells than SCP6 or PD2D-KD1 (Figure S5B-C). Scanning electron microscopy revealed tight attachment of RAW264.7 cells to PD2D, and loose juxtaposition to SCP6 or PD2D-KD1 (Figure 6C). Next, we attempted to analyze in vivo metastases for evidence of osteoclast progenitor attraction. A prerequisite for host cells to interact with VCAM-1+ tumor cells is α4β1 expression. Murine bone marrow analysis revealed α4+β1+ and α4−β1+ subpopulations in the CD11b+ monocytes (Figure 6D). CD11b+ monocytes are known to contain osteoclast progenitors (Ishii et al., 2009). Intriguingly, the α4+β1+ subpopulation differentiated into significantly more multinucleated TRAP+ osteoclasts upon macrophage-colony stimulating factor (M-CSF) and receptor activator for nuclear factor κ B Ligand (RANKL) treatment (Figure 6D). Therefore, VCAM-1+ PD cells may attract the α4+β1+ osteoclast progenitors in vivo to promote bone metastasis. Indeed, we observed the enrichment of osteoclast progenitors, in particular the α4+β1+ subpopulation, in bone metastases formed by VCAM-1+ PD cells (Figure 6E–F). Conversely, mice with PD2D bone metastases contained lower percentage of CD11b+ cells in the peripheral blood (Figure 6G).
Figure 6. VCAM-1-mediated attraction of osteoclast progenitors.
(A) Chemotaxis of RAW264.7 to sVCAM-1 blocked by anti-VCAM-1 or anti-α4 antibodies. (B) Chemotaxis of RAW264.7 to tumor conditioned medium. In (A) and (B), data represent mean ± SD. ***p < 0.001 by Student’s t-test. (C) Scanning EM showing adhesion of RAW264.7 to PD2D but not to SCP6 or VCAM-1-silenced PD2D. (D) FACS of murine bone marrow showing α4+β1+ (P3) and α4− β1+ (P2) cells in CD45+CD11b+ monocytes (P1). Sorted P3, but not P2, differentiated into TRAP+ osteoclasts under induction. Scale bar 500μm. (E) FACS quantification of CD11b+ monocytes in CD45+ bone marrow leukocytes of mice injected with PD2D with or without bone metastases formed, as well as of age-matched mice without injection (control). (F) FACS quantification of α4+β1+ and α4− β1+ subpulations of CD11b+ monocytes in bone marrow of mice injected with PD2D with or without bone metastases formed. (G) FACS quantification of CD11b+ monocyte population in CD45+ peripheral blood leukocytes of mice injected with PD2D with or without bone metastases formed, as well as of age-matched mice with established PD2D primary tumors. In (E), (F) and (G), percentage was reported after excluding tumor cells (positive for human HLA-ABC), if they exist. Data represent mean ± SD. *p < 0.05, ***p < 0.001 by Student’s t-test. (H) Experimental flow of EviBoM. Green dots represent monocytes expressing CX3CR1-EGFP knock-in gene. (I) Representative EviBoM image frames and tracked movement of CX3CR1-EGFP+ monocytes with IgG or anti-VCAM-1 ex vivo treatment. Scale bar 30μm. (J) Comparison of median tracking velocity of CX3CR1-EGFP+ monocytes in (I). Black bar represents the median of all data. Student’s t-test. See also Figure S5, Movie S1 and Movie S2.
If bone metastases attract and immobilize osteoclast progenitors through VCAM-1 activity, transient blocking of VCAM-1 by neutralizing antibodies should release the attached cells from the tumor. However, this hypothesis is difficult to test with the current methodologies since FACS analysis of bone marrow and blood composition may be complicated by VCAM-1-dependent physiological homing of hematopoietic cells (Papayannopoulou et al., 2001). Furthermore, it is difficult to provide information on dynamic cell movement using standard histology. To overcome these technical challenges, we developed a real-time imaging technique, ex vivo imaging bone metastasis (EviBoM), by incorporating BLI with multicolor multiphoton laser-scanning microscopy (MPLSM). To simultaneously detect tumor cells and stromal cells, we stably expressed mKeima (Kogure et al., 2006) to label tumor cells and generated nude mice with EGFP-labeled monocytes by crossing CX3CR1-EGFP knock-in mice (Jung et al., 2000) with nude mice. mKeima showed strong red fluorescence intensity and superior spectral overlap with EGFP at wavelength from 900 to 1000nm, allowing simultaneous detection of mKeima+ tumor cells and EGFP+ monocytes (Figure S5D). After the formation of osteolytic bone metastasis detected by whole mouse BLI, we dissected the long bone, identified the location of metastasis by ex vivo BLI, and embedded the BLI-positive bone section in agarose. We adapted a previously described method for imaging GFP+ hematopoietic stem cells ex vivo in the mouse long bone (Xie et al., 2009) and analyzed the dynamics of tumor-monocyte interaction in bone metastasis (Figure 6H). Using EviBoM, we found that movement velocity of CX3CR1-EGFP+ monocytes in the area of PD2D bone metastases was significantly increased after anti-VCAM-1 antibody treatment (Figure 6I-J, Movie S1 and S2), suggesting the release of VCAM-1 mediated cell-cell adhesion.
VCAM-1 overexpression is dependent on NFκB signaling
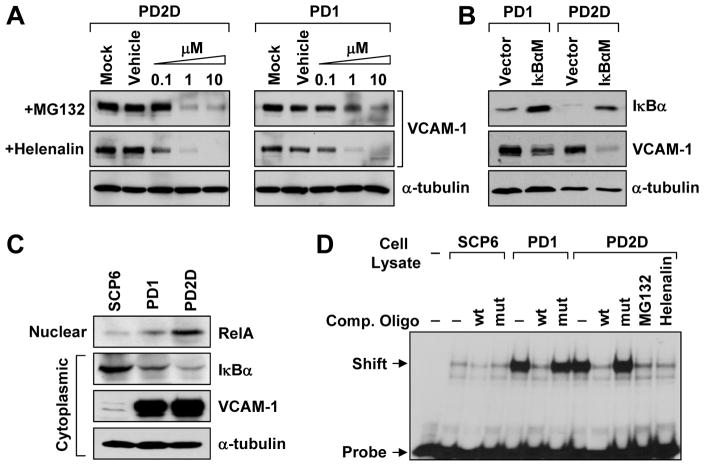
We tested several possible mechanisms for the activation of VCAM-1 expression. We first ruled out gene copy number change based on array CGH analysis at the VCAM-1 locus (Figure S1B) and quantitative PCR (data not shown). Next, we hypothesized that the signaling pathway(s) essential for driving VCAM-1 upregulation is constitutively active in VCAM-1+ PD cells. Previous studies on VCAM-1 regulation identified multiple regulatory pathways or factors, including nuclear factor-κB (NFκB), p300/CBP, PKC, MEK1/2, PLC, mTOR, PI3K, EGFR and miR-126 (Iademarco et al., 1992; Lee et al., 2008; Minami and Aird, 2001). To identify the pathway that might be involved in VCAM-1 upregulation, we used pharmacological inhibitors to inactivate each of these pathways in PD1 and PD2D and looked for consistent decrease of VCAM-1 expression (Figure S6A). Two pathways known to be important for bone metastasis, Src (Zhang et al., 2009) and TGF-β (Kang et al., 2005), were also analyzed pharmacologically (Figure S6B). Among these inhibitors that we tested, only two inhibitors of NFκB pathway, MG132 (Fiedler et al., 1998) and helenalin (Lyβ et al., 1998), blocked VCAM-1 expression at 0.1–1μM concentration (Figure 7A, Figure S6A). To further confirm the role of NFκB signaling in sustaining VCAM-1 expression, we genetically blocked the canonical NFκB signaling with enforced expression of IκBαM, a dominant-negative form of NFκB inhibitor α (IκBα) (Brown et al., 1995). IκBαM effectively reduced VCAM-1 level (Figure 7B). Consistently, PD cells contained less IκBα in the cytoplasm and more RelA in the nucleus compared with SCP6 (Figure 7C). Direct evidence of increased NFκB activity in PD cells was shown by electrophoretic mobility shift assay (EMSA) (Figure 7D). To test if ectopic stimulation of NFκB activity can drive VCAM-1 expression in SCP6, we treated SCP6 with tumor necrosis factor-α (TNF-α), which is a known activator of NFκB signaling, and observed significant increase of VCAM-1 mRNA level, yet the level was still dramatically lower than that in PD cells (Figure S6C-D). This result suggests other mechanisms may function cooperatively with NFκB or independently to activate VCAM-1 expression.
Figure 7. VCAM-1 upregulation is dependent on NFκB activity.
(A) Dose-dependent inhibition of VCAM-1 expression by NFκB pathway inhibitors MG132 and helenalin as detected by western blot. (B) Inhibition of VCAM-1 expression by ectopic IκBαM expression as detected by western blot. (C) Differential abundance of nuclear RelA and cytoplasmic IκBα in SCP6 and VCAM-1+ PD cells as detected by western blot. (D) EMSA showing stronger band shift in PD1 and PD2D compared with SCP6. Wild type competitive probe (wt) and inhibitors MG132 and helenalin, but not mutated competitive probe (mut), blocked radioactive NFκB-specific probe binding. See also Figure S6.
VCAM-1 expression associated with clinical early recurrence
To evaluate the clinical importance of VCAM-1 in breast cancer, especially in the context of recurrence, we analyzed a breast cancer microarray dataset containing metastasis relapse information (Wang et al., 2005). The proportional relapse rate plot showed the typical biphasic distribution of breast cancer relapses with 30-month as the peak (Figure 8A) (Stearns et al., 2007). When VCAM-1 levels were compared between early and late recurrence groups using 30-month as a cutoff to stratify patients, higher VCAM-1 expression was significantly associated with early relapse (Figure 8B). To corroborate conclusion from in silico analysis, we stained VCAM-1 in a breast cancer tissue microarray (TMA) that contains 170 samples (Hu et al., 2009). Higher VCAM-1 expression in cancerous tissue was again correlated with early relapse (Figure 8C). These findings validated the clinical significance of VCAM-1 overexpression in early relapse of breast cancer.
Figure 8. VCAM-1 expression is associated with clinical early recurrence.
(A) Bimodal pattern of metastasis relapse in a breast cancer cohort (Wang et al., 2005). Dotted line separates early and late relapse at 30-month, the peak of the curve. (B) VCAM-1 is expressed at higher level in the early compared with the late metastasis relapse group. Data represent mean ± SEM and are tested by Student’s t-test. (C) VCAM-1 expression correlates with early recurrence in a breast cancer TMA. Also shown are representative breast carcinoma TMA samples stained for VCAM-1. (D) A schematic model for the function of VCAM-1 in the transition from dormant micrometastasis to macrometastasis in bone. Incidental activation of VCAM-1, a process possibly dependent on NFκB signaling and other unidentified mechanism(s) (1), in micrometastasis arrests α4β1-positive osteoclast progenitors through paracrine chemotaxis (2) and adhesion (3). This leads to a localized increase of the osteoclast progenitor population and increased mature osteoclast activity (4). Activated osteoclasts resorb the bone and instigate the formation of bone metastasis vicious cycle (5).
DISCUSSION
Significant progress has been made to elucidate the molecular mechanisms of the multi-step metastasis cascade. However, the mechanism underlying metastatic dormancy and relapse from the dormancy remains one of most challenging but also clinically relevant questions (Aguirre-Ghiso, 2007; Goss and Chambers, 2010). Our characterization of a bone metastasis mouse model has shed light on the molecular understanding of dormancy by showing that VCAM-1 is an essential protein that reactivates indolent micrometastasis in the bone microenvironment (Figure 8D).
A metastasis dormancy animal model
The most frequent sites of breast cancer metastasis include bone, lung, liver and brain. Metastasis relapse can occur in any of these organs, and several attempts have been made to model metastasis dormancy in these sites. Survival of tumor cells during bone metastasis latency was linked to Src activity (Zhang et al., 2009). Progression of lung micrometastasis to macrometastasis in the transgenic MMTV-PyMT model was shown to be triggered by angiogenesis (Gao et al., 2008). Solitary tumor cells in the liver were visualized after intravenous injection of a murine mammary carcinoma cell line (Naumov et al., 2002). However, these models did not investigate the spontaneous in vivo activation of indolent micrometastasis, which represents the last phase of transition from dormancy to overt metastasis.
Our serendipitous observation of rare macrometastatic outgrowths after a long-term latency in mice allowed us to isolate reactivated cancer cells and compare them with the indolent parental cells to identify gene expression variations that may have led to the escape from dormancy. The transition from indolent growth to overt metastasis, as observed in our model, occurs in patients with metastasis recurrence many years after mastectomy. However, it is currently not feasible to prospectively isolate a tumor cell predicted to escape dormancy from a heterogeneous pool of DTCs in patients. In fact, even in basic research, studies on in vivo metastasis dormancy are complicated by the heterogeneous growth properties of most cancer cell lines (Townson and Chambers, 2006): the behavior and characteristics of the minor dormant subpopulations are masked by the coexisting actively proliferating cells. These difficulties highlight the merit of our model. Because of the relatively homogeneous nature of the parental cell SCP6 (derived from a single clone) and the close relatedness of SCP6 with PD sublines, the genome-wide comparison of dormant and reactivated cells is particularly informative. Indeed, we identified VCAM-1 from this model as a key player in metastatic progression from dormancy.
A functional role for VCAM-1 in activating indolent micrometastasis
Our mechanistic investigation of the function of VCAM-1 in promoting the escape from dormancy revealed its connection with osteoclastogenesis. By creating a “molecular sink” to attract circulating monocytic precursors of osteoclasts through VCAM-1-α4κ1 interactions, VCAM-1 instigates a vicious cycle of bone destruction and tumor expansion. As such, VCAM-1 serves as an example of how shifting the physiological process of bone homeostasis can facilitate the expansion of nascent bone metastasis. However, we should mention that, in contrast to other previously identified bone metastasis factors such as Jagged1 (Sethi et al., 2011), VCAM-1 is not sufficient for osteoclast differentiation, as neither RAW264.7 cells nor murine bone marrow co-cultured with VCAM-1+ PD cells or plated on a VCAM-1-coated surface underwent differentiation without RANKL (data not shown). In addition, while it appears that, in this particular model system, VCAM-1 is likely to be a key factor for the recruitment of pre-osteoclasts to nascent bone micrometastasis, we do not rule out other chemoattractants playing contributing roles to this process. It is possible that such factors were upregulated in PD cells posttranscriptionally or posttranslationally, which could not be captured by our microarray analysis. We also want to point out a possible and unexplored regulatory checkpoint of VCAM-1 availability in our system, which is the proteolysis and shedding of sVCAM-1 from tumor cell membranes through the activity of metalloproteinases (Garton et al., 2003).
It is well established that α4β1 is important in bone marrow homing and retention of hematopoietic stem cells through its binding to VCAM-1 expressed in bone marrow endothelial and stromal cells (Papayannopoulou et al., 2001). It is reasonable to speculate that the interaction between VCAM-1 and α4β1 is also part of the program regulating monocyte retention in bone marrow. In support of this hypothesis, VCAM-1-deficient mice showed elevated blood mononuclear leukocytes, possibly due in part to the reduced retention of these cells in the bone marrow (Gurtner et al., 1995). Therefore, it is conceivable that breast cancer cells, by upregulating VCAM-1, could imitate the marrow endothelial and stromal cells to locally retain high density of monocytes as the source of osteoclast differentiation.
We noted that even with the inhibition of VCAM-1, PD2D cells could still expand more than 10-fold to form a micrometastasis with probably a few hundreds cells (Figure 4A and 5A). Our evidence suggests that VCAM-1 is crucial for the expansion of indolent bone micrometastasis but is dispensable for the survival and initial growth of solitary tumor cells, the immediate step of metastatic colonization after seeding. On the other hand, a recent report showed that binding of VCAM-1-overexpressing tumor cells to macrophages directly promotes signal flow through the PI3K-Akt pathway for the survival of breast cancer cells that infiltrate the lung, but is not required for the immediate survival of those that seed the bone marrow (Chen et al., 2011). Nevertheless, in tissue samples of overt metastasis lesions from breast cancer patients, VCAM-1 was found to be overexpressed in both lung and bone metastases (Chen et al., 2011). Taken together, these findings indicate that VCAM-1 plays important functions in promoting metastatic colonization in both bone and lung, although at different stages and through different mechanisms. Therefore, blocking VCAM-1 activity may represent an effective strategy to prevent the formation of overt metastasis in both bone and lung, two of the most frequent sites of distant relapse in breast cancer.
Inflammation, NFκB signaling and VCAM-1 regulation
Inflammation has long been hypothesized to be linked to cancer based on the pathological infiltration of leukocytes in neoplastic tissue as well as the association of chronic inflammation and infectious agents with cancer (Grivennikov et al., 2010). An inflammatory microenvironment, triggered by an infection, trauma or stress, has been proposed to waken dormant tumor cells (Fehm et al., 2008), although experimental evidence is lacking. The canonical NFκB pathway plays a central role in mediating the effect of inflammation on tumor progression (Grivennikov et al., 2010). Here, we found that VCAM-1 expression in PD cells is dependent on constitutive NFκB activity. Therefore, our study suggests a possible link between the NFκB pathway and recurrent bone metastasis, although direct evidence showing the causal role of NFκB activation in the metastatic progression from dormancy is lacking and warrants further experimental validation. Supporting the functional significance of the NFκB pathway in bone metastasis, NFκB has been shown to stimulate granulocyte macrophage-colony stimulating factor (GM-CSF) expression in tumor cells to promote osteoclastogenesis and bone metastasis (Park et al., 2007). Other factors regulated by NFκB may also contribute to metastasis progression, such as CXCL1 and CSF-1 which are also expressed by PD cells, although not at a significantly higher level when compared with SCP6 (data not shown). The other two well-known NFκB targets, CCL2 and CCL5, are expressed at negligible levels in PD cells.
Single-cell imaging of tumor-stromal interactions in bone metastasis
A significant technical contribution of our study is the development of EviBoM (Figure 6H) imaging technique for bone metastasis research. Optical intravital imaging has attracted increasing interest in the field of metastasis research, because it offers high spatial and temporal resolution in vivo and enables direct observation of metastasis at the single cell resolution (Sahai, 2007). Intravital imaging has lent strength to tumor dormancy research in studies characterizing tumor cell fates at secondary organs (Kienast et al., 2009; Naumov et al., 2002). Mouse skull was previously used as the site to study leukemia bone metastasis with in vivo confocal imaging (Sipkins et al., 2005). However, metastases in long bones (in particular, femur and tibia of the legs) are much more commonly associated with clinical and experimental bone metastasis. We developed EviBoM to provide real-time observation of cell movement in long bone metastases, although it is still in an ex vivo setting. A limitation of the current method is that osteoclast progenitors only represent a fraction of the GFP+ cells in the CX3CR1-EGFP mice. More lineage-specific labeling will improve the application of EviBoM and facilitate mechanistic investigation and preclinical drug development for bone metastasis.
EXPERIMENTAL PROCEDURES
Tumor xenografts and analysis
All procedures involving mice were approved by Institutional Animal Care and Use Committee (IACUC) of Princeton University. Intracardiac injections to generate bone metastases was performed in 4-week-old, female nude mice (NCI) as described (Lu and Kang, 2009) with exception for TM40D-BM which was injected into BALB/c mice (NCI). Development of metastasis in bone was monitored by BLI with the IVIS 200 Imaging System (Caliper Life Sciences) and analyzed with the Living Image software as described (Lu and Kang, 2009). X-ray radiography was performed as described (Kang et al., 2003).
Clinical Specimens
A breast cancer tissue microarray (TMA) composed of 170 primary tumors (Hu et al., 2009) was used in our association study. Breast tumor specimens used in the TMA were obtained from the Cancer Institute of New Jersey with informed consent from all subjects in accordance with the institutional review boards of Princeton University and the University of Medicine and Dentistry of New Jersey, and the samples were subsequently deidentified prior to analysis. More detailed information about immunohistochemical analysis of VCAM1-1 expression in TMA can be found in the Supplemental Information.
Ex vivo imaging of bone metastasis (EviBoM)
We stably labeled PD2D with retroviral vectors expressing firefly luciferase (cloned into pMSCVhygro) and mKeima (cloned into pMSCVpuro), and intracardiacally injected tumor cells into CX3CR1-EGFP knock-in mice (Jackson Laboratory) that had been crossed to harbor homozygous nu alleles. After detection of significant bone metastasis formation by BLI, we located the position of bone metastasis via ex vivo BLI, sectioned the bone with QwikStrip Serrated strip (Axis Dental), and embedded them into a thin layer of 1.5% low melting temperature agarose gel that is submerged in phenol red-free DMEM (Thermo Scientific) containing 1x Penicillin-Streptomycin and 10mM HEPES (balancing pH). The bone sections were imaged under a customized upright multiphoton microscope built around a BX51 microscope (Olympus). More detailed methods can be found in the Supplemental Information.
Statistical analysis
Results were reported as mean ± SD (standard deviation) or mean ± SEM (standard error of the mean), indicated in the figure legend. Comparisons between Kaplan-Meier curves were performed using the log-rank test. Other comparisons were performed using unpaired two-sided Student’s t-test without equal variance assumption or nonparametric Mann-Whitney test.
Supplementary Material
HIGHLIGHTS.
VCAM-1 expression is associated with early relapse and activation from dormancy.
VCAM-1 overexpression promotes bone metastasis by activating osteoclastogenesis.
VCAM-1-α 4β1 binding facilitates adhesion of pre-osteoclasts to tumor cells.
VCAM-1 and α4 blocking antibodies reduce the progression of bone metastasis.
SIGNIFICANCE.
The transition from dormant micrometastasis to overt macrometastasis represents a crucial turning point in breast cancer. Significant advances have been made to characterize the complex tumor-microenvironment interactions in overt bone metastasis. However, the molecular and cellular events that drive the early events of bone metastasis — the activation of indolent micrometastasis — remain poorly understood. Our study reveals a mechanism for tumor cells to escape dormancy whereby monocytic osteoclast progenitors are recruited through VCAM-1-α4β1 binding to produce a localized increase of osteoclast activity and initiate the vicious cycle of bone destruction and tumor growth. VCAM-1- or α4- blocking antibodies effectively inhibit bone metastasis, suggesting that VCAM-1 is a promising therapeutic target to restrict the aggressive progression of micrometastasis in bone.
Acknowledgments
We thank S. Thiberge for two-photon microscopy analysis; C. DeCoste and M. Bisher for assistance with flow cytometry and electron microscopy, D. Medina and M. Zhang for TM40D cell lines, L. Cong, J. Friedman and L. Goodell at Tissue Analytic Service Core of Cancer Institute of New Jersey for assistance with TMA analysis and M. Reiss for helpful discussions. The Imaging Core Facility at the Lewis-Sigler Institute is supported by the Center Grant P50GM071508. Y.K. is a Champalimaud Investigator and a Department of Defense Era of Hope Scholar Award recipient. This research was additionally supported by grants from the Breast Cancer Alliance, Susan G. Komen for the Cure, American Cancer Society, and the Brewster Foundation. J.M. is an Investigator of the Howard Hughes Medical Institute and is funded by grants from the National Institutes of health, the Kleberg Foundation, the Hearst Foundation, and the BBVA Foundation. X.L. is a recipient of the Harold W. Dodds Fellowship from Princeton University. Y.H. and B. T. are recipients of the DOD predoctoral fellowships.
Footnotes
Supplemental Information including Supplemental Experimental Procedures, six Supplemental Figures, one Supplemental Table and two Supplemental Movies can be found with this article online.
ACCESSION NUMBERS
The raw and normalized microarray data have been deposited in the Gene Expression Ominbus (GEO) database under accession number GSE20611.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, et al. A Pooled Analysis of Bone Marrow Micrometastasis in Breast Cancer. The New England Journal of Medicine. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IkappaB-alpha Proteolysis by Site-Specific, Signal-Induced Phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Carter RA, Wicks IP. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum. 2001;44:985–994. doi: 10.1002/1529-0131(200105)44:5<985::AID-ANR176>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chambers AF. MDA-MB-435 and M14 Cell Lines: Identical but not M14 Melanoma? Cancer Research. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang XHF, Massagué J. Macrophage Binding to Receptor VCAM-1Transmits Survival Signals in Breast Cancer Cells that Invade the Lungs. Cancer Cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. The Journal of Clinical Investigation. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Seminars in Cancer Biology. 2001;11:297–306. doi: 10.1006/scbi.2001.0385. [DOI] [PubMed] [Google Scholar]
- Demicheli R, Abbattista A, Miceli R, Valagussa P, Bonadonna G. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res Treat. 1996;41:177–185. doi: 10.1007/BF01807163. [DOI] [PubMed] [Google Scholar]
- Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L. Association of VCAM-1 overexpression with oncogenesis, tumor angiogenesis and metastasis of gastric carcinoma. World J Gastroenterol. 2003;9:1409–1414. doi: 10.3748/wjg.v9.i7.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittel BN, McCarthy JB, Wayner EA, LeBien TW. Regulation of human B-cell precursor adhesion to bone marrow stromal cells by cytokines that exert opposing effects on the expression of vascular cell adhesion molecule-1 (VCAM-1) Blood. 1993;81:2272–2282. [PubMed] [Google Scholar]
- Fehm T, Mueller V, Marches R, Klein G, Gueckel B, Neubauer H, Solomayer E, Becker S. Tumor cell dormancy: implications for the biology and treatment of breast cancer. Apmis. 2008;116:742–753. doi: 10.1111/j.1600-0463.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of TNF-alpha -induced NF-kappa B Activation and IL-8 Release in A549 Cells with the Proteasome Inhibitor MG-132. American Journal of Respiratory Cell and Molecular Biology. 1998;19:259–268. doi: 10.1165/ajrcmb.19.2.3149. [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial Progenitor Cells Control the Angiogenic Switch in Mouse Lung Metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17) J Biol Chem. 2003;278:37459–37464. doi: 10.1074/jbc.M305877200. [DOI] [PubMed] [Google Scholar]
- Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Davis V, Li H, McCoy MJ, Sharpe A, Cybulsky MI. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes & Development. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, Reiss M, Au JLS, Haffty BG, Kang Y. MTDH Activation by 8q22 Genomic Gain Promotes Chemoresistance and Metastasis of Poor-Prognosis Breast Cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) 1992. pp. 16323–16329. [PubMed] [Google Scholar]
- Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al. Expression of interleukin-1beta in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(sici)1097-0142(19970801)80:3<421::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of Fractalkine Receptor CX3CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Molecular and Cellular Biology. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massagué J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2009;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- Kogure T, Karasawa S, Araki T, Saito K, Kinjo M, Miyawaki A. A fluorescent variant of a protein from the stony coral Montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat Biotech. 2006;24:577–581. doi: 10.1038/nbt1207. [DOI] [PubMed] [Google Scholar]
- Lee CW, Lin CC, Luo SF, Lee HC, Lee IT, Aird WC, Hwang TL, Yang CM. Tumor necrosis factor-alpha enhances neutrophil adhesiveness: induction of vascular cell adhesion molecule-1 via activation of Akt and CaM kinase II and modifications of histone acetyltransferase and histone deacetylase 4 in human tracheal smooth muscle cells. Mol Pharmacol. 2008;73:1454–1464. doi: 10.1124/mol.107.038091. [DOI] [PubMed] [Google Scholar]
- Li Z, Schem C, Shi Y, Medina D, Zhang M. Increased COX2 expression enhances tumor-induced osteoclastic lesions in breast cancer bone metastasis. Clinical and Experimental Metastasis. 2008;25:389–400. doi: 10.1007/s10585-007-9117-3. [DOI] [PubMed] [Google Scholar]
- Lin KY, Lu D, Hung CF, Peng S, Huang L, Jie C, Murillo F, Rowley J, Tsai YC, He L, et al. Ectopic Expression of Vascular Cell Adhesion Molecule-1 as a New Mechanism for Tumor Immune Evasion. Cancer Research. 2007;67:1832–1841. doi: 10.1158/0008-5472.CAN-06-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Kang Y. Efficient acquisition of dual metastasis organotropism to bone and lung through stable spontaneous fusion between MDA-MB-231 variants. P Natl Acad Sci USA. 2009;106:9385–9390. doi: 10.1073/pnas.0900108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, Massagué J, Kang Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23:1882–1894. doi: 10.1101/gad.1824809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin C, De Smet C, Brasseur F, Muscatelli F, Martelange V, De Plaen E, Brasseur R, Monaco AP, Boon T. Two Members of the HumanMAGEBGene Family Located in Xp21.3 Are Expressed in Tumors of Various Histological Origins. Genomics. 1997;46:397–408. doi: 10.1006/geno.1997.5052. [DOI] [PubMed] [Google Scholar]
- Lyβ G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The Anti-inflammatory Sesquiterpene Lactone Helenalin Inhibits the Transcription Factor NF-κB by Directly Targeting p65. Journal of Biological Chemistry. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- Minami T, Aird WC. Thrombin Stimulation of the Vascular Cell Adhesion Molecule-1 Promoter in Endothelial Cells Is Mediated by Tandem Nuclear Factor-kB and GATA Motifs. Journal of Biological Chemistry. 2001;276:47632–47641. doi: 10.1074/jbc.M108363200. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC, Chambers AF. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of {alpha}4{beta}1 over {beta}2-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al. NF-[kappa]B in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stearns AT, Hole D, George WD, Kingsmore DB. Comparison of breast cancer mortality rates with those of ovarian and colorectal carcinoma. Br J Surg. 2007;94:957–965. doi: 10.1002/bjs.5667. [DOI] [PubMed] [Google Scholar]
- Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5:1744–1750. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- Wang Y, Klijn JGM, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. The Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA. The many faces of tumor dormancy. Apmis. 2008;116:548–551. doi: 10.1111/j.1600-0463.2008.001168.x. [DOI] [PubMed] [Google Scholar]
- Wojtukiewicz MZ, Sierko E, Zimnoch L, Kozlowski L, Kisiel W. Immunohistochemical localization of tissue factor pathway inhibitor-2 in human tumor tissue. Thromb Haemost. 2003;90:140–146. [PubMed] [Google Scholar]
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- Yang G, Zaidi M, Zhang W, Zhu LL, Li J, Iqbal J, Varbanov A, Gross G, Phipps R, Troen BR, Sun L. Functional grouping of osteoclast genes revealed through microarray analysis. Biochemical and Biophysical Research Communications. 2008;366:352–359. doi: 10.1016/j.bbrc.2007.11.106. [DOI] [PubMed] [Google Scholar]
- Zhang XHF, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massagué J. Latent Bone Metastasis in Breast Cancer Tied to Src-Dependent Survival Signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.